Abstract
Background
The current studies explore the effect of omega-3 polyunsaturated fatty acids (PUFAs) on appetite.
Objective
To examine the effect of omega-3 polyunsaturated fatty acids (n-3 PUFAs) on appetite using a systematic review and meta-analysis of controlled clinical trials (CTs).
Patients and methods
Online databases including PubMed, Scopus, ISI Web of Science, and Google Scholar were searched up to January 2022. A random-effects model was used to compare the overall standardized mean difference in appetite scores between n-3 PUFAs supplemented and control individuals.
Results
Fifteen eligible CTs with 1504 participants (872 for n-3 PUFA supplementation and 632 for placebo groups) were included in our systematic review. The meta-analysis showed no significant difference in overall appetite score between n-3 PUFAs supplemented and control groups (standardized mean difference [SMD] = 0.458, 95% confidence interval [CI] − 0.327, 1.242, P value = 0.25). However, the n-3 PUFA supplementation significantly increased the desire to eat (SMD = 1.07, 95% CI 0.116, 2.029, P = 0.02) compared to control.
Conclusion
Although we found no effect of omega-3 supplementation on overall appetite score, it modestly increases the desire to eat. Further CTs evaluating the effect of PUFAs on appetite are still needed to confirm these findings.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13643-023-02430-y.
Keywords: n-3 polyunsaturated fatty acids, Appetite, Visual analog scale, Systematic review, Meta-analysis
Introduction
Dietary recommendations have emphasized the importance of fatty acid type rather than total dietary fat intake [1]. Many studies have explored the association between fatty acids’ chain length, degree of saturation, and position of the double bond of fatty acids consumed with cardiovascular diseases, inflammation, cancer, weight gain, and obesity [2–5]. Studies have also shown that saturated fatty acids (SFAs) are harmful to health, while beneficial health has been offered for monounsaturated (MUFAs) and polyunsaturated fatty acids (PUFAs) [6]. Omega-3 PUFAs’ sources include plant-based products (e.g., nuts, plant seeds, and their oils), seafood, or marine. High attention has been paid to the potential effect of different types of fatty acids on energy balance, weight, and appetite [7, 8].
Appetite is one of the important factors in controlling body weight which is regulated through both physiological and psychological factors [9]. Dietary fat composition could be changed through changes in the type of fatty acid intake which could affect the appetite [10]. Studies have examined appetite responses to meals enriched in different types of fatty acids and suggested that these different effects are via the physiochemical properties of fatty acids [11, 12]. However, there is little consensus on the relative role each may play in controlling food intake. A meta-analysis of controlled trials in patients with cancer cachexia showed n-3 PUFA supplementation did not improve body weight [13]. A study of 18 lean men showed no significant effect of fatty acid chain length on appetite [14]. Another study on 16 obese women reported that fatty acid composition did not differentially affect subjective appetite rating [15]. However, a study on 13 healthy Chinese men illustrated that PUFA-rich meals led to a decrease in appetite compared to MUFA-rich meals [5]. It is also proposed that n-3 PUFA supplementation might affect appetite control [16]. In particular, eicosapentaenoic (EPA) and docosahexaenoic acids (DHA) intake have been reported as appetite modulators [17]. The mechanisms by which n-3 PUFAs reduce appetite are not well understood. The effect of n-3 PUFAs on fat metabolism and plasma concentrations of the appetite-suppressing hormones might explain the effect [18–20]. Several clinical trials have been conducted to examine the effect of n-3 PUFA fatty acids on appetite [11, 21]. However, they have led to inconsistent results. For instance, a study done in Georgia University showed that a diet rich in PUFAs has a greater effect on appetite suppression than a diet rich in monounsaturated fat [22]. Also, consumption of a diet rich in PUFAs in fifteen healthy American men resulted in suppression of postprandial hunger [23]. However, a randomized cross-over study among sixteen healthy American females showed that a liquid meal rich in PUFAs made no significant difference in hunger, fullness, or desire to eat [10].
To address the current controversy on the effect of n-3 PUFA intake on appetite, we conducted a systematic review and meta-analysis of controlled clinical trials (CTs).
Methods
The present study is reported following Preferred Reporting Items for Systematic Reviews and Meta-analyses [24].
Search strategy
We conducted a systematic literature review search in PubMed/MEDLINE, Scopus, and ISI Web of Science (a list of WoS databases is in Supplementary Table 1) without language or any other restriction from the earliest available online indexing year to January 15, 2022. The search strategy included keywords and subject headings about n-3 polyunsaturated fatty acid (“Omega-3 Fatty Acid,” “Eicosapentaenoic Acid,” “EPA,” “DHA,” “docosahexaenoic acid,” “Omega-3,” “n-3,” “fish oil,”) and appetite (“Appetites,” “Appetite Alterations,” “satiety response,” “satiation,” “satiety,” “fullness,” “hunger,” “desire to eat,”). The full list of search terms used is in “Supplementary Table 1”. These searches were supplemented by reviewing the reference lists of trial publications.
Eligibility criteria
Two investigators screened the title and abstract which was followed by the full-text assessment of the eligible articles (BS and FT). All published CTs were included if they met the following inclusion criteria: (1) clinical trials that examined the effects of n-3 PUFAs intake on appetite, (2) the questionnaire for assessing appetite should be valid or clear, (3) n-3 PUFAs consumed as a supplement (not food), (4) individuals consumed n-3 PUFAs were not supplemented with other micro- and macronutrients, (5) the type of received n-3 PUFAs should be specified (EPA and DHA), (6) appetite was reported as a score or side effects, (7) appetite should be assessed by using a valid questionnaire, and (8) participants’ age should be ≥ 18 years. Nonhuman studies were excluded.
Screening process
Two independent authors (BS, FT) conducted the data extraction and evaluated the risk of bias. The possible discrepancies were solved by contacting the third author (ASA).
Data extraction
The following information was extracted: the first author’s last name, the year of publication, geographic location, study design, sample size and attrition, participant’s gender, age, health condition, duration of intervention, intervention dose, and types of n-3 PUFAs, inclusion criteria, and mean (and standard deviation) score of visual analog scale (VAS).
Risk of bias assessment
We assessed study quality using the Cochrane Collaboration’s tool [25] which takes random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selection outcome reporting into account. A judgment of “low risk of bias”, “high risk of bias”, or “unclear risk of bias” was made for each domain based on Cochrane collaboration’s handbook [26].
Statistical analysis
The standardized difference in mean changes ± standard error (SE) in VAS score between participants assigned to n-3 PUFA supplementation and participants assigned to the control group. A random-effects model was used for calculating weighted mean differences (WMDs) and 95% confidence intervals (CIs). Cochran’s Q test was administered to test the statistical heterogeneity between studies. Also, we calculated the ratio of between-study variation to total variation (I2 statistic, range of this estimating is from 0 to 100%). I2 > 50% and P value < 0.05 were considered to indicate a significant heterogeneity between trials. Subgroup analyses based on health status, dose of PUFA supplementation, and risk of bias were administered to detect the source of potential heterogeneity between studies. Sensitivity analyses were conducted to determine if the individual study altered the results of meta-analyses significantly. The possibility of publication bias was assessed by visual inspection of a funnel plot of treatment effects versus their corresponding SE. The asymmetry was also statistically checked by using Egger’s test. The analyses were performed using STATA version 14.1 (Stata Corp, College Station, TX). P values < 0.05 were considered statistically significant.
Results
Study selection
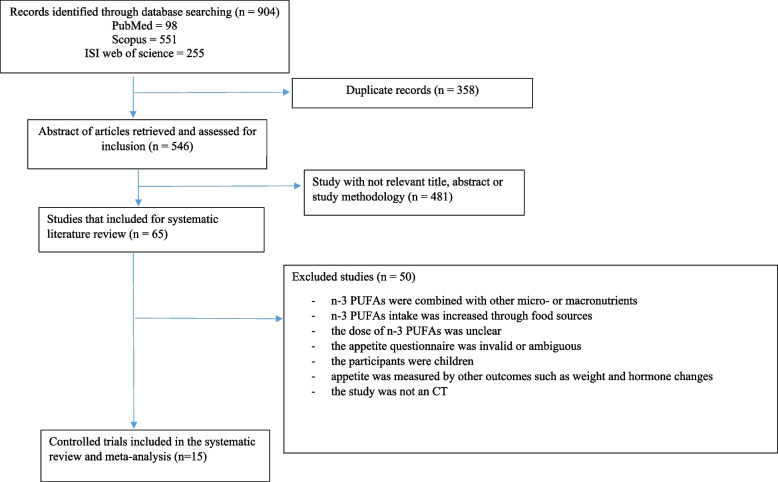
A total of 553 publications were retrieved after duplicates had been removed. After reading the titles and abstracts 481 studies were excluded. We excluded 50 studies for the following reasons: n-3 PUFAs were combined with other micro- or macronutrients (n = 12) [27–38], n-3 PUFA intake was increased through food sources (n = 10) [15, 23, 39–46], the dose of n-3 PUFAs was unclear (n = 2) [36, 47], the appetite questionnaire was invalid or ambiguous (n = 1) [48], the participants were children (n = 7) [49–55], appetite was measured by other outcomes such as weight and hormone changes (n = 16) [7, 37, 56–69], or the study was not a control trial (n = 2) [70, 71]. Finally, 15 studies were included in the systematic review and meta-analysis (Fig. 1) [47, 52, 64, 72–83].
Fig. 1.
Flow diagram of study screening
Study and participants’ characteristics
Characteristics of the 15 included trials are shown in Table 1. 872 participants for n-3 PUFA supplementation and 632 for placebo in our systematic review. The sample size varied from 20 to 421 with an age range from 18 to 90 years. All included CT studies were published between 2003 to 2021. Three studies were conducted in Iran [78, 81, 82], two in Canada [72, 73], two in the USA [64, 77], and two in Israel [47, 52], and the others were conducted in Turkey [76], Denmark [83], Sweden [75], China [74], Brazil [79], and Germany [80]. Of these trials, two studies were cross-over clinical trials and the rest were parallel. The majority of them included both genders, and only two studies were conducted on male adults. The duration of n-3 PUFA supplementation ranged from two to 15 weeks. The dose of n-3 PUFAs ranged from 225 to 4.5 g/d. Six studies reported changes in appetite with the VAS questionnaire [72, 76, 78, 81–83], three studies used another valid questionnaire [47, 73, 75], and the rest reported appetite as a side effect of n-3 PUFA supplementation [52, 64, 77, 79, 80].
Table 1.
Characteristics of studies included in the systematic review
| Source (first author, year of publication) | Country | Number, sex (F/M) | Age (year) | CT design | Duration | Health status | Intervention group | Control group | Reported appetite data |
|---|---|---|---|---|---|---|---|---|---|
| Moradi et al., 2021 [82] | Iran | 72 M | 20–30, Int & con:22.2 | Two arms Parallel | 3 wk | Young male athletes with normal body fat percentage | Two omega-3 soft gel capsules/day (2000 mg omega-3; EPA:360 mg, DHA:240 mg) | Two soft gel capsules/day (1 g of edible paraffin oil) | VAS score |
| Safaeiyan et al., 2018 [81] | Iran | 66 (22M/44F) | 18–45, Int:34.2 Con:33.5 | Two arms Parallel | 4 wk | BMI > 30 kg/m2 | 1000 mg Omega-3 capsules twice a day (180 mg EPA & 120 mg DHA) | Paraffin soft gels twice a day | VAS score |
| Mocelin et al., 2017 [79] | Brazil | 45 (25M/20F) | 18–70, Int:56 Con:51 | Two arms Parallel | 9 wk | Gastrointestinal cancer patients | Two capsules of fish oil/d (3.6 g), each capsule contained 1 g EPA + 0.5 g DHA/d | Two capsules of extra virgin olive oil/d | Adverse event |
| Payahoo et al., 2017 [78] | Iran | 60 (15M/45F) | 18–45, Int:31.9 Con:33.5 | Two arms Parallel | 4 wk | Obese (BMI = 30–40 kg/m2) | 1 g Omega-3 capsules twice a day (180 mg EPA & 120 mg DHA) | Placebo twice a day | VAS score |
| Werner et al., 2017 [80] | Germany | 33 (16M/17F) | > 21 years, Int:70.3 Con:71.3 | Two arms Parallel | 6 wk | Pancreatic cancer | 500 mg soft gel capsules 3 times/d, 60% fish oil & 40% MCT (6.9g/100g EPA + 13.6 g/100g DHA) [0.3 g n-3 fatty acids/d] | Marine phospholipids (MPL), 35% n-3 fatty acid phospholipids + 65% neutral lipids (8.5g/100g EPA + 12.3g/100g DHA) | Adverse event |
| Berg et al., 2014 [64] | USA | 267 (184M/83F) | 21–79, Int:44.2 Con:43.5 | Five arms Parallel | 12 wk | Adults with borderline high or high triglyceride levels | One, two, four or eight 500 mg Krill oil capsules/d (100, 200, 400 or 800 mg EPA + DHA) | Placebo (olive oil) | Adverse event |
| Damsbo-Svendsen, et al., 2013 [83] | Denmark | 20 (10M/10F) | 18–30, Int & con: 24 | Cross-over | 3 wk | Healthy students (> 18 y, normal weight) | 3.5 g n-3 PUFAs (1.9 g EPA & 1.1 g DHA) | 5.2 g soybean oil & 10 IU/g vitamin E | VAS score |
| Kanat et al., 2013 [76] | Turkey | 62 (48M/14F) | 22–84, Int:60.7 | Three arms Parallel | 12 wk | Cancer patients (aged ≥ 18 y) | Megestrol acetate (MA) + Meloxicam + EPA (2.2 g/d) | Megestrol acetate (MA) + Meloxicam | VAS score |
| Miller et al., 2013 [77] | USA | 29 (17M/12F) | > 21 years, 67.4 | Two-period cross-over | Two × 6 wk (2 wk washout) | Adults Diabetes patients with kidney injury | 4 Capsules/d, each 1 g capsules contained PUFAs (85% n-3 [DHA:EPA ratio of 2:1]) | Placebo (corn oil) | Adverse event |
| Vakhapova et al., 2011 [52] | Israel | 131 (66Int/65Con) | 50–90, Int:72.4 Con:72.7 | Two arms Parallel | 15 wk | Elderly | 3 capsules of phosphatidylserine DHA (PS-DHA; 300 mg PS & 79 mg DHA + EPA [DHA:EPA ratio of 3:1]) /d | Placebo (cellulose) | Adverse event |
| Irving et al., 2009 [75] | Sweden | 174 (84M/90F) | Int:73 Con:73 | Two arms Parallel | 24 wk | Patients with mild to moderate Alzheimer | Four 1-g capsules daily (430mg DHA & 150mg EPA) | Four 1-g corn oil (0.6g linoleic acid) | Neuropsychiatric Inventory (NPI) |
| Liu et al., 2007 [74] | Chinese | 22 (13M/9F) | 45–75, Int:56 Con:58 | Two arms Parallel with 1 wk for rest | 7 wk | Gastric cancer cachexia patients | 8 gelatin capsules of fish oil (EPA + DHA 315mg) twice a day | Atracylenolide 6 ml (Atracylenolide Ι 0.11gml−1) twice a day | VAS score |
| Yehuda et al., 2005 [47] | Israel | 126 M | - | Two arms Parallel | 3 wk | Undergraduate college students with anxiety | 225 mg α-linolenic acid & linoleic acid (in ratio of 1:4) for twice daily | placebo (mineral oil) | |
| Jatoi et al., 2004 [73] | Canada | 421 (294M/127F) | 18 > , Int:66 Con:66 | Three arms Parallel | 4 wk | Patients aged > 18 with incurable malignancies | An EPA supplement (1.09 g EPA & 0.46 DHA), two cans/d | Megestrol acetate (MA) | NCCTG questionnaire for appetite |
| Bruera et al., 2003 [72] | Canada | 60 (17M/43F) | Int:63.0 Con:64.6 | Two arms Parallel | 2 wk | Patients with advanced cancer | 18 gelatin capsules containing 1000 mg fish oil (180 mg EPA & 120 mg DHA) | 18 gelatin capsules containing 1000 mg of a placebo (olive oil) | VAS score |
F female, M male, Int intervention, Con control, wk week, CT control trial, PUFAs polyunsaturated fatty acids, DHA docosahexaenoic acid, EPA eicosapentaenoic acid, VAS visual analogue scales, BMI body mass index
Assessment of risk of bias
Of 15 studies, three were determined to have a low risk of bias [79, 82, 83], and the others were evaluated as having a high risk of bias (Table 2). All of the mentioned studies reported random sequence generation, incomplete outcome data, and selective outcome reporting as low risk of bias. However, a study done by Yehuda et al. did not report these domains. Four trials reported the method of allocation concealment [79, 80, 82, 83]. Therefore, the remaining studies were regarded as high or unclear risk of bias.
Table 2.
Study quality and risk of bias assessment of included studies according to the Cochrane Collaboration’s tool
| Fist author (year) | Random Sequence Generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting | Overall qualitya |
|---|---|---|---|---|---|---|---|
| Moradi (2021) [82] | L | L | L | L | L | L | L |
| Safaeiyan (2018) [81] | L | U | L | L | L | L | U |
| Mocellin (2017) [79] | L | L | L | L | L | L | L |
| Payahoo (2017) [78] | L | U | L | L | L | L | U |
| Werner (2017) [80] | L | L | L | U | L | L | U |
| Berge (2014) [64] | L | U | L | U | L | L | U |
| Damsbo-Svendsen (2013) [83] | L | L | L | L | L | L | L |
| Kanat (2013) [76] | U | U | U | U | L | L | U |
| Miller (2013) [77] | L | U | L | L | L | L | U |
| Vakhapova (2011) [52] | L | U | L | U | L | L | U |
| Irving (2009) [75] | L | U | U | U | L | L | U |
| Liu (2007) [74] | L | H | H | H | L | L | H |
| Yehuda (2005) [47] | H | H | H | H | H | H | H |
| Jatoi (2004) [73] | L | U | L | U | L | L | U |
| Bruera (2003) [72] | U | U | L | U | L | L | U |
U unclear risk of bias, L low risk of bias, H high risk of bias
aLow quality: all criteria met; unknown quality: one criterion not met (i.e., high risk of bias for one domain or one criteria unclear); Poor quality: two or more criteria listed as high or unclear risk of bias
Meta-analysis
Eight studies with a total of 636 participants reported data on the effect of n-3 PUFA intake on appetite [47, 72, 75, 76, 78, 81–83]. The meta-analysis showed no significant effect of n-3 PUFA intake and total appetite score (SMD = 0.458, 95% CI − 0.327, 1.242, P = 0.25). There was significant heterogeneity among these studies (Q statistic = 140.49, P = 0.0, I2 = 95.0%). The domains of VAS score including hunger, satiety, and desire to eat were reported in 4 studies. The n-3 PUFA supplementation modestly increased the desire to eat (SMD = 1.07, 95% CI 0.116, 2.029, P = 0.02), and the heterogeneity among these studies was significant (Q statistic = 32.21, P < 0.001, I2 = 91.0%). However, the changes in hunger (SMD = 1.007, 95% CI − 0.139, 2.153, P = 0.08) and satiety (SMD = 0.983, 95% CI − 0.597, 2.564, P = 0.22) were not significant. A significant heterogeneity was observed for both hunger (I2 = 93.7%) and satiety (I2 = 96.4%). Subgroup analysis was conducted based on the health status of the studies’ participants [47, 72, 75, 76, 81–84], the dose of n-3 PUFA intervention [72, 75, 76, 81–84] and studies’ risk of [47, 72, 75, 76, 81–84]. However, no significant effect on appetite was observed in any subgroup (Table 3).
Table 3.
Meta-analysis showing the effects of n-3 PUFA supplementation on appetite in overall analysis as well subgroup analysis (all analyses were conducted using a random effect model)
| Variables | No. of studies | Meta-analysis | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|
| WMD (95% CI) | P effect | Q statistic | P within group | I2 (%) | P between group | ||
| Total visual analog score | |||||||
| All studies | 8 | 0.458 (− 0.327, 1.242) | 0.253 | 140.49 | < 0.001 | 95 | |
| Health status | < 0.001 | ||||||
| Healthy | 2 | 1.522 (− 1.013, 4.057) | 0.23 | 31.18 | < 0.001 | 96.8 | |
| Obese | 2 | − 0.547 (− 2.11, 1.015) | 0.493 | 16.93 | < 0.001 | 94.1 | |
| Cancer | 2 | − 0.137 (− 0.522,0.248) | 0.486 | 0.29 | 0.589 | 0.0 | |
| Anxiety & Alzheimer | 2 | 1.021 (− 0.640, 2.681) | 0.228 | 38.18 | < 0.001 | 97.4 | |
| Dose of intervention | |||||||
| 1000–3000 mg/d | 5 | 0.33 (− 0.92, 1.58) | 0.60 | 91.47 | < 0.001 | 95.6 | < 0.001 |
| ≥ 4000 mg/d | 2 | 0.12 (− 0.13, 0.38) | 0.34 | 0.58 | 0.44 | 0.0 | |
| Risk of bias | < 0.001 | ||||||
| Low risk | 2 | 1.522 (− 1.013, 4.057) | 0.239 | 31.18 | < 0.001 | 96.8 | |
| Unknown | 5 | − 0.22 (− 0.75, 0.30) | 0.40 | 24.33 | < 0.001 | 83.6 | |
| High risk | 1 | 1.87 (1.42, 2.32) | < 0.001 | 51.36 | < 0.001 | 94.2 | |
| Hunger score | 4 | 1.007 (− 0.139, 2.153) | 0.08 | 47.31 | < 0.001 | 93.7 | |
| Satiety score | 4 | 0.983 (− 0.597, 2.564) | 0.22 | 83.68 | < 0.001 | 96.4 | |
| Desire to eat score | 4 | 1.073 (0.116, 2.029) | 0.02 | 33.21 | < 0.001 | 91.0 | |
WMD weighted mean difference
Sensitivity analysis and publication bias
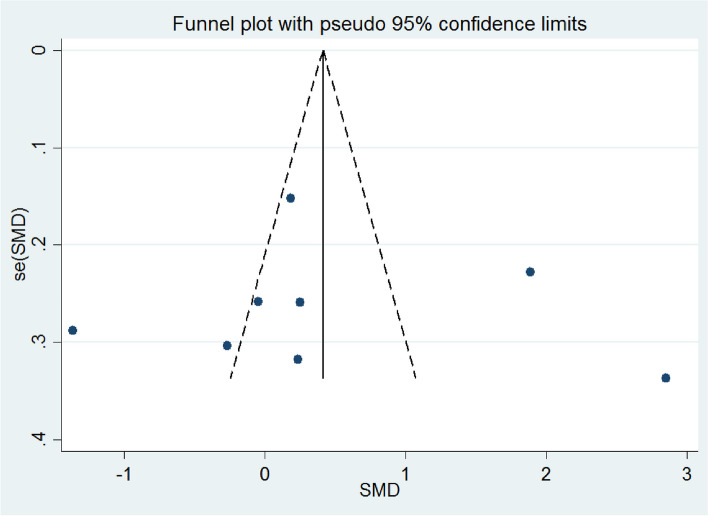
In the sensitivity analysis, none of the included studies significantly influenced the pooled effects. The final result of the sensitivity analysis was shown in Table 4. Visual inspection of the funnel plot (Fig. 2) and Egger’s test (slope = 0.067; CI − 3.82–3.95, intercept value = 0.442) showed no significant publication bias.
Table 4.
Result of sensitivity analysis
| Study omitted | Estimate | 95% confidence interval | |
|---|---|---|---|
| Ozkan Kanat, 2013 [76] | 1.2033992 | − .50218731 | 2.9089859 |
| By Eduardo Bruera, 2003 [72] | 1.2033992 | − .50218731 | 2.9089859 |
| Abdolrasoul Safaeiyan, 2018 [81] | 1.4308839 | − .32518974 | 3.1869574 |
| L.Payahoo, 2017 [78] | 1.2033992 | − .50218731 | 2.9089859 |
| Signe Damsbo-Svendsen, 2013 [83] | 1.2459544 | − .47955447 | 2.9714632 |
| Shlomo Yehuda, 2005 [47] | 1.7502558 | − .02857373 | 3.5290854 |
| Gerd Faxen Irving, 2009 [75] | 1.2033992 | − .50218731 | 2.9089859 |
| Sara Moradi, 2021 [82] | 1.2033992 | − .50218731 | 2.9089859 |
| Combined | 1.2033992 | − .50218731 | 2.9089858 |
Fig. 2.
Funnel plots (with pseudo 95% CI) depicting the effect sizes (difference in means) versus their standard errors (SEs) for controlled trials that assessed the effect of n-3 PUFA supplementation on appetite
Discussion
The present study demonstrated that n-3 PUFAs fatty acid supplementation had no significant effect on overall appetite. However, it modestly increases the desire to eat in adults. To the best of our knowledge, no systematic review and meta-analysis has been published in this regard.
Previous meta-analyses have investigated the relationship between n-3 PUFA intake as supplements or in the context of foods. Furthermore, some studies assessed body weight, appetite hormones, or their gene expressions in humans as markers for appetite. A review by Behroz et al. showed that polyunsaturated fats, such as n-3 and n-6, have a similar effect on increasing energy expenditure, but they differ in how they regulate weight and appetite [85]. A meta-analysis of fifty-two trials illustrated that more than 2000 mg n-3 PUFA intake for more than 10 weeks significantly increased plasma adiponectin levels, but had no significant effect on circulating leptin levels [86]. In a meta-analysis of 22 studies, it is also revealed that omega-3 polyunsaturated fatty acid PUFA supplementation in patients with cancer significantly increases body weight and plasma total ω-3 fatty acids [87]. However, a meta-analysis by Satogami et al. showed that patients with eating disorders had higher levels of n-3 PUFAs in peripheral blood tissues than in controls [88]. In contrast, a recent review on the relationship between dietary fatty acids and appetite reported that an increase in n-3 PUFAs led to higher levels of plasma appetite-suppressing hormones and satiety sensation [85]. However, our study did not find any evidence for this effect.
In our study, n-3 PUFAs fatty acid supplementation significantly increases the desire to eat in adults. However, in a study using sunflower and flaxseed oil as a high-fat diet, PUFA did not find a statistically significant effect on the desire to eat among normal-weight females [10]. The small number of studies included in the meta-analysis might have influenced the effect we observed.
Appetite is controlled via multiple physiologic processes. The mechanism by which PUFAs might change the appetite has not yet been completely explicated. However, several mechanisms were proposed for n-3 PUFA’s effects on appetite. Intracellular long-chain fatty acids of the hypothalamus may increase by n-3 PUFA intake which results in initiating satiety signals and regulating appetite [89]. Also, n-3 PUFAs activate free fatty acid receptor 4, which results in increased intracellular calcium concentration that leads to the secretion of hormones like NPY, which can decrease appetite. Some studies also found that n-3 PUFA supplements stimulate the release of bile acid and cholecystokinin which reduce the appetite [82]. However, not all individuals need to reduce their appetite. For example, patients with cancer might have poor appetite due to cytokine inhibition of neuropeptide Y. On the other hand, supplementation with n-3 PUFAs can decrease the production of interleukin-1 and interleukin-6 cytokines, then may combat the loss of appetite in these patients [90]. Therefore, n-3 PUFAs may play a role in regulating total energy intake, managing both over and under-intake [7, 87].
The current study has several limitations that should be considered. First, the included studies were conducted on participants with different conditions like healthy adults, patients with cancer, and obesity and had different intervention periods. Furthermore, a limited number of studies assessed appetite by using subjective tools. Also, none of the 8 studies in our meta-analysis evaluated the daily omega-3 intake of participants via foods. Therefore, supplementation with n-3 PUFAs may meet daily requirement intake (DRI) and the individual may not have consumed more than DRI. Also, in the included studies the dose of n-3 PUFAs for intervention ranged from 225 to 4.5 g/d. Based on the risk of bias assessment, most of the included studies were judged to be “unclear” regarding their risk of bias. Moreover, only one study belonged to the high-risk group in the subgroup analysis of risk of bias, which might limit the power of subgroup analyses in meta-analyses. Therefore, the results should be treated with caution. Finally, we did not perform the search in Cochrane, so a small number of articles may not have been included in the search results, but we compensated for this by extensive searching in other databases and referencing the included articles.
Conclusion
The findings of the present systematic review and meta-analysis showed that the n-3 PUFA supplementation has no significant effect on appetite; however, it might increase the desire to eat. Regarding the different effects of n-3 PUFAs in healthy and unhealthy subjects with different diseases, more trials that investigate these different outcomes are needed.
Supplementary Information
Additional file 1: Table S1. The search strategy used to search different databases.
Additional file 2: Table S2. Dose of DHA for intervention (studies used for Meta-analysis).
Authors’ contributions
AMA conceived the study; all authors contributed to the design of the study; BS and FT selected studies and extracted studies; BS selected the items and refined them into concepts; BS wrote the draft manuscript; AMA revised the manuscript; all authors edited the manuscript; and all authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The data underlying this article was provided in the Supplementary Table 2.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schwingshackl L, et al. A scoping review of current guidelines on dietary fat and fat quality. Ann Nutr Metab. 2021;77(2):65–82. doi: 10.1159/000515671. [DOI] [PubMed] [Google Scholar]
- 2.Elagizi A, et al. An update on omega-3 polyunsaturated fatty acids and cardiovascular health. Nutrients. 2021;13(1):204. doi: 10.3390/nu13010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritsch J, et al. Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021;19(6):1189–1199. e30. doi: 10.1016/j.cgh.2020.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Mozafarinia M, et al. Association between dietary fat and fat subtypes with the risk of breast cancer in an Iranian population: a case-control study. Lipids Health Dis. 2021;20(1):1–11. doi: 10.1186/s12944-021-01557-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun L, et al. Differential effects of monounsaturated and polyunsaturated fats on satiety and gut hormone responses in healthy subjects. Foods. 2019;8(12):634. doi: 10.3390/foods8120634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siriwardhana N, Kalupahana NS, Moustaid-Moussa N. Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv Food Nutr Res. 2012;65:211–222. doi: 10.1016/B978-0-12-416003-3.00013-5. [DOI] [PubMed] [Google Scholar]
- 7.Harden CJ, et al. Long-chain polyunsaturated fatty acid supplementation had no effect on body weight but reduced energy intake in overweight and obese women. Nutr Res. 2014;34(1):17–24. doi: 10.1016/j.nutres.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Behrouz V, Yari Z. A review on differential effects of dietary fatty acids on weight, appetite and energy expenditure. Crit Rev Food Sci Nutr. 2022;62(8):2235–49. doi: 10.1080/10408398.2020.1852172. [DOI] [PubMed] [Google Scholar]
- 9.van den Akker K, Schyns G, Jansen A. Learned overeating: applying principles of Pavlovian conditioning to explain and treat overeating. Curr Addict Rep. 2018;5(2):223–231. doi: 10.1007/s40429-018-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graybeal AJ, Shah M, Willis JL. Manipulation of fatty acid composition in a high-fat meal does not result in differential alterations in appetite or food intake in normal weight females: a single-blind randomized crossover study. Appetite. 2021;160:105085. doi: 10.1016/j.appet.2020.105085. [DOI] [PubMed] [Google Scholar]
- 11.Strik CM, et al. No evidence of differential effects of SFA, MUFA or PUFA on post-ingestive satiety and energy intake: a randomised trial of fatty acid saturation. Nutr J. 2010;9(1):1–12. doi: 10.1186/1475-2891-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawton CL, et al. The degree of saturation of fatty acids influences post-ingestive satiety. Br J Nutr. 2000;83(5):473–482. doi: 10.1017/S000711450000060X. [DOI] [PubMed] [Google Scholar]
- 13.Jin X, et al. N-3 polyunsaterated fatty acids improve quality of life and survival, but not body weight in cancer cachexia: a systematic review and meta-analysis of controlled trials. Nutr Res. 2022;107:165–178. doi: 10.1016/j.nutres.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Poppitt S, et al. Fatty acid chain length, postprandial satiety and food intake in lean men. Physiol Behav. 2010;101(1):161–167. doi: 10.1016/j.physbeh.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson JL, Clevenger HC, Cooper JA. Hunger and satiety responses to high-fat meals of varying fatty acid composition in women with obesity. Obesity. 2015;23(10):1980–1986. doi: 10.1002/oby.21202. [DOI] [PubMed] [Google Scholar]
- 16.Monnard CR, Dulloo AG. Polyunsaturated fatty acids as modulators of fat mass and lean mass in human body composition regulation and cardiometabolic health. Obes Rev. 2021;22:e13197. doi: 10.1111/obr.13197. [DOI] [PubMed] [Google Scholar]
- 17.Golub N, et al. Greasing the wheels of managing overweight and obesity with omega-3 fatty acids. Med Hypotheses. 2011;77(6):1114–1120. doi: 10.1016/j.mehy.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baillie R, et al. Coordinate induction of peroxisomal acyl-CoA oxidase and UCP-3 by dietary fish oil: a mechanism for decreased body fat deposition. Prostaglandins Leukot Essent Fatty Acids. 1999;60(5–6):351–356. doi: 10.1016/S0952-3278(99)80011-8. [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Matute P, et al. Eicosapentaenoic acid actions on adiposity and insulin resistance in control and high-fat-fed rats: role of apoptosis, adiponectin and tumour necrosis factor-α. Br J Nutr. 2007;97(2):389–398. doi: 10.1017/S0007114507207627. [DOI] [PubMed] [Google Scholar]
- 20.Rossi AS, et al. Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose-fed, insulin-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2005;289(2):R486–R494. doi: 10.1152/ajpregu.00846.2004. [DOI] [PubMed] [Google Scholar]
- 21.Kozimor A, Chang H, Cooper JA. Effects of dietary fatty acid composition from a high fat meal on satiety. Appetite. 2013;69:39–45. doi: 10.1016/j.appet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Prater MC, et al. Hunger and satiety responses to diets enriched with cottonseed oil vs. olive oil. Physiol Behav. 2023;259:114041. doi: 10.1016/j.physbeh.2022.114041. [DOI] [PubMed] [Google Scholar]
- 23.Polley KR, et al. Appetite responses to high-fat diets rich in mono-unsaturated versus poly-unsaturated fats. Appetite. 2019;134:172–181. doi: 10.1016/j.appet.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, et al. Cochrane handbook for systematic reviews of interventions. Hoboken: Wiley; 2019. [Google Scholar]
- 25.Amiot M, Riva C, Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev. 2016;17(7):573–586. doi: 10.1111/obr.12409. [DOI] [PubMed] [Google Scholar]
- 26.Lorzadeh E, et al. The effect of hesperidin supplementation on inflammatory markers in human adults: a systematic review and meta-analysis of randomized controlled clinical trials. Chem Biol Interact. 2019;307:8–15. doi: 10.1016/j.cbi.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Mantovani G, et al. A phase II study with antioxidants, both in the diet and supplemented, pharmaconutritional support, progestagen, and anti-cyclooxygenase-2 showing efficacy and safety in patients with cancer-related anorexia/cachexia and oxidative stress. Cancer Epidemiol Biomark Prev. 2006;15(5):1030–1034. doi: 10.1158/1055-9965.EPI-05-0538. [DOI] [PubMed] [Google Scholar]
- 28.Kapoor N, et al. A prospective randomized controlled trial to study the impact of a nutrition-sensitive intervention on adult women with cancer cachexia undergoing palliative care in India. Integr Cancer Ther. 2017;16(1):74–84. doi: 10.1177/1534735416651968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gómez-Candela C, Horrisberger A, Bermejo L. Efficacy evaluation of an oral powder supplement enriched with eicosapentaenoic acid in cancer patients. Nutr Hosp. 2011;26(6):1385–1393. doi: 10.1590/S0212-16112011000600028. [DOI] [PubMed] [Google Scholar]
- 30.JM GA, et al. Adherence and tolerance as key in brake on weight loss in cancer patients with nutritional risk after intervention with a high calorie nutritional and specific hyperproteic supplement. Nutr Hospit. 2017;34(3):524–531. doi: 10.20960/nh.1331. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani G, et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist. 2010;15(2):200–211. doi: 10.1634/theoncologist.2009-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calder PC, et al. Targeted medical nutrition for cachexia in chronic obstructive pulmonary disease: a randomized, controlled trial. J Cachexia Sarcopenia Muscle. 2018;9(1):28–40. doi: 10.1002/jcsm.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barber M, et al. The effect of an oral nutritional supplement enriched with fish oil on weight-loss in patients with pancreatic cancer. Br J Cancer. 1999;81(1):80–86. doi: 10.1038/sj.bjc.6690654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akita H, et al. The utility of nutritional supportive care with an eicosapentaenoic acid (EPA)-enriched nutrition agent during pre-operative chemoradiotherapy for pancreatic cancer: prospective randomized control study. Clin Nutr ESPEN. 2019;33:148–153. doi: 10.1016/j.clnesp.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani G, et al. Cancer-related anorexia/cachexia syndrome and oxidative stress: an innovative approach beyond current treatment. Cancer Epidemiol Biomark Prev. 2004;13(10):1651–1659. doi: 10.1158/1055-9965.1651.13.10. [DOI] [PubMed] [Google Scholar]
- 36.Sánchez-Lara K, et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr. 2014;33(6):1017–1023. doi: 10.1016/j.clnu.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Ordman AB. Pilot study for an age-and gender-based nutrient signaling system for weight control. Age. 2008;30:201–208. doi: 10.1007/s11357-008-9049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Meij B, et al. Oral nutritional supplements containing n-3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multimodality treatment: an RCT. Eur J Clin Nutr. 2012;66(3):399–404. doi: 10.1038/ejcn.2011.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Probst A, et al. Short-term effect of macronutrient composition and glycemic index of a yoghurt breakfast on satiety and mood in healthy young men. Complement Med Res. 2012;19(5):247–251. doi: 10.1159/000343163. [DOI] [PubMed] [Google Scholar]
- 40.Mathias JR, et al. Relation of endometriosis and neuromuscular disease of the gastrointestinal tract: new insights. Fertil Steril. 1998;70(1):81–88. doi: 10.1016/S0015-0282(98)00096-X. [DOI] [PubMed] [Google Scholar]
- 41.Gholami Z, Akhlaghi M. The effect of flaxseed on physical and mental fatigue in children and adolescents with overweight/obesity: a randomised controlled trial. Br J Nutr. 2021;126(1):151–159. doi: 10.1017/S0007114520003888. [DOI] [PubMed] [Google Scholar]
- 42.Cohen L, et al. Evaluation of the influence of whole and defatted flaxseed on satiety, glucose, and leptin levels of women in the late postoperative stage of bariatric surgery. Obes Surg. 2013;23:157–166. doi: 10.1007/s11695-012-0733-x. [DOI] [PubMed] [Google Scholar]
- 43.Harden CJ, et al. Evaluation of the salivary proteome as a surrogate tissue for systems biology approaches to understanding appetite. J Proteomics. 2012;75(10):2916–2923. doi: 10.1016/j.jprot.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Kratz M, et al. Dietary n-3-polyunsaturated fatty acids and energy balance in overweight or moderately obese men and women: a randomized controlled trial. Nutr Metab. 2009;6(1):1–7. doi: 10.1186/1743-7075-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevenson JL, Paton CM, Cooper JA. Hunger and satiety responses to high-fat meals after a high-polyunsaturated fat diet: a randomized trial. Nutrition. 2017;41:14–23. doi: 10.1016/j.nut.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Tuccinardi D, et al. Mechanisms underlying the cardiometabolic protective effect of walnut consumption in obese people: a cross-over, randomized, double-blind, controlled inpatient physiology study. Diabetes Obes Metab. 2019;21(9):2086–2095. doi: 10.1111/dom.13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yehuda S, Rabinovitz S, Mostofsky DI. Mixture of essential fatty acids lowers test anxiety. Nutr Neurosci. 2005;8(4):265–267. doi: 10.1080/10284150500445795. [DOI] [PubMed] [Google Scholar]
- 48.Mankad D, et al. A randomized, placebo controlled trial of omega-3 fatty acids in the treatment of young children with autism. Mol Autism. 2015;6:1–11. doi: 10.1186/s13229-015-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fristad MA, et al. A randomized controlled trial of individual family psychoeducational psychotherapy and omega-3 fatty acids in youth with subsyndromal bipolar disorder. J Child Adolesc Psychopharmacol. 2015;25(10):764–774. doi: 10.1089/cap.2015.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaid ZA, et al. Fish oil supplementation is beneficial on caloric intake, appetite and mid upper arm muscle circumference in children with leukemia. Asia Pac J Clin Nutr. 2012;21(4):502–510. [PubMed] [Google Scholar]
- 51.López-Alarcón M, et al. The effect of docosahexaenoic acid on the loss of appetite in pediatric patients with pneumonia. Rev Med Inst Mex Seguro Soc. 2006;44(1):5–11. [PubMed] [Google Scholar]
- 52.Vakhapova V, et al. Safety of phosphatidylserine containing omega-3 fatty acids in non-demented elderly: a double-blind placebo-controlled trial followed by an open-label extension. BMC Neurol. 2011;11(1):1–10. doi: 10.1186/1471-2377-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McNamara RK, et al. Detection and treatment of long-chain omega-3 fatty acid deficiency in adolescents with SSRI-resistant major depressive disorder. PharmaNutrition. 2014;2(2):38–46. doi: 10.1016/j.phanu.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daak AA, et al. Effect of omega-3 (n− 3) fatty acid supplementation in patients with sickle cell anemia: randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2013;97(1):37–44. doi: 10.3945/ajcn.112.036319. [DOI] [PubMed] [Google Scholar]
- 55.Sigh S, et al. Effectiveness of a locally produced, fish-based food product on weight gain among Cambodian children in the treatment of acute malnutrition: a randomized controlled trial. Nutrients. 2018;10(7):909. doi: 10.3390/nu10070909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oliveira JM, et al. Effects of a low dose of fish oil on inflammatory markers of brazilian HIV-infected adults on antiretroviral therapy: a randomized, parallel, placebo-controlled trial. Nutrients. 2015;7(8):6520–6528. doi: 10.3390/nu7085294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Natale C, et al. Effects of baked products enriched with n-3 fatty acids, folates, β-glucans, and tocopherol in patients with mild mixed hyperlipidemia. J Am Coll Nutr. 2012;31(5):311–319. doi: 10.1080/07315724.2012.10720427. [DOI] [PubMed] [Google Scholar]
- 58.Freedman S, et al. Increased fat absorption from enteral formula through an in-line digestive cartridge in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2017;65(1):97–101. doi: 10.1097/MPG.0000000000001617. [DOI] [PubMed] [Google Scholar]
- 59.Reis RS, et al. Poor infant inhibitory control predicts food fussiness in childhood–a possible protective role of n-3 PUFAs for vulnerable children. Prostaglandins Leukot Essent Fatty Acids. 2015;97:21–25. doi: 10.1016/j.plefa.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Rahemi M, Alizadeh M. The association of energy intake and expenditure, macronutrients, glycemic index and load, and general characteristics with postprandial peptide YY 3–36 serum levels. Crescent J Med Biol Sci. 2018;5(2):107–114. [Google Scholar]
- 61.Barber MD, Fearon KC. Tolerance and incorporation of a high-dose eicosapentaenoic acid diester emulsion by patients with pancreatic cancer cachexia. Lipids. 2001;36(4):347–351. doi: 10.1007/s11745-001-0726-4. [DOI] [PubMed] [Google Scholar]
- 62.Hellerstein MK, et al. Effects of dietary n-3 fatty acid supplementation in men with weight loss associated with the acquired immune deficiency syndrome: relation to indices of cytokine production. J Acquir Immune Defic Syndr. 1996;11(3):258–270. doi: 10.1097/00042560-199603010-00006. [DOI] [PubMed] [Google Scholar]
- 63.Pu S, et al. Interactions between dietary oil treatments and genetic variants modulate fatty acid ethanolamides in plasma and body weight composition. Br J Nutr. 2016;115(6):1012–1023. doi: 10.1017/S0007114515005425. [DOI] [PubMed] [Google Scholar]
- 64.Berge K, et al. Krill oil supplementation lowers serum triglycerides without increasing low-density lipoprotein cholesterol in adults with borderline high or high triglyceride levels. Nutr Res. 2014;34(2):126–133. doi: 10.1016/j.nutres.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Narverud I, et al. Lack of effects of a single high-fat meal enriched with vegetable n-3 or a combination of vegetable and marine n-3 fatty acids on intestinal peptide release and Adipokines in healthy female subjects. Front Nutr. 2016;3:38. doi: 10.3389/fnut.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burns CP, et al. Phase II study of high-dose fish oil capsules for patients with cancer-related cachexia: a cancer and leukemia group B study. Cancer. 2004;101(2):370–378. doi: 10.1002/cncr.20362. [DOI] [PubMed] [Google Scholar]
- 67.Emsley R, et al. Safety of the omega-3 fatty acid, eicosapentaenoic acid (EPA) in psychiatric patients: results from a randomized, placebo-controlled trial. Psychiatry Res. 2008;161(3):284–291. doi: 10.1016/j.psychres.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 68.Burton-Freeman B. Sex and cognitive dietary restraint influence cholecystokinin release and satiety in response to preloads varying in fatty acid composition and content. J Nutr. 2005;135(6):1407–1414. doi: 10.1093/jn/135.6.1407. [DOI] [PubMed] [Google Scholar]
- 69.Al-Helli A, et al. Effects of fluoxetine, omega 3, or their combination on serum leptin in Iraqi obese subjects. Int J Pharmaceut Sci Rev Res. 2015;35(1):90–95. [Google Scholar]
- 70.Cerchietti LC, Navigante AH, Castro MA. Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids from fish oil and preferential Cox-2 inhibition on systemic syndromes in patients with advanced lung cancer. Nutr Cancer. 2007;59(1):14–20. doi: 10.1080/01635580701365068. [DOI] [PubMed] [Google Scholar]
- 71.Parra D, et al. A diet rich in long chain omega-3 fatty acids modulates satiety in overweight and obese volunteers during weight loss. Appetite. 2008;51(3):676–680. doi: 10.1016/j.appet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 72.Bruera E, et al. Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: a double-blind, placebo-controlled study. Nutr Clin Pract. 2003;18(6):524–524. doi: 10.1177/0115426503018006524. [DOI] [PubMed] [Google Scholar]
- 73.Jatoi A, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer-associated wasting: a North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol. 2004;22(12):2469–2476. doi: 10.1200/JCO.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y, et al. A randomized pilot study of atractylenolide I on gastric cancer cachexia patients. Evid Based Complement Alternat Med. 2008;5(3):337–344. doi: 10.1093/ecam/nem031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faxén Irving G, et al. Omega-3 fatty acid supplementation effects on weight and appetite in patients with Alzheimer’s disease: the omega-3 Alzheimer’s disease study. J Am Geriatr Soc. 2009;57(1):11–17. doi: 10.1111/j.1532-5415.2008.02055.x. [DOI] [PubMed] [Google Scholar]
- 76.Kanat O, et al. Comparison of three different treatment modalities in the management of cancer cachexia. Tumori J. 2013;99(2):229–233. doi: 10.1177/030089161309900218. [DOI] [PubMed] [Google Scholar]
- 77.Miller ER, III, et al. The effects of n-3 long-chain polyunsaturated fatty acid supplementation on biomarkers of kidney injury in adults with diabetes: results of the GO-FISH trial. Diabetes Care. 2013;36(6):1462–1469. doi: 10.2337/dc12-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Payahoo L, et al. Effects of n-3 polyunsaturated fatty acid supplementation on serum leptin levels, appetite sensations, and intake of energy and macronutrients in obese people: a randomized clinical trial. J Diet Suppl. 2018;15(5):596–605. doi: 10.1080/19390211.2017.1360975. [DOI] [PubMed] [Google Scholar]
- 79.Mocellin MC, et al. Fish oil effects on quality of life, body weight and free fat mass change in gastrointestinal cancer patients undergoing chemotherapy: a triple blind, randomized clinical trial. J Funct Foods. 2017;31:113–122. doi: 10.1016/j.jff.2017.01.041. [DOI] [Google Scholar]
- 80.Werner K, et al. Dietary supplementation with n-3-fatty acids in patients with pancreatic cancer and cachexia: marine phospholipids versus fish oil-a randomized controlled double-blind trial. Lipids Health Dis. 2017;16(1):1–12. doi: 10.1186/s12944-017-0495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Safaeiyan A, et al. Effect of omega-3 fatty acids on appetite, energy and macronutrient intake and body weight in obese adults: a randomized clinical trial. Prog Nutr. 2018;20:203–209. [Google Scholar]
- 82.Moradi S, et al. The effect of short-term omega-3 fatty acids supplementation on appetite in healthy men: a randomized double-blinded controlled clinical trial. Nutr Clin Métab. 2022;36(1):46–53. doi: 10.1016/j.nupar.2021.08.004. [DOI] [Google Scholar]
- 83.Damsbo-Svendsen S, Rønsholdt MD, Lauritzen L. Fish oil-supplementation increases appetite in healthy adults. A randomized controlled cross-over trial. Appetite. 2013;66:62–66. doi: 10.1016/j.appet.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 84.Payahoo L, et al. Effects of n-3 polyunsaturated fatty acid supplementation on serum leptin levels, appetite sensations, and intake of energy and macronutrients in obese people: a randomized clinical trial. J Diet Suppl. 2018;15(5):596–605. doi: 10.1080/19390211.2017.1360975. [DOI] [PubMed] [Google Scholar]
- 85.Behrouz V, Yari Z. A review on differential effects of dietary fatty acids on weight, appetite and energy expenditure. Crit Rev Food Sci Nutr. 2022;62(8):2235–2249. doi: 10.1080/10408398.2020.1852172. [DOI] [PubMed] [Google Scholar]
- 86.Sepidarkish M, et al. Effect of omega-3 fatty acids supplementation on adipokines: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2022;62(27):7561–75. doi: 10.1080/10408398.2021.1915743. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, et al. Does omega-3 PUFA-enriched oral nutritional intervention benefit cancer patients receiving chemo (radio) therapy? A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2023;63(18):3081–96. doi: 10.1080/10408398.2021.1984199. [DOI] [PubMed] [Google Scholar]
- 88.Satogami K, et al. Relationship between polyunsaturated fatty acid and eating disorders: systematic review and meta-analysis. Prostaglandins Leukot Essent Fatty Acids. 2019;142:11–19. doi: 10.1016/j.plefa.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 89.Chari M, Lam CK, Lam TK. Hypothalamic fatty acid sensing in the normal and disease states. 2011. [PubMed]
- 90.Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: mechanisms and clinical implications. Gastroenterol Res Pract. 2011;2011:60143. doi: 10.1155/2011/601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The search strategy used to search different databases.
Additional file 2: Table S2. Dose of DHA for intervention (studies used for Meta-analysis).
Data Availability Statement
The data underlying this article was provided in the Supplementary Table 2.