Abstract
Background
Concomitant type 2 diabetes (T2DM) and cardiovascular disease (CVD) is frequent with a poor prognosis with high risk of comorbidities. Strict risk factor control reduces the risk for complications – yet many people do not achieve treatment targets. The complexity and fragmentation of the healthcare system may, together with the vulnerability of these patients, be a reason.
Objective
The purpose of this paper is to describe the protocol of a non-randomized interventional pilot study testing the feasibility and effect of a multidisciplinary, shared care clinic using personalized medicine and coordinated care in people living with concomitant T2D and CVD.
Methods
Participants were included from the Holbaek area in Denmark. People suffered from T2DM and CVD and were dysregulated regarding to HbA1c, cholesterol, micro/macroalbuminuaria or blood pressure. Participants went through a thorough evaluation to identify their needs and resources and received consultations every three months for one year.
Results
A total of 63 participants with T2DM and CVD were enrolled in the clinic. The participants had a mean age of 69 years and a BMI of 30.9 kg/m2. Almost 50 % had heart failure, 95 % dyslipidemia and 91 % hypertension. Around 54 % received GLP-1 agonists and 39 % received SGLT-2-inhibitors.
Perspectives
To our knowledge, a similar study with a multidisciplinary, shared care, outpatient clinic treating people living with concomitant T2DM and CVD, has not been performed previously. This study will provide information about the feasibility and efficacy of a multidisciplinary clinic based on changes in cardiovascular risk factors and medication.
Keywords: Diabetes Mellitus, Type 2, Cardiovascular Diseases, Risk Factors, Pilot Projects, Prospective Studies, Comorbidity, Vulnerable Patients
1. Introduction
The prevalence of type 2 diabetes (T2DM) as well as cardiovascular disease (CVD) has reached epidemic levels. Globally, it is estimated that 463 million people live with T2DM and 523 million people live with CVD in 2019, and CVD remain the leading cause of disease burden in the world. (Bjorner, 1998, Wenting, 2021) In Denmark, with a population of 5,8 million people (Befolkning Danmark, Rungby, 2017), 280.000 people were estimated to live with T2DM in 2018, whereas the prevalence of CVD in 2021 was estimated to be 524.000 people. (Roth, 2020, Saeedi, 2019) A Danish nationwide survey from 2017 also showed, that the prevalence of CVD in people living with T2DM was as high as 21.4 % (Rungby, 2017).
The association between T2DM and CVD is well established. Globally, CVD is a common comorbidity in T2DM. Overall, CVD affects 32,2% of patients living with T2DM, and is thereby a major cause of mortality among people living with T2DM, accounting for about half of all deaths in this patient group (Einarson, 2018).
The epidemiological association between T2DM and CVD is partly explained by shared underlying risk factors as given by the metabolic syndrome, and partly by other commonly occurring risk factors such as age, male sex and smoking (Cosentino, 2020, Classification and Diagnosis of Diabetes, 2021). Nonetheless, T2DM is an independent risk factor for CVD, driven by hyperglycemia and the progressive decrease in insulin sensitivity (Wan, 2018, Doessing and Burau, 2015, Low Wang, 2016).
National guidelines for treatment of T2DM recommend strict risk factor control, including reaching target goals for blood pressure, cholesterol levels and HbA1c. Renin-Angiotension-Aldosterone-System blockage (RAAS-blockage) and lipid-lowering drugs are widely used in primary care, but in a Danish study, only half of the people living with T2DM are at target with regards to blood pressure (BP), and 2/3 for low-density lipoprotein cholesterol (LDL-C) (Knudsen, 2013).
Despite the knowledge of the importance of regulating these risk factors to prevent CVD in people living with T2DM, the proportion of people reaching recommended treatment targets are still suboptimal (Stark Casagrande, 2013).
The reasons for this suboptimal treatment are unknown, but the organization of the Danish healthcare system, the lack of coordination between primary and secondary sector, and the specialized outpatient clinics may all contribute to this. For people living with T2DM, it is well known, that CVD is a key driver for the increased number of contacts at both the general practitioners (GP), in various outpatient clinics and hospital clinics (Struijs, 2006).
A contributing factor may be that the group of people living with T2DM and CVD is a vulnerable population with lower socioeconomic status and a shorter education (Doessing and Burau, 2015) that often interact with and oppose health-beneficial actions and changes (Vedsted, 2017).
Finally, there may be a need for tailoring treatment goals to the individual persońs capacity, situation, preferences, wishes and needs - as recommended by Danish and international guidelines. The extent to which this is done is uncertain. However, it may be difficult to personalize medical treatment and interventions for multimorbid people due to complex health-related and socio-economic conditions, as well as organizational fragmentation in the healthcare system (Barnett, 2012).
The RAMP-DM study from Hong Kong (Wan, 2018) which recruited 121.584 people with T2DM from primary care and consisted of an intervention with multidisciplinary risk assessment and management program, lead to significantly greater reductions in CVD, microvascular complications and mortality as compared to usual care. However, the people living with diabetes with already diagnosed CVD were excluded and focus was on diabetes treatment.
Our study is a pilot study with a non-randomized intervention setup to assess the feasibility of a new multidisciplinary outpatient clinic. The purpose of the new outpatient clinic for people living with concomitant T2DM and CVD is to individualize and optimize treatment management by using a small team of nurses and physicians with experience within multiple disease management. With a people centered approach, we hope to achieve better risk factor control, initiation of preventive drug according to guidelines and improved quality of life. In this paper we report the study design and baseline characteristics.
2. Method
2.1. Study design
The study was designed as a prospective, non-randomized intervention study of people living with concomitant T2DM and CVD, being treated in an outpatient clinic at Holbaek hospital for both their T2DM and CVD. The paticipants were enrolled from June 2020 until June 2021. Participants were recruited consecutively mostly from the outpatient clinics of diabetes, nephrology and cardiology, who met the inclusion criteria. Participants were also invited through the GPs in the area.
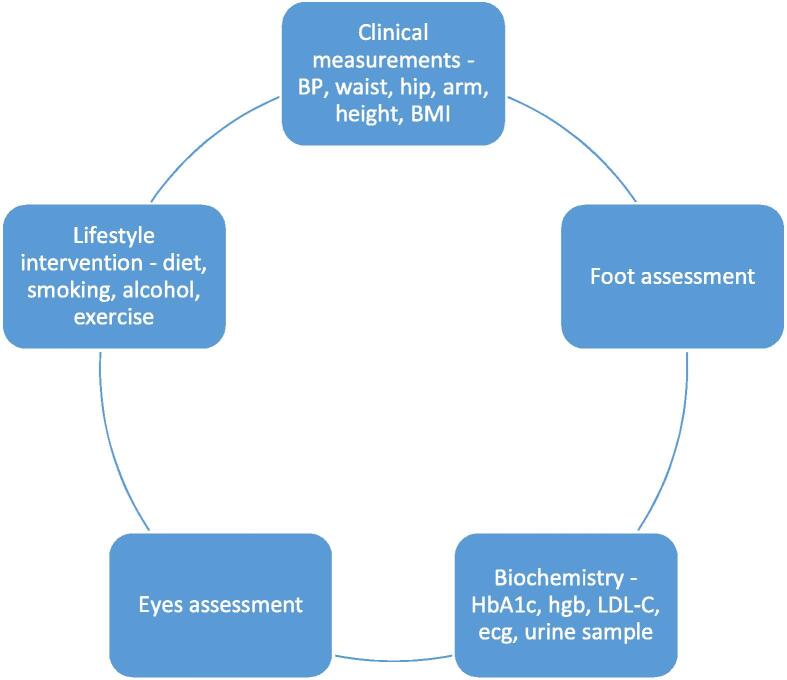
Fig. 1, Fig. 2 show the flow-chart for the setup and an overview for the examinations.
Fig. 1.
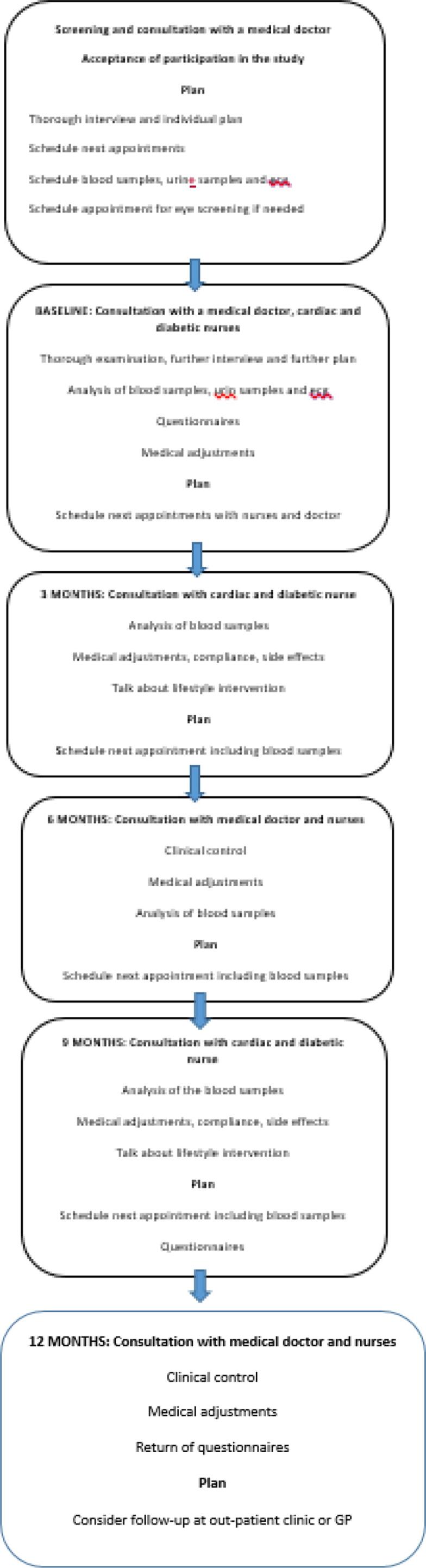
Flowchart of assessments at different visits in the multidisciplinary clinic. A multidisciplinary clinic including 63 adult participants included from June 2020 until June 2021, with type 2 diabetes and cardiovascular disease, in an outpatient clinic located at Holbaek, Denmark.
Fig. 2.
Examinations and initial thorough examination at baseline in the multidisciplinary clinic: A multidisciplinary clinic including 63 adult participants included from June 2020 until June 2021, with type 2 diabetes and cardiovascular disease, in an outpatient clinic located at Holbaek, Denmark. BP: Blood pressure; BMI: Body mass index; HbA1c: Haemoglobin A1c; Hgb: Haemoglobin; LDL-c: Low Density Lipoprotein-cholesterol.
2.2. Aims and study hypothesis
The primary aim of this pilot study is to investigate the feasibility of the outpatient clinic and the setup for this. In addition, we aim to demonstrate that the multidisciplinary, personalized care can reduce cardiovascular risk factors further and improve quality of life.
The objectives of this pilot study are to
-
1)
Assess the feasibility of delivering a multi- and interdisciplinary intervention consisting of personalized medicine and coordinated care in people living with concomitant T2DM and CVD.
-
2)
Describe the characteristics of the study population.
-
3)
Evaluate the performance of the multi- and interdisciplinary team and the coordination of care
-
4)
Evaluate the effect on cardiovascular risk factors (HbA1c, BP, LDL-C, smoking, physical activity, physical capacity) medical changes and markers of subclinical damage (albuminuria, arterial stiffness, cardiac structure and function)
-
5)
Evaluate the effect on Patient Reported Outcome, Quality of Life (SF-36) and health understanding using Health literacy questionnaire (HLQ)
2.3. Participants
Potential participants were contacted by phone to inform about the study and assess eligibility. Interested and eligible people were invited to a physical meeting. The people had a consultation with respectively the doctor and the nurses, were informed again about the clinic and the study, and signed the approved consent form for the study. Subsequently they were enrolled in the study with a follow-up time of 12 months. The inclusion and exclusion-criteria are shown in Table 1.
Table 1.
Inclusion and exclusion criteria of the participants in the multidisciplinary clinic. Inclusion of 63 adult participants in the multidisciplinary outpatient clinic, included during from June 2020 until June 2021 from the Holbaek area of Denmark.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
T2DM: Type 2 Diabetes Mellutis.
CVD: Cardiovascular Disease.
2.4. Sample size
The current trial is a feasibility trail, and as such no power calculations were performed (Billingham et al., 2013). However, to ensure sufficient power, we planned to enroll 100 consecutive people living with concomitant T2DM and CVD from the outpatient clinics, fulfilling inclusion criteria. Due to closing of outpatients clinics during the outbreak of Covid-19, we had to close enrolment after including 63 patients.
2.5. Intervention
The shared care clinic was launched as a collaboration between Holbaek Hospital, Slagelse Hospital and Steno Diabetes Center Zealand (SDCS) in June 2020. All people fulfilling the inclusion and exclusion criteria were eligible to join the study.
The workflow of the study is shown in Fig. 1, Fig. 2. Initially the participants received a consultation and thorough examination by a doctor to get a complete overview of the clinical and medical profile, together with the participant́s treatment preference and preferable goals leading to a treatment plan, that was discussed with a specialist doctor in cardiology and endocrinology. Afterwards the participant met with the diabetes- and cardiac nurses who explained the plan in detail. The nurses provided individual diabetes education, lifestyle education (diet, smoking, alcohol and exercise) and an explanation of the link between T2DM and CVD, as well as individualized information about and training in risk factor control.
Prior to the first visit in the shared care outpatient clinic, the participants had an electrocardiogram (ECG) performed and blood samples measured (haemoglobin, creatinine, estimated glomerular filtration rate, lipids (total cholesterol (TC), LDL-C, high density lipoprotein cholesterol (HDL-C)), potassium, sodium, albumin, calcium, C-reactive protein (CRP), c-peptide and thyroid gland related hormones). At the first visit clinical parameters including weight, height, body mass index (BMI), waist, hip and arm circumference, as well as blood pressure and heart rate were measured. Information on smoking status, alcohol consumption, education and working status, exercise, diabetic neuropathy, diabetic retinopathy, diabetic nephropathy, previous arteriosclerotic events and medication were collected.
The treatment plan was made as a multidisciplinary intervention including both the participant, the doctor, the nurses and the specialist team (doctors who specialized in respectively endocrinology and cardiology), and were thoroughly described in the electronic journal. The nurses were trained in T2DM and CVD, respectively, and were given rights to adjust medication according to Danish guidelines resembling current American Diabetes Association (ADA/EASD) guidelines (ElSayed, 2023). When needed, there was easy access to consulting the clinics medical doctor. At the doctors’ appointments, further clinical assessments and medical adjustments were made. The nurses were also responsible for most phone consultations.
The participants were planned to be seen both by a doctor and the nurses at baseline, 6 months and 12 months, and in addition by the nurses at 3 months and 9 months. Additional consultations were conducted according to the needs of the individual participant. The participants were offered phone consultations if necessary.
The participants were also given 4 different questionnaires at baseline and after 12 months; SF-36v2 (Anderson et al., 1996) (Bjorner, 1998), HQL 2014 (Zhang, 2007), a modified PRO and a self-designed questionnaire designed to evaluate participant satisfaction with the shared care clinic.
2.6. Outcome
The shared clinic will be evaluated from an organization and feasibility perspective, a participant perspective and a clinical perspective. All factors for primary and secondary outcome will be measured at both baseline and after 12 months of follow-up.
-
1)
The clinical perspective will be the primary outcome consisting of the percentage of people reaching target goals for HbA1c, systolic and diastolic blood pressures, cholesterol levels, weight, BMI and medication.
-
2)
The organization perspective and thereby feasibility will be one of the secondary outcomes, evaluating the percentages of no-shows, percentages of dropouts, percentages of patients receiving evidence-based medication and the average time used by the staff.
-
3)
The patient perspective will be another secondary outcome evaluating the questionnaires concerning quality of life, understanding of health, the experience of living with a chronic disease and the participant́s assessment of the shared care clinic.
2.7. Assessment of biochemistry variables
At baseline, venous blood samples, ECG and urine sample were collected, fasting. The samples were analyzed at the local biochemistry department making the results available at the consultations one hour later, reducing the number of visits.
2.8. Statistical methods
All biochemistry data and the measured parameters were entered in the StataBE 17 database for statistical analyses. Continuous variables are presented as mean ± SD. Categorical variables are presented as percentages. The data from the questionnaires were entered in a RedCap database developed for the study.
Paired Student t-test will be used to compare continuous variables between groups, and macnemar test will be used to compare categorical variables. A p-value < 0.05 will be considered significant.
We compared male vs female, and according to a variety of clinical and biochemistral variables.
2.9. Ethics and dissemination
This study has been approved by the Medical Ethics Committee at Region Zealand, Denmark (SJ-808) and comply with the Declaration of Helsinki. Only evidence based, approved and implemented medication was used. All participants gave written informed consent before recruitment.
3. Results
Over an eight months period, participants were screened from the nephrology, diabetes and cardiac out-patient clinics, 73 were invited to participate, but 10 chose to decline, leaving 63 included in the study. There were several other eligible participants, but due to corona lockdowns in Denmark, we had to stop the inclusion earlier than anticipated.
Our study revealed a pattern of highly comorbid participants (Table 2). As expected, the participants had many comorbidities and were obese with an average BMI of almost 31. The mean age was 69 years and the patients consisted mostly of male persons (77,8%). Almost 91.5 % had hypertension and about 94.9 % had dyslipidemia. Half of the participants were diagnosed with heart failure before the inclusion (49,2%), and many of the participants had atrial fibrillation (40,7%). It also showed that 93 % had coronary artery disease, and 76 % had coronary revascularisation performed. The diabetes duration was 17.8 years.
Table 2.
Baseline characteristics of the 63 adult participants included from June 2020 until June 2021 in the multidisciplinary outpatient clinic, included from the Holbaek area in Denmark.
| All (N = 63) | Male | Female | P-value | |
|---|---|---|---|---|
| BMI | 30.9 ± 5.16 | 30.8 ± 5.5 | 31.1 ± 4 | 0.85 |
| Age | 69 ± 9.8 | 69.1 ± 9.8 | 67.5 ± 9.8 | 0.6 |
| Diabetes duration | 17.8 ± 9.5 | 18.9 ± 9.2 | 13.5 ± 9.7 | 0.06 |
| Waist circumference | 113.9 ± 14.9 | 114.9 ± 15 | 109.7 ± 13 | 0.34 |
| Hip | 109.4 ± 11.2 | 111.6 ± 10.2 | 109 ± 11.5 | 0.53 |
| Right arm | 32.3 ± 6.7 | 32.7 ± 7.2 | 30.5 ± 3.7 | 0.38 |
| Systolic BP | 135.1 ± 18.4 | 134.2 ± 15.9 | 137.9 ± 25.4 | 0.51 |
| Diastolic BP | 77.3 ± 10.5 | 77.6 ± 9 | 76.4 ± 14.6 | 0.72 |
| Pulse | 72.4 ± 11,1 | 70.7 ± 10.9 | 78 ± 10.6 | 0.03 |
| Smoking | 16.9 % | 15.6 % | 21.4 % | 0.61 |
| HbA1c | 63.8 ± 12.9 | 63.9 ± 12.7 | 63.3 ± 14.6 | 0.88 |
| Cholesterol total | 3.9 ± 1.2 | 3.81 ± 1.2 | 4.1 ± 1.3 | 0.45 |
| LDL | 1.7 ± 1.1 | 1.6 ± 1.1 | 2 ± 1.1 | 0.29 |
| HDL | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.30 |
| Triglyceride | 2.6 ± 1.9 | 2.7 ± 2.1 | 2.3 ± 1.4 | 0.47 |
| Creatinine | 123 ± 98 | 134.4 ± 107.9 | 86.6 ± 47.9 | 0.12 |
| eGFR | 64.8 ± 25.9 | 63.2 ± 26.7 | 69.7 ± 23.3 | 0.42 |
| Albuminuria ratio | 356.5 ± 989 | 442.4 ± 1123 | 91.8 ± 201 | 0.29 |
| Dysregulated hypertension | 40.7 % | 40 % | 42.86 % | 0.85 |
| Dysregulated hypercholesterolemia | 28.8 % | 24.44 % | 42.84 % | 0.18 |
| Atrial fibrillation | 40.7 % | 48.9 % | 14.3 % | 0.02 |
BMI: Body Mass Index.
BP: Blood pressure.
HbA1c: Haemoglobin A1c.
LDL: Low Density Lipoprotein
HDL: High Density Lipoprotein
eGFR: Estimated Glomerular Filtration Rate.
All participants received antidiabetic medication, 63 % received Metformin, 54 % received GLP-1, 39 % received SGLT-2-I, and only 5 % received DPP4 inhibitors. Almost half of the participants were also treated with insulin (48 %). Most of the participants received statins (86 %) and a large proportion was treated with RAAS-blockage (86 %) (Table 3). Twentyfive percent of participants received 1 antidiabetic medication, 40.7 % received 2 antidiabetic medications, 28.8 % received 3 antidiabetic medications and 5.1 % received 4 antidiabetic medications.
Table 3.
Baseline pharmalogical treatment of the 63 adult participants included from June 2020 until June 2021 in the multidisciplinary outpatient clinic, included from the Holbaek area in Denmark.
| All (%) | Male (%) | Female (%) | P-value | |
|---|---|---|---|---|
| Metformin | 62.7 | 53.3 | 92.9 | 0.01 |
| SGLT-2 | 39 | 42.2 | 28.6 | 0.80 |
| GLP-1 | 54.4 | 53.3 | 57.1 | 0.36 |
| Insulin | 50.8 | 53.5 | 42.9 | 0.49 |
| DPP4 | 6.8 | 0 | 8.9 | 0.25 |
| ACEI | 45.8 | 51.1 | 28.6 | 0.14 |
| ARB | 40.7 | 44.4 | 28.6 | 0.29 |
| Betablocks | 79.7 | 77.8 | 85.7 | 0.52 |
| Statins | 86.4 | 86.7 | 85.7 | 0.93 |
SGLT-2: Sodium-glucose Cotransporter 2 inhibitor.
GLP-1: Glucagon Like Peptid-1.
DPP4: Dipeptidyl-peptidase-4-inhibitorACEI: Angiotensin Converting Enzym Inihibitor.
ARB: Angiotensin-II-receptorantagonist.
Participants without heart failure were shown to have a significant lower BMI than participants with heart failure (P-value = 0,04). With the exception of BMI, perhaps underlining the importance of specific weight reduction interventions in this group of people, no other significant differences were found in terms of any other biochemical variables or stratification parameters. When comparing respectively participants with different HbA1c stratifications, and male compared to female, none of these showed any significant difference, with the exception that the females had a higher pulse than the male (P-value = 0,03), and the lower HbA1c category had lower pulse (P-value < 0,01). It could be a random finding, but higher heart rate has been associated with higher HbA1c before (Wenting, 2021). Participants without heart failure had a significantly lower BMI (P = 0.04) and lower waist circumference (P = 0.03).
4. Discussion
Our study underlines the poor risk factor profile seen in people living with concomitant T2DM and CVD with almost all having dyslipidemia and hypertension, and heart failure in around half of participants enrolled.
It is well established that risk factor management plays an important role in the prevention of CVD in people living with T2DM (Newman, 2017). Several studies have also shown, that early intensive risk factor control reduces CVD risk and should be a main focus in management of T2DM (Nørgaard, 2019). However, a recent cross-sectional study assessed the trends in longitudinal use of glucose-, blood pressure- and lipidlowering drugs in people diagnosed with T2DM, finding that 20 % of participants did not maintain continuity in use of the recommended medication (Chehal, 2023) highlighting the need of further examining the reasons for this.
Several trials with multi/interdisciplinary interventions have been performed in either people living with T2DM or with CVD, but not in people with both conditions. In 2019, a systematic review and meta-analysis on multidisciplinary interventions in people living with T2DM was published including 16 studies (Siaw and Lee, 2019). It showed, that HbA1c and systolic blood pressure (SBP) were significantly improved. Also, a systematic review and meta-analysis from 2021 including 39 studies in people with T2DM and hypertension, both RCT and non-RCT, with inter-professional collaborative practices showed that there was a positive association between multidisciplinary interventions and HbA1c, diastolic blood pressure (DBP) and SBP (Lee, 2021). A study from 2010 has shown that nurses, with a highly specialized training, have a high success rate in running a significant part of the consultations in hypertension treatment, leading to a higher percentage reaching recommended goals (Opgaveglidning ved behandling af hypertension., 2010).
The RAMP-DM study (Wan, 2018), was conducted as a population-based and propensity-matched 5-year prospective cohort study with 121.584 people with T2DM from primary care. A multidisciplinary risk assessment and management program for T2DM consisting of individualized care plans based on the individuaĺs risk factors according to a standardized risk-stratified guide was made, leading to a significantly greater reduction in CVD, microvascular complications and mortality as compared to usual care. The benefits of the RAMP-DM intervention extended beyond simply improving conventional disease parameters, and poeple with the lower baseline CVD risk had a higher relative risk reduction compared to poeple with higher baseline CVD risk highlighting the importance of early intervention.
An analysis of the STENO2 study also showed, that the benefits of the multifactorial intervention extended beyond the reduction of the individual risk factors (Gaede, 2008).
There are several possible explanations for the success of the RAMP-DM study: The RAMP-DM study had initial risk stratification, coordinated care managed by the RAMP nurses, health care professionals with experience in treating diabetes, and reminder systems for all appointments leading to higher compliance and earlier identification of diabetic complications enabling early appropriate adjustment of pharmacological treatment.
However, limitations in the RAMP study were respectively the prospective setup, possible lack of validation of complications coding, mental lifestyle behavior and drug adherence, leading to misinterpretation of CVD and mortality risk. The RAMP participants were matched using a propensity score based on all baseline covariates. The challenges with the matched cohort was, that there was a limited number of participants receiving usual care, whereby not all RAMP-DM participants were matched, leading to potential biases.
The RAMP-DM showed, that the participants with a low risk had a higher relative risk reduction compared to participants with high risk. The authors concluded, that a possible explanation was, that the participants with at high risk already had developed complications making them more resistant to treatment whereas the low-risk participants were more sensitive to treatment making prevention of complications easier.
Our study consisted of participants with known macrovascular complications in form of diagnosed CVD which to some degree are irreversible conditions. People with known CVD or microvascular disease were excluded in the RAMP study. Therefore, our participants were in even higher risk than participants in the RAMP study. This consideration alone, makes it relevant to test the effect of a multidisciplinary intervention in our multimorbid patient group. To our knowledge, there is still lack of specific studies addressing multidisciplinary intervention in out-patient clinics in people living with concomitant T2DM and CVD, treated by educated medical doctors and nurses, and aiming to improve quality of life and reduce risk factors and ultimately micro- and macrovascular complications.
We hypothesize, that the multidisciplinary shared care clinic, despite intentionally including patients with concomitant T2DM and CVD will be as successful as the RAMP-DM intervention supporting these multimorbid people to reach more of their many recommended treatment goals and at the time improve their quality of life.
5. Conclusion
To our knowledge, a trial testing a multidisciplinary outpatient clinic treating people living with concomitant T2DM and CVD has not previously been performed. The study will provide information on a multidisciplinary clinic and outcome, from both a clinical, organizational and participant́s perspective with a setup of treatment planned by educated nurses and specialists. Measurements will include changes in cardiovascular risk factors, analyses of questionnaires regarding quality of life, and evaluation of percentages of absences, dropouts, patients receiving evidence-based medication and the time consumption for the staff.
The outcome of the trial will hopefully result in the preparation of a large-scale multicenter randomized controlled study to substantiate the results.
6. Novelty statement
A trial testing a multidisciplinary outpatient clinic treating people living with concomitant T2DM and CVD has not previously been performed. Our study will provide information about feasibility and efficacy of an outpatient clinic treating people living with T2DM and CVD in a multidisciplinary setup. The implications of this study for further research are discussed in the later outcome articles.
Funding
This work was supported by the local research committee at NSR Hospitals, by Holbaek and Slagelse hospital and by research grant from Steno Diabetes Center Sjælland.
CRediT authorship contribution statement
Julie Rye Noer Pontoppidan: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Data curation. Emil Eik Nielsen: . Michael Hecht Olsen: . Michael Kriegbaum Skjødt: . Jesper Olund Christensen: . Ilan Esra Raymond: . Sara Hedlund Møller: . Anne Merete Boas Soja: . Peter Haulund Gæde: .
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- Anderson C., Laubscher S., Burns R. Validation of the Short Form 36 (SF-36) health survey questionnaire among stroke patients. Stroke. 1996;27(10):1812–1816. doi: 10.1161/01.str.27.10.1812. [DOI] [PubMed] [Google Scholar]
- Barnett K., et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- Befolkning Danmark. Danmarks statistik.
- Billingham S.A., Whitehead A.L., Julious S.A. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med. Res. Method. 2013;13:104. doi: 10.1186/1471-2288-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorner J.B., et al. The Danish SF-36 Health Survey: translation and preliminary validity studies. J. Clin. Epidemiol. 1998;51(11):991–999. doi: 10.1016/s0895-4356(98)00091-2. [DOI] [PubMed] [Google Scholar]
- Chehal P.K., et al. Continuity of Medication Use by US Adults With Diabetes, 2005–2019. JAMA Netw. Open. 2023;6(1) doi: 10.1001/jamanetworkopen.2022.53562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care, 2021. 44(Suppl 1): p. S15-s33. [DOI] [PubMed]
- Cosentino F., et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- Doessing A., Burau V. Care coordination of multimorbidity: a scoping study. J. Comorb. 2015;5:15–28. doi: 10.15256/joc.2015.5.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarson T.R., et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018;17(1):83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSayed N.A., et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Supplement_1):S158–S190. doi: 10.2337/dc23-S010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaede P., et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 2008;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- Kannel W.B., McGee D.L. Diabetes and cardiovascular disease. The Framingham Study. Jama. 1979;241(19):2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- Knudsen S.T., et al. Difficulties in reaching therapeutic goals for hypertension and dysplipidaemia in patients with type 2 diabetes in general practice. Dan. Med. J. 2013;60(12):A4740. [PubMed] [Google Scholar]
- Lee J.K., et al. Assessment of Interprofessional Collaborative Practices and Outcomes in Adults With Diabetes and Hypertension in Primary Care: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2020.36725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low Wang C.C., et al. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms, Management, and Clinical Considerations. Circulation. 2016;133(24):2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J.D., et al. Primary Prevention of Cardiovascular Disease in Diabetes Mellitus. J. Am. Coll. Cardiol. 2017;70(7):883–893. doi: 10.1016/j.jacc.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nørgaard C.H., et al. The Importance and Role of Multiple Risk Factor Control in Type 2 Diabetes. Curr. Cardiol. Rep. 2019;21(5):35. doi: 10.1007/s11886-019-1123-y. [DOI] [PubMed] [Google Scholar]
- Opgaveglidning ved behandling af hypertension. 2010; Available from: https://ugeskriftet.dk/videnskab/opgaveglidning-ved-behandling-af-hypertension. [PubMed]
- Prevalence of CVD in Denmark. Available from: https://hjerteforeningen.dk/alt-om-dit-hjerte/noegletal/?gclid=EAIaIQobChMIiZ-E_7Hy9gIVTo9oCR19ywPmEAAYASAAEgI2xvD_BwE.
- Prevalence of type 2 diabetes in denmark. Available from: https://diabetes.dk/forskning/viden-om-diabetes/diabetes-i-danmark.
- Roth G.A., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungby J., et al. Prevalence of cardiovascular disease and evaluation of standard of care in type 2 diabetes: a nationwide study in primary care. Cardiovasc. Endocrinol. 2017;6(4):145–151. doi: 10.1097/XCE.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- Siaw M.Y.L., Lee J.Y. Multidisciplinary collaborative care in the management of patients with uncontrolled diabetes: A systematic review and meta-analysis. Int. J. Clin. Pract. 2019;73(2) doi: 10.1111/ijcp.13288. [DOI] [PubMed] [Google Scholar]
- Stark Casagrande S., et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36(8):2271–2279. doi: 10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struijs J.N., et al. Comorbidity in patients with diabetes mellitus: impact on medical health care utilization. BMC Health Serv. Res. 2006;6:84. doi: 10.1186/1472-6963-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedsted, P., Dokumentation af multisygdom i det danske samfund - fra silotænkning til sammenhæng. 2017.
- Wan E.Y.F., et al. Five-Year Effectiveness of the Multidisciplinary Risk Assessment and Management Programme-Diabetes Mellitus (RAMP-DM) on Diabetes-Related Complications and Health Service Uses-A Population-Based and Propensity-Matched Cohort Study. Diabetes Care. 2018;41(1):49–59. doi: 10.2337/dc17-0426. [DOI] [PubMed] [Google Scholar]
- Wenting W., et al. Increased resting heart rate and glucose metabolism in a community population. J. Int. Med. Res. 2021;49(11) doi: 10.1177/03000605211053754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., et al. The effects of interventions on health-related quality of life among persons with diabetes: a systematic review. Med. Care. 2007;45(9):820–834. doi: 10.1097/MLR.0b013e3180618b55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.