Abstract
Introduction
The sural neuro-fasciocutaneous flap is widely used for reconstructing skin defects in the lower calf. Variations of the sural nerve in the calf are infrequent, which may require a variation in the traditional surgical procedure.
Case presentation
A 76-year-old male patient had soft tissue defect of the right lateral ankle and lower leg caused by an accident 18 years ago. He had exposed bones and had osteomyelitis. He underwent two primary operations, and finally, we used a sural neuro-fasciocutaneous flap to effectively cover the defect. We observed that the course of the sural nerve was atypical during the surgery, and we adjusted the flap axis laterally to bring the lateral sural cutaneous nerve inside the flap to improve the success rate of the surgery. The flap entirely survived, and there was no sensory impairment in the calf. The patient was discharged from the hospital after 10 days.
Clinical discussion
Some type of variant of the sural nerve makes the flap harvest without the neurovascular component of the sural nerve and the cutaneous chain, which might decrease flap survival. Moving the flap axis laterally and bringing in the lateral sural nerve or peroneal communicating nerve offers an adequate blood supply to the vascular territory and the flap region.
Conclusion
In patients with sural nerve variants, the procedure does not have to follow the traditional theory of the sural neuro-fasciocutaneous flap. Preoperative and intraoperative protection of the sural nerve variant should also be considered.
Keywords: Sural neuro-fasciocutaneous flap, Variant, Lateral calf defect, Reconstruction, Case report
Highlights
-
•
The sural neuro-fasciocutaneous flap is commonly used to reconstruct soft tissue defects in the distal calf.
-
•
Adjusting the axis of the flap to bring the nerve branches into the flap may improve flap survival.
-
•
Preoperative and intraoperative protection of the sural nerve variant should also be considered.
1. Introduction
With the expansion of an aging population, the frequency of chronic ulcers is increasing every year, which poses a tremendous challenge to plastic surgeons. Chronic distal calf ulcers are most common, with trauma, diabetes, and varicose veins being the primary causative factors [[1], [2], [3]]. Autologous skin grafts or free flaps are acceptable choices; however, local fasciocutaneous flaps are more commonly used for soft tissue defects. The sural neurofasciocutaneous flap is relatively common in lower limb reconstruction [4,5].
The variant sural nerve may be excluded when the flap is harvested intraoperatively. Impairment of the blood supply to the flap and necrosis may occur, although these variants are rarely common [6]. Adjusting the flap axis and attempting to bring the lateral sural cutaneous nerve or peroneal communicating nerve into the flap might increase the reliable blood supply to the flap, especially when the flap area is large.
In this case, we report that a patient received a sural neuro-fasciocutaneous flap for a chronic lower extremity ulcer. The variant sural nerve was found intraoperatively, and the procedure was promptly modified to ensure reliable coverage of the patient's soft tissue deficit. This case report has been reported in line with the SCARE criteria [7].
2. Presentation of case
A 76-year-old man with a history of depression came to our department with a right lateral ankle and lower leg soft tissue defect measuring 8 cm*6 cm caused by a farm implement injury 18 years earlier(Fig. 1a). But he hadn't gone to the hospital because of his mental difficulties, and he had been treated conservatively in the local community for years. After admission, a series of pre-operative tests were routinely carried out. We found no abnormalities in the results of the blood tests. His inflammatory markers were not elevated in any way. The CTA of the lower extremities demonstrated atherosclerosis of both lower extremities along with mild luminal stenosis and tiny arteries of the right anterior and posterior tibial arteries. Based on the preoperative DR results and physical examination, his right fibula was partially absent and his ankle was fused and immobile(Fig. 1b and c). The patient was limping and unable to perform weight-bearing strenuous activities. In addition, he suffered from osteomyelitis due to bone exposure. He did not have any symptoms except effusion from the ulcers. Notably, he was insensitive to pain from the ulcer wound. The patient's medical and surgical history was unremarkable except for depression.
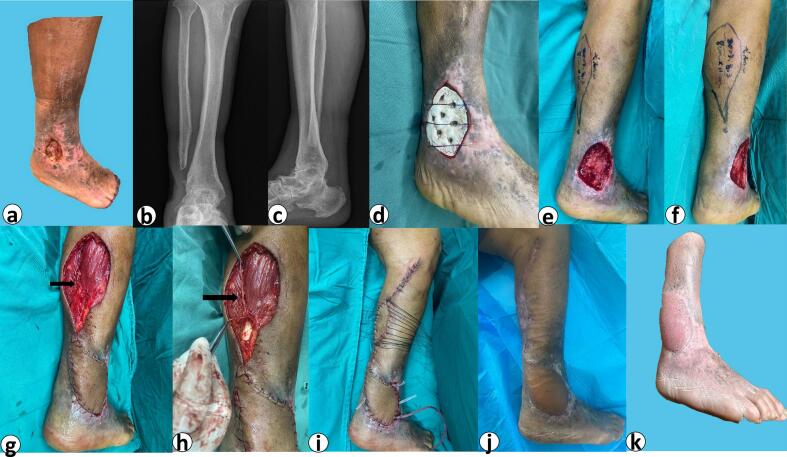
Fig. 1.
The patient's course of treatment.
a: Preoperative view of the right lateral calf defect. b: Preoperative DR image of the right ankle (frontal view). c: Preoperative DR image of the right ankle(lateral view). d: Filling the defect with bone cement in the first-stage of surgery e: Preoperative markings for second-stage operation (lateral view). f: Preoperative markings for second-stage operation (posterior view). g: Intraoperative flap harvest and coverage. Black arrow: sural nerve penetrating into the muscle. h: intraoperative dissection of the medial sural cutaneous nerve. Black arrow: raised sural nerve, not penetrated from the Achilles tendon i: Immediate postoperative result. The donor site was closed by grafting excess “dog ear” skin from the upper calf. j: The postoperative result was cosmetically and functionally acceptable. k: Appearance at 6 months postoperatively.
After the initial evaluation, we supplied the patient with a two-stage reconstructive procedure. We obtained the patient's agreement and signed an informed consent for surgery. The preoperative bacterial culture of the patient revealed the presence of Staphylococcus aureus. The ceftiamidine 2 g IV q12h was given until the bacterial culture in the wound was negative. The first stage of surgery was extended to remove both the necrotic bone (stump of the fibula) and the ulcer tissue on the fourth day following admission. Simultaneously, we filled the defect wound with antibiotic bone cement and covered it with a Vaccum Sealing Drainage (VSD) device(Fig. 1d). The excised tissue was determined by pathology to be scar tissue rather than cancerous tissue.
The wound condition improved significantly and bacterial cultures were negative after one week of negative pressure therapy. We were planning the second stage of the reconstruction. Given the exposed bone of the ulcer, the considerable amount of surrounding scar tissue, the poor vascularity of the lower extremities, the history of depression and many other factors, autologous skin grafts and free flaps were not considered as they would have made the procedure more difficult and less successful. Sural neuro-fasciocutaneous flap repair of calf ulcers was an appropriate surgical procedure(Fig. 1e and f). We incised the distal region of the flap at the level of the midcalf according to the design line and did not look for the medial sural nerve below the deep fascial layer. Instead, the short saphenous vein ran solitary in the deep fascial layer in the posterior midline of the leg. Intraoperatively, the medial sural nerve was found deep in the muscle between the medial and lateral heads of the gastrocnemius muscle and under the tendon portion of the gastrocnemius muscle(Fig. 1g and h), making it impossible to bring the neurovascular component of the sural nerve and the cutaneous chain into the flap. [8]
A thicker nerve was found on the surface of the lateral head of the gastrocnemius muscle after lateral probing along the flap's original incision. This nerve proceeded downward along the surface of the lateral head of the gastrocnemius muscle to its superficial insertion into the skin at the posterior lower calf. We believe it was the lateral sural cutaneous nerve and was the principal component of the variant sural nerve in this patient. [9,10] We turned the axis of the flap about 30° to the lateral side and cut a flap of about 11 cm*8 cm(Fig. 2). Approach above the peroneus longus area and bring the nerve into the flap. The subcutaneous fascia was modified to preserve roughly 2 cm. The excess skin from the “dog ear” was excised and transplanted into the flap donor area(Fig. 1i). Postoperative anticoagulation was routinely provided for 5 days, and our regimen was subcutaneous injection of enoxaparin 40 mg daily. The rehabilitation protocol included 10 days of bed rest and elevation. The flap survived completely and the patient was discharged two weeks after surgery without complications(Fig. 1j). After 6 months of follow-up, the flap had become flatter and the patient was satisfied with the shape of his lower limbs as it did not affect his mobility or sensation(Fig. 1k).
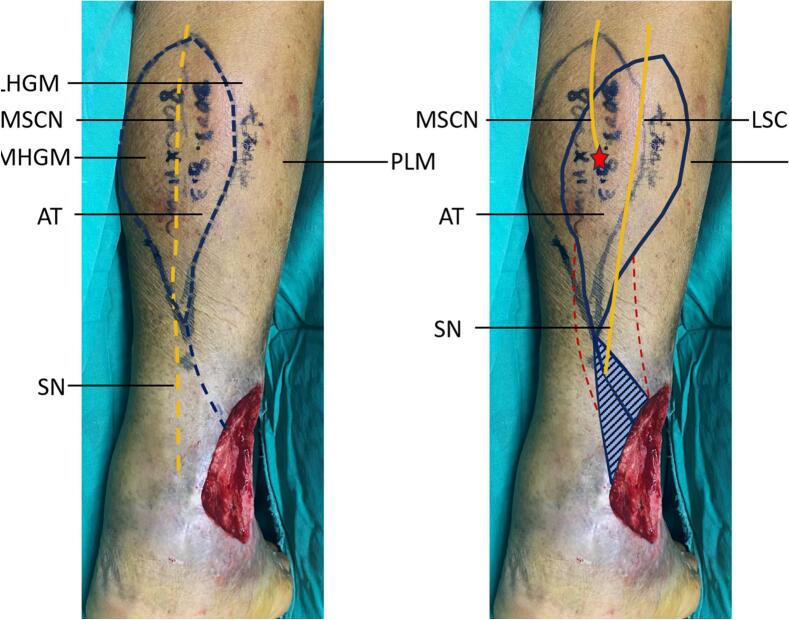
Fig. 2.
Intraoperative adjustment of the flap axis.
A: The conventional preoperative marking of the procedure. B: The actual intraoperative marking of the procedure. The axis was displaced laterally to bring the lateral sural cutaneous nerve into the flap. Yellow dashed line: normal sural nerve route and flap design axis; Red dashed line: pedicle area of the flap; Blue triangle: the ineffective donor site of the flap; Red star: Point of insertion of the lateral sural cutaneous nerve into muscle. LHGM - lateral head of gastrocnemius muscle; MHGM - medial head of gastrocnemius muscle; AT - Achilles tendon; PLM - peroneus longus muscle; SN - sural nerve; MSCN - medial sural cutaneous nerve; LSCN - lateral sural cutaneous nerve.
3. Disscussion
Although the fasciocutaneous flap is less thick than the musculocutaneous flap, it is also capable of vascularisation and good coverage of soft tissue defects with exposed bone [11,12]. On the contrary, the postoperative appearance is generally satisfactory due to the exclusion of muscle tissue. Sural neuro-fasciocutaneous flaps are widely utilized to reconstruct soft tissue defects of the foot and ankle. Since Masquelet et al. first described this flap in 1992, it has undergone various modifications and achieved attractive clinical results [[13], [14], [15]]. According to perforator flap theory and the concept of the angiosome, the sural neuro-fasciocutaneous flap is derived from the perforating vessels of the peroneal artery as well as the accompanying vascular axis around the sural nerve [16]. The connecting vessels of the perforator vessel with its cutaneous branch chain and the accompanying vascular axis around the sural nerve, contribute to the blood flow throughout the choke zones [17]. There is an adequate supply of blood, which serves to increase the flap region and vascular territory. It becomes especially critical in the case of a large flap area.
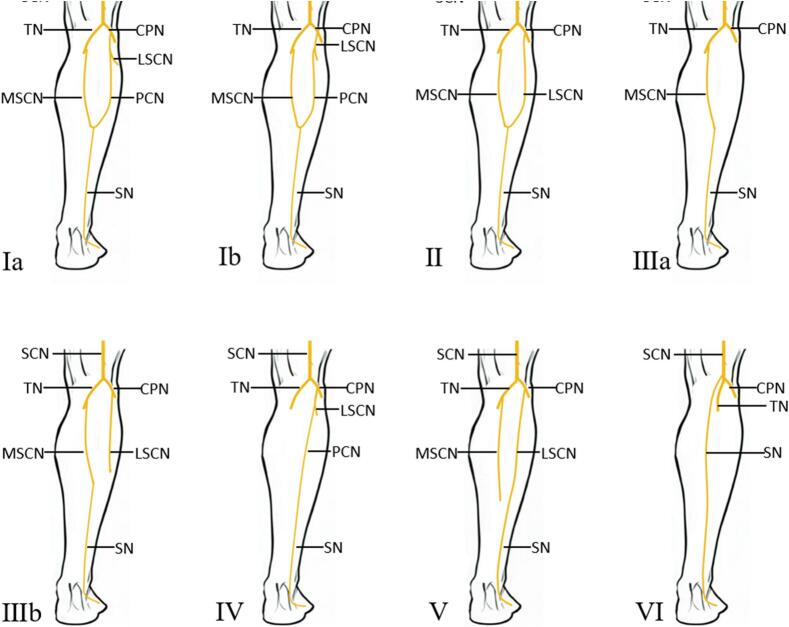
The conventional procedure axis is designed to draw a longitudinal line from the midpoint of the popliteal fossa to the midpoint between the Achilles tendon and the lateral malleolus, representing the course of the sural nerve in the posterior aspect of the lower leg, more specifically the course of the medial sural cutaneous nerve, as this is the anatomical structure of the majority of the population. However, variations in the sural nerve are usually not considered. Numerous anatomical studies have demonstrated the existence of sural nerve variability, which has been divided into six types(Fig. 3). The lateral sural cutaneous nerve may be absent or not involved in the composition of the sural nerve because of individual anatomical diversity. Although, this frequency was only reported in about 4 % of cases [10].
Fig. 3.
Classification of sural nerve variations.
SCN - sciatic nerve; TN - tibial nerve; CPN - common peroneal nerve; SN - sural nerve; MSCN - medial sural cutaneous nerve; LSCN - lateral sural cutaneous nerve; PCN - peroneal communicating nerve; LDCN - lateral dorsal cutaneous nerve.
(Reprinted with permission from ref. [10]. Copyright(2015) Annals of Anatomy.)
The case we show corresponds to the latter kind. The medial sural nerve penetrates the medial-lateral head of the gastrocnemius muscle in the upper section of the calf, and the small saphenous vein travels fully independently in the posterior aspect of the calf. Intraoperatively, a nerve was discovered in the lateral calf and terminated subcutaneously in the distal calf, which may be identified as the lateral sural nerve. It is extremely rare for this patient to possess a type V variation of the sural nerve. The flap axis was laterally rotated to accommodate the lateral sural cutaneous nerve, allowing the flap to display both the nutrient vessels around the nerve and its communication branches with the cutaneous chains of the perforating vessels(Fig. 2).
The pedicle was remained approximately 2 cm to ensure venous return, while allowing the flap to be flexibly rotated. The triangular skin paddle was retained above the pedicle to reduce tension on the skin covering. Moreover, we didn't neglect the donor site morbidity after flap harvest. After confirming that the skin donor site could not be closed directly, we harvested skin from an adjacent site rather than from another uninjured limbs. The excess “dog ear” along the margin of the donor site was considered a better supply of skin, and an appropriately expanded incision was acceptable.
Normal sural nerve anatomy is the basis for the traditional method of the sural neuro-fasciocutaneous flap. The main aim of this report is to describe how to improve the viability of sural neuro-fasciocutaneous flaps when a variation of the sural nerve is recognized, especially when the flap area is large. Satisfactory postoperative results can be achieved by properly adjusting the surgical approach. On the other hand, the prevention of intraoperative injury to the aberrant sural nerve should be a consideration for plastic surgeons.
4. Conclusion
In patients with the sural nerve variety, the flap design does not remain unaltered. A favorable result can be attained intraoperatively by adjusting the procedure to the position of the lateral sural cutaneous nerve. Preoperative and intraoperative protection of the sural nerve variation should also be considered.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Ethical exemption was given due to the single case study with patient data occultation and written consent for publication.
Funding
This work was supported by the Construction of Standardized Treatment System for Acute Wounds in Southern Anhui Province, China (No. 662202204007).
Author contribution
Wei Ding: Conceptualising the plan for surgery, performing the surgery, reviewing the manuscript.
Kun Qian: Assisting in planning and in the surgery, writing the literature review for case report.
Na Zuo: Writing the draft for case report.
Shuai Wang: Assisting the surgery, prepare the necessary equipments.
Fulin Deng: Taking note and data visualisation perioperatively.
Guarantor
Wei Ding.
Research registration number
1.Name of the registry: None.
2.Unique identifying number or registration ID: None.
3.Hyperlink to your specific registration (must be publicly accessible and will be checked): None.
Declaration of competing interest
The authors declare no conflict of interest.
Contributor Information
Kun Qian, Email: qiankuncz@wnmc.edu.cn.
Wei Ding, Email: 13956174927@163.com.
References
- 1.Martinengo L., Olsson M., Bajpai R., Soljak M., Upton Z., Schmidtchen A., Car J., Järbrink K. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019;29:8–15. doi: 10.1016/j.annepidem.2018.10.005. January. [DOI] [PubMed] [Google Scholar]
- 2.Vos T., Allen C., Arora M., Barber R.M., Bhutta Z.A., Brown A., Carter A., Casey D.C., Charlson F.J., Chen A.Z., Coggeshall M., Cornaby L., Dandona L., Dicker D.J., Dilegge T., Erskine H.E., Ferrari A.J., Fitzmaurice C., Fleming T., Murray C.J.L. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/s0140-6736(16)31678-6. October. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raffetto J.D., Ligi D., Maniscalco R., Khalil R.A., Mannello F. Why venous leg ulcers have difficulty healing: overview on pathophysiology, clinical consequences, and treatment. J. Clin. Med. 2020;10(1):29. doi: 10.3390/jcm10010029. December 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gould L., Abadir P., Brem H., Carter M., Conner-Kerr T., Davidson J., DiPietro L., Falanga V., Fife C., Gardner S., Grice E., Harmon J., Hazzard W.R., High K.P., Houghton P., Jacobson N., Kirsner R.S., Kovacs E.J., Margolis D., Schmader K. Chronic wound repair and healing in older adults: current status and future research. J. Am. Geriatr. Soc. 2015;63(3):427–438. doi: 10.1111/jgs.13332. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S.L., Chen T.M., Wang H.J. The distally based sural fasciomusculocutaneous flap for foot reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2006;59(8):846–855. doi: 10.1016/j.bjps.2005.10.013. , August. [DOI] [PubMed] [Google Scholar]
- 6.Wang C., Xiong Z., Xu J., Zhang L., Huang H., Li G. The distally based lateral sural neuro-lesser saphenous veno-fasciocutaneous flap: anatomical basis and clinical applications. J. Orthop. Traumatol. 2012;15(3):215–223. doi: 10.1007/s10195-012-0202-2. June 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2023;109(5):1136–1140. doi: 10.1097/js9.0000000000000373. April 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi Z., Chen Y., Chu T., Gao W., Li Z., Yan H., Song Y. Distally based sural neuro-fasciocutaneous perforator flap for foot and ankle reconstruction: surgical modifications for flap pedicle and donor site closure without skin graft. J. Plast. Reconstr. Aesthet. Surg. 2018;71(2):224–231. doi: 10.1016/j.bjps.2017.10.021. February. [DOI] [PubMed] [Google Scholar]
- 9.Vuksanovic-Bozaric A., Radunovic M., Radojevic N., Abramovic M. The bilateral anatomical variation of the sural nerve and a review of relevant literature. Anat. Sci. Int. 2013;89(1):57–61. doi: 10.1007/s12565-013-0195-9. August 6. [DOI] [PubMed] [Google Scholar]
- 10.Ramakrishnan P.K., Henry B.M., Vikse J., Roy J., Saganiak K., Mizia E., Tomaszewski K.A. Anatomical variations of the formation and course of the sural nerve: a systematic review and meta-analysis. Ann. Anat. 2015;202:36–44. doi: 10.1016/j.aanat.2015.08.002. November. [DOI] [PubMed] [Google Scholar]
- 11.Faenza M., Di Pace B., Di Costanzo P., Brongo S., Pieretti G., Verdura V., Nicoletti G., Rubino C. Serratus fascial flap in immediate breast reconstruction with tissue expander: is all that glitters gold? J. Plast. Reconstr. Aesthet. Surg. 2020;73(2):391–407. doi: 10.1016/j.bjps.2019.09.037. February. [DOI] [PubMed] [Google Scholar]
- 12.Faenza M., Pieretti G., Lamberti R., Di Costanzo P., Napoletano A., Di Martino M., Casale F., Ferraro G.A., Nicoletti G.F. Limberg fasciocutaneous transposition flap for the coverage of an exposed hip implant in a patient affected by Ewing sarcoma. Int. J. Surg. Case Rep. 2017;41:516–519. doi: 10.1016/j.ijscr.2017.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masquelet A.C., Romana M.C., Wolf G. Skin island flaps supplied by the vascular axis of the sensitive superficial nerves. Plast. Reconstr. Surg. 1992;89(6):1115–1121. doi: 10.1097/00006534-199206000-00018. June. [DOI] [PubMed] [Google Scholar]
- 14.Yilmaz M., Karatas O., Barutcu A. The distally based superficial sural artery island flap: clinical experiences and modifications. Plast. Reconstr. Surg. 1998;102(7):2358–2367. doi: 10.1097/00006534-199812000-00013. December. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F.H., Chang S.M., Lin S.Q., Song Y.P., Zheng H.P., Lineaweaver W.C., Zhang F. Modified distally based sural neuro-veno-fasciocutaneous flap: anatomical study and clinical applications. Microsurgery. 2005;25(7):543–550. doi: 10.1002/micr.20162. [DOI] [PubMed] [Google Scholar]
- 16.Morris S.F., Taylor I.G. Predicting the survival of experimental skin flaps with a knowledge of the vascular architecture. Plast. Reconstr. Surg. 1993;92(7):1352–1361. [PubMed] [Google Scholar]
- 17.Taylor G., Palmer J. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br. J. Plast. Surg. 1987;40(2):113–141. doi: 10.1016/0007-1226(87)90185-8. March. [DOI] [PubMed] [Google Scholar]