Abstract
Supersulfides, which are defined as sulfur species with catenated sulfur atoms, are increasingly being investigated in biology. We recently identified pyridoxal phosphate (PLP)-dependent biosynthesis of cysteine persulfide (CysSSH) and related supersulfides by cysteinyl-tRNA synthetase (CARS). Here, we investigated the physiological role of CysSSH in budding yeast (Saccharomyces cerevisiae) by generating a PLP-binding site mutation K109A in CRS1 (the yeast ortholog of CARS), which decreased the synthesis of CysSSH and related supersulfides and also led to reduced chronological aging, effects that were associated with an increased endoplasmic reticulum stress response and impaired mitochondrial bioenergetics. Reduced chronological aging in the K109A mutant could be rescued by using exogenous supersulfide donors. Our findings indicate important roles for CARS in the production and metabolism of supersulfides—to mediate mitochondrial function and to regulate longevity.
Keywords: Supersulfides, Cysteinyl-tRNA synthetase, ER stress, Mitochondrial energy metabolism, Longevity
Graphical abstract
Highlights
-
•
Yeast CARS has supersulfide synthetic activity similar to mammals.
-
•
CARS-deficient yeast has enhanced ER stress and abnormal mitochondrial energy metabolism.
-
•
Supersulfides increase the lifespan of budding yeast.
1. Introduction
The presence of supersulfides as low-molecular-weight thiols such as cysteine persulfide (CysSSH) and glutathione persulfide (GSSH) or as persulfidated cysteine residues in proteins has increasingly been discovered in both prokaryotic and eukaryotic cells [[1], [2], [3], [4], [5], [6], [7]]. The presence of multiple sulfur atoms in supersulfides enhances their nucleophilicity and antioxidant ability compared with their corresponding thiols. We recently demonstrated that the mitochondrial isoform of cysteinyl-tRNA synthetase (CARS2) acts as a primary source of CysSSH biosynthesis (and we therefore named it cysteine persulfide synthase, CPERS). We also determined that such CysSSH-producing activity may be critically involved in the regulation of mitochondrial function. The functional role of CARS2 was clarified by means of in vivo analysis including using Cars2 mutant mice and demonstrating the involvement of supersulfides in processes such as mitochondrial energy metabolism, embryonic development, cardiac function, tumorigenesis, innate immunity, antiviral defense mechanisms, and prevention of chronic pulmonary disorders [2,[7], [8], [9], [10], [11], [12]]. CARS/CPERS can also mediate direct incorporation of CysSSH into proteins during translation and result in the formation of protein supersulfides (protein persulfidation), which may affect protein stability, conformation, or activity [[1], [2], [3], [4], [5],[13], [14], [15], [16]]. However, the in vivo physiological functions of supersulfides produced by CARS/CPERS are still poorly understood.
In this study, we investigated the physiological functions of CysSSH by using the budding yeast Saccharomyces cerevisiae. Our analysis, both in vitro and in vivo, showed that CysSSH production by CARS is conserved in this yeast. Unlike certain other amino acids, the yeast genome lacks specific mitochondrial aminoacyl-tRNA synthetases (ARSs) for Ala, Gln, His, Val, and Cys tRNA aminoacylation [[17], [18], [19], [20], [21]]. Instead, it uses alternative pathways, including alternative transcription initiation and noncanonical initiation codon usage, to translate both cytosolic and mitochondrial isoforms from a single gene. As a notable result, our prior research demonstrated that in the case of yeast CARS/CPERS, which encodes both cytosolic and mitochondrial isoforms, the mitochondrial CARS/CPERS is activated via alternative transcription initiation by a key transcription factor called the heme activator protein (Hap) complex [21].
For this study, we constructed a yeast mutant strain with a K109A mutation in one of the pyridoxal phosphate (PLP)-binding sites on CRS1 (yeast ortholog of CARS), which could synthesize proteins but had reduced CysSSH production. The K109A mutant had a shorter chronological lifespan than the wild-type (WT) yeast, along with an abnormal endoplasmic reticulum (ER) stress response and impaired mitochondrial bioenergetics. Supersulfide donors, however, rescued the decrease in the chronological lifespan in the K109A mutant. More important, supersulfide donors significantly increased the longevity of the WT yeast. These results suggest that reactive supersulfides may play important roles in regulating longevity and maintaining the lifespan of the yeast. To this end, we conducted loss-of-function studies of CRS1 in budding yeast, which demonstrated that CRS1 plays a fundamental role in longevity: maintaining mitochondrial energy production and protein folding. Our studies provide robust evidence for the critical requirement of supersulfide-mediated mitochondrial energy metabolism, i.e. sulfur respiration, in eukaryotes under physiological conditions.
2. Materials and methods
2.1. Media, yeast strains, and plasmids
Supplementary Table S1 provides the strains of the yeast S. cerevisiae and Supplementary Table S2 lists the primers used in this study. The media used for growth were yeast extract-peptone-dextrose (YPD), synthetic complete (SC), and Sehgal and Gibbon's (SG). YPD contains 1 % yeast extract (Difco Laboratories, Detroit, MI, USA), 2 % peptone (Difco Laboratories), and 2 % glucose. SC contains 2 % glucose, 0.67 % yeast nitrogen base without amino acids (Difco Laboratories), and 0.2 % dropout supplement (Takara Bio, Shiga, Japan). SG contains 2 % galactose instead of the glucose in SC. For auxotrophic markers, the appropriate amino acids and/or nucleotides were removed. When necessary, 2 % agar (Nacalai Tesque, Kyoto, Japan) was added to solidify the medium.
To generate a heterozygous deletion mutant of CRS1, HTS1 (yeast ortholog of histidyl-tRNA synthetase [HARS]), or GUS1 (yeast ortholog of glutamyl-tRNA synthetase [EARS]) (BY4743 Δcrs1, BY4743 Δhts1, or BY4743 Δgus1), a deletion cassette containing a G418-resistant gene was amplified by means of polymerase chain reaction (PCR) using primers (CRS1 dis-Fw and CRS1 dis-Rv; HTS1 dis-Fw and HTS1 dis-Rv; or GUS1 dis-Fw and GUS1 dis-Rv) and pFA6a-kanMX6 (purchased from the AddGene repository; Plasmid #39296). The PCR fragments were integrated into the genome of the BY4743 WT strain by transformation. The G418-resistant phenotype was screened, and the correct integration event was verified by using PCR with chromosomal DNA. For the construction of IRE1-or HAC1-disruptant strains, gene-specific deletion cassettes were prepared from the BY4741-derived deletion library (EUROSCARF, Oberursel, Germany) by using PCR with primers (IRE1 dis-Fw and IRE1 dis-Rv, or HAC1 dis-Fw and HAC1 dis-Rv), which were then integrated into the genome of the BY4742 WT strain by transformation. The G418-resistant phenotype was screened, and the correct integration event was verified by means of PCR with chromosomal DNA.
The CRISPR-Cas9 system was used to construct a mutant (BY4742 K109A) that chromosomally expresses Crs1 K109A [21]. A gRNA expression plasmid (p426-gRNA-CRS1) targeting the CRS1 locus was constructed by using the QuikChange method with primers (gRNA-YNL247W KIIL motif Fw and gRNA-YNL247W KIIL motif Rv) and with p426-SNR52p-gRNA.CAN1.Y-SUP4t (purchased from the AddGene repository) as a template. The BY4742 WT strain harboring p415-GalL-Cas9-CYC1t (purchased from the AddGene repository) was grown in SG medium without Leu and used for transformation with p426-gRNA-CRS1 (700 μg) and double-stranded oligonucleotides (1 nmol) by means of the LiAc/SS carrier DNA/PEG method (volume: 400 μL) [21]. Double-stranded oligonucleotides were generated by means of PCR with primers (AIIL mutation DNA PCR Fw and AIIL mutation DNA PCR Rv) and with a template DNA (AIIL mutation DNA). The transformed cells were incubated in SG without uracil and Leu at 30 °C for 4 h and plated on YPD medium. The correct mutation was confirmed by sequencing.
For expression of S. cerevisiae Crs1 (scCrs1) or Schizosaccharomyces pombe Crs1 (spCrs1) proteins in Escherichia coli (E. coli), plasmids were constructed by using Gateway technology (Thermo Fisher Scientific, Rockford, IL, USA). The modified CRS1 DNA sequence designed by GeneOptimizer expert software [22] was used in this study because the original CRS1 DNA sequence was not cloned in any bacterium such as E. coli. pDONR-Crs1 or pDONR-spCrs1 was generated by a BP reaction with pDONR221 (entry vector) and PCR-amplified fragments prepared by using primers (designed CRS1 gateway Fw and designed CRS1 gateway Rv, or spCRS1 gateway Fw and spCRS1 gateway Rv) and pCrs1full-GFP [23], or an S. pombe genomic DNA, respectively. pDONR-Crs1 K109A was prepared by means of the QuikChange method with primers (designed CRS1 K109A Fw and designed CRS1 K109A Rv) and pDONR-scCrs1. pET53-Crs1, pET53-Crs1 K109A, and pET53-spCRS1 were constructed via an LR reaction from pET53-DEST (C-terminal StrepII tag for E. coli expression; Merck Millipore, Watford, UK). For expression of CRS1 with the original promoter in yeast, the expression plasmid pRS416-CRS1 was constructed. The PCR-amplified fragment containing the original promoter of CRS1 was prepared with primers (CRS1 up-1kbp NEBuilder Fw and CRS1 up-1kbp NEBuilder Rv) and a with a genomic DNA. The PCR-amplified fragment containing the original terminator of CRS1 was prepared with primers (CRS1 down-1kbp NEBuilder Fw and CRS1 down-1kbp NEBuilder Rv) and with a genomic DNA. The CRS1 open reading frame (ORF) was prepared with primers (designed CRS1 ORF NEBuilder Fw and designed CRS1 ORF NEBuilder Rv) and with pDONR-Crs1 as a template DNA. The PCR-amplified fragments with the original promoter, the PCR products containing the original terminator, and CRS1 ORF were mixed, and the CRS1 with the promoter and terminator were inserted into HindIII/XbaI-digested pRS416 (Agilent Technologies, Santa Clara, CA, USA) by using the NEBuilder HiFi DNA Assembly kit (New England Biolabs, Ipswich, MA, USA), according to the manufacturer's instructions.
2.2. Purification of recombinant proteins
To prepare recombinant Crs1, Crs1 K109A, and spCrs1 proteins, E. coli strain T7 Express (New England Biolabs) transformed with pET53-Crs1, pET53-Crs1 K109A, or pET53-spCRS1 was grown at 37 °C in LB medium containing 100 μg/mL carbenicillin. When the optical density OD600 value was 0.6, 0.2 mM isopropyl-β-d-thiogalactopyranoside was added to the culture medium to induce gene expression. After cultivation for 18 h at 17 °C, cells were harvested and suspended in ice-cold buffer A (100 mM Tris-HCl, 150 mM NaCl, pH 8.0) containing a protein inhibitor cocktail and disrupted by sonic oscillation under cooling. After centrifugation, the supernatant was loaded with Strep-Tactin Sepharose (IBA Lifesciences, Goettingen, Germany) and washed with 20 column volumes of buffer A. The recombinant StrepII-tagged Crs1, Crs1 K109A, and spCrs1 were eluted with buffer A containing 2.5 mM desthiobiotin (IBA Lifesciences). Protein concentration was determined by using the Bradford method with bovine serum albumin as the standard protein.
2.3. Detection of supersulfides
Supersulfides produced by Crs1 were analyzed by using liquid chromatography (LC)-electrospray ionization (ESI)-tandem mass spectrometry (MS/MS) (LC-ESI-MS/MS), according to our previous method [2]. To measure cysteine polysulfide generated from recombinant Crs1, the recombinant Crs1 was incubated with cysteine in 50 mM HEPES buffer (pH 7.5) at 37 °C. The mixtures were then reacted with 1 mM β-(4-hydroxyphenyl) ethyl iodoacetamide (HPE-IAM) in methanol at 37 °C for 20 min to form CysS-(S)n-HPE-IAM adducts. After centrifugation, aliquots of the supernatants were diluted 10–100 times with 0.1 % formic acid containing known amounts of isotope-labeled internal standards, after which they were subjected to LC-ESI-MS/MS.
For analyses of intracellular supersulfide levels, cultured yeasts were lysed by means of sonication in a cold methanol solution containing 1 mM HPE-IAM, and then the cell lysates were incubated at 37 °C for 20 min. After centrifugation, aliquots of the supernatants of the lysates were diluted 20 times with 0.1 % formic acid containing known amounts of isotope-labeled standards, and they were then analyzed via LC-ESI-MS/MS for persulfide and polysulfide determination.
2.4. Chronological lifespan assay
Chronological lifespan was determined according to a previously described method with simple modifications [19]. Briefly, fresh colonies were inoculated into SC medium. Overnight culture was then seeded into SC medium with an initial OD600 of 0.1, and the samples were cultured with shaking at 200 rpm at 30 °C under humid conditions. Starting on day 3 and then 2–3 days thereafter, a small amount of culture was taken out and diluted and plated on YPD plates to determine colony formation after 2 days of incubation at 30 °C. Day 3 was considered to be the point of 100 % survival. Supersulfide donors were added every 6 days.
2.5. Western blotting analysis
Western blotting analysis was performed with anti-3-phosphoglycerate kinase (Pgk1) (Abcam, Cambridge, UK; clone 22C5D8) and anti-subunit II of cytochrome c oxidase (Cox2) (Abcam; clone 4B12A5). Samples were solubilized by using Laemmli lysis buffer (125 mM Tris HCl, 2 % sodium dodecyl sulfate [SDS], 20 % glycerol, and 10 % 2-mercaptoethanol, pH 6.8), loaded on SDS-polyacrylamide gel electrophoresis gels and transferred to polyvinylidene difluoride membranes (GE Healthcare, Little Chalfont, England). The membranes were blocked with Blocking One (Nacalai Tesque) for 60 min at room temperature and were then incubated with primary antibodies (1/2000) diluted with Can Get Signal Immunoreaction Enhancer Solution 1 (TOYOBO, Osaka, Japan) at 4 °C overnight. After the membranes were washed with Tris-buffered saline containing Tween 20 buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1 % Tween 20, pH 7.4), they were incubated with horseradish peroxidase-conjugated secondary antibody diluted (1/5000) with Can Get Signal Immunoreaction Enhancer Solution 2 (TOYOBO) for 1 h at room temperature. Immunoreactive bands were detected by using an ECL Prime Western Blotting Detection Reagent (GE Healthcare) with a luminescent image analyzer (ImageQuant LAS 500; GE Healthcare).
2.6. Growth test
Yeast cells were cultured at 30 °C in SC medium starting from 0.1 of the OD600. Cell growth was monitored by measuring the OD600.
2.7. Real-time quantitative PCR analysis
Cultured cells were disrupted by using the Multi-Beads Shocker (Yasui Kikai, Osaka, Japan) with 0.5-mm glass beads, and total RNA was extracted via the NucleoSpin RNA Plus kit (Takara Bio) according to the manufacturer's instructions. cDNA was synthesized from total RNA by means of the PrimeScript RT Reagent Kit (Takara Bio). Real-time quantitative PCR was performed with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA), the synthesized cDNA, and primers for each gene by using the CFX Connect Real-Time System (Bio-Rad Laboratories). Supplementary Table S2 lists the primers used in this study. The following PCR protocol was used: 95 °C for 4 min followed by 40 cycles each at 95 °C for 15 s and at 60 °C for 30 s. The expression level of each gene was normalized to ACT1 as the reference gene.
2.8. Analysis of HAC1 mRNA splicing
We detected HAC1 mRNA according to the method in a previous report [24]. Briefly, cultured cells were disrupted by using the Multi-Beads Shocker (Yasui Kikai) with 0.5-mm glass beads, and total RNA was extracted by means of the NucleoSpin RNA Plus kit (Takara Bio) according to the manufacturer's instructions. cDNA was synthesized from total RNA via the ReverTra-Plus Kit (Toyobo); it was then used as the template for individual PCR with primers (HAC1 Fw and HAC1 Rv). The PCR products were run on 2 % agarose gels. Tunicamycin-treated cells (1 μg/mL, for 1 h) were used as a positive control.
2.9. Protein disulfide isomerase (PDI) activity
PDI activity was determined by using the in vitro refolding assay with reduced RNase (rRNase) as a substrate. A solution of RNase, 20 mg/mL (Sigma, St. Louis, MO, USA), was incubated in 100 mM Tris-HCl buffer (pH 8.0) containing 6 M guanidine-HCl, 2 mM EDTA, and 50 mM tris(2-carboxyethyl)phosphine (TCEP) at 37 °C for 16 h. The unfolded RNase solution was buffer-exchanged by using the PD10 desalting column equilibrated with 50 mM Tris-HCl (pH 7.4) with 1 mM EDTA and 0.1 % acetic acid. The rRNase concentration was estimated by using a molar extinction coefficient of 9300 M−1cm−1 at 280 nm. The isomerase activity of the recombinant yeast PDI (yPDI) was analyzed by using the formation of native RNase, which was measured spectrophotometrically by monitoring the hydrolysis of the RNase substrate cCMP at 296 nm. rRNase (10 μM) was added to 3 μM recombinant yPDI in 100 mM Tris-HCl buffer (pH 7.6) containing 4.5 mM cCMP, 1 mM glutathione (GSH), 0.2 mM glutathione disulfide (GSSG), and 100 mM Tris-HCl (pH 7.6). Assays were performed at 30 °C in 50 mM Tris-HCl buffer (pH 7.6) containing yPDI (0.7–1.4 mM), 1 mM GSH, and 0.2 mM GSSG. Reactions were initiated by the addition of reduced RNase (1.0 mL of a 0.50 mg/mL solution in 100 mM acetic acid). At 10-min intervals, aliquots (10 mL) were removed and added to 500 mL of 100 mM 2-(N-morpholino)ethanesulfonic acid buffer, pH 6.0, containing poly(C) (10 mM). RNase activity was monitored by noting the change in absorbance at 238 nm.
2.10. PDI proteome analysis
Recombinant yPDI proteins(20 μg/mL) were reduced with or without 100 μM TCEP in 250 mM sodium acetate buffer, pH 6.5, at 37 °C for 30 min. Samples were treated with 100 μM sodium disulfide (Na2S2) at 37 °C for 15 min and were then alkylated with 1 mM iodoacetamide (37 °C, 30 min). Samples were digested with Trypsin Gold (2 μg/mL) in 0.025 % ProteaseMAX Surfactant (37 °C, 3 h) and subjected to LC-ESI-quadrupole time-of-flight MS/MS (LC-ESI-Q-TOF MS/MS). LC-ESI-Q-TOF MS/MS analysis was performed by using 6545XT AdvanceBio LC/Q-TOF (Agilent Technologies) connected to the Agilent HPLC-Chip system. Modification analysis of the active center cysteine was performed by means of Agilent MassHunter BioConfirm software. The SS- and SSS-binding levels of C61 and C64 within the LATDSFNEYIQSHDLVLAEFFAPWCGHCK peptide were detected by observations at m/z 835.6369 and 843.8814, respectively.
2.11. Determination of intracellular ATP levels
About 1 × 107 cultured cells (OD = 1.0) were harvested, washed three times with ice-cold PBS, and resuspended in 200 μL of sterilized water. After being frozen with liquid nitrogen, the frozen cells were heated at 95 °C for 10 min so that ATP was released to the outside of the cells. ATP levels in 100 μL of the supernatant were determined by using the IntraCellular ATP assay kit (Toyo B-Net, Tokyo, Japan).
2.12. Statistical analysis
Results are presented as means ± standard deviations (SDs) of at least three independent measurements unless otherwise specified. Student's t-test was used for comparisons of continuous variables, and Multiple t-test/two-way analysis of variance (ANOVA) with Tukey's test was utilized for multiple comparisons in GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). p < 0.05 was set as the level of statistical significance.
3. Results
3.1. Supersulfide biosynthesis is confirmed in yeast
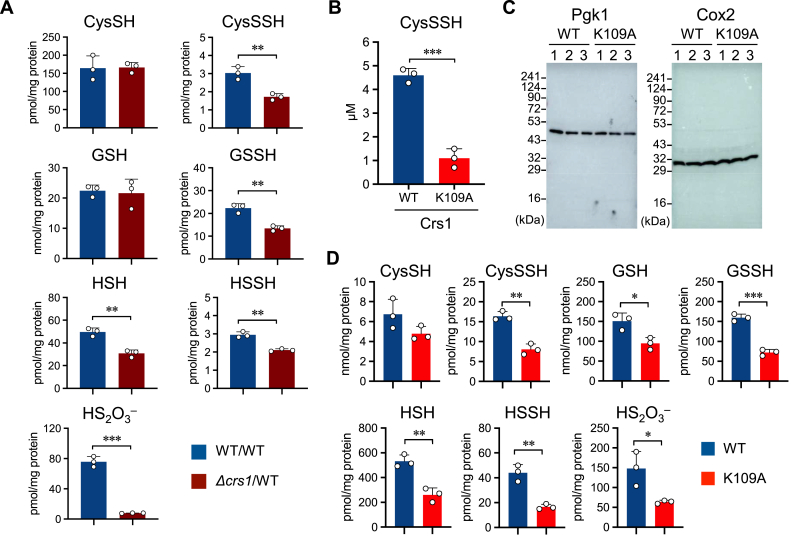
To evaluate the production of supersulfides in yeast, we first generated a heterologous deletion mutant of CRS1 (yeast ortholog of CARS/CPERS). Indeed, although overall production of cysteine and GSH was not altered in the Δcrs1 mutant, the intracellular supersulfide content (CysSSH, GSSH, HSS−, and their oxidation product HS2O3−) significantly decreased (Fig. 1A), which indicates a role of Crs1 in supersulfide biosynthesis similar to that of CARS/CPERS in mammals and bacteria. Moreover, Δcrs1 mutant yeast also had decreased levels of hydrogen sulfide (HSH), which presumably originated from reduction of supersulfides by endogenous reducing systems. To directly confirm the ability of Crs1 to synthesize CysSSH, we incubated recombinant Crs1 from two yeast species, the budding yeast S. cerevisiae (scCrs1) and the fission yeast S. pombe (spCrs1), in the presence of cysteine. Recombinant Crs1 proteins from both budding yeast and fission yeast had CPERS activity (Figs. S1A and B). Because Crs1 has two enzymatic functions, namely cysteinyl-tRNA synthetase activity involved in protein synthesis and CPERS activity [2], any phenotype associated with Crs1 deficiency may be associated with the loss of either enzymatic function. We therefore took advantage of previous studies of recombinant CARS from E. coli showing that mutation of a PLP-binding lysine (K73A) reduced CPERS activity without affecting cysteinyl-tRNA synthetase activity [2]. We generated the corresponding lysine-to-alanine mutant in recombinant yeast Crs1 (K109A), which similarly showed a marked reduction in CPERS activity compared with WT Crs1 (Fig. 1B and S1C). We then constructed a yeast strain containing the K109A mutation by using the CRISPR/Cas9 system, and we confirmed that overall protein translation activity was not affected (Fig. 1C). Overall production of supersulfides (CysSSH, GSSH, HSS−) and related oxidation products (HS2O3−), however, was markedly lower in the K109A mutant compared with that in the WT in late log phase (Fig. 1D), similar to findings with the CRS1 deletion mutant. However, upon introducing the WT Crs1 into the K109A mutant, the overall production of supersulfides and related oxidation products reached levels comparable to those observed in the WT (Fig. 2A). Moreover, the quantification of GSH and GSSG levels was performed using mass spectrometry analysis, followed by the calculation of the GSSG/GSH ratio. In the K109A mutant yeast, the GSSG/GSH ratio was observed to be higher compared to that in the WT yeast, indicating a potential imbalance in the redox status of the mutant strain. Additionally we have observed the upregulation of Glutathione Peroxidase 2 (GPX2) gene expression levels in the K109A mutant strain. GPX2 is a downstream gene of the reactive oxygen species-responsive transcription factor Yap 1 and recognized as a standard marker for oxidative stress (Fig. 2B). On the introduction of WT Crs1 into the mutant strain, the GSSG/GSH ratio and the expression of GPX2 returned to levels comparable to those observed in the WT (Fig. 2A and B). These findings suggest an elevation of oxidative stress in the Crs1 K109A mutant, which is rescued by the introduction of WT Crs1 into the mutant strain. Also, we observed a marked reduction in HSH, which suggests that its endogenous production depends largely on the initial production of supersulfides followed by their reduction by endogenous reducing systems. Therefore, the K109A mutant is an ideal model to directly assess the biological functions of supersulfides in vivo.
Fig. 1.
Supersulfide production by yeast cysteinyl-tRNA synthetase (Crs1). (A)In vivo formation of various supersulfides in the yeast WT and Δcrs1 strain. Endogenous production of supersulfides was identified by means of HPE-IAM labeling LC-ESI-MS/MS analysis (n = 3). **p < 0.01, ***p < 0.001; Student's t-test. (B) CysSSH production by the recombinant WT and K109A mutant protein (n = 3). ***p < 0.001; Student's t-test. (C) Translation activity of the yeast K109A strain as determined by Western blot analysis. Protein samples were prepared for detection of Pgk1 (nuclear encoded gene) and Cox2 (mitochondrial genome-encoded gene). (D) Supersulfide metabolome analysis with the yeast WT and K109A strain. *p < 0.05, **p < 0.01, ***p < 0.001; Student's t-test. CysSH, cysteine; CysSSH, cysteine persulfide; GSH, reduced glutathione; GSSH, glutathione persulfide; HSH, hydrogen sulfide; HSSH, hydrogen disulfide; HS2O3−, thiosulfate.
Fig. 2.
In vivo formation of various supersulfides in the yeast WT, K109 mutant, and K109A + Crs1 strain. (A)In vivo formation of various supersulfides in the yeast WT, K109A mutant, and K109A + Crs1 strain. K109A + Crs1 strain was generated by introduction of the WT Crs1 transgene into the mutant strain. Endogenous production of supersulfides was identified by means of HPE-IAM labeling LC-ESI-MS/MS analysis (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 ****p < 0.0001; Multiple t-test. n.s., not significant. (B) Gene expression of oxidative stress marker in the WT, K109 mutant, and K109A + Crs1 strain. The mRNA of Gpx2, the oxidative stress marker, was quantified using qPCR in WT, K109A, and K109A + Crs1 strain at late log phase. *p < 0.05, ***p < 0.001; Multiple t-test. n.s., not significant.
3.2. Crs1 supersulfide synthase activity promotes longevity in yeast
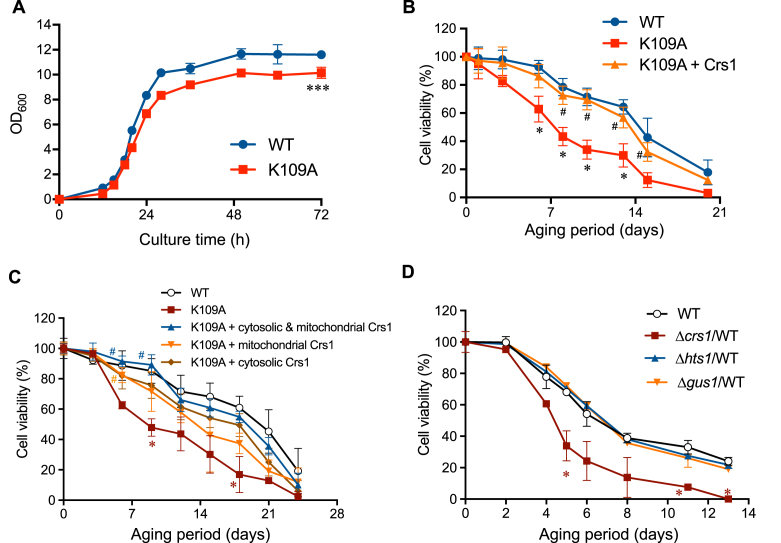
To investigate the functions of Crs1 supersulfide synthase activity, we studied the impact of the K109A mutation on yeast growth. Indeed, S. cerevisiae containing CRS1 K109A showed slightly impaired growth compared with the WT (Fig. 3A), and the chronological lifespan of K109A yeast was significantly shorter than that of the WT strain, which could be reversed by expression of WT Crs1 (Fig. 3B). In contrast to mammals and their CARS genes, S. cerevisiae contains only one CRS1 gene, which encodes both cytoplasmic and mitochondrial isoforms that can be expressed via alternative transcription [21]. With an exogenous promoter (alcohol dehydrogenase 1), we overexpressed either full-length Crs1 (expressed in only mitochondria) or N-terminal truncated Crs1 (expressed in only cytosol), which in both cases partially rescued the lifespans of K109A strains (Fig. 3C), thereby indicating that the observed shortened lifespans of K109A mutants were caused by deficient supersulfide synthesis in both cytosol and mitochondria. Heterozygous deletion of CRS1 in yeast also produced shortened lifespans, although similar heterozygous deletion of other tRNA synthetases, such as histidyl-tRNA synthetase HTS1 (yeast ortholog of HARS) and glutamyl-tRNA synthetase GUS1 (yeast ortholog of EARS), did not reduce the lifespan (Fig. 3D). These collective findings indicate that the shortened lifespan of CRS1 mutants is due to reduced supersulfide synthase activity rather than inhibition of translational function.
Fig. 3.
Phenotypic analysis of the WT and the CRS1 K109A mutant strain. (A) Growth curves of the WT and the CRS1 K109A mutant. Cell growth was determined at the indicated time points by measuring the OD600. ***p < 0.001; two-way ANOVA with Tukey's test. (B) A chronological lifespan assay of the WT and the K109A mutant. Chronological lifespan was determined by using colony formation units. The K109A mutant showed a significant variance in survival rates compared with the WT. The K109A mutant strain with plasmid expressed WT Crs1 showed the same survival rate as the WT. *p < 0.05 vs. WT, #p < 0.05 vs. K109A; two-way ANOVA with Tukey's test. (C) Mitochondrial or cytosolic Crs1 expression vectors were transformed into the K109A mutants, and the chronological lifespans of the mutants were measured. As a control, pRS416 was transformed into the K109A mutant. *p < 0.05 vs. WT, #p < 0.05 vs. K109A; two-way ANOVA with Tukey's test. (D) Chronological lifespans were measured in heterozygous mutants of CARS (Δcrs1/WT), HARS (Δhts1/WT), and EARS (Δgus1/WT). *p < 0.05 vs. WT; two-way ANOVA with Tukey's test.
3.3. Reduced lifespan of the K109A mutant is associated with increased ER stress
Our observations of a partial rescue of the lifespan as caused by both cytosolic and mitochondrial Crs1 expression in K109A mutant yeast suggest the importance of the synthesis of both cytosolic and mitochondrial supersulfides. Because mammalian CARSs catalyze CysS–(S)n–H formation from cysteine and co-translational protein persulfidation, inhibition of these processes may induce protein misfolding and trigger an unfolded protein response (UPR) or ER stress pathways. In yeast, IRE1 and HAC1 genes are two key components of ER stress. HAC1 mRNA is normally present in a precursor form, and activation of Ire1 during ER stress induces a splicing reaction that results in the formation of mature HAC1 mRNA encoding a nuclear transcription factor that mediates ER stress responses [25]. Analysis of HAC1 mRNA splicing variants in WT and K109A yeast strains showed enhanced HAC1 splicing during chronological aging, which was observed at earlier time points (day 9) in the K109A mutant compared with the WT strain (day 15); this finding suggests accelerated ER stress in the K109A mutant strain (Fig. 4A upper and middle panels). Therefore, one of the target genes for HAC1, KAR2, which encodes for the binding immunoglobulin protein chaperone protein that is involved in the translocation, folding, and assembly of secretory and transmembrane proteins [25], was induced during aging and was observed at earlier time points in the K109A mutants (day 6) compared with the WT strain (Fig. 4B). Consistent with the importance of both Ire1 and Hac1 in UPR/ER stress pathways because of incorrect protein folding, deletion of either gene significantly shortened the yeast lifespan (Fig. S2). The introduction of WT Crs1 into K109A mutant demonstrated a suppression in the splicing of HAC1 (Fig. 4A lower panel), alleviating ER stress.
Fig. 4.
Crs1-dependent ER stress response in aging. (A) HAC1 splicing during aging. cDNAs were made from the cultured cells, followed by individual PCR with primers in upper panel (HAC1 Fw and HAC1 Rv). The PCR products were electrophoresed to determine the HAC1 splicing (middle and lower panels). Arrows indicate primer positions. M: DNA marker, Tm: tunicamycin-treated sample as the positive control of ER stress. (B)KAR2 induction during aging. Conventional real-time quantitative PCR analysis was used, and cDNAs from the WT and the K109A strain were measured by using the KAR2 primers (given in Supplementary Table S2). **p < 0.01 vs. WT; two-way ANOVA with Tukey's test. (C) Alteration of PDI enzyme activity by means of reduction. Recombinant PDI protein was reduced with TCEP and treatment with or without Na2S2 treatment, followed by measurement of PDI enzymatic activity by using RNase as a substrate. (D) LC-ESI-Q-TOF-MS chromatograms obtained via proteome analysis of peptide fragments from the recombinant PDI protein, which included cysteine residues. The disulfide bond or trisulfide bond of cysteine in the active center of the PDI protein was identified by monitoring at m/z 835.6369 and 843.8814, respectively. Peptide fragments containing cysteine residues are shown at the tops of the panels. Cysteine residues in the fragments are indicated by red type. CysS-SCys, disulfide bond. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Our findings with CRS1 K109A mutants suggest that defective supersulfide synthesis contributes to enhanced protein misfolding and UPR pathways. In addition, defective supersulfide synthesis in K109A mutants may disrupt mechanisms for the repair of protein misfolding by PDI. We therefore investigated the impact of persulfidation on the enzymatic activity of recombinant Pdi1p (yeast homolog of PDI) with RNase A as a substrate. As expected, disruption of Pdi1p with TCEP strongly suppressed PDI enzymatic activity, which was partially restored by subsequent treatment with the supersulfide donor Na2S2 (Fig. 4C); this finding suggests that protein persulfidation of Pdi1p can enhance its enzymatic activity. To study this, we investigated the persulfidation of active centers of the recombinant protein yPDI, and we found enhanced persulfidation of active centers in native PDI after Na2S2 administration (Fig. 4D). Collectively, these findings suggest that defective supersulfide synthesis in CRS1 mutant yeast may contribute to an increased misfolded protein burden and impaired proteostasis during aging and may lead to reduced longevity [26].
3.4. Shortened lifespan of the K109A mutant is also associated with reduced mitochondrial function
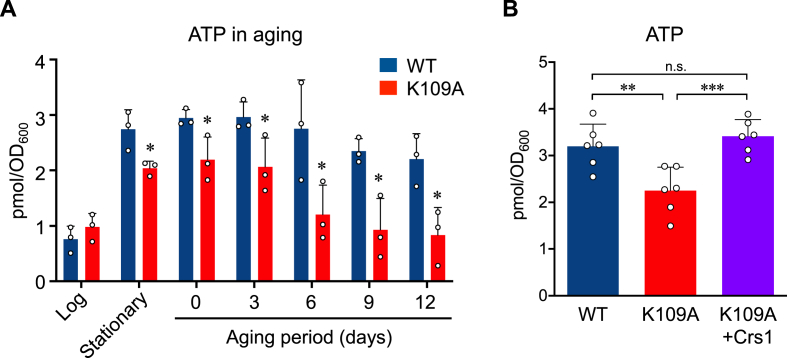
We recently proposed that mitochondrial CARS contributes to mitochondrial energy metabolism by producing CysSSH [2], and we therefore hypothesized that the K109A yeast mutants also have impaired mitochondrial energy metabolism. Indeed, although cellular the ATP contents were similar in both WT and K109A strains during the log phase of growth, ATP contents were significantly reduced in K109A mutants in the stationary phase and progressively decreased during aging (Fig. 5A), which suggests that CysSSH production by mitochondrial Crs1 contributes to the maintenance of mitochondrial membrane potential and ATP production, especially during the stationary phase and aging. K109A mutant expressed Crs1 showed an ATP level equivalent to that of the WT (Fig. 5B).
Fig. 5.
Crs1-dependent mitochondrial energy metabolism in aging.
(A) ATP contents of the WT and the K109A mutant were measured during aging. *p < 0.05 vs. WT; two-way ANOVA with Tukey's test. (B) ATP contents of the WT, the K109A mutant, and K109A mutant + Crs1 were measured at stationary phase. **p < 0.01, ***p < 0.01; Multiple t-test. n.s., not significant.
3.5. Longevity is regulated by exogenous supersulfides
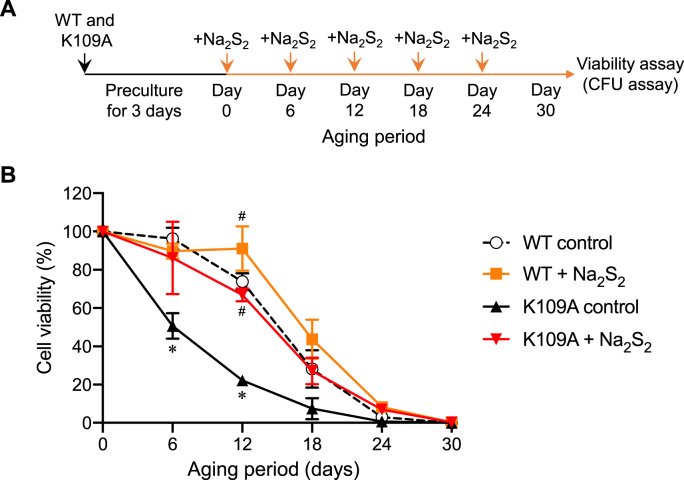
We next studied whether shortened longevity in K109A mutant yeast can be rescued by treatment with various exogenous sulfur donors, including the supersulfide donor Na2S2, the tetrasulfide donor sodium tetrasulfide (Na2S4), the hydrogen sulfide donor sodium hydrosulfide (NaHS), and the supersulfide oxidation product thiosulfate (Fig. 6 and S3). As a notable result, administration of Na2S2 markedly lengthened the lifespan of the K109A mutant to the level of the WT yeast, whereas it minimally affected the longevity of WT yeast (Fig. 6B). By comparison, administration of Na2S4 also restored the longevity of the K109A mutant yeast, whereas NaHS only modestly affected longevity, and administration of thiosulfate did not change the lifespan at all (Fig. S3). These findings thus confirm that shortened longevity of K109A mutants is due to reduced supersulfide synthesis and can be restored by addition of exogenous supersulfides.
Fig. 6.
Effects of supersulfide donors on longevity. (A) Schematic showing the method used for regulation of longevity by exogenous supersulfide. Yeast cells were cultured at 30 °C for 3 days in SC medium. Thereafter, the persulfide donor Na2S2 (20 μM) was added to the culture every 6 days until day 30. A small amount of the culture was removed and diluted and plated on YPD plates to determine colony formation after 2 days of incubation at 30 °C. (B) Chronological survival curves of the WT and the K109A mutant with or without Na2S2 were constructed by counting the number of colony-forming units. *p < 0.05 vs. WT control, #p < 0.05 vs. K109A control; two-way ANOVA with Tukey's test.
4. Discussion
This study showed unequivocally the presence of essential function of cytosolic and mitochondrial supersulfides and metabolic enzymes CARS/CPERS, which suggests that eukaryotes from yeast to mammals utilize sulfur respiration under physiological conditions. CARS/CPERS has a crucial role in endogenous CysSSH production, which suggests that the enzyme serves as the principal CPERS in vivo, as found in our previous studies [2,8,27] In a previous report, we identified one CARS gene in yeast, named YNL247W or CRS1, which appears to be responsible for transcribing both cytosolic and mitochondrial forms of the CARS protein; we also discovered that the transcription factor Hap complex, which is involved in mitochondrial biogenesis, determined the transcription start sites of the CRS1 gene [21].
In this study, we investigated the role of the CRS1 gene in the yeast S. cerevisiae and the relationship of CRS1 to sulfur respiration and lifespan. We generated a mutant strain of S. cerevisiae (K109A) that carried a mutant CRS1 gene, and we found that this strain had reduced levels of CysSSH and impaired sulfur respiration, as we previously reported for E. coli and mammals [2,6]. In our experiments, the decrease in supersulfides that was linked to CARS deficiency manifested as a shortened lifespan. This observed phenotype correlates with the age-associated reduction in persulfidation, a phenomenon that indicates evolutionary conservation across diverse species [28]. No significant differences in cell growth were found between WT and K109A mutant strains during the log phase (Fig. 3A). Similarly, ER stress response and ATP levels showed consistent patterns between the two yeast types during log-phase growth, in line with proliferation analysis results (Fig. 4, Fig. 5A). These results indicate that there is no hamper to proliferation during the log phase, where glycolysis prevails, while supersulfides become important in the stationary phase, where mitochondrial energy metabolism takes precedence. Taken together, the results underscore the pivotal role of supersulfides in mitochondrial energy metabolism.
When Crs1 was expressed simultaneously in the cytoplasm and the mitochondria, the CRS1 mutant was rescued completely, but such cytosolic or mitochondrial Crs1 expression could be only partially rescued (Fig. 3C). Administration of Na2S2 to the K109A mutant rescued the lifespan of the mutant so that it reached the WT level (Fig. 6B). These findings suggest that Crs1 may have crucial roles in both the mitochondria and the cytosol.
Supersulfides can be found not only in low-molecular-weight compounds such as CysSH and GSH but also in numerous cysteine residues in proteins, and they may regulate protein functions and protect protein thiols against irreversible oxidation [14,[29], [30], [31]]. This unique supersulfide-associated protein modification, known as protein persulfidation, is believed to contribute to redox signaling and the regulation of cell functions as a novel mechanism of protein function regulation [[32], [33], [34]]. We previously demonstrated that protein persulfidation occurs during translation, when CysSSH is introduced in place of cysteine during translation via CysSSH-binding tRNA [2]. Although the function of protein persulfidation is still largely unknown, defects in protein persulfidation may result in protein misfolding. Indeed, Hac1, a transcription factor involved in the ER stress response, is strongly activated in the K109A mutant strain during the aging phase of its lifespan. Therefore, Crs1-derived supersulfide may be involved in the regulation of ER protein refolding, and the K109A mutant may be susceptible to ER stress related to disruption of this mechanism. ER stress is often caused by protein refolding defects in the ER, frequently resulting from abnormal disulfide bonds [35,36]. To prevent disulfide bond failure, eukaryotes including yeast have a PDI that introduces the correct disulfide bonds. PDI has two thioredoxin-like catalytic domains, each containing a typical Cys-X-X-Cys motif in its catalytic domain. As a result, PDI activity may be regulated by persulfidation.
An interesting finding is that PDI activity, which was observed after the purification of recombinant proteins, was almost completely lost after treatment with agents that reduce supersulfides and convert them to thiols (Fig. 4C). Also, PDI activity was restored by adding Na2S2, a supersulfide donor, to the reduced PDI, most likely by persulfidating cysteines in PDI active centers (Fig. 4D). These results would suggest that PDI is persulfidated to enhance its activity. This would also suggest that PDI in the K109A strain has a lower persulfidation level of the active site, perhaps also leading to a defective disulfide bond in the protein in the ER. The reduced longevity observed in the K109A mutant may, in part, be attributable to a synergistic outcome arising from the heightened ER stress that emanates from increased protein misfolding and a concurrent decrease in the enzymatic activity of PDI, which is a critical enzyme for repair of protein misfolding. In fact, strains deficient in the ER stress-related genes IRE1 and HAC1 have shorter lifespans than the WT (Fig. S2). That the initial persulfidation event catalyzes subsequent disulfide formation in PDI is plausible, thereby inducing its active conformation. This suggestion is intriguing, given that PDI is conventionally regarded as being converted to the oxidized form via endoplasmic reticulum oxidase 1 (ERO1) activity [37]. The supersulfide-mediated mechanism proposed here introduces a compelling alternative hypothesis for a novel PDI protein recycling system.
Taken together, our findings indicate that yeast CARS/CPERS has different roles in both cytosol and mitochondria. Supersulfides produced by CARS/CPERS in mitochondria make a substantial contribution to the maintenance of mitochondrial energy metabolism. In addition, cytosolic CARS/CPERS contributes protein persulfidation, and its deficiency causes protein misfolding and ER stress. Abnormal mitochondrial energy metabolism and increased ER stress have been implicated in various diseases such as cardiac disease, autoimmune diseases, chronic pulmonary disorders, neurodegenerative diseases including Alzheimer's and Parkinson's diseases, inflammation, cancers, and digestive diseases [2,[6], [7], [8], [9], [10], [11],[38], [39], [40]]. It is thus suggested that supersulfides may have a therapeutic potential for various diseases. For example, some sulfide-releasing agents are currently under development as analgesic and anti-inflammatory drugs for therapeutics of gastrointestinal diseases [40]. Supersulfides rather than hydrogen sulfide are likely to be responsible for the beneficial consequences of application of sulfide-releasing agents, as revealed herein and reported earlier [[41], [42], [43], [44]]. Given the promising implications of the present experimental findings and the remarkable potency of supersulfides, continued investigation of supersulfides will surely aid the discovery and development of new drugs for these diseases.
Funding
This work was supported in part by Transformative Research Areas, International Leading Research, Scientific Research [(S), (A), (B), (C), Challenging Exploratory Research] from the Ministry of Education, Culture, Sports, Sciences and Technology (MEXT), Japan, to T. Akaike (21H05258, 21H05263, 23K20040, 18H05277, and 22K19397), H. Motohashi (21A303, 21H05258, 21H05264, and 21H04799), A. Nishimura (19H05639 and 21K05504), T. Matsunaga (22K06893), T. Ida (23K06386), M. Jung (23K14341), S. Ogata (23K14333), Y. Unno (22K11796), T. Takata (23K06094) and M. Morita (23K06145); by Japan Society for the Promotion of Science (JSPS) fellowship to U. Barayeu (PE23749); by Japan Science and Technology Agency (JST), Japan, CREST Grant Number JPMJCR2024, to T. Akaike; and by a grant from the Japan Agency for Medical Research and Development (AMED) to H. Motohashi and T. Akaike (JP21zf0127001).
CRediT authorship contribution statement
Akira Nishimura: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Funding acquisition. Sunghyeon Yoon: Investigation. Tetsuro Matsunaga: Investigation. Tomoaki Ida: Investigation. Minkyung Jung: Investigation. Seiryo Ogata: Investigation. Masanobu Morita: Investigation. Jun Yoshitake: Methodology, Investigation, Formal analysis. Yuka Unno: Validation, Methodology. Uladzimir Barayeu: Writing – review & editing, Writing – original draft, Investigation. Tsuyoshi Takata: Investigation. Hiroshi Takagi: Investigation. Hozumi Motohashi: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Funding acquisition, Conceptualization. Albert van der Vliet: Writing – review & editing, Writing – original draft, Supervision, Conceptualization. Takaaki Akaike: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
We thank J. B. Gandy for her excellent editing of the manuscript before submission.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.103018.
Contributor Information
Akira Nishimura, Email: nishimura@bs.naist.jp.
Takaaki Akaike, Email: takaike@tohoku.med.ac.jp.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
The data that has been used is confidential.
References
- 1.Ida T., Sawa T., Ihara H., Tsuchiya Y., Watanabe Y., Kumagai Y., Suematsu M., Motohashi H., Fujii S., Matsunaga T., Yamamoto M., Ono K., Devarie-Baez N.O., Xian M., Fukuto J.M., Akaike T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA. 2014;111(21):7606–7611. doi: 10.1073/pnas.1321232111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akaike T., Ida T., Wei F.Y., Nishida M., Kumagai Y., Alam M.M., Ihara H., Sawa T., Matsunaga T., Kasamatsu S., Nishimura A., Morita M., Tomizawa K., Nishimura A., Watanabe S., Inaba K., Shima H., Tanuma N., Jung M., Fujii S., Watanabe Y., Ohmuraya M., Nagy P., Feelisch M., Fukuto J.M., Motohashi H. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017;8(1):1177. doi: 10.1038/s41467-017-01311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ono K., Akaike T., Sawa T., Kumagai Y., Wink D.A., Tantillo D.J., Hobbs A.J., Nagy P., Xian M., Lin J., Fukuto J.M. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: implications of their possible biological activity and utility. Free Radic. Biol. Med. 2014;77:82–94. doi: 10.1016/j.freeradbiomed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung M., Kasamatsu S., Matsunaga T., Akashi S., Ono K., Nishimura A., Morita M., Abdul Hamid H., Fujii S., Kitamura H., Sawa T., Ida T., Motohashi H., Akaike T. Protein polysulfidation-dependent persulfide dioxygenase activity of ethylmalonic encephalopathy protein 1. Biochem. Biophys. Res. Commun. 2016;480(2):180–186. doi: 10.1016/j.bbrc.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Kasamatsu S., Nishimura A., Morita M., Matsunaga T., Abdul Hamid H., Akaike T. Redox signaling regulated by cysteine persulfide and protein polysulfidation. Molecules. 2016;21(12):1721. doi: 10.3390/molecules21121721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogata S., Matsunaga T., Jung M., Barayeu U., Morita M., Akaike T. Persulfide biosynthesis conserved evolutionarily in all organisms. Antioxidants Redox Signal. 2023;39(13–15):983–999. doi: 10.1089/ars.2023.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuto J.M., Ignarro L.J., Nagy P., Wink D.A., Kevil C.G., Feelisch M., Cortese-Krott M.M., Bianco C.L., Kumagai Y., Hobbs A.J., Lin J., Ida T., Akaike T. Biological hydropersulfides and related polysulfides - a new concept and perspective in redox biology. FEBS Lett. 2018;592(12):2140–2152. doi: 10.1002/1873-3468.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsunaga T., Sano H., Takita K., Morita M., Yamanaka S., Ichikawa T., Numakura T., Ida T., Jung M., Ogata S., Yoon S., Fujino N., Kyogoku Y., Sasaki Y., Koarai A., Tamada T., Toyama A., Nakabayashi T., Kageyama L., Kyuwa S., Inaba K., Watanabe S., Nagy P., Sawa T., Oshiumi H., Ichinose M., Yamada M., Sugiura H., Wei F.Y., Motohashi H., Akaike T. Supersulphides provide airway protection in viral and chronic lung diseases. Nat. Commun. 2023;14(1):4476. doi: 10.1038/s41467-023-40182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishida M., Sawa T., Kitajima N., Ono K., Inoue H., Ihara H., Motohashi H., Yamamoto M., Suematsu M., Kurose H., van der Vliet A., Freeman B.A., Shibata T., Uchida K., Kumagai Y., Akaike T. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat. Chem. Biol. 2012;8(8):714–724. doi: 10.1038/nchembio.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan S., Fujii S., Matsunaga T., Nishimura A., Ono K., Ida T., Ahmed K.A., Okamoto T., Tsutsuki H., Sawa T., Akaike T. Reactive persulfides from Salmonella Typhimurium downregulate autophagy-mediated innate immunity in macrophages by inhibiting electrophilic signaling. Cell Chem. Biol. 2018;25(11):1403–1413.e4. doi: 10.1016/j.chembiol.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Numakura T., Sugiura H., Akaike T., Ida T., Fujii S., Koarai A., Yamada M., Onodera K., Hashimoto Y., Tanaka R., Sato K., Shishikura Y., Hirano T., Yanagisawa S., Fujino N., Okazaki T., Tamada T., Hoshikawa Y., Okada Y., Ichinose M. Production of reactive persulfide species in chronic obstructive pulmonary disease. Thorax. 2017;72(12):1074–1083. doi: 10.1136/thoraxjnl-2016-209359. [DOI] [PubMed] [Google Scholar]
- 12.Erdélyi K., Ditrói T., Johansson H.J., Czikora Á., Balog N., Silwal-Pandit L., Ida T., Olasz J., Hajdú D., Mátrai Z., Csuka O., Uchida K., Tóvári J., Engebraten O., Akaike T., Børresen Dale A.L., Kásler M., Lehtiö J., Nagy P. Reprogrammed transsulfuration promotes basal-like breast tumor progression via realigning cellular cysteine persulfidation. Proc. Natl. Acad. Sci. USA. 2021;118(45) doi: 10.1073/pnas.2100050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarosz A.P., Wei W., Gauld J.W., Auld J., Özcan F., Aslan M., Mutus B. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is inactivated by S-sulfuration in vitro. Free Radic. Biol. Med. 2015;89:512–521. doi: 10.1016/j.freeradbiomed.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Millikin R., Bianco C.L., White C., Saund S.S., Henriquez S., Sosa V., Akaike T., Kumagai Y., Soeda S., Toscano J.P., Lin J., Fukuto J.M. The chemical biology of protein hydropersulfides: studies of a possible protective function of biological hydropersulfide generation. Free Radic. Biol. Med. 2016;97:136–147. doi: 10.1016/j.freeradbiomed.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasamatsu S., Nishimura A., Alam M.M., Morita M., Shimoda K., Matsunaga T., Jung M., Ogata S., Barayeu U., Ida T., Nishida M., Nishimura A., Motohashi H., Akaike T. Supersulfide catalysis for nitric oxide and aldehyde metabolism. Sci. Adv. 2023;9(33) doi: 10.1126/sciadv.adg8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dóka É., Pader I., Bíró A., Johansson K., Cheng Q., Ballagó K., Prigge J.R., Pastor-Flores D., Dick T.P., Schmidt E.E., Arnér E.S., Nagy P. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci. Adv. 2016;2(1) doi: 10.1126/sciadv.1500968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natsoulis G., Hilger F., Fink G.R. The HTS1 gene encodes both the cytoplasmic and mitochondrial histidine tRNA synthetases of S. cerevisiae. Cell. 1986;46(2):235–243. doi: 10.1016/0092-8674(86)90740-3. [DOI] [PubMed] [Google Scholar]
- 18.Wang C.C., Chang K.J., Tang H.L., Hsieh C.J., Schimmel P. Mitochondrial form of a tRNA synthetase can be made bifunctional by manipulating its leader peptide. Biochemistry. 2003;42(6):1646–1651. doi: 10.1021/bi025964c. [DOI] [PubMed] [Google Scholar]
- 19.Chatton B., Walter P., Ebel J.P., Lacroute F., Fasiolo F. The yeast VAS1 gene encodes both mitochondrial and cytoplasmic valyl-tRNA synthetases. J. Biol. Chem. 1988;263(1):52–57. [PubMed] [Google Scholar]
- 20.Tang H.L., Yeh L.S., Chen N.K., Ripmaster T., Schimmel P., Wang C.C. Translation of a yeast mitochondrial tRNA synthetase initiated at redundant non-AUG codons. J. Biol. Chem. 2004;279(48):49656–49663. doi: 10.1074/jbc.M408081200. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura A., Nasuno R., Yoshikawa Y., Jung M., Ida T., Matsunaga T., Morita M., Takagi H., Motohashi H., Akaike T. Mitochondrial cysteinyl-tRNA synthetase is expressed via alternative transcriptional initiation regulated by energy metabolism in yeast cells. J. Biol. Chem. 2019;294(37):13781–13788. doi: 10.1074/jbc.RA119.009203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raab D., Graf M., Notka F., Schödl T., Wagner R. The GeneOptimizer Algorithm: using a sliding window approach to cope with the vast sequence space in multiparameter DNA sequence optimization. Synth. Syst. Biotechnol. 2010;4(3):215–225. doi: 10.1007/s11693-010-9062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longtine M.S., McKenzie A., 3rd, Demarini D.J., Shah N.G., Wach A., Brachat A., Philippsen P., Pringle J.R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14(10):953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 24.Oikawa D., Kimata Y., Kohno K. Self-association and BiP dissociation are not sufficient for activation of the ER stress sensor Ire1. J. Cell Sci. 2007;120(Pt 9):1681–1688. doi: 10.1242/jcs.002808. [DOI] [PubMed] [Google Scholar]
- 25.Patil C., Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr. Opin. Cell Biol. 2001;13(3):349–355. doi: 10.1016/s0955-0674(00)00219-2. [DOI] [PubMed] [Google Scholar]
- 26.Chadwick S.R., Fazio E.N., Etedali-Zadeh P., Genereaux J., Duennwald M.L., Lajoie P. A functional unfolded protein response is required for chronological aging in Saccharomyces cerevisiae. Curr. Genet. 2020;66(1):263–277. doi: 10.1007/s00294-019-01019-0. [DOI] [PubMed] [Google Scholar]
- 27.Zainol Abidin Q.H., Ida T., Morita M., Matsunaga T., Nishimura A., Jung M., Hassan N., Takata T., Ishii I., Kruger W., Wang R., Motohashi H., Tsutsui M., Akaike T. Synthesis of sulfides and persulfides is not impeded by disruption of three canonical enzymes in sulfur metabolism. Antioxidants. 2023;12(4):868. doi: 10.3390/antiox12040868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zivanovic J., Kouroussis E., Kohl J.B., Adhikari B., Bursac B., Schott-Roux S., Petrovic D., Miljkovic J.L., Thomas-Lopez D., Jung Y., Miler M., Mitchell S., Milosevic V., Gomes J.E., Benhar M., Gonzalez-Zorn B., Ivanovic-Burmazovic I., Torregrossa R., Mitchell J.R., Whiteman M., Schwarz G., Snyder S.H., Paul B.D., Carroll K.S., Filipovic M.R. Selective persulfide detection reveals evolutionarily conserved antiaging effects of S-sulfhydration. Cell Metabol. 2019;30(6) doi: 10.1016/j.cmet.2019.10.007. 1152-1170.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie L., Gu Y., Wen M., Zhao S., Wang W., Ma Y., Meng G., Han Y., Wang Y., Liu G., Moore P.K., Wang X., Wang H., Zhang Z., Yu Y., Ferro A., Huang Z., Ji Y. Hydrogen sulfide induces Keap 1 S-sulfhydration and suppresses diabetes-accelerated atherosclerosis via Nrf 2 activation. Diabetes. 2016;65(10):3171–3184. doi: 10.2337/db16-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bestetti S., Medraño-Fernandez I., Galli M., Ghitti M., Bienert G.P., Musco G., Orsi A., Rubartelli A., Sitia R. A persulfidation-based mechanism controls aquaporin-8 conductance. Sci. Adv. 2018;4(5) doi: 10.1126/sciadv.aar5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuevasanta E., Reyes A.M., Zeida A., Mastrogiovanni M., De Armas M.I., Radi R., Alvarez B., Trujillo M. Kinetics of formation and reactivity of the persulfide in the one-cysteine peroxiredoxin from Mycobacterium tuberculosis. J. Biol. Chem. 2019;294(37):13593–13605. doi: 10.1074/jbc.RA119.008883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dóka É., Ida T., Dagnell M., Abiko Y., Luong N.C., Balog N., Takata T., Espinosa B., Nishimura A., Cheng Q., Funato Y., Miki H., Fukuto J.M., Prigge J.R., Schmidt E.E., Arnér E.S.J., Kumagai Y., Akaike T., Nagy P. Control of protein function through oxidation and reduction of persulfidated states. Sci. Adv. 2020;6(1) doi: 10.1126/sciadv.aax8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vignane T., Filipovic M.R. Emerging chemical biology of protein persulfidation. Antioxidants Redox Signal. 2023;39(1–3):19–39. doi: 10.1089/ars.2023.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedre B., Talwar D., Barayeu U., Schilling D., Luzarowski M., Sokolowski M., Glatt S., Dick T.P. 3-Mercaptopyruvate sulfur transferase is a protein persulfidase. Nat. Chem. Biol. 2023;19(4):507–517. doi: 10.1038/s41589-022-01244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 36.Appenzeller-Herzog C., Ellgaard L. The human PDI family: versatility packed into a single fold. Biochim. Biophys. Acta. 2008;1783(4):535–548. doi: 10.1016/j.bbamcr.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Cao S.S., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxidants Redox Signal. 2014;21(3):396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhandary B., Marahatta A., Kim H.R., Chae H.J. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int. J. Mol. Sci. 2012;14(1):434–456. doi: 10.3390/ijms14010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benham A.M. The protein disulfide isomerase family: key players in health and disease. Antioxidants Redox Signal. 2012;16(8):781–789. doi: 10.1089/ars.2011.4439. [DOI] [PubMed] [Google Scholar]
- 40.Chan M.V., Wallace J.L. Hydrogen sulfide-based therapeutics and gastrointestinal diseases: translating physiology to treatments. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305(7):G467–G473. doi: 10.1152/ajpgi.00169.2013. [DOI] [PubMed] [Google Scholar]
- 41.Sawa T., Motohashi H., Ihara H., Akaike T. Enzymatic regulation and biological functions of reactive cysteine persulfides and polysulfides. Biomolecules. 2020;10(9):1245. doi: 10.3390/biom10091245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khodade V.S., Aggarwal S.C., Eremiev A., Bao E., Porche S., Toscano J.P. Development of hydropersulfide donors to study their chemical biology. Antioxidants Redox Signal. 2022;36(4–6):309–326. doi: 10.1089/ars.2021.0149. [DOI] [PubMed] [Google Scholar]
- 43.Yu B., Kang T., Xu Y., Liu Y., Ma Y., Ke B. Prodrugs of persulfide and sulfide: is there a pharmacological difference between the two in the context of rapid exchanges among various sulfur species in vivo? Angew. Chem., Int. Ed. 2022;61 doi: 10.1002/anie.202201668. [DOI] [PubMed] [Google Scholar]
- 44.Barayeu U., Sawa T., Nishida M., Wei F.Y., Motohashi H., Akaike T. Supersulfide biology and translational medicine for disease control. Br. J. Pharmacol. 2023;23 doi: 10.1111/bph.16271. advance online publication. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.