Abstract
Purpose
Allergic rhinitis (AR) and migraine are among the most common public health problems worldwide. Observational studies on the correlation between AR and migraine have reported inconsistent results. This study aimed to investigate the causal relationship of AR with migraine and its subtypes, including migraine with aura (MA) and migraine without aura (MO).
Methods
Bidirectional two-sample Mendelian randomization (MR) analysis was performed with publicly available summary-level statistics of large genome-wide association studies to estimate the possible causal effects. The inverse variance-weighted method was selected for primary analysis and was supplemented with the weighted median, weighted mode, and MR-Egger methods. The causal analysis using summary effect estimates (CAUSE) were further performed to verify the causality. Several sensitivity tests, including the leave-one-out, Cochran’s Q, MR-Egger intercept, and MR-PRESSO tests, were performed to assess the robustness of the results.
Results
AR did not exhibit a significant causal correlation with the elevated risk of any migraine (odd ratio (OR), 0.816; 95% confidence interval (CI), 0.511–1.302; P = 0.394), MA (OR, 0.690; 95% CI 0.298–1.593; P = 0.384), or MO (OR, 1.022; 95% CI 0.490–2.131; P = 0.954). Consistently, reverse MR analysis did not reveal causal effects of any migraine or its subtypes on AR. Almost all sensitivity analyses supported the robustness of the results.
Conclusions
This MR study did not reveal a clear causal association between AR and migraine risk. More research is warranted to reveal the complex association between AR and migraine.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-024-01682-1.
Keywords: Mendelian randomization, Allergic rhinitis, Migraine, Causal relationship, Genetic correlation
Introduction
Allergic rhinitis (AR), a common chronic inflammatory disease, is characterized by an enhanced response of the immune system to exogenous allergens. The pathophysiological mechanisms of AR are complicated and are related to an interplay between genetic predispositions and environmental factors [1]. The classic symptoms of AR include nasal itching, nasal congestion, watery rhinorrhea, and frequent sneezing, which have a negative impact on patients' health and quality of life [2]. The prevalence of AR has rapidly increased worldwide in recent decades. Globally, AR is estimated to affect 10–40% of adults and 5–15% of children [3].
Migraine is a highly prevalent neurovascular headache disorder associated with decreased productivity and marked disability [4]. According to the Global Burden of Disease Study 2016, the global prevalence of migraine is estimated to be 14% [5]. The typical clinical features of this neurological disorder include unilateral, throbbing headache attacks accompanied by nausea, vomiting, photophobia, and phonophobia [4]. However, migraine is a highly heterogeneous disorder and can be classified as migraine with aura (MA), migraine without aura (MO), and several rare subphenotypes based on the International Classification of Headache Disorders (ICHD) diagnostic criteria [6]. Although the pathogenesis of migraine has not been elucidated, it may be related to genetic factors and the activation of the trigeminovascular system [7].
Allergists and neurologists have been exploring the relationship between AR and migraine as these diseases exhibit high prevalence worldwide and overlapping symptoms [8]. Some pathophysiological features of AR are similar to those of migraine. For example, AR and migraine have a strong genetic component and similar triggers (e.g., inhaled irritants and weather changes) and inflammatory mechanisms [9]. However, the current clinical evidence for the association between AR and migraine has been inconsistent. Several population-based studies have demonstrated the correlation between AR and an increased risk and frequency of migraine [10–12], whereas other studies have suggested no correlation between AR and migraine [13, 14]. These previous observational studies could not demonstrate a causal relationship between AR and migraine owing to potential confounding factors and reverse causality [15]. The elucidation of the causal relationship between AR and migraine will enable the effective management of AR and migraine.
Mendelian randomization (MR) is a statistical approach that utilizes genetic variants, especially single-nucleotide polymorphisms (SNPs), to evaluate the potential causality between exposure and outcome [16]. The MR strategy is based on the principle of random assignment of alleles at meiosis. Thus, MR methods are independent of external factors confounding observational epidemiological studies [16]. Compared with traditional observational studies, MR analysis avoids confounders, reverse causality, and other biases. Furthermore, MR is recognized as an effective alternative to randomized controlled trials (RCTs) in determining causality in cases where economical, practical, and ethical RCTs are lacking [17]. Recently, the enhanced accessibility of human genetic data has increased the application of MR to infer causality in medical fields, such as etiological and drug target validation studies [18, 19]. This study conducted a bidirectional two-sample MR analysis to assess the potential causality between AR and migraine and its subtypes MA and MO.
Materials and methods
Study design
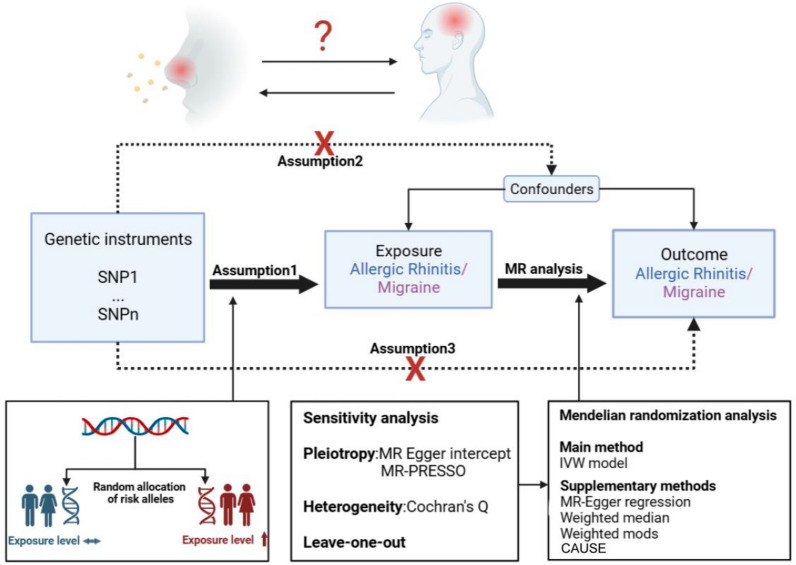
SNPs were used as instrumental variables (IVs) to estimate the causal effect of exposure on outcome in this study. All SNPs included as IVs should fulfill the following three key assumptions for MR analysis [15] (Fig. 1): Assumption 1, IVs are strongly correlated with exposure; Assumption 2, IVs are not associated with any confounding factors related to both exposure and outcome; Assumption 3, IVs affect outcome only through exposure.
Fig. 1.
Schematic of MR study on the association between AR with migraine
Data sources
We obtained eligible summary-level statistics for each trait from large-scale, public GWAS datasets (Table 1). The GWAS summary data associated with AR, which were extracted from the UK biobank, comprised data of 25,486 patients diagnosed with AR and 87,097 controls. Summary-level statistics of migraine and its subtypes, including MA and MO, were extracted from the FinnGen consortium. The three summary-level statistics on migraine came from the same study, which involved 8,547 migraine cases, including 3,541 MA cases and 3,215 MO cases, and 176,107 controls. All participants were of European ancestry without overlap of exposure and outcome samples. No additional ethical approval was required as all data used in this MR study were obtained from previously published GWAS datasets.
Table 1.
Summary of the data sources used in this MR study
| Exposure or outcome | Sample size | Numbers of cases | Numbers of controls | Ancestry | Consortium | GWAS ID |
|---|---|---|---|---|---|---|
| AR | 112,583 | 25,486 | 87,097 | European | UK biobank | ukb-b-7178 |
| Migraine | 184,654 | 8547 | 176,107 | European | FinnGen | finn-b-G6_MIGRAINE |
| MA | 179,648 | 3541 | 176,107 | European | FinnGen | finn-b-G6_MIGRAINE_WITH_AURA |
| MO | 179,322 | 3215 | 176,107 | European | FinnGen | finn-b-G6_MIGRAINE_NO_AURA |
AR, allergic rhinitis; MA, migraine with aura; MO, migraine without aura; MR, Mendelian randomization analysis
Selection of genetic variants as IVs
A series of quality control procedures was performed to identify genetic IVs that met these three MR assumptions [16]. The genome-wide significance threshold was set at P < 5 × 10−8 to screen for SNPs strongly associated with exposure. If SNPs did not satisfy the threshold, the P-value was relaxed to < 5 × 10−6 according to previous MR studies. Additionally, the clumping procedure (R2 < 0.001 and clumping distance > 10,000 kb) was performed to eliminate the effect of linkage disequilibrium between the included SNPs. Furthermore, traits related to SNPs were examined after the clumping process by querying the PhenoScanner database [20]. Common risk factors for migraines include smoking, alcohol consumption, systolic and diastolic blood pressure, body mass index, and major depression [21]. In addition, common risk factors for AR include bronchial asthma, nasal polyps, sinusitis, epistaxis, otitis media, and allergic strep throat according to previous MR studies [22]. SNPs associated with the confounders of outcome were excluded. Palindromic SNPs with intermediate allele frequencies were excluded by harmonizing exposure and outcome data to align SNPs on the same effect allele for both exposure and outcome. Moreover, the strength of all SNPs as genetic IVs was quantified using the F-statistic (F = β2 / se2). IVs with F-statistics less than 10 were considered weak IVs and were not used in subsequent MR analyses [23]. After the filtering procedure described above, these rigorously screened SNPs served as the final IVs for subsequent MR analysis.
MR analysis
The causal association between AR and migraine was evaluated using four MR analysis methods. The inverse variance-weighted (IVW) method was the preferred method to determine the causality between exposure and outcome, while the MR-Egger, weighted median, and weighted mode methods served as alternative MR methods [16]. The IVW model can provide unbiased causal estimates when all IVs are valid [24]. The weighted median method offers an unbiased estimation in cases when up to 50% of the IVs are invalid [25]. When all IVs are invalid, the MR-Egger regression provides a conservative estimate of causality but with decreased statistical accuracy [26]. The weighted mode method can also be applied to evaluate the robustness of the MR results [27]. We also employed a Bayesian posterior probabilities-based MR method namely causal analysis using summary effect estimates (CAUSE) [28], as a further validation analysis of causality. The odds ratios (ORs) with corresponding 95% confidence interval (CI) were used to present the effect estimates from MR analyses. In addition, causal effects (i.e., OR) between AR and migraine were converted from a logit scale to a liability scale using the approach described by Byrne et al. [29], with an assumed population prevalence of 23% for AR [30] and 15% for migraine [31], respectively. The causal effect of exposure on the outcome was considered significant at P < 0.05.
Furthermore, a series of sensitivity analyses, including the pleiotropy test and heterogeneity test, was performed. The MR-Egger intercept test and MR-Pleiotropy Residual Sum and Outlier (PRESSO) global tests were used to detect potential horizontal pleiotropy [32, 33]. P > 0.05 indicated the lack of pleiotropy in IVs. The presence of horizontal pleiotropy can also be visualized with a funnel plot in which a symmetric graph suggests the lack of pleiotropy [34]. Heterogeneity between IVs was assessed using Cochran’s Q-test in the IVW and MR-Egger methods [35]. The effect of heterogeneity was disregarded if P > 0.05. Additionally, we performed the leave-one-out (LOO) analysis to check whether a single SNP was responsible for the causal association [36].
All these analyses were performed using R software (version 4.1.2) with the R packages TwoSample MR (version 0.5.7), MR-PRESSO (version 1.0), and CAUSE (version 1.2.0).
Results
Characteristics of the selected IVs
After screening, 31 significant SNPs related to AR (P < 5 × 10−8) that fulfilled the inclusion criteria were identified as valid IVs (Additional file 1: Table S1). As SNPs associated with migraine were not identified at the genome-wide significance threshold of P < 5 × 10−8, a less stringent threshold of P < 5 × 10−6 was used. Consequently, 12, 8, and 10 SNPs related to migraine, MA, and MO, respectively, were obtained as valid IVs (Additional file 1: Tables S2–S4). Of these, none of the SNPs were associated with relevant confounders. The F-statistics for all IVs were > 10, suggesting that the IVs included in this study were unlikely to be affected by weak instrument bias (Additional file 1: Tables S1–S4).
Causal effects of AR on migraine
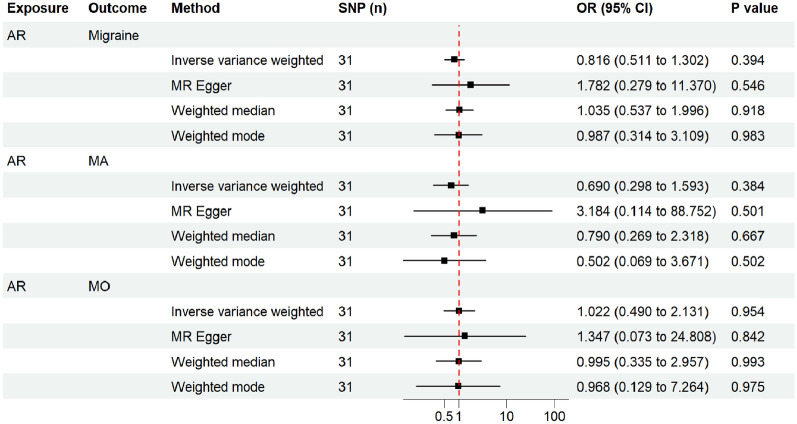
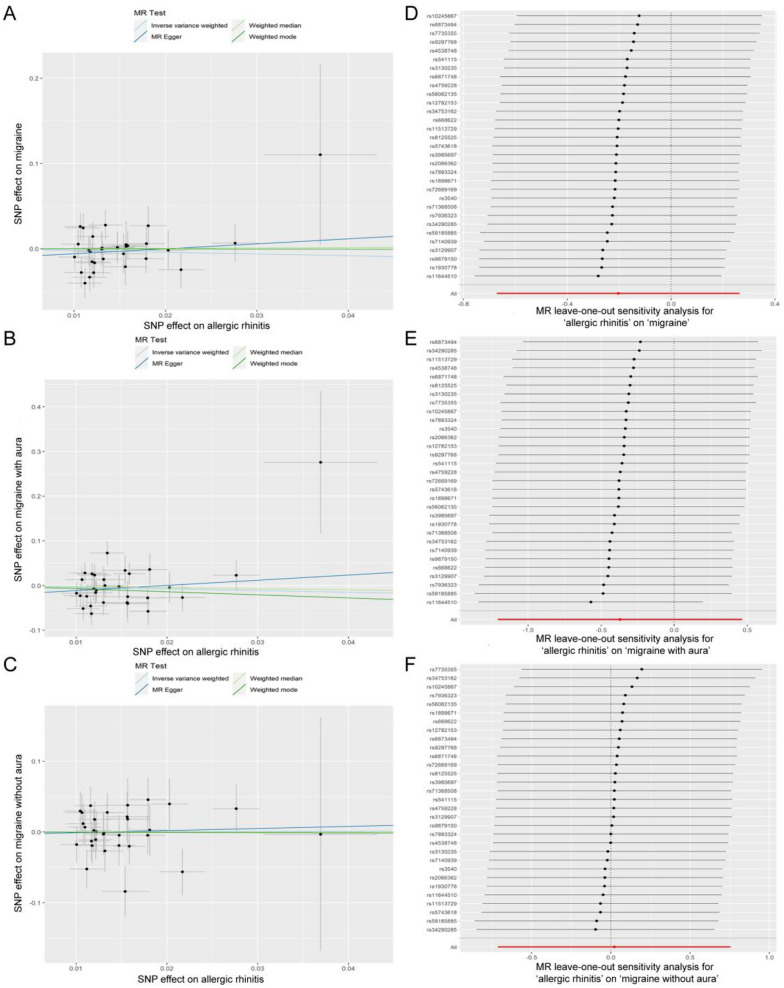
The results from the IVW model revealed that genetic predispositions to AR were not associated with an increased risk of migraine (OR, 0.816; 95% CI 0.511–1.302; P = 0.394) and its subtypes MA (OR, 0.690; 95% CI 0.298–1.593; P = 0.384) and MO (OR, 1.022; 95% CI 0.490–2.131; P = 0.954) (Fig. 2). The liability ORs calculated by each method are provided in Additional file 1: Table S5. Analysis with the weighted median, weighted mode, and MR-Egger methods also indicated that AR lacked a genetic causal association with migraine and its subtypes (all P > 0.05) (Fig. 2). The results of the MR-PRESSO global test and the MR-Egger intercept test did not reveal the presence of horizontal pleiotropy (all P > 0.05) (Table 2). The funnel plots showing heterogeneity are illustrated in Additional file 2: Fig. S1. The Cochran’s Q-test results revealed no evidence of heterogeneity in the IVs (all P > 0.05) (Table 2). The LOO sensitivity analysis demonstrated that the causal association between exposure and outcome was not biased by any single SNP (Fig. 3). These sensitivity analyses confirmed the robustness of the conclusions.
Fig. 2.
Estimated causal effects of AR on migraine and its subtypes using different MR methods. The scale of x-axis is logarithmic
Table 2.
Sensitivity analyses of the forward MR study
| Exposure | Outcome | SNP (n) | Heterogeneity tests | Cochran’s Q | P value | Horizontal pleiotropy tests | Intercept | P value |
|---|---|---|---|---|---|---|---|---|
| AR | Migraine | 31 | IVW | 29.28 | 0.503 | MR Egger intercept test | − 0.012 | 0.400 |
| MR Egger | 28.551 | 0.489 | MR-PRESSO global test | NA | 0.395 | |||
| AR | MA | 31 | IVW | 43.241 | 0.056 | MR Egger intercept test | − 0.023 | 0.359 |
| MR Egger | 41.986 | 0.056 | MR-PRESSO global test | NA | 0.391 | |||
| AR | MO | 31 | IVW | 26.918 | 0.628 | MR Egger intercept test | − 0.004 | 0.849 |
| MR Egger | 26.881 | 0.578 | MR-PRESSO global test | NA | 0.952 |
AR, allergic rhinitis; MA, migraine with aura; MO, migraine without aura; SNP, single nucleotide polymorphism; IVW, inverse variance weighted; MR-PRESSO, Mendelian randomization pleiotropy residual sum and outlier; NA, not available
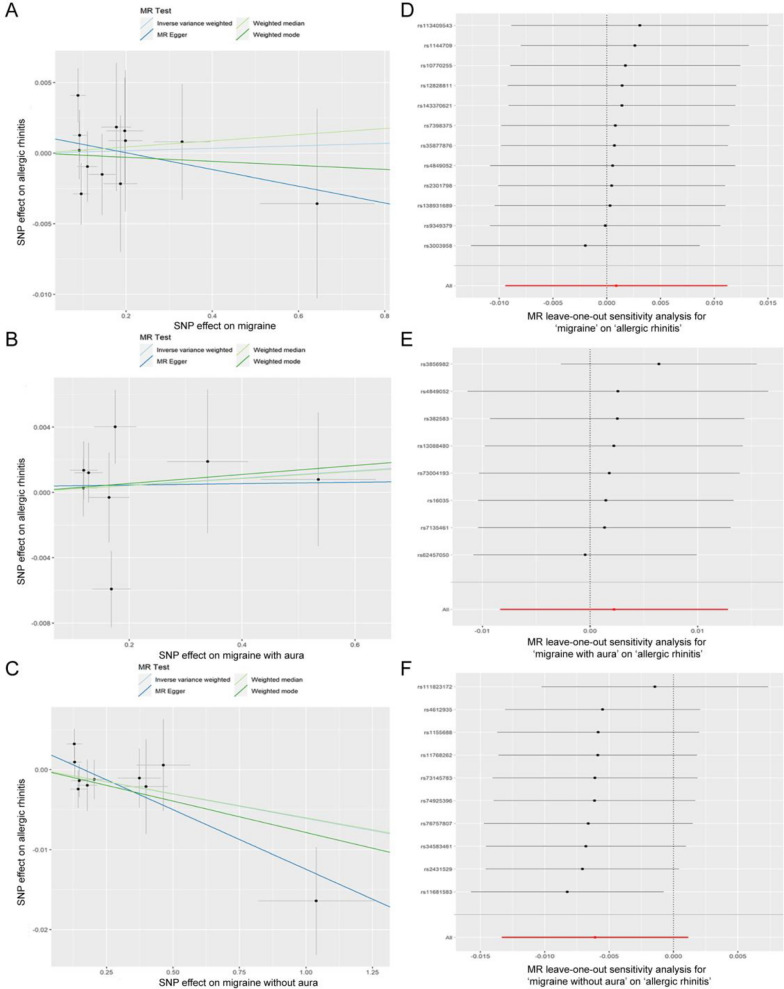
Fig. 3.
Visualization of the causal effects of AR on migraine and its subtypes. A Scatter plot of the causal effect of AR on migraine. B Scatter plot of the causal effect of AR on MA. C Scatter plot of the causal effect of AR on MO. D Leave-one-out sensitivity analysis of the causal effect for AR on migraine. E Leave-one-out sensitivity analysis of the causal effect for AR on MA. F Leave-one-out sensitivity analysis of the causal effect for AR on MO
Causal effects of migraine on AR
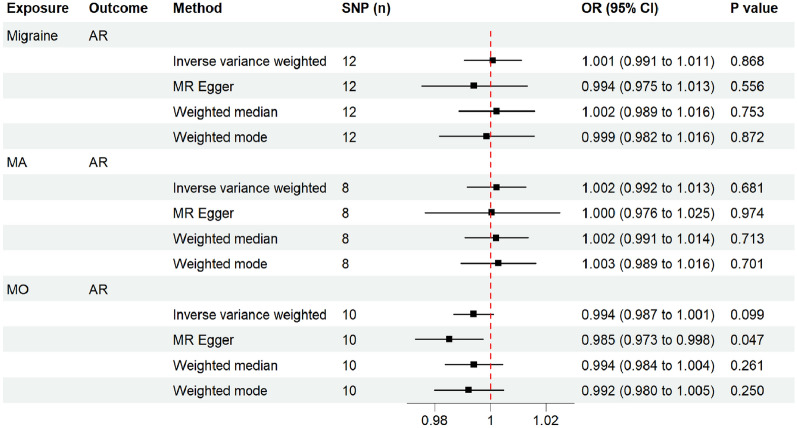
To further explore the exact causal relationship between AR and migraine, a reverse MR analysis was performed with migraine, MA, and MO as the exposures and AR as the outcome. Based on the IVW model, migraine (including its subtypes) was not causally correlated with AR (P > 0.05) (Fig. 4). The liability ORs calculated by each method are provided in Additional file 1: Table S5. The results of analysis with the weighted median and weighted mode methods were consistent with those of analysis with the IVW method (all P > 0.05) (Fig. 4). The MR-Egger method revealed a possible causal effect of migraine on AR (P = 0.043). However, the MR-Egger method suffers from an obvious limitation, namely its weak statistical power for detecting a causation [26]. The results of causal analysis with the IVW method are considered the main findings of this study. To further confirm the causal effects of migraine and its subtypes on AR, CAUSE analysis was then performed. The results indicated that the sharing model was better than the causal model, with no significant causal effect of migraine (including its subtypes) on AR (all P > 0.05) (Additional file 2: Fig. S2–S4). Furthermore, all sensitivity analyses revealed no significant heterogeneity among the IVs (all P > 0.05) and no marked horizontal pleiotropy (all P > 0.05) (Table 3). The LOO sensitivity analysis of the causal effect for migraine and MA on AR indicated that no specific SNPs promoted the causal association (Fig. 5). However, the LOO sensitivity analysis assessing the causal effect of MO on AR showed that there was a potentially influential SNP (Fig. 5). Thus, we may need to interpret the conclusion with caution.
Fig. 4.
Estimated causal effects of migraine and its subtypes on AR using different MR methods
Table 3.
Sensitivity analyses of the reverse MR study
| Exposure | Outcome | SNP (n) | Heterogeneity tests | Cochran’s Q | P value | Horizontal pleiotropy tests | Intercept | P value |
|---|---|---|---|---|---|---|---|---|
| Migraine | AR | 12 | IVW | 8.118 | 0.703 | MR Egger intercept test | 0.001 | 0.426 |
| MR Egger | 7.430 | 0.684 | MR-PRESSO global test | NA | 0.850 | |||
| MA | AR | 8 | IVW | 10.557 | 0.159 | MR Egger intercept test | 0.000 | 0.874 |
| MR Egger | 10.509 | 0.105 | MR-PRESSO global test | NA | 0.693 | |||
| MO | AR | 10 | IVW | 8.774 | 0.458 | MR Egger intercept test | 0.002 | 0.126 |
| MR Egger | 5.858 | 0.663 | MR-PRESSO global test | NA | 0.129 |
AR, allergic rhinitis; MA, migraine with aura; MO, migraine without aura; SNP, single nucleotide polymorphism; IVW, inverse variance weighted; MR-PRESSO, Mendelian randomization pleiotropy residual sum and outlier; NA, not available
Fig. 5.
Visualization of the causal effects of migraine and its subtypes on AR. A Scatter plot of the causal effect of migraine on AR. B Scatter plot of the causal effect of MA on AR. C Scatter plot of the causal effect of MO on AR. D Leave-one-out sensitivity analysis of the causal effect for migraine on AR. E Leave-one-out sensitivity analysis of the causal effect for MA on AR. F Leave-one-out sensitivity analysis of the causal effect for MO on AR
Discussion
AR and migraine are prominent public health problems worldwide [15]. Many studies have reported the epidemiological overlap between AR and migraine. However, the causal association between AR and migraine is inconclusive [16]. To the best of our knowledge, this is the first bidirectional MR study to investigate the causal association between AR and migraine. The findings of this study indicated no clear causal association between genetic susceptibility to AR and the risk of migraine (including any migraine, MA, and MO). Additionally, migraine (including any migraine, MA, and MO) did not exert a significant causal effect on AR.
Several studies have examined the possible link between AR and migraine. In particular, various observational investigations have revealed a strong epidemiological association between AR and migraine. One case–control study from America involving 76 patients with AR and 57 patients with non-AR revealed that the prevalence of migraine in the AR group was significantly higher than that in the non-AR group [37]. Similar findings were reported by Ozturk et al. [38] who demonstrated that the frequency of migraine in patients with AR was approximately four times higher than that in controls. In 2007, a large cross-sectional questionnaire-based study from Norway revealed that the probability of migraine in patients with hay fever was approximately 1.5 times higher than that in patients with non-hay fever and that this link strengthened with an increase in headache frequency [39]. Additionally, results from the American Migraine Prevalence and Prevention Study (published in 2013) revealed an increased frequency of migraines and disability in patients with AR [12]. Recently, Han et al. [10] performed a nationwide cohort study in a Korean population to investigate the relationship between allergic diseases and migraine risk. Consistent with previous findings, the authors demonstrated that patients with AR had a significantly higher risk of migraine than controls. Additionally, the number of concurrent allergic diseases was positively associated with the risk of migraine. These studies were focused mainly on adults. Some studies have reported a close association between AR and migraine risk in children. Wang et al. [11] performed a nationwide cohort study in a Chinese population involving 461,850 children with AR and 460,718 non-AR controls. The authors demonstrated that the prevalence and subsequent risk of migraine in the AR cohort were significantly higher than those in the non-AR cohort. Additionally, the susceptibility to MO was higher than that to MA in children with AR [11]. Furthermore, several epidemiological investigations have reported that AR may be a potential risk factor for migraine [40]. A retrospective case–control study from Spain reported that the incidence and disease severity of AR in children with migraine were higher than those in children without migraine [41]. A large clinical study conducted by Eross et al. demonstrated that 54% of migraineurs diagnosed according to the criteria of the ICHD had a medical history of AR [42].
The findings of this study are in contrast to those of many observational studies but are consistent with those of some epidemiological studies [13, 14]. This discrepancy may be due to the inherent limitations of observational studies. First, many observational studies in the field are based on case–control and cross-sectional designs, which are ambiguous in terms of chronology, preventing the inference of clear causal relationships. Second, observational studies are susceptible to a variety of confounding factors, even prospective and population-based studies, which may lead to biased results. Third, reverse causation could also lead to observational associations. Finally, the diagnostic criteria used in some studies may reduce the reliability of the findings.
This study did not reveal significant genetic causality between AR and migraine, which must be interpreted with caution. We believe that the association between AR and migraine observed in the clinical setting may be driven by similar pathogenetic mechanisms. Autonomic dysfunction is reported to be one of the potential biological mechanisms contributing to this association [8, 9]. Patients with AR and migraine experience cranial autonomic symptoms, such as rhinorrhea, nasal congestion, lacrimation, and conjunctival injection, which reflect parasympathetic hyperfunction and sympathetic hypofunction [8, 9]. Several basic and clinical studies have reported the important role of parasympathetic hyperactivity in the development of AR and migraine. Furthermore, AR and migraine share the same pathogenic mechanisms, which are based on immune dysfunction and inflammation [10, 11]. The presence of the inflammatory microenvironment in AR may contribute to the aggravation and development of migraine. For example, the levels of many pro-inflammatory mediators involved in AR pathogenesis, such as prostaglandins (PGs), leukotrienes, histamine, nitric oxide (NO), and calcitonin gene-related peptide (CGRP) are significantly upregulated during migraine attack [10, 11]. In addition to mediating allergic inflammation, PGs contribute to pain and inflammation in migraine [43, 44]. CGRP, a neuropeptide released by the trigeminal nerve, plays a crucial role in migraine pathophysiology [4]. Monoclonal antibodies targeting the CGRP system are effective in treating migraine and are considered a breakthrough in migraine pharmacotherapy [45]. CGRP also promotes allergic inflammation by modulating various immune cells, such as group 2 innate lymphoid cells (ILC2s), dendritic cells, and Th2 cells [10]. Histamine released by mast cells is a well-known inflammatory mediator that mediates allergic reactions. Additionally, histamine can increase vascular permeability and NO levels, inducing vasodilation and consequently altering the blood–brain barrier permeability and eliciting localized neurogenic inflammation [11]. These pathophysiological changes are key factors in the development of migraine [4]. Furthermore, the activation and sensitization of the trigeminal system is critical for the development of migraine and AR [46, 47].
One possible explanation for the association between AR and migraine is they share several confounders. Previous studies have demonstrated that several psychiatric disorders, such as depression, anxiety, bipolar disorder, and sleep disturbances are frequently co-prevalent in individuals with migraine and regulate migraine evolution [48, 49]. These psychiatric co-morbidities are strongly associated with increased headache frequency, medication overuse, disability, and poor quality of life in patients with migraine [48, 49]. Furthermore, large-scale GWAS revealed genetic overlap between migraine and psychiatric disorders. Yang et al. [50] reported some same genetic susceptibility loci and significant cross-disorder genetic correlation between migraine and major depressive disorder based on GWAS genotype data. Epidemiological studies have suggested that AR is closely associated with common psychiatric disorders, such as anxiety, depression, bipolar disorder, and attention-deficit/hyperactivity disorder [51, 52]. In both the pediatric and adult populations, the risk of developing psychiatric disorders in patients with AR was significantly higher than that in controls [52, 53]. The severity and duration of AR symptoms were related to poor mental health [52]. Additionally, psychological stress is a common trigger for both AR and migraine. High stress loads induce migraine in susceptible people and increase the frequency of migraine attacks in patients with migraine [54]. Additionally, high stress loads increase the severity of AR symptoms and decrease the efficacy of standard treatments [55].
The major advantage of this study is the application of a robust MR design that minimizes reverse causality and confounding factors associated with traditional observational research. Furthermore, the large-scale GWAS dataset and multiple sensitivity analyses augmented the reliability of the findings. The singular population distribution also effectively diminished the population stratification bias. However, this study has some limitations. First, the GWAS cases in this study were all of European ancestry. Therefore, further studies are needed to determine if the findings of this study can be generalized to other human populations. Second, in-depth stratified analyses, such as sex-stratified and age-stratified analyses, were not performed due to the limitations of the GWAS data. Third, the relatively small number of GWAS samples of migraine subtypes analyzed in this study may lead to decreased statistical power in the reverse MR study.
Conclusion
This MR study did not provide conclusive evidence to support a direct causal effect between the genetic predisposition to AR and the elevated risk of migraine (including any migraine, MA, and MO). Further studies are needed to elucidate the causal association between AR and migraine risk. We emphasize the benefit of screening patients with AR for migraine and adopting optimal management strategies.
Supplementary Information
Additional file 1: Table S1. Detailed information on the valid IVs associated with AR. Table S2. Detailed information on the valid IVs associated with migraine. Table S3. Detailed information on the valid IVs associated with MA. Table S4. Detailed information on the valid IVs associated with MO. Table S5. Liability-scale MR estimates of causal effect between AR and migraine.
Additional file 2: Figure S1. Funnel plot of the MR analysis. A AR on migraine; B AR on MA; C AR on MO; D migraine on AR; E MA on AR; F MO on AR. Figure S2. Estimated causal effects of migraine on AR using CAUSE analysis. A Results of expected log pointwise posterior density (ELPD) and plots of the posterior distributions of the parameters for the sharing model and causal model; B Scatter plots of the data showing for each model, the probability that each variant is acting through the shared factor and the contribution of each variant to the ELPD test statistic. Figure S3. Estimated causal effects of MA on AR using CAUSE analysis. A Results of ELPD and plots of the posterior distributions of the parameters for the sharing model and causal model. B Scatter plots of the data showing for each model, the probability that each variant is acting through the shared factor and the contribution of each variant to the ELPD test statistic. Figure S4. Estimated causal effects of MO on AR using CAUSE analysis. A Results of ELPD and plots of the posterior distributions of the parameters for the sharing model and causal model. B Scatter plots of the data showing for each model, the probability that each variant is acting through the shared factor and the contribution of each variant to the ELPD test statistic.
Acknowledgements
We thank Mr. Yu Hu from Hubei University of Technology for his assistance with using the R software. We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Author contributions
HL and KYL contributed to conceptualization, methodology, and writing draft. YLX, YFW, SYC, PQL contributed to methodology, visualization and formal analysis. MTG and JCC contributed to formal analysis and validation. YX contributed to review, editing, supervision, project administration. All authors read and approved the final version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (NSFC): No. 82071017 (to Yu Xu); No. 82271134 (to Yu Xu) and the Fundamental Research Funds for the Central Universities: No. 2042020kf1044 (to Yu Xu).
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao Lv and Kunyu Liu contributed equally to this work.
References
- 1.Zhang Y, Lan F, Zhang L. Update on pathomechanisms and treatments in allergic rhinitis. Allergy. 2022;77(11):3309–3319. doi: 10.1111/all.15454. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nat Rev Dis Primers. 2020;6(1):95. doi: 10.1038/s41572-020-00227-0. [DOI] [PubMed] [Google Scholar]
- 3.Wise SK, Damask C, Roland LT, et al. International consensus statement on allergy and rhinology: allergic rhinitis—2023. Int Forum Allergy Rhinol. 2023;13(4):293–859. doi: 10.1002/alr.23090. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari MD, Goadsby PJ, Burstein R, et al. Migraine. Nat Rev Dis Primers. 2022;8(1):2. doi: 10.1038/s41572-021-00328-4. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2016 Neurology Collaborators Neurology Collaborators Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashina M, Terwindt GM, Al-Karagholi MA, et al. Migraine: disease characterisation, biomarkers, and precision medicine. Lancet. 2021;397(10283):1496–1504. doi: 10.1016/S0140-6736(20)32162-0. [DOI] [PubMed] [Google Scholar]
- 7.Hovaguimian A, Roth J. Management of chronic migraine. BMJ. 2022;379:e067670. doi: 10.1136/bmj-2021-067670. [DOI] [PubMed] [Google Scholar]
- 8.Özge A, Uluduz D, Bolay H. Co-occurrence of migraine and atopy in children and adolescents: myth or a casual relationship? Curr Opin Neurol. 2017;30(3):287–291. doi: 10.1097/WCO.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 9.Gryglas A. Allergic rhinitis and chronic daily headaches: is there a link? Curr Neurol Neurosci Rep. 2016;16(4):33. doi: 10.1007/s11910-016-0631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han JH, Lee HJ, Yook HJ, et al. Atopic disorders and their risks of migraine: a nationwide population-based cohort study. Allergy Asthma Immunol Res. 2023;15(1):55–66. doi: 10.4168/aair.2023.15.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang IC, Tsai JD, Lin CL, et al. Allergic rhinitis and associated risk of migraine among children: a nationwide population-based cohort study. Int Forum Allergy Rhinol. 2016;6(3):322–327. doi: 10.1002/alr.21654. [DOI] [PubMed] [Google Scholar]
- 12.Martin VT, Fanning KM, Serrano D, et al. Chronic rhinitis and its association with headache frequency and disability in persons with migraine: results of the American Migraine Prevalence and Prevention (AMPP) Study. Cephalalgia. 2014;34(5):336–348. doi: 10.1177/0333102413512031. [DOI] [PubMed] [Google Scholar]
- 13.Forcelini CM, Gradaschi RTS, Tonin GA, et al. Is allergic rhinitis related to migraine disability in adults? Arq Neuropsiquiatr. 2019;77(6):424–428. doi: 10.1590/0004-282x20190063. [DOI] [PubMed] [Google Scholar]
- 14.Güvenç IA, Acar M, Muluk NB, et al. Is there an association between migraine and allergic rhinitis? Ear Nose Throat J. 2017;96(6):E18–e23. doi: 10.1177/014556131709600604. [DOI] [PubMed] [Google Scholar]
- 15.Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614–1621. doi: 10.1001/jama.2021.18236. [DOI] [PubMed] [Google Scholar]
- 16.Richmond RC, Davey SG. Mendelian randomization: concepts and scope. Cold Spring Harb Perspect Med. 2022;12(1):a040501. doi: 10.1101/cshperspect.a040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekula P, Del Greco MF, Pattaro C, et al. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. 2016;27(11):3253–3265. doi: 10.1681/ASN.2016010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Wang P, Zhang RD, et al. Mendelian randomization as a tool to gain insights into the mosaic causes of autoimmune diseases. Autoimmun Rev. 2022;21(12):103210. doi: 10.1016/j.autrev.2022.103210. [DOI] [PubMed] [Google Scholar]
- 19.Khasawneh LQ, Al-Mahayri ZN, Ali BR. Mendelian randomization in pharmacogenomics: the unforeseen potentials. Biomed Pharmacother. 2022;150:112952. doi: 10.1016/j.biopha.2022.112952. [DOI] [PubMed] [Google Scholar]
- 20.Kamat MA, Blackshaw JA, Young R, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851–4853. doi: 10.1093/bioinformatics/btz469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bi Y, Zhu Y, Tang S, et al. Lipids, lipid-modifying drug target genes and migraine: a Mendelian randomization study. J Headache Pain. 2023;24(1):112. doi: 10.1186/s10194-023-01633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin C, Li J, Deng Y, et al. Effect of obesity, lipids and adipokines on allergic rhinitis risk: a Mendelian randomization study. Braz J Otorhinolaryngol. 2023;89(5):101306. doi: 10.1016/j.bjorl.2023.101306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–764. doi: 10.1093/ije/dyr036. [DOI] [PubMed] [Google Scholar]
- 24.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowden J, Davey Smith G, Haycock PC, et al. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–389. doi: 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess S, Davey Smith G, Davies NM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 2019;4:186. doi: 10.12688/wellcomeopenres.15555.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison J, Knoblauch N, Marcus JH, et al. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat Genet. 2020;52(7):740–747. doi: 10.1038/s41588-020-0631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrne EM, Zhu Z, Qi T, et al. Conditional GWAS analysis to identify disorder-specific SNPs for psychiatric disorders. Mol Psychiatry. 2021;26(6):2070–2081. doi: 10.1038/s41380-020-0705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trikojat K, Buske-Kirschbaum A, Schmitt J, et al. Altered performance in attention tasks in patients with seasonal allergic rhinitis: seasonal dependency and association with disease characteristics. Psychol Med. 2015;45(6):1289–1299. doi: 10.1017/S0033291714002384. [DOI] [PubMed] [Google Scholar]
- 31.Stovner LJ, Andree C. Prevalence of headache in Europe: a review for the Eurolight project. J Headache Pain. 2010;11(4):289–299. doi: 10.1007/s10194-010-0217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verbanck M, Chen CY, Neale B, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lv X, Xu B, Tang X, et al. The relationship between major depression and migraine: a bidirectional two-sample Mendelian randomization study. Front Neurol. 2023;14:1143060. doi: 10.3389/fneur.2023.1143060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greco MF, Minelli C, Sheehan NA, et al. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926–2940. doi: 10.1002/sim.6522. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Gu X, Wang X, et al. Exploring genetic associations between allergic diseases and indicators of COVID-19 using mendelian randomization. iScience. 2023;26(6):106936. doi: 10.1016/j.isci.2023.106936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ku M, Silverman B, Prifti N, et al. Prevalence of migraine headaches in patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97(2):226–230. doi: 10.1016/S1081-1206(10)60018-X. [DOI] [PubMed] [Google Scholar]
- 38.Ozturk A, Degirmenci Y, Tokmak B, et al. Frequency of migraine in patients with allergic rhinitis. Pak J Med Sci. 2013;29(2):528–531. doi: 10.12669/pjms.292.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aamodt AH, Stovner LJ, Langhammer A, et al. Is headache related to asthma, hay fever, and chronic bronchitis? The Head-HUNT study. Headache. 2007;47(2):204–212. doi: 10.1111/j.1526-4610.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- 40.Ferretti A, Gatto M, Velardi M, et al. Migraine, allergy, and histamine: is there a link? J Clin Med. 2023;12(10):3566. doi: 10.3390/jcm12103566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muñoz-Jareño N, Fernández-Mayoralas DM, Martínez-Cervell C, et al. Relationship between migraine and atopy in childhood: a retrospective case-control study. Rev Neurol. 2011;53(12):713–720. [PubMed] [Google Scholar]
- 42.Eross E, Dodick D, Eross M. The sinus, allergy and migraine study (SAMS) Headache. 2007;47(2):213–224. doi: 10.1111/j.1526-4610.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 43.Peebles RS., Jr Prostaglandins in asthma and allergic diseases. Pharmacol Ther. 2019;193:1–19. doi: 10.1016/j.pharmthera.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antonova M, Wienecke T, Olesen J, et al. Prostaglandins in migraine: update. Curr Opin Neurol. 2013;26(3):269–275. doi: 10.1097/WCO.0b013e328360864b. [DOI] [PubMed] [Google Scholar]
- 45.Cohen F, Yuan H, DePoy EMG, et al. The arrival of anti-CGRP monoclonal antibodies in migraine. Neurotherapeutics. 2022;19(3):922–930. doi: 10.1007/s13311-022-01230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Undem BJ, Taylor-Clark T. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol. 2014;133(6):1521–1534. doi: 10.1016/j.jaci.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashina M, Hansen JM, Do TP, et al. Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol. 2019;18(8):795–804. doi: 10.1016/S1474-4422(19)30185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergman-Bock S. Associations between migraine and the most common psychiatric co-morbidities. Headache. 2018;58(2):346–353. doi: 10.1111/head.13146. [DOI] [PubMed] [Google Scholar]
- 49.Pelzer N, de Boer I, van den Maagdenberg A, et al. Neurological and psychiatric comorbidities of migraine: concepts and future perspectives. Cephalalgia. 2023;43(6):3331024231180564. doi: 10.1177/03331024231180564. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y, Zhao H, Boomsma DI, et al. Molecular genetic overlap between migraine and major depressive disorder. Eur J Hum Genet. 2018;26(8):1202–1216. doi: 10.1038/s41431-018-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tzeng NS, Chang HA, Chung CH, et al. Increased risk of psychiatric disorders in allergic diseases: a nationwide, population-based, cohort study. Front Psychiatry. 2018;9:133. doi: 10.3389/fpsyt.2018.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim DH, Han K, Kim SW. Relationship between allergic rhinitis and mental health in the general Korean adult population. Allergy Asthma Immunol Res. 2016;8(1):49–54. doi: 10.4168/aair.2016.8.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112(6):525–532. doi: 10.1016/j.anai.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Sauro KM, Becker WJ. The stress and migraine interaction. Headache. 2009;49(9):1378–1386. doi: 10.1111/j.1526-4610.2009.01486.x. [DOI] [PubMed] [Google Scholar]
- 55.El Hennawi DD, Ahmed MR, Farid AM. Psychological stress and its relationship with persistent allergic rhinitis. Eur Arch Otorhinolaryngol. 2016;273(4):899–904. doi: 10.1007/s00405-015-3641-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Detailed information on the valid IVs associated with AR. Table S2. Detailed information on the valid IVs associated with migraine. Table S3. Detailed information on the valid IVs associated with MA. Table S4. Detailed information on the valid IVs associated with MO. Table S5. Liability-scale MR estimates of causal effect between AR and migraine.
Additional file 2: Figure S1. Funnel plot of the MR analysis. A AR on migraine; B AR on MA; C AR on MO; D migraine on AR; E MA on AR; F MO on AR. Figure S2. Estimated causal effects of migraine on AR using CAUSE analysis. A Results of expected log pointwise posterior density (ELPD) and plots of the posterior distributions of the parameters for the sharing model and causal model; B Scatter plots of the data showing for each model, the probability that each variant is acting through the shared factor and the contribution of each variant to the ELPD test statistic. Figure S3. Estimated causal effects of MA on AR using CAUSE analysis. A Results of ELPD and plots of the posterior distributions of the parameters for the sharing model and causal model. B Scatter plots of the data showing for each model, the probability that each variant is acting through the shared factor and the contribution of each variant to the ELPD test statistic. Figure S4. Estimated causal effects of MO on AR using CAUSE analysis. A Results of ELPD and plots of the posterior distributions of the parameters for the sharing model and causal model. B Scatter plots of the data showing for each model, the probability that each variant is acting through the shared factor and the contribution of each variant to the ELPD test statistic.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.