Abstract
UDP-glucuronosyltransferases (UGTs) catalyze the conjugation of glucuronic acid with endogenous and exogenous lipophilic small molecules to facilitate their inactivation and excretion from the body. This represents approximately 35 % of all phase II metabolic transformations. Fatty acids and their oxidized eicosanoid derivatives can be metabolized by UGTs. F2-isoprostanes (F2-IsoPs) are eicosanoids formed from the free radical oxidation of arachidonic acid. These molecules are potent vasoconstrictors and are widely used as biomarkers of endogenous oxidative damage. An increasing body of evidence demonstrates the efficacy of measuring the β-oxidation metabolites of F2-IsoPs rather than the unmetabolized F2-IsoPs to quantify oxidative damage in certain settings. Yet, the metabolism of F2-IsoPs is incompletely understood. This study sought to identify and characterize novel phase II metabolites of 15-F2t-IsoP and 5-epi-5-F2t-IsoP, two abundantly produced F2-IsoPs, in human liver microsomes (HLM). Utilizing liquid chromatography-mass spectrometry, we demonstrated that glucuronide conjugates are the major metabolites of these F2-IsoPs in HLM. Further, we showed that these molecules are metabolized by specific UGT isoforms. 15-F2t-IsoP is metabolized by UGT1A3, 1A9, and 2B7, while 5-epi-5-F2t-IsoP is metabolized by UGT1A7, 1A9, and 2B7. We identified, for the first time, the formation of intact glucuronide F2-IsoPs in human urine and showed that F2-IsoP glucuronidation is reduced in people supplemented with eicosapentaenoic and docosahexaenoic acids for 12 weeks. These studies demonstrate that endogenous F2-IsoP levels can be modified by factors other than redox mechanisms.
Keywords: Isoprostanes, Metabolism, Mass spectrometry, Glucuronidation, Fish oil
Graphical abstract
Highlights
-
•
F2-Isoprostanes (F2-IsoPs) are metabolized to glucuronide conjugates by human liver microsomes.
-
•
Individual F2-IsoP isomers are metabolized by specific UDP-glucuronosyltransferases (UGTs).
-
•
Intact F2-IsoP glucuronide conjugates were identified in human urine.
-
•
Supplementation with eicosapentaenoic acid and docosahexaenoic acid decreases F2-IsoP glucuronidation.
1. Introduction
More than 30 years ago, Morrow and Roberts discovered that F2-isoprostanes (F2-IsoPs) are formed via the free radical (nonenzymatic) oxidation of arachidonic acid [1,2]. The Biomarkers of Oxidative Stress Study (BOSS) [[3], [4], [5]] demonstrated that these molecules are formed in a dose- and time-dependent manner following administration of a controlled disruption in redox homeostasis. This finding revolutionized the ability of oxidative stress (OS) to be evaluated in human and animal models of disease [6,7]. F2-IsoPs are also increased in numerous OS related diseases, including cardiovascular disease [[8], [9], [10]], neurodegeneration [[11], [12], [13], [14], [15]], frailty [[16], [17], [18], [19]], diabetes [[20], [21], [22]], and SARS-CoV-2 [[23], [24], [25], [26]].
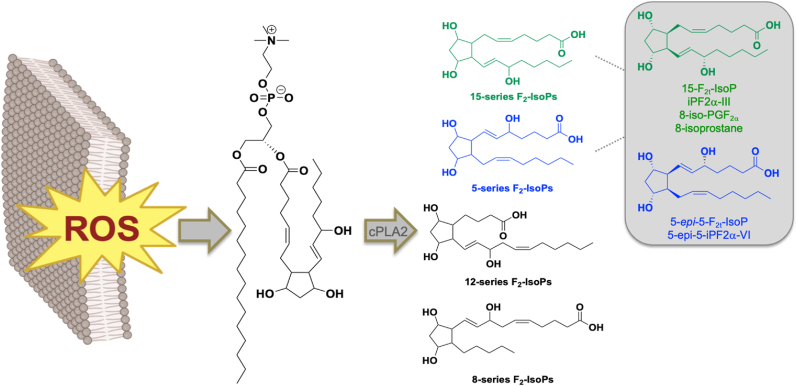
F2-IsoPs are separated into four sets of regioisomers identified by the position of the hydroxyl group on the side chain (Fig. 1). Oxidation of arachidonic acid in vitro indicates that 5- and 15-series F2-IsoPs are equally produced in greater abundance than 8- and 12-series F2-IsoPs [27]. The most well-studied F2-IsoP is 15-F2t-IsoP (also referred to as iPF2α-III, 8-iso-prostaglandin F2α or 8-iso-PGF2α, and 8-isoprostane, Fig. 1 inset). This molecule was the first F2-IsoP to be chemically synthesized, and immunoassay (ELISA) and mass spectrometric (MS) methodologies have commonly been used for its’ quantification [7]. More than twenty years ago, Lawson and colleagues reported that the 5-series F2-IsoP isomers 5-F2c-IsoP (8,12-iso-iPF2α-VI) and 5-epi-5-F2c-IsoP (5-epi-8,12-iso-iPF2α-VI) are more abundant in human urine than 15-F2t-IsoP [28]. Yet, most literature reports still focus solely on quantification of 15-F2t-IsoP.
Fig. 1.
F2-isoprostanes are generated from the free radical oxidation of esterified arachidonic acid in membrane lipids. F2-IsoPs are released by the action of lipases to yield the free fatty acids. Four different series of F2-IsoP regioisomers are named based on the position of the hydroxyl group on the side chain. 15-series F2-IsoPs are the most well studied isomers, while 5-series F2-IsoPs are formed more abundantly in human biological fluids.
F2-IsoPs in urine are less affected by autoxidation during sample collection and long-term storage than those in plasma or tissue [29]. Roberts et al. identified the major urinary metabolite of 15-F2t-IsoP is 2,3-dinor-5,6-dihydro-15-F2t-IsoP (F2-IsoP-M) [30], a phase I metabolite generated via β-oxidation and subsequent double bond reduction.
Chiabrando and colleagues also identified 2,3-dinor-15-F2t-IsoP to be present in human and rat urine [31]. Further, Chiabrando et al. explored the metabolism of 15-F2t-IsoP and other 15-series F2-IsoPs by primary rat hepatocytes. They demonstrated that 15-series F2-IsoPs can undergo an additional round of β-oxidation to yield tetranor-15-F2-IsoPs) and can be metabolized by phase II mechanisms to yield taurine conjugates. Notably, the metabolism of 5-series F2-IsoPs remains unexplored.
An increasing body of evidence demonstrates the efficacy of F2-IsoP-M as a biomarker of OS. In one of the first studies to evaluate F2-IsoP-M in a large population, F2-IsoP-M but not unmetabolized 15-F2t-IsoP was positively associated with age and postmenopausal status among middle-aged and older women [32]. In other studies, urinary F2-IsoP-M showed associations with plasma antioxidant levels, carbohydrate intake, and glycemic index while urinary 15-F2t-IsoP did not [[33], [34], [35], [36]]. A variety of environmental factors, including lower socioeconomic status and exposures to phthalates and herbicides, are also positively associated with F2-IsoP-M but not unmetabolized 15-F2t-IsoP [[37], [38], [39]]. Taken together, these studies support the idea that understanding the metabolism of these widely-used biomarkers is imperative for interpreting the F2-IsoPs as biomarkers of OS.
Indirect evidence supports the idea that F2-IsoPs can be metabolized to yield glucuronide conjugates. In 1996, Awad and Morrow first suggested this possibility when studying the production of biliary F2-IsoPs in rats administered carbon tetrachloride [40]. Other groups have shown that treatment of human urine with β-glucuronidase enzymes, which would degrade F2-IsoP glucuronide conjugates to release free F2-IsoPs and glucuronic acid, leads to an increase in the level of free F2-IsoPs [[41], [42], [43]]. Yet, no study to date has demonstrated the formation of an intact F2-IsoP glucuronide conjugate. To better understand F2-IsoP metabolism by glucuronide conjugation, we have, for the first time, investigated the metabolic transformation of 15-F2t-IsoP and 5-epi-5-F2t-IsoP (5-epi-5-iPF2α-VI) following incubation with human liver microsomes and specific UGT isoforms. The presence of F2-IsoP glucuronide conjugates were analyzed in these incubations and in human urine samples using liquid chromatography-mass spectrometry (LC/MS).
2. Materials and methods
2.1. Reagents and supplies
15-F2t-IsoP and [2H4]-15-F2t-IsoP were obtained from Cayman Chemical. 5-epi-5-F2t-IsoP was generous gift from the laboratory of Thierry Durand (University of Montpellier, Montpellier, France). General lab supplies and solvents were obtained Fischer-Scientific (Hampton, NH). All solvents were of LC/MS grade. B-One® was obtained from Kura Biotech (Puerto Varas, Los Lagos, Chile). Human liver microsomes (HLM) were obtained from BioIVT, LLC (Westbury, NY USA). Reagents for HLM incubations were purchased from Sigma-Aldrich (St. Louis, MO USA). Recombinant UDP-glucuronosyltransferase (UGT) isoforms and UGT reaction mix reagents were obtained from (GENTEST, Woburn, MA USA).
2.2. Urine sample collection
The Fatty Acid Desaturase Activity, Fish Oil, and Colorectal Cancer Prevention study was approved by the Vanderbilt University Medical Center Institutional Review Board and registered at ClinicalTrials.gov (NCT01661764) [44,45]. Participants were recruited from individuals who were identified as colorectal adenoma cases within the Tennessee Colorectal Polyp Study or were identified through electronic medical record reviews to have undergone a colonoscopy at Vanderbilt University Medical Center and diagnosed with an adenoma. Eligible participants were between the ages of 40 and 80 years, had a past medical history of one or more adenomas, and had a known genotype for the rs174535 SNP of the fatty acid desaturase 1 enzyme (FADS1). Participants were excluded if they had a previous resected CRC, congestive heart failure or coronary artery disease, inflammatory bowel disease, any cancer (except nonmelanoma skin cancer), advanced kidney disease, cirrhosis, were pregnant or breast feeding, using fish oil supplements or anticoagulants, or allergic to fish products.
For the purposes of the work presented herein, 10 subjects who received the fish oil-sourced supplement Lovaza were selected. Each participant received three daily pills, each containing 465 mg of eicosapentaenoic acid (EPA) and 375 mg docosahexaenoic acid (DHA) for a total daily dose of 1395 mg EPA plus 1125 mg DHA. Adherence was assessed by weekly telephone calls, pill counts, and red blood cell n-3 LC-PUFA measurements. Study participation lasted 24 weeks with three in-person visits: at baseline, 12 weeks, and 24 weeks. At each visit, participants arrived after an overnight fast, and 30 mL of spot urine were collected and stored at −80 °C.
2.3. Human liver microsome glucuronidation of F2-IsoP
The metabolism of F2-IsoPs by HLM was performed according to the protocol of Hill et al. with modifications [46]. Briefly, 15-F2t-IsoP, [2H4]-15-F2t-IsoP, or 5-epi-5-F2t-IsoP (0.005 mL of a 10 μM solution in ethanol) was mixed with 0.432 mL PBS pH 7.4, 0.050 mL NADPH-regenerating system (NRS) containing 93 mg glucose-6-phosphate, 100 mg NADP, 38U glucose-6-phosphate dehydrogenase, 67 mg magnesium chloride hexahydrate, 48 mg UDPGA and 0.250 mg alamethicin in 1 mL of PBS. Samples were mixed and incubated at 37 °C for 5min. After this incubation, 0.013 mL of HLM (20 mg/mL, InVitroCYPTM 25 mixed-gender pooled donors) were added to each sample. The samples were mixed again and incubated at 37 °C for 0–120 min. The reaction was stopped by the addition of 0.275 mL ice cold methanol and vortexed. Samples were centrifuged (10,000g for 10 min at 4 °C) and the supernatant was collected for extraction and LC/MS analysis.
2.4. F2-IsoP incubation with UDP-glucuronosyltransferase isoforms
Incubations of F2-IsoPs with UDP-glucuronosyltransferase (UGT) enzymes was performed according to the manufacturer's instructions. 15-F2t-IsoP or 5-epi-5-F2t-IsoP (0.005 mL of a 10 μM solution in ethanol) was mixed with 0.147 mL DI water, 0.020 mL UGT Reaction Mix Solution A, 0.050 mL UGT Reaction Mix Solution B, and 0.030 mL of the recombinant UGT, Samples were incubated at 37 °C for 15 min. The reaction was stopped by the addition of 0.125 mL ice cold methanol and vortexed. Samples were centrifuged (10,000g for 10 min at 4 °C) and the supernatant was collected for extraction and LC/MS analysis.
2.5. Sample extraction for analysis of F2-IsoP glucuronide conjugates by LC/MS
Sample supernatant (0.5 mL) or urine (0.5 mL) was diluted with 9.5 mL 0.1 N aqueous HCl containing [2H4]-15-F2t-IsoP (5.2 ng). The sample pH was adjusted to 3 with 1 N aqueous HCl. Solid phase extraction was performed using a C-18 Sep-Pak cartridge (Waters Corp., Milford, MA USA) preconditioned with 10 mL acetonitrile and 10 mL 50 mM ammonium acetate (pH 3.4). After samples were loaded, the cartridge was washed with 10 mL 50 mM ammonium acetate (pH 3.4) and 10 mL heptane. Metabolites were eluted with 10 mL 95 % ethanol. Samples were dried under a stream of nitrogen and resuspended in a 5:1 mixture of mobile phases A and B (0.06 mL). Mobile phase A was water containing 0.2 % acetic acid while mobile phase B was acetonitrile:water (95:5) containing 0.1 % acetic acid.
2.6. Liquid chromatography-mass spectrometry (LC/MS) analysis of F2-IsoP glucuronide conjugates
LC/MS was performed on a Waters Xevo TQ-XS triple quadrupole mass spectrometer connected to a Waters Acquity I-Class UPLC (Waters Corp., Milford, MA USA). Separation of analytes was obtained using a Waters Acquity UPLC HSS T3 (1.0 × 100 mm, 1.8 μm particle size) with mobile phases A and B as described above. The LC gradient and MS settings are described in the Supplemental Materials.
2.7. Extraction of human urine for analysis of F2-IsoPs by LC/MS
[2H4]-15-F2t-IsoP (1ng/0.005 mL ethanol, internal standard) was added to a 0.050 mL aliquot of urine. The sample was diluted with 0.4 mL 5 % methanol in water containing 0.1 % formic acid (Solution A) and acidified to pH 3 with 0.1 N HCl. Solid phase extraction was performed using an Oasis HLB 96-well μElution plate (Waters, Milford, MA, USA) preconditioned with 0.4 mL methanol and 0.4 mL Solution A. Samples were loaded onto the μElution plate, and wells were washed with 0.4 mL Solution A and then 0.2 mL hexane. Analytes were eluted with 0.030 mL isopropanol/acetonitrile (1:1) into a 96-well LC/MS collection plate containing 0.030 mL LC/MS grade water in each well.
2.8. Treatment of HLM supernatant or human urine with B-One®
[2H4]-15-F2t-IsoP (1ng/0.005 mL ethanol, internal standard) was added to a 0.050 mL aliquot of HLM supernatant or a 0.050 mL aliquot of urine. The β-glucuronidase enzyme B-One® (0.100 mL, Kura Biotech, Puerto Varas, Chile) was added to each sample. Samples were mixed and incubated at room temperature for 5 min. The samples were then diluted with 0.4 mL 5 % methanol in water containing 0.1 % formic acid, acidified to pH 3 with 0.1 N HCl, and extracted as described above for F2-IsoP LC/MS analysis.
2.9. Liquid chromatography-mass spectrometry (LC/MS) analysis of F2-IsoPs
LC/MS was performed on a Waters Xevo TQ-XS triple quadrupole mass spectrometer connected to a Waters Acquity I-Class UPLC (Waters Corp., Milford, MA USA). Separation of analytes was obtained using a Waters Acquity UPLC BEH C18 (1.0 × 100 mm, 1.7um particle size) with using water containing 0.01 % acetic acid (mobile phase A) and acetonitrile (mobile phase B). The LC gradient and MS settings are described in the Supplemental Materials.
3. Results
3.1. Incubation of F2-IsoPs with human liver microsomes
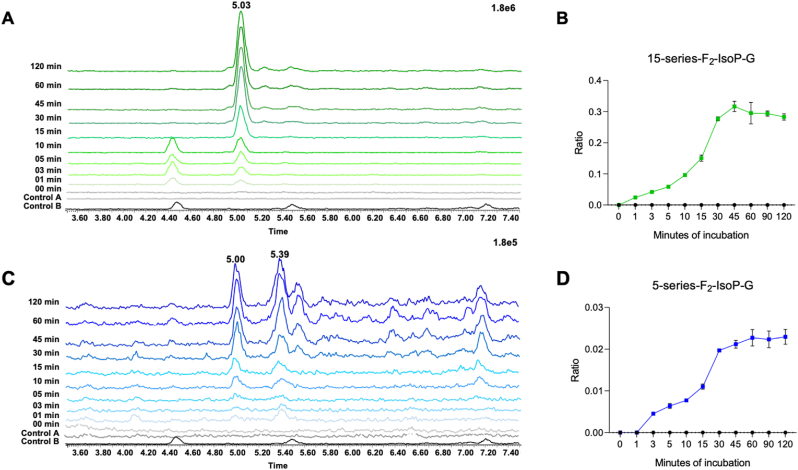
15-F2t-IsoP and 5-epi-5-F2t-IsoP were separately incubated with HLM and the NADP-regenerating system (NRS) at 37 °C. Individual tubes were incubated for 0–120 min; the reaction was stopped by the addition of ice-cold methanol. Control incubations were performed (A) without any F2-IsoP or (B) without the NRS. Using targeted LC-MS/MS with multiple reaction monitoring (MRM), samples were analyzed for the presence of phase II F2-IsoP metabolites including F2-IsoP glucuronide conjugates, F2-IsoP sulfate conjugates, and glucuronide and sulfate conjugates of dinor-F2-IsoP metabolites that result from β-oxidation. Only F2-IsoP glucuronide conjugates were detected. Chromatograms showing the time-dependent formation of 15-F2t-IsoP and 5-epi-5-F2t-IsoP glucuronide conjugates are shown in Fig. 2A and C, respectively. HLM incubations with 15-F2t-IsoP show the formation of glucuronide metabolites after just 1 min (Fig. 2B). 5-epi-5-F2t-IsoP is metabolized to these polar conjugates within 3 min (Fig. 2D). To confirm that these metabolites were glucuronide conjugates, the 5-epi-5-F2t-IsoP HLM supernatant (t = 2hrs) were treated with a β-glucuronidase enzyme (B-One®, Kura Biotech, Puerto Varas, Chile). Metabolite peaks were no longer detected.
Fig. 2.
15-F2t-IsoP and 5-epi-5-F2t-IsoP were incubated with human liver microsomes (HLM) and the NADPH regenerating system mix for 0–120 min. Aliquots of the microsome reaction were extracted and analyzed by LC/MS. (A) Chromatograms showing the formation of a peak consistent with the expected m/z transition (m/z 529 → m/z 353) for a 15-F2t-IsoP glucuronide conjugate from the incubation with HLM. (B) Time-dependent formation of the 15-F2t-IsoP glucuronide conjugate. (C) Chromatograms showing the formation of a peak consistent with the expected m/z transition (m/z 529 → m/z 353) for a 5-epi-5-F2t-IsoP glucuronide conjugate from the incubation with HLM. (D) Time-dependent formation of the 5-epi-5-F2t-IsoP glucuronide conjugate. Control A HLM incubations contained 15-F2t-IsoP or 5-epi-5-F2t-IsoP but no NADPH, while Control B incubations contained NADPH but no F2-IsoP isomer.
3.2. Product ion scan of F2-IsoP glucuronide metabolites
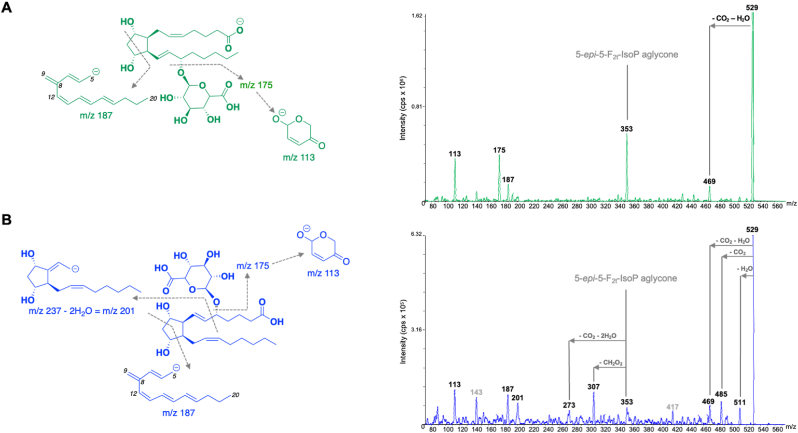
To further confirm the structure of these metabolites as glucuronide conjugates, the extracted supernatants of 15-F2t-IsoP and 5-epi-5-F2t-IsoP HLM incubation (t = 30 min) were analyzed by LC-MS/MS product ion scanning of m/z 529. The resulting spectra are shown in Fig. 3. Product ions detected in both the 15-F2t-IsoP and 5-epi-5-F2t-IsoP glucuronide conjugate spectra are m/z 469, 353, 175, and 113. The low-abundance product ion m/z 469 results from the loss of CO2 and H2O from the m/z 529 [M − H]- ion. Neutral loss of 176, which is a common loss to glucuronide conjugates, results in the product ion at m/z 353. The product ions m/z 175 and 113 result from the glucuronide directly. The product ion spectra of the 5-epi-5-F2t-IsoP glucuronide conjugate also shows low abundance fragments at m/z 335, 317, and 299 resulting from sequential loss of H2O from m/z 353. Unfortunately, none of these fragments allow determination of the exact position of glucuronide conjugation.
Fig. 3.
(A) Product ion spectra of the 15-F2t-IsoP glucuronide conjugate eluting at 5.03 min in Fig. 3A. (B) Product ion spectra of the 5-epi-5-F2t-IsoP glucuronide conjugate eluting at 5.00 min in Fig. 3B.
3.3. Incubation of 15-F2t-IsoP and 5-epi-5-F2t-IsoP with individual UGT isoforms
Nine recombinant UGT isoforms were used for the determination of the contribution of UGTs to the formation of F2-IsoP glucuronidation. Single concentrations of 15-F2t-IsoP and 5-epi-5-F2t-IsoP (100 nM) were used to screen the contribution of possible UGT isoforms to F2-IsoP metabolism. UGT1A9 and UGT2B7 are involved in the metabolism of both 15-F2t-IsoP and 5-epi-5-F2t-IsoP to their corresponding glucuronide metabolites (Fig. 4). UGT1A3 was shown to have activity for only 15-F2t-IsoP, while UGT1A7 was shown to have activity for only 5-epi-5-F2t-IsoP. UGT1A1, UGT1A4, UGT1A6, UGT1A10, and UGT2B15 did not show activity for either F2-IsoP isomer.
Fig. 4.
15-F2t-IsoP and 5-epi-5-F2t-IsoP were incubated with individual recombinant UGT enzymes for 60 min. Aliquots of the microsome reaction were extracted and analyzed by LC/MS. (A) Chromatograms showing the formation of a peak consistent with the expected m/z transition (m/z 529 → m/z 353) for a 15-F2t-IsoP glucuronide conjugate from the incubation with UGT enzymes. (B) Chromatograms showing the formation of a peak consistent with the expected m/z transition (m/z 529 → m/z 353) for a 5-epi-5-F2t-IsoP glucuronide conjugate from the incubation with UGT enzymes. The blank incubations contained no F2-IsoPs.
3.4. Analysis of F2-IsoPs in human urine after treatment with B-One®
Other laboratories have utilized traditional β-glucuronidase enzyme preparations to explore the possibility that F2-IsoPs are metabolized via glucuronide conjugation. Each of these enzyme preparations required heating of the samples at 37 °C as well as optimization of pH and incubation time. The effect of pH and heat on the stability of urinary F2-IsoPs remains undetermined as F2-IsoP glucuronide standards do not exist. B-One® is advantageous compared to other β-glucuronidase enzymes due its broad substrate reactivity and the quick reaction time at ambient temperatures. Since a 5-min incubation of the HLM supernatant with B-One® at room temperature eliminated the presence of the F2-IsoP glucuronide, we utilized these same conditions for the urine analysis.
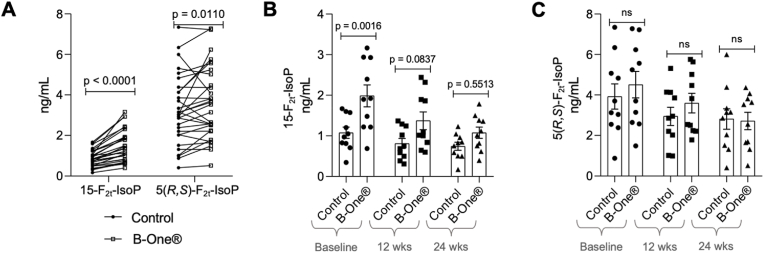
Urine samples were previously collected from subjects within the Fatty Acid Desaturase Activity, Fish Oil, and Colorectal Cancer Prevention study. Each of these participants had morning, fasted urine samples collected at baseline and following 12- and 24- week supplementation with the fish oil supplement Lovasa. Levels of F2-IsoPs were measured before and after treatment if B-One® using LC-MS/MS. 5-epi-5-F2t-IsoP is not resolved from 5-F2t-IsoP under these conditions, thus we measure isomers simultaneously as 5(R,S)–F2t-IsoP. When all samples (baseline plus treatment) were compared together, levels of both 15-F2t-IsoP and 5(R,S)–F2t-IsoP were significantly increased in the urine samples following treatment with B-One® (Fig. 5A) but to different extents. 15-F2t-IsoP levels were increased on average 69 % while levels of 5-epi-5-F2t-IsoP were increased by ∼20 %. However, when samples were stratified by time point, only baseline levels of 15-F2t-IsoP were significantly increased following B-One® treatment (Fig. 5B). We further compared the effect of Lovasa on F2-IsoPs levels in the urine samples. Measurement of unconjugated urinary 15-F2t-IsoP (no B-One® treatment) showed no significant change after 12 or 24 weeks of treatment, while measurement of total urinary 15-F2t-IsoP (urine treated with B-One®) showed a time-dependent decrease during the treatment period. Both unconjugated and total 5(R,S)–F2t-IsoP showed a significant decrease following 24 weeks of treatment.
Fig. 5.
(A) Unmetabolized F2-IsoPs were measured in human urine using LC-MS/MS before and after treatment with β-glucuronidase – all subjects and all time points. (B) Levels of unmetabolized 15-F2t-IsoP in urine before and after treatment β-glucuronidase stratified by time of sample collection. (C) Levels of unmetabolized 5(R,S)–F2t-IsoP in urine before and after treatment with β-glucuronidase stratified by time of sample collection.
3.5. Detection of intact F2-IsoP glucuronide conjugates in human urine
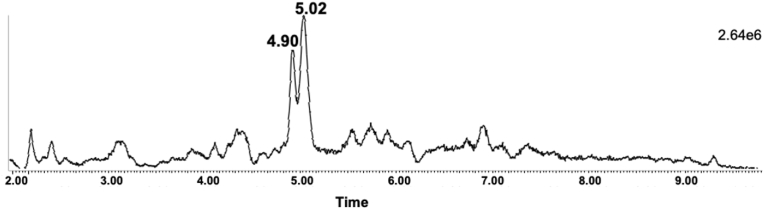
Finally, we sought to detect intact F2-IsoP glucuronide conjugates in human urine. Fig. 6 shows that MRM analysis of the transition m/z 529 → m/z 353 detected multiple peaks at the expected retention time for F2-IsoP glucuronide conjugates. F2-IsoP glucuronide conjugates do not have a MS/MS fragmentation pattern that allows for distinction of 15-F2t-IsoP-G and 5-epi-5-F2t-IsoP-G, but the presence of multiple peaks indicates multiple isomers as would be expected. Product scan analysis of these peaks shows a fragmentation pattern similar to that detected for the F2-IsoP-G produced by HLM.
Fig. 6.
Detection of intact F2-IsoP glucuronide conjugates in a human urine sample (m/z 529 → m/z 353) using LC-MS/MS.
4. Discussion
F2-IsoPs are biologically active molecules generated primarily from the free radical catalyzed, non-enzymatic oxidation of arachidonic acid. The Biomarkers of Oxidative Stress Study (BOSS) demonstrated that F2-IsoPs are formed in a time- and dose-dependent manner following administration of CCl4, a potent inducer of oxidative stress. These molecules are widely used as biomarkers of endogenous oxidative injury in a variety of human conditions and diseases. Currently, clinicaltrials.gov reports F2-IsoPs evaluation in more than 60 active clinical trials. Even though most studies focus on F2-IsoPs as simply biomarkers of oxidative injury, these molecules also exhibit potent biological activity. 15-F2t-IsoP is a potent vasoconstrictor, activates platelets, and has recently been shown to promote lung fibroblast proliferation. These biological activities, at least in part, result from activation of the thromboxane-prostanoid receptor TBXA2R. It is thus imperative to fully understand mechanisms by which this molecule and other F2-IsoPs are inactivated and cleared from the body.
Glucuronidation, catalyzed by UGTs, represents approximately 35 % of all phase II metabolic transformations. In these reactions, glucuronic acid is conjugated to polar molecular functional groups, including hydroxyls and carboxylic acids. Other eicosanoids, including leukotriene B4, 20-hydroxy-eicosatetraenoic acid (20-HETE), and prostaglandin E2 (PGE2), are metabolized via this mechanism [[47], [48], [49], [50], [51]]. Other laboratories have provided indirect evidence that F2-IsoPs are metabolized by β-glucuronidation, however this study is the first to directly demonstrate formation of intact F2-IsoP glucuronide conjugates. Herein, we found that human liver microsomes metabolize two F2-IsoP isomers, 15-F2t-IsoP and 5-epi-5-F2t-IsoP, to glucuronide conjugates as determined using LC/MS. Further, we demonstrated that specific UGTs are responsible for this metabolic transformation and specificity differences exist between F2-IsoP regioisomers. While UGT1A9 and UGT2B7 metabolically transform both 15-F2t-IsoP and 5-epi-5-F2t-IsoP, 15-F2t-IsoP is transformed by UGT1A3 and UGT1A7 shows activity toward the metabolism of 5-epi-5-F2t-IsoP. 15-F2t-IsoP and 5-epi-5-F2t-IsoP are spatially quite different molecules due to the proximity of the side chain hydroxyl group to the carboxyl acid and the end terminus of the chain. This spatial difference likely influences the positioning of the molecules in the active sites of individual UGT isoforms; yet factors that influence UGT isoform substrate specificity are poorly understood [52].
The metabolism of F2-IsoPs via glucuronidation has important implications toward the quantification of these molecules in vivo. F2-IsoPs have long-been considered ideal oxidative stress biomarkers due to their relative chemical stability compared to ROS, such as hydrogen peroxide and superoxide, and other highly transient species [7]. Yet, changes in UGT expression and availability could potentially lead to changes in concentrations of endogenous F2-IsoP glucuronide conjugates and, consequentially, changes in levels of unmetabolized F2-IsoPs. For instance, valproic acid (VPA) is a drug commonly used to treat epileptic seizures. VPA is metabolized through glucuronidation by UGT1A9 and UGT2B7, two of the UGTs that metabolized F2-IsoPs [53]. Tong et al. have shown that administration of VPA to rats is associated with a concomitant increase in 15-F2t-IsoP. The authors indicated in the paper that the mechanism by which VPA increased levels of 15-F2t-IsoP were unclear but implicated a role for oxidative stress. Our results suggest that VPA could preferentially compete with 15-F2t-IsoP for the UGT enzymes, thereby reducing 15-F2t-IsoP glucuronidation and, in turn, increasing levels of unmetabolized 15-F2t-IsoP. In addition to VPA, other eicosanoids, estrogens, bile acids, fatty acids, numerous pharmaceuticals, and foods can all influence the activity of UGTs. Further, UGT expression is altered in many diseases, and disruption of redox homeostasis also leads to changes in UGT expression [54,55].
In this work, participants in the Fatty Acid Desaturase Activity, Fish Oil, and Colorectal Cancer Prevention study received a daily dose of Lovasa, containing the fatty acids EPA and DHA, for a period of 24 weeks. EPA and DHA have previously been shown to inhibit UGT activity and consequentially decrease glucuronidation of endogenous UGT substrates such as estradiol [56]. Herein, we observed that levels of total urinary 15-F2t-IsoP (measured following β-glucuronidase treatment) decreased following treatment with Lovasa, whereas levels of free urinary 15-F2t-IsoP were unchanged. This result indicates a decrease in 15-F2t-IsoP glucuronidation following consumption of high dose EPA/DHA. Over the past decade, there has been significant interest in the effect of fish oil supplementation on human health and the resulting effect on the production of endogenous oxidized lipids, including not only isoprostanes but also enzymatically generated prostaglandins, epoxy-fatty acids, and specialized pro-resolving lipid mediators. The results overall have been mixed and overall disappointing [57,58]. Most studies of fish oil supplementation have shown no decrease in F2-IsoPs as originally hypothesized, thus concluding that EPA/DHA supplementation has no effect on OS. Although this study is small, these results demonstrate that urinary levels of F2-IsoPs are not only affected by endogenous OS but also by metabolism induced by EPA/DHA supplementation. Together these results highlight the need for more comprehensive assessment of OS and F2-IsoP formation in a follow-up study with a larger population.
In summary, we report that two F2-IsoP isomers, 15-F2t-IsoP and 5-epi-5-F2t-IsoP, are rapid metabolized to glucuronic acid conjugates via action of UGTs in human liver microsomes. These novel findings provide direct evidence for the inactivation and elimination of F2-IsoPs via specific UGTs. Further, these studies represent the first detection of intact F2-IsoP glucuronide conjugates in human urine. Importantly, our findings demonstrate that endogenous levels of urinary free F2-IsoPs can be affected by mechanisms that are independent of oxidative stress and lipid peroxidation. Quantification of unmetabolized F2-IsoPs is commonly used in both experimental and clinical research and may result in a biased, inaccurate assessment of true F2-IsoP production. Development of chemically synthesized standards for F2-IsoP glucuronide conjugates will be essential for the further characterization and quantitation of these metabolites.
CRediT authorship contribution statement
Ginger L. Milne: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. Marina S. Nogueira: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Benlian Gao: Data curation, Methodology. Stephanie C. Sanchez: Data curation, Investigation, Project administration, Validation, Writing – review & editing. Warda Amin: Methodology, Writing – review & editing. Sarah Thomas: Methodology. Camille Oger: Data curation, Resources, Writing – original draft, Writing – review & editing. Jean-Marie Galano: Data curation, Resources, Writing – original draft, Writing – review & editing. Harvey J. Murff: Resources, Writing – review & editing. Gong Yang: Conceptualization, Resources, Supervision, Writing – original draft, Writing – review & editing. Thierry Durand: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is dedicated to the memory of Dr. Benlian Gao, co-author, who passed away from ovarian cancer on October 24, 2023. GLM is supported by the Vanderbilt Brain Institute and the Vanderbilt Diabetes Research Center (P30 DK020593). GLM and MSN are supported by a grant from the Cayman Biomedical Research Institute. GY and MSN are supported by R01 CA237895.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.103020.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Morrow J.D., et al. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrow J.D., Harris T.M., Roberts L.J. Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal. Biochem. 1990;184:1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- 3.Kadiiska M., et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Kadiiska M., et al. Biomarkers of oxidative stress study: III. Effects of the nonsteroidal anti-inflammatory agents indomethacin and meclofenamic acid on measurements of oxidative products of lipids in CCl4 poisoning. Free Radic. Biol. Med. 2005;38:711–718. doi: 10.1016/j.freeradbiomed.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Kadiiska M.B., et al. Biomarkers of oxidative stress study V: ozone exposure of rats and its effect on lipids, proteins, and DNA in plasma and urine. Free Radic. Biol. Med. 2013;61:408–415. doi: 10.1016/j.freeradbiomed.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milne G.L., Yin H., Hardy K.D., Davies S.S., Roberts L.J. Isoprostane generation and function. Chem. Rev. 2011;111:5973–5996. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy M.P., et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022;4:651–662. doi: 10.1038/s42255-022-00591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santilli F., Guagnano M.T., Vazzana N., La Barba S., Davi G. Oxidative stress drivers and modulators in obesity and cardiovascular disease: from biomarkers to therapeutic approach. Curr. Med. Chem. 2015;22:582–595. doi: 10.2174/0929867322666141128163739. [DOI] [PubMed] [Google Scholar]
- 9.Anderson C., Milne G.L., Park Y.-M.M., Sandler D.P., Nichols H.B. Cardiovascular disease risk factors and oxidative stress among premenopausal women. Free Radic. Biol. Med. 2018;115:246–251. doi: 10.1016/j.freeradbiomed.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunapuli P., Lawson J.A., Rokach J.A., Meinkoth J.L., FitzGerald G.A. Prostaglandin F2α (PGF2α) and the isoprostane, 8,12-iso-Isoprostane F2α-III, induce cardiomyocyte hypertrophy: differential activation of downstream signaling pathways. J. Biol. Chem. 1998;273:22442–22452. doi: 10.1074/jbc.273.35.22442. [DOI] [PubMed] [Google Scholar]
- 11.Raefsky S.M., et al. Deuterated polyunsaturated fatty acids reduce brain lipid peroxidation and hippocampal amyloid β-peptide levels, without discernible behavioral effects in an APP/PS1 mutant transgenic mouse model of Alzheimer's disease. Neurobiol. Aging. 2018;66:165–176. doi: 10.1016/j.neurobiolaging.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peña-Bautista C., et al. Plasma isoprostanoids assessment as Alzheimer's disease progression biomarkers. J. Neurochem. 2021;157:2187–2194. doi: 10.1111/jnc.15183. [DOI] [PubMed] [Google Scholar]
- 13.Peña-Bautista C., et al. New screening approach for Alzheimer's disease risk assessment from urine lipid peroxidation compounds. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-50837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Blanco A., et al. Reliable determination of new lipid peroxidation compounds as potential early Alzheimer Disease biomarkers. Talanta. 2018;184:193–201. doi: 10.1016/j.talanta.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Peña-Bautista C., et al. Clinical utility of plasma lipid peroxidation biomarkers in alzheimer's disease differential diagnosis. Antioxid. Basel Switz. 2020;9:649. doi: 10.3390/antiox9080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C.K., et al. Biomarkers of oxidative stress are associated with frailty: the Framingham Offspring Study. AGE. 2016;38:1. doi: 10.1007/s11357-015-9864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kane A.E., Sinclair D.A. Frailty biomarkers in humans and rodents: current approaches and future advances. Mech. Ageing Dev. 2019;180:117–128. doi: 10.1016/j.mad.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soysal P., et al. Oxidative stress and frailty: a systematic review and synthesis of the best evidence. Maturitas. 2017;99:66–72. doi: 10.1016/j.maturitas.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Laosa O., Alonso C., Castro M., Rodriguez-Manas L. Pharmaceutical interventions for frailty and sarcopenia. Curr. Pharmaceut. Des. 2014;20:3068–3082. doi: 10.2174/13816128113196660705. [DOI] [PubMed] [Google Scholar]
- 20.Simeone P., et al. Thromboxane-dependent platelet activation in obese subjects with prediabetes or early type 2 diabetes: effects of liraglutide- or lifestyle changes-induced weight loss. Nutrients. 2018;10:1872. doi: 10.3390/nu10121872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schöttker B., Xuan Y., Gào X., Anusruti A., Brenner H. Oxidatively damaged DNA/RNA and 8-isoprostane levels are associated with the development of type 2 diabetes at older age: results from a large cohort study. Diabetes Care. 2020;43:130–136. doi: 10.2337/dc19-1379. [DOI] [PubMed] [Google Scholar]
- 22.Li Q., Wang M., Li X., Shao Y. Aging and diabetic retinopathy: inherently intertwined pathophysiological processes. Exp. Gerontol. 2023;112138 doi: 10.1016/j.exger.2023.112138. [DOI] [PubMed] [Google Scholar]
- 23.Biagini D., et al. MS-based targeted profiling of oxylipins in COVID-19: a new insight into inflammation regulation. Free Radic. Biol. Med. 2022;180:236–243. doi: 10.1016/j.freeradbiomed.2022.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koenis D.S., et al. Disrupted resolution mechanisms favor altered phagocyte responses in COVID-19. Circ. Res. 2021;129 doi: 10.1161/CIRCRESAHA.121.319142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawelzik S.-C., et al. Decreased oxidative stress and altered urinary oxylipidome by intravenous omega-3 fatty acid emulsion in a randomized controlled trial of older subjects hospitalized for COVID-19. Free Radic. Biol. Med. 2023;194:308–315. doi: 10.1016/j.freeradbiomed.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Žarković N., et al. The impact of severe COVID-19 on plasma antioxidants. Mol. Basel Switz. 2022;27:5323. doi: 10.3390/molecules27165323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin H., Porter N.A., Morrow J.D. Separation and identification of F2-isoprostane regioisomers and diastereomers by novel liquid chromatographic/mass spectrometric methods. J. Chromatogr. B. 2005;827:157–164. doi: 10.1016/j.jchromb.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 28.Lawson J.A., et al. Identification of two major F2 isoprostanes, 8, 12-iso-and 5-epi-8, 12-iso-isoprostane F2α-VI, in human urine. J. Biol. Chem. 1998;273:29295–29301. doi: 10.1074/jbc.273.45.29295. [DOI] [PubMed] [Google Scholar]
- 29.Awad J.A., Morrow J.D., Takahashi K., Roberts L.J. Identification of non-cyclooxygenase-derived prostanoid (F2-isoprostane) metabolites in human urine and plasma. J. Biol. Chem. 1993;268:4161–4169. [PubMed] [Google Scholar]
- 30.Roberts L.J., Moore K.P., Zackert W.E., Oates J.A., Morrow J.D. Identification of the major urinary metabolite of the F2-isoprostane 8-iso-prostaglandin F2α in humans. J. Biol. Chem. 1996;271:20617–20620. doi: 10.1074/jbc.271.34.20617. [DOI] [PubMed] [Google Scholar]
- 31.Chiabrando C., et al. Identification and measurement of endogenous beta-oxidation metabolites of 8-epi-Prostaglandin F2alpha. J. Biol. Chem. 1999;274:1313–1319. doi: 10.1074/jbc.274.3.1313. [DOI] [PubMed] [Google Scholar]
- 32.Dorjgochoo T., et al. Obesity, age, and oxidative stress in middle-aged and older women. Antioxidants Redox Signal. 2011;14:2453–2460. doi: 10.1089/ars.2010.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai Q., et al. Oxidative stress, obesity, and breast cancer risk: results from the Shanghai Women's Health Study. J. Clin. Oncol. 2009;27:2482. doi: 10.1200/JCO.2008.19.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Y., et al. Quality of dietary carbohydrate is more important than its quantity in lipid peroxidation. Am. J. Clin. Nutr. 2022;116:189–196. doi: 10.1093/ajcn/nqac047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li M., et al. Lipid peroxidation biomarkers associated with height and obesity measures in the opposite direction in women. Obesity. 2022;30:1257–1267. doi: 10.1002/oby.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lara-Guzmán Ó.J., et al. Dietary antioxidant intake is inversely associated with 2,3-dinor oxylipin metabolites, the major excreted oxylipins in overweight and obese subjects. Free Radic. Biol. Med. 2022;190:42–54. doi: 10.1016/j.freeradbiomed.2022.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Eick S.M., et al. Associations between social, biologic, and behavioral factors and biomarkers of oxidative stress during pregnancy: findings from four ECHO cohorts. Sci. Total Environ. 2022;835 doi: 10.1016/j.scitotenv.2022.155596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eick S.M., et al. Associations between socioeconomic status, psychosocial stress, and urinary levels of 8-iso-prostaglandin-F2α during pregnancy in Puerto Rico. Free Radic. Biol. Med. 2019;143:95–100. doi: 10.1016/j.freeradbiomed.2019.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van ′t Erve T.J., et al. Phthalates and phthalate alternatives have diverse associations with oxidative stress and inflammation in pregnant women. Environ. Sci. Technol. 2019;53:3258–3267. doi: 10.1021/acs.est.8b05729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Awad J.A., Morrow J.D. Excretion of F2-isoprostanes in bile: a novel index of hepatic lipid peroxidation. Hepatology. 1995;22:962–968. [PubMed] [Google Scholar]
- 41.Yan Z., Mas E., Mori T.A., Croft K.D., Barden A.E. A significant proportion of F2-isoprostanes in human urine are excreted as glucuronide conjugates. Anal. Biochem. 2010;403:126–128. doi: 10.1016/j.ab.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Gómez C., et al. Quantitative metabolic profiling of urinary eicosanoids for clinical phenotyping. J. Lipid Res. 2019;60:1164–1173. doi: 10.1194/jlr.D090571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holder C., et al. High-throughput and sensitive analysis of free and total 8-isoprostane in urine with isotope-dilution liquid chromatography-tandem mass spectrometry. ACS Omega. 2020;5:10919–10926. doi: 10.1021/acsomega.0c00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murff H.J., et al. N-3 long chain fatty acids supplementation, fatty acids desaturase activity, and colorectal cancer risk: a randomized controlled trial. Nutr. Cancer. 2022;74:1388–1398. doi: 10.1080/01635581.2021.1955286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White M.N., et al. Effects of fish oil supplementation on eicosanoid production in patients at higher risk for colorectal cancer. Eur. J. Cancer Prev. 2019;28:188. doi: 10.1097/CEJ.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill J.R. In vitro drug metabolism using liver microsomes. Curr. Protoc. Pharmacol. 2003;23 doi: 10.1002/0471141755.ph0708s23. [DOI] [PubMed] [Google Scholar]
- 47.Wheelan P., Hankin J.A., Bilir B., Guenette D., Murphy R.C. Metabolic transformations of leukotriene B4 in primary cultures of human hepatocytes. J. Pharmacol. Exp. Therapeut. 1999;288:326–334. [PubMed] [Google Scholar]
- 48.Berry K.A.Z., Borgeat P., Gosselin J., Flamand L., Murphy R.C. Urinary metabolites of leukotriene B4 in the human subject. J. Biol. Chem. 2003;278:24449–24460. doi: 10.1074/jbc.M300856200. [DOI] [PubMed] [Google Scholar]
- 49.Prakash C., Zhang J.Y., Falck J.R., Chauhan K., Blair I.A. 20-Hydroxyeicosatetraenoic acid is excreted as a glucuronide conjugate in human urine. Biochem. Biophys. Res. Commun. 1992;185:728–733. doi: 10.1016/0006-291x(92)91686-k. [DOI] [PubMed] [Google Scholar]
- 50.Little J.M., et al. Glucuronidation of oxidized fatty acids and prostaglandins B1 and E2 by human hepatic and recombinant UDP-glucuronosyltransferases. J. Lipid Res. 2004;45:1694–1703. doi: 10.1194/jlr.M400103-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Allain E.P., et al. Inactivation of prostaglandin E2 as a mechanism for UGT2B17-mediated adverse effects in chronic lymphocytic leukemia. Front. Oncol. 2019;9:606. doi: 10.3389/fonc.2019.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong D., Ako R., Hu M., Wu B. Understanding substrate selectivity of human UDP-glucuronosyltransferases through QSAR modeling and analysis of homologous enzymes. Xenobiotica. 2012;42:808–820. doi: 10.3109/00498254.2012.663515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tong V., Teng X.W., Karagiozov S., Chang T.K.H., Abbott F.S. Valproic acid glucuronidation is associated with increases in 15-F2t-isoprostane in rats. Free Radic. Biol. Med. 2005;38:1471–1483. doi: 10.1016/j.freeradbiomed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 54.Gradinaru D., Minn A.-L., Artur Y., Minn A., Heydel J.-M. Effect of oxidative stress on UDP-glucuronosyltransferases in rat astrocytes. Toxicol. Lett. 2012;213:316–324. doi: 10.1016/j.toxlet.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 55.Kalthoff S., Ehmer U., Freiberg N., Manns M.P., Strassburg C.P. Interaction between oxidative stress sensor Nrf2 and xenobiotic-activated aryl hydrocarbon receptor in the regulation of the human phase II detoxifying UDP-glucuronosyltransferase 1A10. J. Biol. Chem. 2010;285:5993–6002. doi: 10.1074/jbc.M109.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shibuya A., Itoh T., Tukey R.H., Fujiwara R. Impact of fatty acids on human UDP-glucuronosyltransferase 1A1 activity and its expression in neonatal hyperbilirubinemia. Sci. Rep. 2013;3:2903. doi: 10.1038/srep02903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skarke C., et al. Bioactive products formed in humans from fish oils. J. Lipid Res. 2015;56:1808–1820. doi: 10.1194/jlr.M060392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaloga G.P. Narrative review of n-3 polyunsaturated fatty acid supplementation upon immune functions, resolution molecules and lipid peroxidation. Nutrients. 2021;13:662. doi: 10.3390/nu13020662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.