Abstract
Overactivation of the NLRP3 inflammasome is implicated in chronic low-grade inflammation associated with various disease states, including obesity, type 2 diabetes, atherosclerosis, Alzheimer's disease, and Parkinson's disease. Emerging evidence, mostly from cell and animal models of disease, supports a role for ketosis in general, and the main circulating ketone body beta-hydroxybutyrate (BHB) in particular, in reducing NLRP3 inflammasome activation to improve chronic inflammation. As a result, interventions that can induce ketosis (e.g., fasting, intermittent fasting, time-restricted feeding/eating, very low-carbohydrate high-fat ketogenic diets) and/or increase circulating BHB (e.g., exogenous ketone supplementation) have garnered increasing interest for their therapeutic potential. The purpose of the present review is to summarize our current understanding of the literature on how ketogenic interventions impact the NLRP3 inflammasome across human, rodent and cell models. Overall, there is convincing evidence that ketogenic interventions, likely acting through multiple interacting mechanisms in a cell-, disease- and context-specific manner, can reduce NLRP3 inflammasome activation. The evidence supports a direct effect of BHB, although it is important to consider the myriad of other metabolic responses to fasting or ketogenic diet interventions (e.g., elevated lipolysis, low insulin, stable glucose, negative energy balance) that may also impact innate immune responses. Future research is needed to translate promising findings from discovery science to clinical application.
Keywords: Ketogenic diets, NLRP3 inflammasome, Fasting, Beta-hydroxybutyrate, Inflammation
Highlights
-
•
Fasting and ketogenic interventions suppress NLRP3 activation, likely via BHB
-
•
BHB inhibits NLRP3 activation through multiple mechanisms
-
•
BHB inhibits K+ efflux, ROS, and ER stress-induced NLRP3 inflammasome activation
-
•
BHB likely acts differentially across species, diseases and physiological contexts
1. Introduction
1.1. Relevance of the NLRP3 inflammasome in health & disease
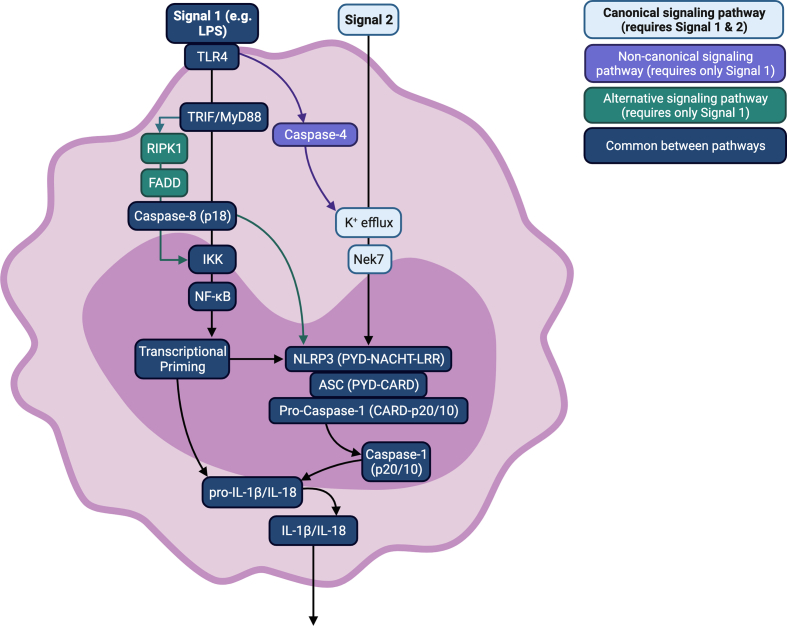
Persistent low-grade inflammation, largely driven by overactivation of cells of the innate immune system, is now recognized to play a significant role in many chronic and aging-associated diseases, including obesity, type 2 diabetes (T2D), cardiovascular disease (CVD), and Alzheimer's disease. Understanding the cellular and molecular processes underpinning such chronic inflammatory disease states is therefore important as it may guide potential (anti-inflammatory) treatments. Indeed, such understanding has led to anti-inflammatory therapy such as interleukin (IL)-1 receptor blockade or IL-1β antagonists, which block pro-inflammatory IL-1β signaling, with randomized controlled trials showing slower progression of CVD and T2D using such agents [1,2]. The NOD-like receptor (NLR) family pyrin domain containing 3 (NLRP3) inflammasome is one molecular pathway involved in innate immune activation that has been repeatedly implicated in chronic inflammation associated with various disease states [3]. The detailed molecular mechanisms of NLRP3 signaling and activation have been extensively described in several recent reviews [[4], [5], [6], [7], [8]] and a summary is presented in [Fig. 1].
Fig. 1.
Mechanistic overview of NLRP3 inflammasome signalling. Abbreviations:ASC: associated speck-like protein; CARD: caspase activation and recruitment domain; FADD:Fas-associated death domain protein; IKK:IkB kinase; IL: interleukin; LPS: lipopolysaccharide; MyD88: myeloid differentiation primary response 88; NACHT: NAIP (neuronal apoptosis inhibitor protein), C2TA (class 2 transcription activator, of the MHC), HET-E (heterokaryon incompatibility) and TP1 (telomerase-associated protein 1); Nek7: never in mitosis gene A (NIMA)-related kinase; NF-kB: nuclear factor k-light-chain-enhancer of activated B cells; NLRP3:NOD-like receptor pyrin domain containing 3; PYD: pyrin domain; LRR: C-terminal receptor domain of leucine-rich repeats; RIPK1: receptor-interacting serine/threonine-protein kinase 1; TLR4: toll-like receptor 4; TRIF: toll-interleukin-1 receptor (TIR) domain containing adaptor inducing interferon beta.
Briefly, a danger- or pathogen-associated molecular pattern (DAMP or PAMP) binds its ligand (e.g., toll-like receptor (TLR)) which induces activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and consequent transcription of NLRP3 and IL1B mRNA. In canonical NLRP3 signalling, a second activating signal is required following the initial priming signal. The second signal induces NLRP3 oligomerization and recruitment of NLRP3, associated speck-like protein (ASC) and pro-caspase-1. Pro-caspase-1 is cleaved autoproteolytically, and the resulting caspase-1 cleaves pro-IL-1β and pro-IL-18 into their active forms, which are then secreted from the cell [3]. It is important to acknowledge that our understanding of the mechanisms by which the NLRP3 inflammasome is activated is largely based on studies in rodents, and evidence suggests that alternative mechanisms of activation exist in human immune cells in addition to the canonical two-step model [9]. Importantly, in human monocytes, a single TLR4-mediated stimulus is sufficient to activate the NLRP3 inflammasome, which can occur independently of caspase-4 and K+ efflux. This single-step model of activation, coined “alternative activation”, induces a more moderated inflammatory response in which pyroptosis does not occur and less IL-1β is secreted [9]. A final “non-canonical” mode of activation has also been identified in which rodent caspase-11 is also activated by a single TLR4-mediated stimulus and activates NLRP3 directly via K+ efflux [[9], [10], [11], [12]]. Despite these multiple modes of NLRP3 inflammasome activation, the ultimate release of pro-inflammatory cytokines remains a tightly regulated process.
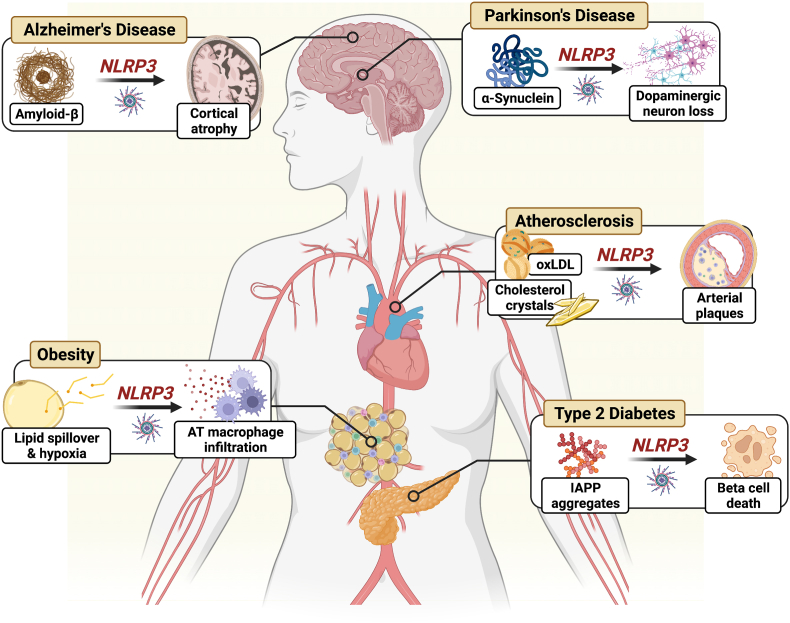
Numerous studies, in both rodent and human models, have reported heightened NLRP3 activation in conditions where chronic low-grade inflammation is implicated in disease pathophysiology [Fig. 2]. This includes NLRP3 in adipose tissue-infiltrating macrophages, which contribute to the development of insulin resistance and low-grade inflammation in obesity [3] and in pancreas-infiltrating macrophages, which respond to a myriad of metabolites (e.g., ceramides, saturated fatty acids, hyperglycemia), reactive oxygen species (ROS), and aggregated islet amyloid polypeptide (IAPP) contributing to beta cell death/dysfunction in T2D [3,13]. NLRP3 is also implicated in atherosclerotic cardiovascular disease. Macrophage NLRP3 in atherosclerotic plaques are activated by oxidized LDL, cholesterol crystals, and calcium phosphate crystals [3,14]. Additionally, NLRP3 in microglia contribute to the pathogenesis of Alzheimer's disease in response to amyloid-β and aggregated Tau [3,15], and microglia and astrocyte NLRP3 respond to α-synuclein via TLR2 in Parkinson's disease (PD), which is likely compounded by the loss of dopaminergic neurons which negatively regulate NLRP3 activation [3]. In many instances, the effects observed in cells and tissues obtained from intact animals and humans can be recapitulated in cell culture models using disease-specific stimuli (e.g., IAPP for T2D, amyloid-β for AD) suggesting that overactivation of the NLRP3 inflammasome can be specific to disease pathology. Therefore, reducing activation of the NLRP3 inflammasome is hypothesized to help lower inflammation in many chronic disease states [3].
Fig. 2.
The NLRP3 inflammasome as a central mediator of disease. AT, adipose tissue; NLRP3, NOD-like receptor pyrin domain containing 3; oxLDL, oxidized low-density lipoprotein; IAPP, islet amyloid polypeptide. Abbreviations:AT: adipose tissue; IAPP: islet amyloid polypeptide; NLRP3: NOD-like receptor pyrin domain containing 3; oxLDL: oxidized low-density lipoprotein.
The purpose of the present review is to summarize our current understanding of the literature on ketogenic interventions and NLRP3 across human, rodent and cell models. This review also aims to bring nuance to the broader physiological effects of ketogenic interventions and their potential effects on signalling through the NLRP3 inflammasome.
2. Methods
Here we present a narrative review with defined search criteria. All searches were conducted in the MEDLINE database. To adequately encompass the variety of names used to describe ketogenic interventions, studies were identified if they contained any of the following MeSH terms: intermittent fasting; fasting; caloric restriction; diet, carbohydrate-restricted; diet, high-protein low carbohydrate; diet, ketogenic; ketones; ketone bodies; 3-hydroxybutyric acid; or the key words β-hydroxybutyrate, high-fat low-carbohydrate diet. Studies directly quantifying NLRP3 inflammasome activation were identified if they contained any of the following MeSH terms: NLR Family, Pyrin Domain-Containing 3 Protein; Inflammasomes; or the key word NLRP3 inflammasome. Combined, these search criteria identified 102 papers. Studies were included if they were original research articles and were conducted in human or rodent cell lines, ex vivo or in vivo rodent models, and ex vivo or in vivo human models, and directly quantified NLRP3 inflammasome activation in response to ketogenic interventions in the context of non-infectious disease. Additionally, reference lists of the included papers were checked for studies that may have been missed with these search criteria. Following exclusions, 30 studies were identified as relevant by these criteria [Table 1].
Table 1.
Summary of included studies investigating the effect of ketogenic interventions in cell lines, rodent, or human models.
| Model species or cell type | Sex | Disease model | Intervention |
Major Conclusion | Ref | |||
|---|---|---|---|---|---|---|---|---|
| Direct treatment with BHB | Ketogenic diet | Fasting/ADF/TRE | ||||||
| Bae et al., 2016 | Human hepatoma HepG2 cell line; Sprague-Dawley rats | Males | Aging, ER stress | ✓ | – | ✓ | BHB treatment suppresses ER stress-induced NLRP3 activation via activation of AMPK and upregulation of catalase and MnSOD. | [16] |
| Chakraborty et al., 2018 | Salt-sensitive (S) Dahl rats | Males | Hypertension | ✓ | – | – | BHB treatment reduces hypertension and protects against renal injury likely via inhibition of renal NRLP3 inflammasome activation. | [17] |
| Deora et al., 2017 | Primary microglia | Not reported | Parkinson's Disease | ✓ | – | – | BHB inhibits MSU/ATP- but not synuclein fibril-induced NLRP3 activation indicating that BHB does not suppress fibril-induced microglial NLRP3 activation. | [18] |
| Goldberg et al., 2017 | BMDMs; human- & C57BL/6 mouse-derived neutrophils; Sprague-Dawley rats | Male & female humans; rodent sex not reported | Gout, aging | ✓ | ✓ | – | BHB inhibits S100A9/MSU-induced NLRP3 activation and protects against gouty flare. | [19] |
| Goldberg et al., 2023 | HmgclAlb−Cre mice | Males & females | N/A | ✓ | – | – | Liver- but not myeloid cell-derived ketogenesis is most important for suppressing NLRP3 inflammasome activation and regulating metabolism. | [20] |
| Guo et al., 2018 | SH-SY-5Y cell line; C57BL/6 mice | Males | Stroke | ✓ | ✓ | – | KD & BHB protects against cerebral occlusion/reperfusion injury by suppression of ER- and oxidative stress-induced NLRP3 activation | [21] |
| Harun-Or-Rashid & Inman, 2018 | DBA/2J (D2) & DBA/2JGpnmb+ (D2G) mice | Males & females | Glaucoma | – | ✓ | – | KD inhibits retinal microglial NLRP3 activation via inhibition of AMPK activation and the HCAR1/ARRB2 signalling axis. | [22] |
| Jiang et al., 2022 | BV2 cell line; C57BL/6 mice | Males | Parkinson's Disease | ✓ | – | – | BHB treatment improves PD symptoms and protects against microglial pyroptosis by downregulating STAT3/NLRP3/GSDMD signalling. | [23] |
| Kim et al., 2022 | Healthy humans; isolated human macrophages | Males & females | N/A | – | ✓ | – | Acute KD and ex vivo BHB treatment inhibit NLRP3 activation. | [24] |
| Kong et al., 2021 | BV2 cell line; Sprague-Dawley rats | Males | Spinal Cord Injury (SCI) | ✓ | – | – | KD improves outcomes after SCI, inhibits NLRP3 activation in macrophages/microglia, and promote M2a differentiation following SCI. | [25] |
| Kong et al., 2022 | Sprague-Dawley rats | Males | Osteoarthritis | – | ✓ | – | KD protects against knee osteoarthritis and decreases NLRP3 activation. | [26] |
| Liang et al., 2021 | Mouse 3T3-L1 pre-adipocyte cell line; Sprague-Dawley rats | Males | Insulin resistance | – | – | ✓ | Intermittent fasting improves insulin sensitivity via inhibition of NLRP3 activation. | [27] |
| Luo et al., 2022 | HK-2 cell line; C57BL/6 mice | Males | Acute Kidney Injury | ✓ | – | – | BHB treatment protects against acute kidney injury and decreases in vivo and in vitro NLRP3 activation in renal tubular cells. | [28] |
| Miyauchi et al., 2019 | C57BL/6 mice | Males | Acute Liver Injury | ✓ | – | ✓ | Fasting for 12 h protects against liver ischemia and reperfusion injury likely via increasing autophagy and suppressing NLRP3 activation. | [29] |
| Neudorf et al., 2019 | Healthy humans | Males & females | N/A | ✓ | – | – | Acute in vivo BHB treatment via ketone monoester or ketone salt supplementation increases NLRP3 activation. | [30] |
| Neudorf et al., 2020 | Humans with obesity | Males & females | Obesity | ✓ | – | – | Acute in vivo BHB treatment decreases plasma IL-1β but does not protect against hyperglycemia-induced NLRP3 activation. | [31] |
| Poh et al., 2021 | C57BL/6NTac mice | Males | Chronic Cerebral Hypoperfusion | – | – | ✓ | Intermittent fasting protects against hypoperfusion-induced NLRP3 activation, apoptosis and pyroptosis. | [32] |
| Qian et al., 2017 | C57BL/6J mice | Males | Spinal Cord Injury | ✓ | – | – | Treatment with BHB improves outcomes from SCI by protecting against loss of motor neurons and suppressing microglial NLRP3 activation and oxidative stress. | [33] |
| Sahin et al., 2021 | Wistar albino rats | Males | Pancreatitis | ✓ | – | – | BHB treatment improves histology following acute pancreatitis and decreases NLRP3 activation. | [34] |
| Shang et al., 2018 | G6 glioma cell line | N/A | Glioma | ✓ | – | – | BHB treatment suppresses NLRP3 activation and migration in C6 glioma cells. | [35] |
| Shippy et al., 2020 | Post-mortem AD & control patient tissue samples; C57BL/6J & 5XFAD mice | Male & female mice & humans | Alzheimer's Disease (AD) | ✓ | – | – | Post-mortem RBCs and brain parenchyma contain less BHB in AD patients, and BHB treatment inhibits NLRP3 activation and protects against plaque formation in a mouse model of AD. | [36] |
| Thomsen et al., 2018 | Healthy humans | Males | N/A | ✓ | – | – | Concurrent infusion of BHB and LPS increases plasma IL-1β, IL-16, and TNF-α compared to concurrent LPS and placebo infusion. | [37] |
| Traba et al., 2015 | Healthy humans | Males & females | N/A | – | – | ✓ | Fasting for 24 h decreases NLRP3 activation while feeding increases NLRP3 activation. | [38] |
| Traba et al., 2017 | THP-1 human monocyte cell line; C57BL/6SIRT3−/- mice | Not reported | N/A | – | – | ✓ | Fasting inhibits NLRP3 activation via activation of SIRT3/SOD2. | [39] |
| Trotta et al., 2019 | C57BL/6 mice | Not reported | Retinopathy | ✓ | – | – | Treatment with BHB protects from diabetes-induced retinopathy via decreases in retinal ER stress, apoptosis, and NLRP3 activation. | [40] |
| Walsh et al., 2021 | Humans with obesity | Males & females | Obesity | ✓ | – | – | Repeated ketone monoester supplementation for 14 days decreases ex vivo LPS-stimulated NLRP3 activation. | [41] |
| Wu et al., 2022 | THP-1 macrophage cell line; C57BL/6J mice | Males | Osteolysis | ✓ | – | – | BHB treatment suppresses CoCrMo alloy particle-induced NLRP3 activation and reduces osteoclast differentiation and activity. | [42] |
| Wu et al., 2023 | C57BL/6J mice | Males | Cognitive Impairment | – | – | ✓ | Alternate-day fasting protects against whole-brain radiotherapy-induced cognitive dysfunction via decreases in NLRP3 activation and increases in neuron and astrocyte abundance. | [43] |
| Fann et al., 2014 | C57BL/6J mice | Males | Stroke | – | – | ✓ | Intermittent fasting protects against ischemia-reperfusion brain injury and suppresses NLRP3 activation. | [44] |
| Youm et al., 2015 | BMD macrophages & neutrophils; human-derived CD14+ monocytes; Nlrp3−/−, Gpr109a−/−, Ucp2−/− & Sirt2−/− mice; NLRP3L351PneoR/+ & NLRP3A350VneoR/+ mice | Male & female humans; mouse sex not reported | FACS, MWS, peritonitis | ✓ | ✓ | – | In vivo and in vitro treatment with BHB suppresses NLRP3 activation in a K+ efflux-dependent, AMPK-, ROS-, GRP109A-, and SIRT2-, UCP2-independent manner. | [45] |
Abbreviations: AD: Alzheimer's disease; ADF: alternate-day fasting; BHB: beta-hydroxybutyrate; BMD: bone-marrow derived; BMDM: bone-marrow derived macrophages; ER: endoplasmic reticulum; FACS: familial cold autoinflammatory syndrome; KD: ketogenic diet; MWS: Muckle-Wells syndrome; PD: Parkinson's Disease; SCI: spinal cord injury.
3. Metabolism during ketogenic interventions
3.1. Fasting & low-carbohydrate dietary interventions
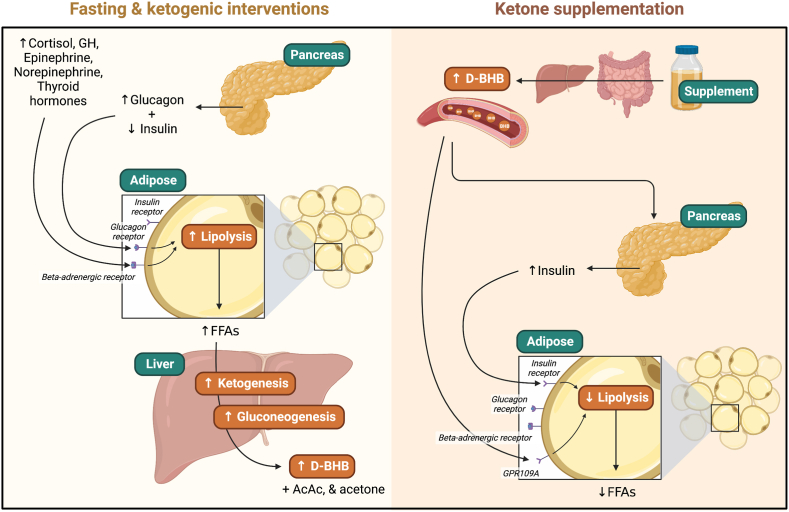
Fasting and modified-fasting interventions (e.g., intermittent fasting, time-restricted feeding/eating) induce a myriad of hormonal and metabolic changes. These changes primarily function to cue physiological systems to efficiently deriving metabolic substrates from endogenous rather than exogenous sources in order to meet energy demand in key tissues. Importantly, the hormonal response to fasting preserves limited hepatic glycogen stores and valuable amino acids by limiting carbohydrate and protein metabolism, and increasing systemic fat oxidation, hepatic gluconeogenesis, and hepatic ketogenesis. This topic has been reviewed in excellent detail elsewhere [46]. Briefly, following the cessation of the immediate postprandial period (3–5 h), circulating insulin falls over the subsequent 12–24 h. This environment of consistently low insulin, in combination with a steady increase in catecholamines, cortisol, and glucagon, promotes adipose tissue lipolysis with gradually decreasing reliance on glucose [46]. The increase in free fatty acid (FFA) mobilization causes hepatic fat oxidation to increase. As flux through the tricarboxylic acid (TCA) cycle approaches its maximal rate, acetyl CoA is preferentially used for hepatic ketogenesis such that blood ketone (acetoacetate, beta-hydroxybutyrate (BHB), and acetone) concentration increases, with blood BHB reaching approximately 1.5 mM within 48–72 hours of fasting [47]. Increasing glucagon and epinephrine in combination with low insulin also increases hepatic cAMP, which downregulates hepatic glucose uptake and glycolysis via inhibition of phosphofructokinase and consequent decreased flux through glucokinase. Increasing cAMP is also a key signal to increasing hepatic glycogenolysis by covalent modification of glycogen phosphorylase. Finally, amino acids, lactate, and glycerol (from increased lipolysis) are used by the liver for gluconeogenesis. Thus, this coordinated hormonal response facilitates glucose sparing while maintaining a minimum blood glucose level coincident with upregulated fat and ketone oxidation.
While fasting and modified-fasting interventions such as time-restricted feeding/eating and intermittent fasting can induce various levels of this altered hormonal and metabolic state with energy restriction, a very low-carbohydrate high-fat ketogenic diet (KD) can also achieve a similar state of sustained low insulin, high availability of FFAs, increased reliance on fat oxidation, and increased hepatic ketogenesis without a requirement for a caloric deficit. Although there will be differences in overall metabolic state between fasting/intermittent fasting and a very low-carbohydrate high-fat KD (e.g., pulsatile or intermittent vs. sustained ketosis, differences in postprandial triglycerides, level of energy balance), the mechanism underlying production of ketone bodies appears similar. Levels of blood BHB typically reach 0.3–0.7 mM when individuals follow a modern KD [48].
3.2. Exogenous ketone supplementation
Given the myriad of metabolic changes resulting from fasting and KD, it is difficult to determine which effects (if any) are due to ketones per se. To circumvent this, researchers have been experimenting with exogenously-induced ketosis – primarily via infusion of BHB – since as early as the 1950's [[49], [50], [51], [52]]. The last decade has seen substantial innovations and improvements in the tolerability and efficacy of orally-ingested ketone supplements such that ingestion of a ketone monoester (R-(3)-hydroxybutyl-(R)-hydroxybutyrate) and to a lesser degree, ketone salts (primarily BHB bound to a cation such as Na+, Ca2+, or Mg2+), are now an accessible means of exogenously-inducing ketosis in humans.
Following ingestion of 12–30 g of the R-(3)-hydroxybutyl-(R)-hydoxybutyrate ketone monoester supplement, blood BHB is raised to approximately 2.5 mM within 30–60 min and remains above 0.5 mM for as long as 3–4 hours [53,54]. Despite their utility in isolating the direct effects of BHB from the effects induced by severe caloric or carbohydrate restriction, ketone supplements induce a unique metabolic state of their own (depicted in [Fig. 3]). Contrary to fasting or KD, ketone supplements directly suppress lipolysis via G-protein coupled receptor (GPR)109a and induce a mild elevation in insulin [55]. Ketone supplements also consistently lower glucose [53], which is likely the result of multiple mechanisms including the increase in insulin and reduced hepatic glucose output, despite modestly elevated glucagon which is likely to be a counterregulatory response to decreased blood glucose. Thus, ketone supplements transiently induce ketosis equivalent to up to several days of fasting [47,54], but induce a metabolic state which is clearly distinct from that of endogenously-induced ketosis.
Fig. 3.
Major metabolic perturbations and differences of endogenous versus exogenous ketogenic interventions. Abbreviations: AcAc: acetoacetate; D-BHB: D-isoform of beta-hydroxybutyrate; FFA: free fatty acid; GH: growth hormone; GPR109A: G protein-coupled receptor 109A.
4. Effects of ketogenic and fasting interventions on the NLRP3 inflammasome
Fasting and modified fasting interventions have been observed to improve disease outcomes in diseases wherein the NLRP3 inflammasome is implicated. In LDLR−/− mice (a model of atherosclerotic CVD), intermittent fasting (1 day fasting; 3 days ad libitum) improves liver lipid content, reduces lesions in the aorta, and increases plaque stability [56]. Long-term (∼6 years) caloric restriction improves risk factors for atherosclerosis, including blood lipids, blood pressure, and carotid artery intima-media thickness [57]. With respect to cognitive function, older adults without memory impairment who restricted caloric intake by 30 % for 3 months improved their verbal memory scores [58]. However, whether fasting directly modified NLRP3 inflammasome activation per se was not shown until within the last decade. Traba and colleagues found that compared to after refeeding, the unprimed NLRP3 inflammasome in monocytes was less sensitive to activation following 24 h of fasting in healthy humans, as quantified by reduced secretion of IL-1β and IL-18 into culture, decreased IL1B, IL18, and NLRP3 mRNA, and decreased phosphorylation of NF-κB and Iκ-Bα [38]. A follow-up study in mice found that this suppressive effect of fasting on NLRP3 inflammasome activation was dependent on sirtuin (SIRT)3-mediated deacetylation of superoxide dismutase (SOD)2 [39]. Furthermore, acute starvation of rats decreased caspase-1, ASC, NLRP3, IL-1β, and IL-18 with a simultaneous reduction of markers of endoplasmic reticulum (ER) stress, including phosphorylated protein kinase R (PKR)-like endoplasmic reticulum kinase (pPERK), phosphorylated inositol-requiring enzyme 1 (pIRE1), and activating transcription factor (ATF)6α [16]. Other studies have shown similar suppressive effects of fasting on NLRP3 activation. Alternate-day fasting for 6–8 weeks in high-fat diet-fed and streptozotocin-treated rats [27] and mice with cognitive impairment [43], 12 hours of fasting in mice with acute liver injury [29], and 4 months of time-restricted feeding (16 hours daily food deprivation) in mice with chronic cerebral hypoperfusion [32] or stroke [44] have all been shown to effectively suppress NLRP3 activation and the pathologic symptoms associated with each disease model. While these limited data evaluating the direct effect of fasting on NLRP3 inflammasome activation clearly show that fasting decreases transcription and translation of the NLRP3 inflammasome signalling components, it is less clear if this effect is due to the removal of an activating molecule (e.g., lowered blood glucose, negative energy balance) or the addition of a suppressive signal (e.g., elevated BHB).
Intriguingly, many of the same beneficial outcomes that can be achieved with fasting may also be achievable with KD. This interesting observation indicates that severe caloric restriction per se is sufficient but not required for induction of the suppressive signals responsible for mediating the down-regulated sensitivity of the NLRP3 inflammasome to activation. In a model of gout, rats fed KD for one week had lower serum IL-1β and less knee swelling compared to mice fed normal chow [19]. In a stroke model, mice were fed an isocaloric KD for three weeks, following which an occlusion and reperfusion injury was administered to the middle cerebral artery. Compared to mice fed normal chow or a high carbohydrate diet, mice fed a KD exhibited lower thioredoxin-interacting protein (TXNIP) and NLRP3 protein, and decreased caspase-1 activity and IL-1β which coincided with smaller infarct volume, and better preserved blood-brain barrier permeability and cognitive function [21]. Similarly, 8 weeks of KD in mice with glaucoma exhibited decreased NLRP3 and IL1B mRNA and protein in retinal microglia [22] and decreased markers of NLRP3 activation resulting in reduced osteoarthritis-associated damage [26]. Macrophages isolated from healthy humans who were fed an isocaloric KD for three days secreted less IL-1β and participants had improved insulin sensitivity [24]. Thus, isocaloric severe carbohydrate restriction (i.e., a very low-carbohydrate KD) appears to mimic the suppressive effect of fasting on markers of NLRP3 inflammasome activation and mediates a protective effect against certain non-infectious diseases.
While fasting, modified fasting interventions, and KD interventions have distinct caloric differences, one of the primary commonalities of these interventions is the elevation of circulating ketone bodies. Interestingly, several of the above studies using a fasting or KD intervention were able to replicate their findings by either infusing rodents with BHB or treating cells with BHB. Bae and colleagues found that in addition to the acutely starved rats having higher serum BHB, rats that were injected with BHB (200 mg/kg) exhibited comparable suppression of the same markers of ER stress (pPERK, pIRE1, and ATF6α), improved markers of insulin sensitivity (decreased phosphorylation of c-Jun N-terminal kinase (JNK) and increased phosphorylation of protein kinase B (Akt)), and markers of NLRP3 inflammasome activation [16]. Similarly, Goldberg and colleagues found that caspase-1 activation and IL-1β secreted from bone marrow-derived macrophages (BMDMs), rodent neutrophils, and human neutrophils in response to priming and activation by S100A8 fibrils and monosodium urate (MSU) crystals, lipopolysaccharide (LPS) and ATP, and ceramides [19] was dose-dependently suppressed by BHB treatment (1-10 mM BHB). Guo and colleagues found that cells from a stroke model treated with BHB prior to oxygen and glucose deprivation followed by reoxygenation exhibited improved viability, mitochondrial morphology, and mitochondrial function, decreased NLRP3 protein, caspase-1 activity, and IL-1β secretion [21]. The finding that BHB per se suppresses NLRP3 inflammasome activation has been widely reproduced in cell and rodent models and in a variety of diseases including models of Parkinson's disease, stroke, Alzheimer's disease, spinal cord injury, acute kidney injury, liver injury, hypertension, pancreatitis, diabetes-induced retinopathy, type 1 diabetes, and insulin resistance [17,18,20,23,25,26,28,29,[33], [34], [35], [36],40,42,45]. Interestingly, the related circulating ketone body, acetoacetate, and the related short chain fatty acids, butyrate and acetate, do not appear to inhibit NLRP3 inflammasome activation, such that this suppressive effect appears specific to BHB [45]. Additionally, the effect of BHB is specific to the NLRP3 inflammasome, as studies show that BHB does not impact the NOD-like receptor family CARD domain containing protein 4 (NLRC4) or absent in melanoma 2 (AIM2) inflammasomes [45]. Thus, BHB exhibits a robust and reproducible inhibitory effect on the NLRP3 inflammasome in cell and rodent models.
In contrast, the studies utilizing directly elevated BHB in vivo in humans are limited, and the effect of BHB in vivo in healthy humans remains equivocal. Notably, concurrent infusion of BHB (2.4 ml/kg/hr) and LPS (1 ng/kg) into healthy humans actually increased circulating IL-1β compared to both placebo and lipid infusion [37]. Similarly, in two separate studies by our group, one of which used ketone salts (0.3 g/kg) and the other which used a ketone monoester (0.482 g/kg), LPS-stimulated caspase-1 activation from ex vivo whole blood cultures was increased 30 min following acute ingestion of the supplement compared to placebo. Accordingly, IL-1β secreted into the same LPS-stimulated cultures was also increased following ingestion of the ketone monoester supplement, although NLPR3 and IL1B mRNA were unchanged [30]. Thus, these data indicate that in healthy humans, concurrent exposure to BHB and LPS is in fact an NLRP3 inflammasome-activating stimulus rather than an anti-inflammatory suppressive signal.
These data are clearly in stark contrast to the consistent and robust effect reported in cell and rodent models. A major difference between the rodent/cell data and these human data is the choice of stimulus used to activate the NLRP3 inflammasome. Rodent leukocytes require a two-step NLRP3 inflammasome activation protocol in which cells are traditionally primed with LPS and activated with ATP. Human leukocytes are unique in that they can be activated using the canonical two-step protocol or alternatively they may be simultaneously primed and activated in response to LPS stimulation alone [9]. These activation methods produce distinct signalling cascades. The previously described human studies were conducted in lean, healthy humans, leading us to hypothesize that given the generally higher levels of pro-inflammatory molecules in individuals with obesity (which may prime NLRP3 [59]), the anti-inflammatory action of BHB may only manifest in individuals with chronic, low-grade inflammation. While we did observe in a subsequent study that hyperglycemia from an oral glucose tolerance test (OGTT) did augment caspase-1 activation, acutely elevated BHB from ketone monoester supplementation did not blunt this [31]. However, when individuals with obesity ingested a ketone monoester supplement thrice daily for 14 days, we observed that LPS-stimulated caspase-1 activation and secretion of IL-1β into cultures was significantly blunted, despite no differences in fasting plasma cytokines [41]. Notably, blood samples from this study were collected in the fasted state on the morning following completion of the 14-day intervention such that blood BHB was not elevated beyond normal basal concentrations (0.1–0.2 mM) at the time of measurement. Therefore, these data suggest that consistent exposure to BHB may have an inhibitory effect in humans, but may require a sufficient background of low-grade inflammation to be effective, perhaps due to the NLRP3 inflammasome being activated in a manner more representative of canonical NLRP3 signalling. Intriguingly, these inhibitory effects may be maintained for some length of time following return of BHB to basal levels.
5. Proposed mechanisms of BHB-mediated suppression of NLRP3
The mechanisms responsible for NLRP3 inflammasome assembly and the post-translational modifications that regulate its activation are incompletely understood. Given the diversity of NLRP3 activators, many of which we do not yet fully understand, it is unsurprising that there exists diverse mechanisms and pathways by which the NLRP3 inflammasome may be inhibited. Accordingly, it is difficult to pinpoint the precise event that BHB may be acting upon, and if that inhibitory event is common between different activators relevant to diverse disease states. Nevertheless, it has become clear that regardless of the mechanism(s) by which BHB is acting, BHB is acting upstream of NLRP3 complex assembly. As such, BHB must be inhibiting a component of the priming signal responsible for inducing transcription and translation of NLRP3, IL1B, and IL18, and/or a component of the signalling cascade responsible for inducing assembly of the NLRP3 complex. Here we describe the most robustly-supported mechanisms by which BHB may be acting, although it should be recognized that the relevant mechanism(s) may differ by species, disease model, and activation protocol.
5.1. Inhibition of K+ efflux
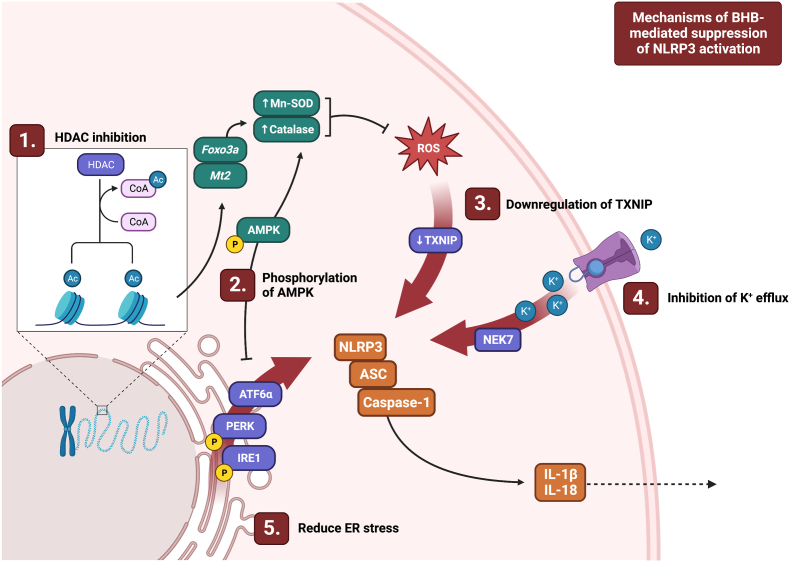
The most commonly studied activation cascade of the NLRP3 inflammasome is the two-step model in which a TLR4-mediated priming signal induces transcription of NLRP3, IL1B, and IL18, and an activating signal induces assembly of the NLRP3 complex in a K+ efflux-dependent manner. Interestingly, KD and BHB treatment not only dose-dependently prevent ASC oligomerization [23,42,45] and phosphorylation of NF-κB [19], but do so by maintaining the intracellular K+ concentration in this model [45]. However, while most NLRP3 stimuli induce K+ efflux, this is not a signalling event which is shared by all activating pathways (5). Accordingly, this potential mechanism is relevant to most commonly used activation models. Thus, BHB appears to be acting at or upstream of K+ efflux, thereby preventing the flux in intracellular ions that signal to induce downstream formation of the NLRP3 complex.
5.2. Increased oxidative stress resistance
ROS are important activators of the NLRP3 inflammasome and likely signal by interacting with TXNIP which subsequently binds NLRP3 directly [60]. Oxidative stress, which induces an inflammatory response and is implicated in many disease states, reflects an imbalance in the generation of ROS and the availability of anti-oxidant defence systems to neutralize these ROS. Interestingly, BHB has been found to function as an histone deacetylase (HDAC) inhibitor and robustly inhibits HDAC1, HDAC3, HDAC4 at elevated but physiologically-relevant concentrations (IC50 2.4–5.3 mM; [61]). Thus, BHB treatment increases acetylation of histone H3 [33,61] on lysine residues 9 and 14, which results in upregulating transcription of forkhead box (Fox)o3a and metallothionein (Mt)2, which are transcription factors that regulate the expression of anti-oxidant enzymes [61]. Subsequently, the expression of key targets of these transcription factors, manganese superoxide dismutase (Mn-SOD) and catalase, are also upregulated, coincident with reduced markers of ROS, lipid peroxidation, and NLRP3 inflammasome activation following BHB treatment [21,61]. Despite these data, some studies in rat myotubes and bovine somatic cells and cumulus-oocyte complexes have failed to show an inhibitory effect of BHB on histone deacetylation [62,63]. Interestingly, this same study found that BHB hyperacetylates bovine somatic cell nuclear transfer embryos suggesting that BHB may have a differential effect by cell type [63]. BHB has also been found to increase phosphorylation of AMPK, which can also increase expression of Mn-SOD and catalase [16]. Interestingly, Wu and colleagues found that BHB-mediated suppression of CoCrMo alloy particle-stimulated NLRP3 activation occurred independently of BHB-mediated HDAC inhibition [42]. Finally, Guo and colleagues provided evidence that BHB may downregulate expression of TXNIP, which interacts with NLRP3 in response to ROS stimulation [21]. Thus, BHB may alleviate NLRP3-activating signals by increasing resistance to oxidative stress or by downregulation of key transducer proteins, but the relevant mechanism at play likely differs based on the mechanism of activation.
5.3. Protection against ER stress
Products of ER stress are important activators of the NLRP3 inflammasome in various conditions and tissues including liver disease, kidney disease and T2D. The products of ER stress do not seem to directly activate NLRP3, but rather seem to act primarily by inducing ROS production, which in turn activates NLRP3, a process which has been excellently reviewed by Li and colleagues [64]. Both KD and BHB per se have been found to inhibit markers of ER stress and consequent NLRP3 inflammasome activation [16,21,40]. Bae and colleagues found that in vivo injection of mice with BHB reduced the key ER stress markers, pPERK, ATF6a, and pIRE1 in HepG2 cells. Accordingly, levels of NLRP3, ASC, pro-caspase-1, caspase-1, IL-1β and IL-18 protein were subsequently reduced. Interestingly, BHB treatment also suppressed markers of insulin resistance in rat liver, including pJNK, Akt, and serine 307 phosphorylation of insulin receptor substrate (IRS)-1. These improvements were attributable to a BHB-mediated increase in pAMPK [16], although whether pAMPK is required for BHB-mediated improvements in ER stress is less clear. Additionally, if BHB is inhibiting NLRP3 complex formation directly, it is difficult to tease apart its suppressive effect on ER stress-induced NLRP3 activation from its direct interaction with signalling events immediately upstream of NLRP3. Regardless, treatment with BHB effectively reduces markers of ER stress and consequently or simultaneously suppresses NLRP3 inflammasome activation.
5.4. Emerging mechanisms
Despite robust evidence that BHB acts by the aforementioned mechanisms, inhibition of K+ efflux, reduced ROS, and lower ER stress do not appear to be required for the suppressive effect of BHB on NLRP3 activation in all models. While it has been suggested that BHB may be mediating its anti-inflammatory effects by ligation of GPR109a, it has been reported that the inhibitory effect of BHB occurs independently of GPR109a [19,45]. The NLRP3-suppressor function of BHB is not dependent on SIRT2 or autophagy pathways, including uncoupling protein (UCP)2, and BHB does not need to be oxidized in the TCA cycle [19,42,45]. Additionally, Goldberg and colleagues reported that BHB acts independent of additional enzymes which can cleave IL-1β including elastase and cathepsin [19]. New mechanisms by which BHB may be acting continue to emerge in the literature. Jiang and colleagues reported that BHB may be working through the signal transducer and activator of transcription (STAT)3/GSDMD pathway [23] which has been partially supported by Shang and colleagues [35]. Kim and colleagues reported that 3 days of KD increased serum fibroblast growth factor (FGF)21, and that subsequent treatment of human macrophages with FGF21, BHB, or FGF21 in combination with BHB inhibited IL-1β secretion to a similar extent, likely working by improving autophagy [24]. There is also recent evidence that BHB can induce modification of histones by β-hydroxybutyrylation, a process which has been thoroughly reviewed by Zhou and colleagues including its potential implications [65]. However, whether β-hydroxybutyrylation is a mediator of the suppressive effects of BHB on the NLRP3 inflammasome remains to be determined. Taken together, these studies indicate that BHB is likely acting on multiple points in the activating cascade downstream of the ligand but upstream of the NLRP3 complex (summarized in[Fig. 4]). Additionally, these studies suggest that it is unlikely that there is a single central mechanism common across disease and species models on which BHB is acting.
Fig. 4.
Depiction of recently-characterized mechanisms of BHB-mediated suppression of NLRP3 inflammasome activation. Abbreviations: AMPK: 5' AMP-activated protein kinase; ASC: associated speck-like protein; ATF6a: activating transcription factor 6 a; ER: endoplasmic reticulum; Foxo3a: forkhead box O3a; HDAC: histone deacetylase; IL: interleukin; IRE1: phosphorylated inositol-requiring enzyme 1; Mn-SOD: manganese superoxide dismutase; Mt2: metallothionein 2; Nek7: never in mitosis gene A (NIMA)-related kinase; NLRP3: NOD-like receptor (NLR) family pyrin domain containing 3; PERK: protein kinase R (PKR)-like endoplasmic reticulum kinase; ROS: reactive oxygen species; TXNIP: thioredoxin-interacting protein.
6. Conclusions and future directions
Overall, there is strong evidence that induction of ketosis, likely mediated at least partially through the direct effects of BHB per se, can suppress activation of the NLRP3 inflammasome. Given the plethora of potential mechanisms involving intracellular signalling, ROS, ER stress, post-translational modifications, receptor-mediated action, autophagy, and/or mitochondrial metabolism, it is likely that any effect of BHB on the NLRP3 inflammasome and related inflammatory cascades does not work through a single mechanism. Rather, BHB likely acts through multiple synergies, interactions, and molecular mechanisms in different cell types across different time courses and different diseases and physiological contexts. This is likely why different mechanisms predominate in different models and study designs, and why human studies do not align as closely with cell and animal studies. Future studies with clever designs focusing on specific disease states and cells/tissues will need to take this into consideration in order to translate the exciting basic discovery science to clinical application.
Although BHB may act directly to inhibit overactivation of the NLRP3 inflammasome, it is important to recognize that physiological ketosis, induced through fasting and/or a KD, has a myriad of metabolic effects of which an increase in circulating BHB is just one. In vivo, an elevated rate of lipolysis, stabilized or lower glycemia, elevated fat oxidation, reduced insulin, and overall negative energy balance are likely to interact with elevated BHB to induce effects on immune cells. Given the increased interest in fasting and ketogenic dietary interventions for the treatment and/or prevention of chronic diseases, elucidating how BHB impacts inflammation promises to be an area of continued interest from a mechanistic and clinical perspective.
Funding sources
JPL was supported by a Killam Accelerator Research Fellowship and the Natural Sciences and Engineering Research Council of Canada (RGPIN-2019-05204). HN was supported by the Natural Sciences and Engineering Research Council of Canada (CGSD3-547438-2020).
Acknowledgments
All figures in this article were created with BioRender.com.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Anti-inflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 2.Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13(6):465–476. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- 3.Guo H, Callaway J, Ting J. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10(2):128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akbal A, Dernst A, Lovotti M, Mangan MSJ, McManus RM, Latz E. How location and cellular signaling combine to activate the NLRP3 inflammasome. Cell Mol Immunol. 2022;19(11):1201-14 doi: 10.1038/s41423-022-00922-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blevins HM, Xu Y, Biby S, Zhang S. The NLRP3 inflammasome pathway: a review of mechanisms and inhibitors for the treatment of inflammatory diseases. Front Aging Neurosci. 2022;14:879021. doi: 10.3389/fnagi.2022.879021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu J, Wu H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu Rev Immunol. 2023;41:301–316. doi: 10.1146/annurev-immunol-081022-021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaidt M, Ebert T, Chauhan D, Schmidt T, Schmid-Burgk J, Rapino F, et al. Human monocytes engage an alternative inflammasome pathway. Immunity. 2016;44(4):833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Schmid‐Burgk JL, Gaidt MM, Schmidt T, Ebert TS, Bartok E, Hornung V. Caspase‐4 mediates non‐canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur J Immunol. 2015;45(10):2911–2917. doi: 10.1002/eji.201545523. [DOI] [PubMed] [Google Scholar]
- 12.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 13.Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol. 2013;4:37. doi: 10.3389/fendo.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karasawa T, Takahashi M, Jichi Medical University Division of inflammation research, center for molecular medicine. Role of NLRP3 inflammasomes in atherosclerosis. J Atherosclerosis Thromb. 2017;24(5:443–451. doi: 10.5551/jat.RV17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang T, Zhang Y, Wu S, Chen Q, Wang L. The role of NLRP3 inflammasome in Alzheimer’s disease and potential therapeutic targets. Front Pharmacol. 2022;13:845185. doi: 10.3389/fphar.2022.845185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae HR, Kim DH, Park MH, Lee B, Kim MJ, Lee EK, et al. β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget. 2016;7(41):66444–66454. doi: 10.18632/oncotarget.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakraborty S, Galla S, Cheng X, Yeo JY, Mell B, Singh V, et al. Salt-responsive metabolite, beta-hydroxybutyrate, attenuates hypertension. Cell Rep. 2018;25(3):677–689.e4. doi: 10.1016/j.celrep.2018.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deora V, Albornoz EA, Zhu K, Woodruff TM, Gordon R. The ketone body beta-hydroxybutyrate does not inhibit synuclein mediated inflammasome activation in microglia. J Neuroimmune Pharmacol. 2017;12(4):568–574. doi: 10.1007/s11481-017-9754-5. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg EL, Asher JL, Molony RD, Shaw AC, Zeiss CJ, Wang C, et al. β-Hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep. 2017;18(9):2077–2087. doi: 10.1016/j.celrep.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg EL, Letian A, Dlugos T, Leveau C, Dixit VD. Innate immune cell-intrinsic ketogenesis is dispensable for organismal metabolism and age-related inflammation. J Biol Chem. 2023;299(3):103005. doi: 10.1016/j.jbc.2023.103005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo M, Wang X, Zhao Y, Yang Q, Ding H, Dong Q, et al. Ketogenic diet improves brain ischemic tolerance and inhibits NLRP3 inflammasome activation by preventing drp1-mediated mitochondrial fission and endoplasmic reticulum stress. Front Mol Neurosci. 2018;11:86. doi: 10.3389/fnmol.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harun-Or-Rashid M, Inman DM. Reduced AMPK activation and increased HCAR activation drive anti-inflammatory response and neuroprotection in glaucoma. J Neuroinflammation. 2018;15(1):313. doi: 10.1186/s12974-018-1346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Z, Yin X, Wang M, Wang Y, Li F, Gao Y, et al. beta-Hydroxybutyrate alleviates pyroptosis in MPP+/MPTP-induced Parkinson’s disease models via inhibiting STAT3/NLRP3/GSDMD pathway. Int Immunopharm. 2022;113(Pt B) doi: 10.1016/j.intimp.2022.109451. [DOI] [PubMed] [Google Scholar]
- 24.Kim ER, Kim SR, Cho W, Lee SG, Kim SH, Kim JH, et al. Short term isocaloric ketogenic diet modulates NLRP3 inflammasome via B-hydroxybutyrate and fibroblast growth factor 21. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.843520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong G, Liu J, Li R, Lin J, Huang Z, Yang Z, et al. Ketone metabolite beta-hydroxybutyrate ameliorates inflammation after spinal cord injury by inhibiting the NLRP3 inflammasome. Neurochem Res. 2021;46(2):213–229. doi: 10.1007/s11064-020-03156-2. [DOI] [PubMed] [Google Scholar]
- 26.Kong G, Wang J, Li R, Huang Z, Wang L. Ketogenic diet ameliorates inflammation by inhibiting the NLRP3 inflammasome in osteoarthritis. Arthritis Res Ther. 2022;24(1):113. doi: 10.1186/s13075-022-02802-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang BJ, Liao SR, Huang WX, Huang C, Liu HS, Shen WZ. Intermittent fasting therapy promotes insulin sensitivity by inhibiting NLRP3 inflammasome in rat model. Ann Palliat Med. 2021;10(5):5299–5309. doi: 10.21037/apm-20-2410. [DOI] [PubMed] [Google Scholar]
- 28.Luo S, Yang M, Han Y, Zhao H, Jiang N, Li L, et al. beta-Hydroxybutyrate against Cisplatin-Induced acute kidney injury via inhibiting NLRP3 inflammasome and oxidative stress. Int Immunopharm. 2022;111 doi: 10.1016/j.intimp.2022.109101. [DOI] [PubMed] [Google Scholar]
- 29.Miyauchi T, Uchida Y, Kadono K, Hirao H, Kawasoe J, Watanabe T, et al. Up-regulation of FOXO1 and reduced inflammation by beta-hydroxybutyric acid are essential diet restriction benefits against liver injury. Proc Natl Acad Sci U S A. 2019;116(27):13533–13542. doi: 10.1073/pnas.1820282116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neudorf H, Durrer C, Myette‐Cote E, Makins C, O’Malley T, Little JP. Oral ketone supplementation acutely increases markers of NLRP3 inflammasome activation in human monocytes. Mol Nutr Food Res. 2019;63(11) doi: 10.1002/mnfr.201801171. [DOI] [PubMed] [Google Scholar]
- 31.Neudorf H, Myette-Cote E, P Little J. The impact of acute ingestion of a ketone monoester drink on LPS-stimulated NLRP3 activation in humans with obesity. Nutrients. 2020;12(3):854 doi: 10.3390/nu12030854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poh L, Rajeev V, Selvaraji S, Lai MKP, Chen CLH, Arumugam TV, et al. Intermittent fasting attenuates inflammasome-associated apoptotic and pyroptotic death in the brain following chronic hypoperfusion. Neurochem Int. 2021;148 doi: 10.1016/j.neuint.2021.105109. [DOI] [PubMed] [Google Scholar]
- 33.Qian J, Zhu W, Lu M, Ni B, Yang J. D-beta-hydroxybutyrate promotes functional recovery and relieves pain hypersensitivity in mice with spinal cord injury. Br J Pharmacol. 2017;174(13):1961–1971. doi: 10.1111/bph.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahin E, Bektur Aykanat NE, Kacar S, Bagci R, Sahinturk V. Beta-hydroxybutyrate, one of the three main ketone bodies, ameliorates acute pancreatitis in rats by suppressing the NLRP3 inflammasome pathway. Turk J Gastroenterol. 2021;32(8):702–711. doi: 10.5152/tjg.2021.191062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shang S, Wang L, Zhang Y, Lu H, Lu X. The beta-hydroxybutyrate suppresses the migration of glioma cells by inhibition of NLRP3 inflammasome. Cell Mol Neurobiol. 2018;38(8):1479–1489. doi: 10.1007/s10571-018-0617-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shippy DC, Wilhelm C, Viharkumar PA, Raife TJ, Ulland TK. beta-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J Neuroinflammation. 2020;17(1):280. doi: 10.1186/s12974-020-01948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomsen HH, Rittig N, Johannsen M, Møller AB, Jørgensen JO, Jessen N, et al. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: anticatabolic impact of ketone bodies. Am J Clin Nutr. 2018;108(4):857–867. doi: 10.1093/ajcn/nqy170. [DOI] [PubMed] [Google Scholar]
- 38.Traba J, Kwarteng-Siaw M, Okoli T, Li J, Huffstutler R, Bray A, et al. Fasting and refeeding differentially regulate NRLP3 inflammasome activation in human subjects. J Clin Invest. 2015;125(12):4592–4600. doi: 10.1172/JCI83260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traba J, Geiger SS, Kwarteng-Siaw M, Han K, Ra OH, Siegel RM, et al. Prolonged fasting suppresses mitochondrial NLRP3 inflammasome assembly and activation via SIRT3-mediated activation of superoxide dismutase 2. J Biol Chem. 2017;292(29):12153–12164. doi: 10.1074/jbc.M117.791715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trotta MC, Maisto R, Guida F, Boccella S, Luongo L, Balta C, et al. The activation of retinal HCA2 receptors by systemic beta-hydroxybutyrate inhibits diabetic retinal damage through reduction of endoplasmic reticulum stress and the NLRP3 inflammasome. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0211005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh JJ, Neudorf H, Little JP. 14-Day ketone supplementation lowers glucose and improves vascular function in obesity: a randomized crossover trial. J Clin Endocrinol Metabol. 2021;106(4):e1738–54. doi: 10.1210/clinem/dgaa925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Y, Teng Y, Zhang C, Pan Y, Zhang Q, Zhu X, et al. The ketone body beta-hydroxybutyrate alleviates CoCrMo alloy particles induced osteolysis by regulating NLRP3 inflammasome and osteoclast differentiation. J Nanobiotechnol. 2022;20(1):120. doi: 10.1186/s12951-022-01320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Q, Yu M, Wang Z, Ai X, Liu Z, Zeng J, et al. Alternate-day fasting for the protection of cognitive impairment in c57BL/6J mice following whole-brain radiotherapy. Neurochem Int. 2023;162 doi: 10.1016/j.neuint.2022.105463. [DOI] [PubMed] [Google Scholar]
- 44.Fann DYW, Santro T, Manzanero S, Widiapradja A, Cheng YL, Lee SY, et al. Intermittent fasting attenuates inflammasome activity in ischemic stroke. Exp Neurol. 2014;257:114–119. doi: 10.1016/j.expneurol.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 45.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dote-Montero M, Sanchez-Delgado G, Ravussin E. Effects of intermittent fasting on cardiometabolic health: an energy metabolism perspective. Nutrients. 2022;14(3):489. doi: 10.3390/nu14030489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hashim SA, VanItallie T. Ketone body therapy: from the ketogenic diet to the oral administration of ketone esters. J Lipid Res. 2014;55(9):1818–1826. doi: 10.1194/jlr.R046599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hallberg SJ, McKenzie AL, Williams PT, Bhanpuri NH, Peters AL, Campbell WW, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 Year: an open-label, non-randomized, controlled study. Diabetes Therapy. 2018;9(2):583–612. doi: 10.1007/s13300-018-0373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madison LL, Mebane D, Unger RH, Lochner A. The hypoglycemic action of ketones. II. Evidence for a stimulatory feedback of ketones on the pancreatic beta cells. J Clin Investig. 1964;43(3):408–415. doi: 10.1172/JCI104925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Senior B, Loridan L. Direct regulatory effect of ketones on lipolysis and on glucose concentrations in man. Nature. 1968;219(5149):83–84. doi: 10.1038/219083a0. [DOI] [PubMed] [Google Scholar]
- 51.Sherwin RS, Hendler RG, Felig P. Effect of diabetes mellitus and insulin on the turnover and metabolic response to ketones in man. Diabetes (New York, NY) 1976;25(9):776–784. doi: 10.2337/diab.25.9.776. [DOI] [PubMed] [Google Scholar]
- 52.Neptune EM. Changes in blood glucose during metabolism of ß hydroxybutyrate. The American Journal of Physiology. 1956;187(3):451–453. doi: 10.1152/ajplegacy.1956.187.3.451. [DOI] [PubMed] [Google Scholar]
- 53.Falkenhain K, Daraei A, Forbes SC, Little JP. Effects of exogenous ketone supplementation on blood glucose: a systematic review and meta-analysis. Adv Nutr. 2022;13(5):1697–1714. doi: 10.1093/advances/nmac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stubbs BJ, Cox PJ, Evans R, Santer P, Miller JJ, Faull OK, et al. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:848 doi: 10.3389/fphys.2017.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falkenhain K, Islam H, Little JP. Exogenous ketone supplementation: an emerging tool for physiologists with potential as a metabolic therapy. Exp Physiol. 2023;108(2):177–187. doi: 10.1113/EP090430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Y, Su J, Yan Y, Zhao Q, Ma J, Zhu M., et al. Intermittent fasting inhibits high-fat diet–induced atherosclerosis by ameliorating hypercholesterolemia and reducing monocyte chemoattraction. Front Pharmacol. 2021;12:719750. doi: 10.3389/fphar.2021.719750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fontana L.=, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proceedings of the National Academy of Sciences - PNAS. 2004;101(17):6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Witte AV, Fobker M, Gellner R, Knecht S, Flöel A. Caloric restriction improves memory in elderly humans. Proceedings of the National Academy of Sciences - PNAS. 2009;106(4):1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 61.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Moan NL, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chriett S, Dąbek A, Wojtala M, Vidal H, Balcerczyk A, Pirola L. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci Rep. 2019;9(1):742. doi: 10.1038/s41598-018-36941-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sangalli JR, Sampaio RV, Del Collado M, da Silveira JC, De Bem THC, Perecin F, et al. Metabolic gene expression and epigenetic effects of the ketone body β-hydroxybutyrate on H3K9ac in bovine cells, oocytes and embryos. Sci Rep. 2018;8(1):13766. doi: 10.1038/s41598-018-31822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li W, Cao T, Luo C, Cai J, Zhou X, Xiao X, et al. Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl Microbiol Biotechnol. 2020;104(14):6129–6140. doi: 10.1007/s00253-020-10614-y. [DOI] [PubMed] [Google Scholar]
- 65.Zhou T, Cheng X, He Y, Xie Y, Xu F, Xu Y, et al. Function and mechanism of histone β-hydroxybutyrylation in health and disease. Front Immunol. 2022;13:981285. doi: 10.3389/fimmu.2022.981285. [DOI] [PMC free article] [PubMed] [Google Scholar]