Abstract
Background
The treatment of hidradenitis suppurativa (HS) has always been a real challenge for dermatologists; to date, adalimumab the only biologic drug approved for HS is adalimumab, an anti-tumor necrosis factor (TNF)-α drug, the approval of this drug dates to 2015, data provided by real life show an effectiveness rate of about 60% percent. Recently (31 October 2023) FDA approves secukinumab for moderate-severe HS. The treatment and management of HS is very challenging as available treatments are very limited and show very variable outcomes.
Methods
We conducted a prospective monocentric study designed to evaluate the efficacy and safety of secukinumab treatment in HS patients in a real-life setting.
Results
The initial cohort of patients recruited included 21 HS patients including 12 females and 9 males. About 57.1% of patients achieved the primary endpoint and recorded significant decrease in all the severity assessment scales (IHS4, DLQI and VAS pain scale) at week 16 and 52, when HiSCR reached 71.4%.
Conclusion
The results of our study highlight that treatment with secukinumab in patients with severe HS who failed adalimumab may be a safe and effective therapeutic weapon.
Keywords: hidradenitis suppurativa, secukinumab, adalimumab, treatments
Introduction
Hidradenitis suppurativa (HS), is a chronic, inflammatory, recurrent and debilitating skin disease affecting hair follicles, the manifestations are characterized by inflammatory, painful and localized lesions in body districts where apocrine glands are present.1,2 The underlying pathogenic mechanism of HS is not yet known certainly follicular hyperkeratosis within the pilosebaceous-apocrine unit represents the initial lesion. New studies show new insights into the role of pro-inflammatory cytokines in the pathogenesis of HS, helping to fill some existing gaps in the knowledge of the development of this pathology.3,4
The treatment of HS has always been a real challenge for dermatologists; to date, the first FDA biologic drug approved for HS is adalimumab, an anti-tumor necrosis factor (TNF)-α drug, whose approval dates to 2015. Several real-life studies highlight a mean clinical success rate of about 60%.5–8 However, primary or secondary lack of efficacy may occur.9–13 Secukinumab, an anti-interleukin 17A drug approved for moderate to severe psoriasis, psoriatic arthritis and ankylosing spondylitis has shown effective results in clinical trials improving signs and symptoms of HS with a good safety profile and response up to 52 weeks.14 Even few real-life data are already in line with the studies carried out.14 Recently, European Commission (EC) has approved secukinumab, for use in adults with active moderate to severe HS and an inadequate response to conventional systemic HS therapy,15 while FDA approval has been reached recently (31 October 2023).
The treatment and management of HS is very challenging as available treatments are very limited and show very variable outcomes. Indeed, different new drugs are being tested for HS (eg, spesolimab and porvocitinib).
It is therefore important for the future to select new therapeutic targets for HS establishing a universally and shared treatment algorithm. Among future drugs, secukinumab certainly represents the most immediate novelty.
Methods
We conducted a prospective monocentric study designed to evaluate the efficacy and safety of secukinumab treatment in HS patients in a real-life setting. The inclusion criteria followed the criteria established during the clinical trials conducted for HS.15,16
Inclusion criteria were: i) age ≥ 18 years; ii) diagnosis of moderate to severe HS (Hurley II or III); particularly eligible patients were required to be diagnosed at least 1 year before the baseline visit and have a total abscess and inflammatory nodule (AN) count ≥ 3 at baseline, HS lesions in ≥ 2 distinct anatomical locations, a draining fistula count ≤ 20 at baseline, and inadequate response to oral antibiotics for the treatment of HS. iii) Adalimumab primary or secondary inefficacy, and/or Adalimumab discontinuation due to adverse events or Adalimumab contraindication (eg multiple sclerosis, severe heart failure); iv) secukinumab treatment according to the classical schedule for psoriasis: 300 mg administered subcutaneously weekly for 5 weeks, then every 4 weeks. Exclusion criteria: relevant medical conditions (such as hepatitis B, hepatitis C, HIV or tuberculosis) or pregnancy or lactation. The real-life study was carried out up to 52 weeks of treatment. At baseline, demographic data, HS clinical data, duration, treatment history and severity [Hurley score, International HS Severity Scoring System (IHS4), Dermatology Life Quality Index (DLQI) and VAS pain scale] were registered. HS clinical data, treatment history and disease severity as well as eventual adverse events and were performed at each follow-up (week 16, 32 and 52) in addition with laboratory analysis. Safety was assessed by treatment-emergent adverse events, physical examinations, and laboratory monitoring. The primary objective was the proportion of patients reaching Hidradenitis Suppurativa Clinical Response (HiSCR) score at 16 weeks. The secondary objective included HiSCR, International HS Severity Scoring System (IHS4), Dermatology Life Quality Index (DLQI) and VAS pain scale16–18 at week 16, 32 and 52.
The study was conducted in accordance with the protocol and applicable ethical guidelines and principles derived from the 1964 Declaration of Helsinki. The study protocol was reviewed and approved by Local Ethic Committee University of Naples Federico II. Patients provided written informed consent before undergoing the specific study procedures.
Statistical Analysis
The collected data were processed using Graph-Pad Prism software (GraphPad Inc., La Jolla, CA, USA). Parameters were calculated for each variable (number of days of therapy performed, IHS4, VAS, DLQI) using mean ± standard deviation. Paired t-test was used to compare data collected before starting Secukinumab and after 1 year of therapy. In addition, paired t-test was used to compare the same variables (data collected at the beginning of Secukinumab treatment with data collected after 1 year of therapy (T12) within the original sample. P-value <0.05 with a 95% confidence interval was considered statistically significant in all analyses performed.
Results
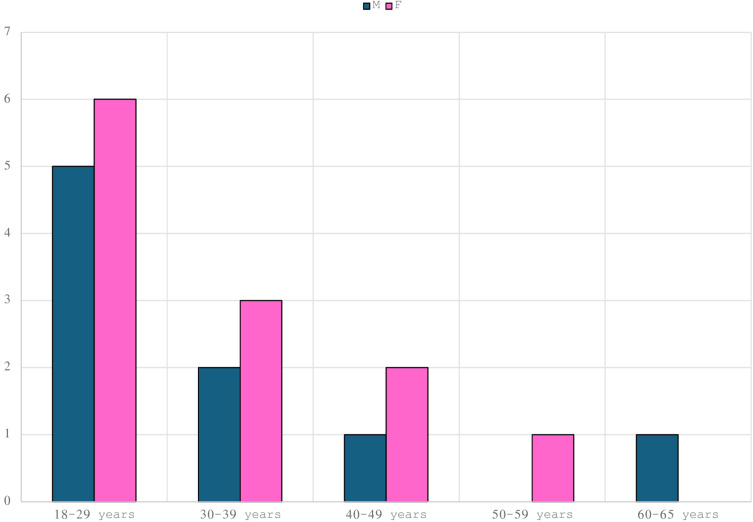
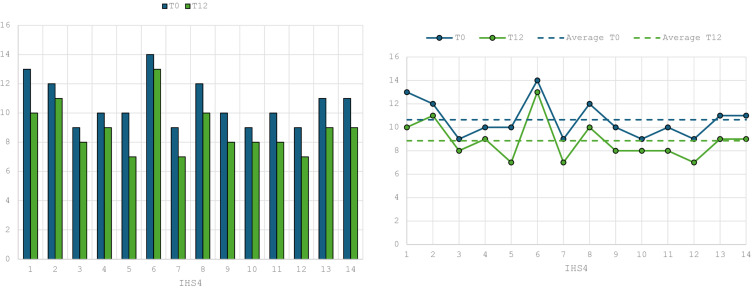
The initial cohort of patients recruited included 21 HS patients including 12 females and 9 males with a total mean age of 30.7 years. The majority included people aged 18 to 29 (11 patients, 52.4%) (Figure 1). Seven of the 21 patients initially recruited (33%) did not complete the study until week 52 (as treated analysis). Particularly, two patients (9.5%) showed primary inefficacy (week 16), one (4.7%) developed an adverse event (pneumonia) at week 12 and 4 (19%) did not present for scheduled follow-ups and were therefore excluded. Hence, treatment discontinuation for inefficacy or adverse events was 14.3%. Consequently, evaluations at week 52 were performed on the remaining 14 patients (66.7%). The enrolled patients had HS for at least 10 years (mean HS history was 14.7). All patients had been on at least one line of treatment (antibiotic therapy with clindamycin plus rifampicin or tetracyclines). Among the 21 patients enrolled, 17 patients (81%) had previously failed due to secondary inefficacy treatment with Adalimumab (anti TNF-alpha), the other 4 patients were bio-naïve (19%) but showing contraindication to adalimumab. As regards disease severity, the IHS4 score calculated at baseline showed a moderate-severe disease in all patients, with an average value at baseline of 10.4. Indeed, the patient had a severe impact on quality of life showing an average DLQI value of 27.6. The huge severity of HS at baseline is highlighted by the mean average of VAS pain scale which was 9.2. As regards the primary endpoint, HiSCR was achieved at week 16 by 57.1% (8/14) of patients. This score continued to improve at every follow-up reaching 71.4% (10/14) at week 52. As regards, the other severity index, IHS4 was statistically significantly improved at week 52 reaching 8.5 (P<0.05). Specifically, 7 patients obtained a decrease in value of 4 points and 7 patients obtained a decrease in value of 3 points at the end of the study (week 52) (Figure 2).
Figure 1.
Data and baseline characteristics of the sample.
Figure 2.
Data regarding IHS4 from week 0 to week 52.
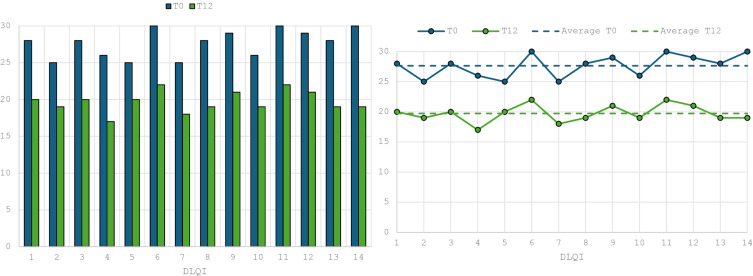
DLQI greatly improved at week 52 showing the average value dropped by 7 points to 19.7 (p<0.001). Although this decrease was significant, the recorded DLQI value still remained high, indicating that HS shows a constant strong psychological impact on patients even their clinical situation improves (Figure 3).
Figure 3.
Data regarding DLQI from week 0 to week 52.
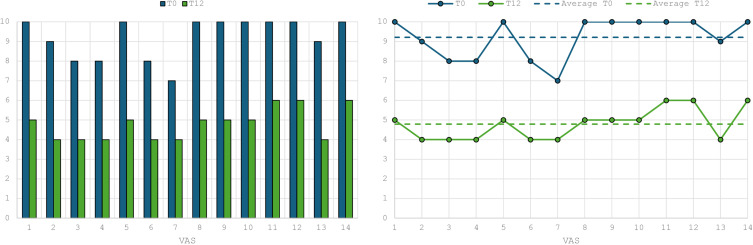
As regards VAS pain, the average value found at week 52 was 4.8, therefore there was a decrease of approximately 50% compared to the initial value (P<0.001) (Figure 4).
Figure 4.
Data regarding VAS pain score from week 0 to week 52.
In conclusion, 33% of patients did not obtain met the primary endpoint of the study (HiSCR at week 16) and did not obtain a significant decrease in IHS4 values at baseline.
However, 57.1% of patients achieved the primary endpoint and recorded significant decrease in all the severity assessment scales (IHS4, DLQI and VAS pain scale) at week 16 and 52, when HiSCR reached 71.4%.
Discussion
The results of our study highlight that treatment with secukinumab in patients with severe HS who failed Adalimumab may be a safe and effective therapeutic weapon. Adalimumab was the only FDA biologic drug approved for HS until 31 October 2023, when secukinumab received FDA approval for HS.10–14
Data from the SUNSHINE and SUNRISE trials compared HS patients treated with secukinumab every 2 weeks vs patients treated with secukinumab every 4 weeks vs placebo; the HiSCR rates ranged from 42% to 46% under different regimens up to 52 weeks.13
The efficacy of secukinumab in real life has been widely discussed in the literature through case series, case reports, and retrospective studies. In particular, clinical data to date show encouraging results. Particularly, Prussick et al18 described of 9 patients 55.5% (5/9) achieved HiSCR at week 16 and that 67% (6/9) at week 24 continued to have this result. Casseres et al19 found that 13 of 20 patients (65%) achieved HiSCR at week 16. In the study by Reguiai et al20 involving 20 patients, 75% of patients (15/20) achieved HiSCR at week 16. Ribero et al21 concluded that 26% (8/24) of patients achieved HiSCR at week 16 and 41% (7/17) at week 28. Finally, Melgosa et al22 recruited 23 patients, of whom 73.9% (17/23) achieved HiSCR at week 16, 71.4% (15/21) at week 24, 71.4% (10/14) at week 36, and 83.3% (10/12) at week 52 of secukinumab treatment.
Hence, from all real-life data, a mean of 58.4% was achieved at week 16 while a mean of 75% at week 52. Our study is in line with these results: indeed, HiSCR at week 16 was 57.1% while at week 52 was 71.4%.
In addition, good results have also been reported by several case reports reporting data on the efficacy and safety of the drug in patients suffering from HS.23–26. However, real life data with high number of patients are still limited. In our opinion, there are still few real-life data in the literature especially the number of samplings is too small, so it is necessary to have more and more real-life studies that can confirm this positive trend of Secukinumab in HS.
The elevated levels of IL17 found in the serum of individuals with hidradenitis suppurativa (HS), the increased presence of IL-17A/C/F transcripts in the affected tissue, the detection of IL-17A in serum, and the identification of CD4+ IL-17+ T cells in both the affected and surrounding tissues have all been extensively reported in scientific literature. These findings potentially underlie the rationale behind the observed effectiveness of Secukinumab treatment in managing HS.27,28 However, the validation of this hypothesis warrants extensive large-scale studies in the future. These studies should aim to corroborate this hypothesis, further elucidating data regarding potential variations in treatment efficacy between Adalimumab-naive and Adalimumab-treated HS patients, as well as across different clinical presentations of HS. Such comprehensive investigations will be pivotal in refining our understanding of the disease pathogenesis and optimizing therapeutic strategies for diverse HS patient populations.
Patients with HS require long-term management and frequent follow-up due to the highly recalcitrant nature of the disease; to date, first-line therapies such as antibiotics fail to guarantee results in severe forms of HS.16,29,30
Unfortunately, adalimumab may experience primary or secondary lack of efficacy and paradoxical reactions have been described; in these patients an alternative therapy needs to be found.31–35 In this scenario, secukinumab which has shown promising results in clinical trials and real life with active moderate to severe HS and an inadequate response to conventional systemic HS therapy becomes very important to ensure a successful therapeutic alternative.13
The evolving understanding of hidradenitis suppurativa’s (HS) pathogenesis has spurred the creation of novel, targeted medications characterized by high efficacy and safety. Recent years have seen extensive discourse regarding the pivotal role of IL-23 in HS. Multiple scholarly articles have delved into the involvement of the IL-23/TH17 pathways, elucidating their potential significance in the disease’s development. Notably, research has highlighted the overexpression of IL-23 in lesions present in HS patients, alongside the discovery of elevated serum levels of IL-23 among individuals affected by HS. This accumulating body of evidence signifies a promising avenue for exploring the mechanisms underlying HS, offering potential targets for therapeutic interventions aimed at modulating IL-23-related pathways to mitigate disease progression.36–40
There will certainly be an increase in the number of therapies available for HS over the next few years to ensure an individualized approach for each patient also based on comorbidities. Several publications of ongoing clinical trials discuss the use of spesolimab (anti-IL-36 receptor) and JAK inhibitors, but so far there are too few reports to express a definitive opinion.41–58
Larger Phase 3 studies will be needed to truly understand the efficacy and safety of these drugs for HS.
Strengths and Limitations
The main point is certainly the real-life experience until 52 weeks, to date there are few data with a long period. The limitations include the small sample size, the open-label design with no control group.
Conclusion
In conclusion, the results of our real life study show that treatment with secukinumab in patients with severe HS who failed adalimumab may be safe and effective, in particular showed HiSCR was achieved at week 16 by 57.1% of patients. This score continued to improve at every follow-up reaching 71.4% at week 52. These data confirm previous data regarding efficacy and safety of secukinumab in treatment of HS available in literature.16–29
Funding Statement
There is no funding to report.
Data Sharing Statement
Data are reported in the current study and are on request by corresponding author.
Ethic Statement
The study was conducted in accordance with the protocol and applicable ethical guidelines and principles derived from the 1964 Declaration of Helsinki. The study protocol was reviewed and approved by Local Ethic Committee University of Naples Federico II. Patients provided written informed consent before undergoing the specific study procedures.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The author(s) report no conflicts of interest in this work.
References
- 1.Chiricozzi A, Veraldi S, Fabbrocini G, et al. The Hidradenitis Suppurativa (HS) “Multidisciplinary Unit”: a rationale and practical proposal for an organised clinical approach. Eur J Dermatol. 2018;28(2):274–275. doi: 10.1684/ejd.2018.3254 [DOI] [PubMed] [Google Scholar]
- 2.Martora F, Martora L, Fabbrocini G, Marasca C. A case of pemphigus vulgaris and hidradenitis suppurativa: may systemic steroids be considered in the standard management of hidradenitis suppurativa? SkinAppendageDisord. 2022;8(3):265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82(5):1045–1058. [DOI] [PubMed] [Google Scholar]
- 4.Stephan C, Kurban M, Abbas O. Reply to: “Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis”. J Am Acad Dermatol. 2020;83(5):e371. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TV, Damiani G, Orenstein LAV, Hamzavi I, Jemec GB. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur AcadDermatolVenereol. 2021;35(1):50–61. [DOI] [PubMed] [Google Scholar]
- 6.Martora F, Marasca C, Battista T, Fabbrocini G, Ruggiero A. Management of patients with hidradenitis suppurativa during COVID-19 vaccination: an experience from southern Italy. Comment on: ‘Evaluating the safety and efficacy of COVID-19 vaccination in patients with hidradenitis suppurativa’. Clin Experimental Dermatol. 2022;47(11):2026–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maronese CA, Moltrasio C, Genovese G, Marzano AV. Biologics for Hidradenitis suppurativa: evolution of the treatment paradigm. Expert Rev Clin Immunol. 2023;1–21. doi: 10.1080/1744666X.2023.2298356 [DOI] [PubMed] [Google Scholar]
- 8.Maronese CA, Ingram JR, Marzano AV. Has the time come to assess small-molecule/biologic drug combinations for the management of moderate-to-severe hidradenitis suppurativa? Br J Dermatol. 2023;189(4):467–468. doi: 10.1093/bjd/ljad224 [DOI] [PubMed] [Google Scholar]
- 9.Kimball AB, Okun MM, Williams DA, et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med. 2016;375(5):422–434. doi: 10.1056/NEJMoa1504370 [DOI] [PubMed] [Google Scholar]
- 10.Jemec GBE, Okun MM, Forman SB, et al. Adalimumab medium-term dosing strategy in moderate-to-severe hidradenitis suppurativa: integrated results from the Phase III randomized placebo-controlled PIONEER trials. Br J Dermatol. 2019;181(5):967–975. doi: 10.1111/bjd.17919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zouboulis CC, Okun MM, Prens EP, et al. Long-term Adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80(1):60–69.e2. doi: 10.1016/j.jaad.2018.05.040 [DOI] [PubMed] [Google Scholar]
- 12.Martora F, Megna M, Battista T, et al. Adalimumab, Ustekinumab, and Secukinumab in the Management of Hidradenitis Suppurativa: a review of the real-life experience. ClinCosmetInvestigDermatol. 2023;16:135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimball AB, Jemec GBE, Alavi A, et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. Lancet. 2023;401(10378):747–761. doi: 10.1016/S0140-6736(23)00022-3 [DOI] [PubMed] [Google Scholar]
- 14.Novartis Europharm Limited. Cosentyx® (secukinumab): summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/cosentyx-epar-product-information_en.pdf. Accessed May, 2023.
- 15.Zouboulis CC, Tzellos T, Kyrgidis A, et al. Development and validation of the International Hidradenitis Suppurativa severity score system (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177(5):1401–1409. doi: 10.1111/bjd.15748 [DOI] [PubMed] [Google Scholar]
- 16.Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur AcadDermatolVenereol. 2015;29(4):619–644. doi: 10.1111/jdv.12966 [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Crehuet P, Haselgruber S, Padial-Gomez A, et al. Short-term effectiveness, safety, and potential predictors of response of secukinumab in patients with severe hidradenitis suppurativa refractory to biologic therapy: a multicenter observational retrospective study. Dermatol Ther. 2023;13(4):1029–1038. doi: 10.1007/s13555-023-00906-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prussick L, Rothstein B, Joshipura D, et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol. 2019;181(3):609–611. doi: 10.1111/bjd.17822 [DOI] [PubMed] [Google Scholar]
- 19.Casseres RG, Prussick L, Zancanaro P, et al. Secukinumab in the treatment of moderate to severe hidradenitis suppurativa: results of an open-label trial. J Am Acad Dermatol. 2020;82(6):1524–1526. doi: 10.1016/j.jaad.2020.02.005 [DOI] [PubMed] [Google Scholar]
- 20.Reguiaï Z, Fougerousse AC, Maccari F, Bécherel PA. Effectiveness of secukinumab in hidradenitis suppurativa: an open study (20 cases). J Eur AcadDermatolVenereol. 2020;34(11):e750–e751. [DOI] [PubMed] [Google Scholar]
- 21.Ribero S, Ramondetta A, Fabbrocini G, et al. Effectiveness of secukinumab in the treatment of moderate–severe hidradenitis suppurativa: results from an Italian multicentric retrospective study in a real‐life setting. AcadDermatolVenereol. 2021;35(7):e441–e442. [DOI] [PubMed] [Google Scholar]
- 22.Melgosa Ramos FJ, García Ruiz R, Estébanez Corrales A, MateuPuchades A. Long-term secukinumab efficacy in patients with moderate to severe hidradenitis suppurativa: a retrospective single-centre case series (23 patients). J Eur Acad Dermatol Venereol. 2022;37(4):e517–e519. [DOI] [PubMed] [Google Scholar]
- 23.Marasca C, Megna M, Balato A, Balato N, Napolitano M, Fabbrocini G. Secukinumab and hidradenitis suppurativa: friends or foes? JAAD Case Rep. 2019;5(2):184–187. doi: 10.1016/j.jdcr.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorlacius L, Theut Riis P, Jemec GBE. Severe hidradenitis suppurativa responding to treatment with secukinumab: a case report. Br J Dermatol. 2018;179(1):182–185. doi: 10.1111/bjd.15769 [DOI] [PubMed] [Google Scholar]
- 25.Schuch A, Fischer T, Boehner A, Biedermann T, Volz T. Successful treatment of severe recalcitrant hidradenitis suppurativa with the interleukin-17A antibody secukinumab. Acta Derm Venereol. 2018;98(1):151–152. doi: 10.2340/00015555-2794 [DOI] [PubMed] [Google Scholar]
- 26.Jørgensen AR, Yao Y, Thomsen SF. Therapeutic response to secukinumab in a 36-year-old woman with hidradenitis suppurativa. Case Rep Dermatol Med. 2018;2018:8685136. doi: 10.1155/2018/8685136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matusiak Ł, Szczęch J, Bieniek A, Nowicka-Suszko D, Szepietowski JC. Increased interleukin (IL)-17 serum levels in patients with hidradenitis suppurativa: implications for treatment with anti-IL-17 agents. J Am Acad Dermatol. 2017;76(4):670–675. doi: 10.1016/j.jaad.2016.10.042 [DOI] [PubMed] [Google Scholar]
- 28.Saraçöztürk G, Ergun T, PekerEyüboğlu İ, Akkiprik M. Serum high-sensitivity C-reactive protein, tumor necrosis factor-α, interleukin (IL)-1β, IL-17A and IL-23 levels in patients with hidradenitis suppurativa. Cytokine. 2021;144:155585. doi: 10.1016/j.cyto.2021.155585 [DOI] [PubMed] [Google Scholar]
- 29.Martora F, Marasca C, Picone V, Fornaro L, Megna M, Fabbrocini G. How adalimumab impacts antibiotic prescriptions in patients affected by hidradenitis suppurativa: a 1-year prospective study and retrospective analysis. J Clin Med. 2023;12(3):837. doi: 10.3390/jcm12030837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunger RE, Laffitte E, Läuchli S, et al. Swiss practice recommendations for the management of hidradenitis suppurativa/acne inversa. Dermatology. 2017;233(2–3):113–119. doi: 10.1159/000477459 [DOI] [PubMed] [Google Scholar]
- 31.Ruggiero A, Martora F, Picone V, Marano L, Fabbrocini G, Marasca C. Paradoxical hidradenitis suppurativa during biologic therapy, an emerging challenge: a systematic review. Biomedicines. 2022;10(2):455. doi: 10.3390/biomedicines10020455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvador-Rodriguez L, Montero-Vílchez T, Arias-Santiago S, Molina-Leyva A. Paradoxical hidradenitis suppurativa in patients receiving TNF-α inhibitors: case series, systematic review, and case meta-analysis. Dermatology. 2020;236(4):307–313. doi: 10.1159/000506074 [DOI] [PubMed] [Google Scholar]
- 33.Delobeau M, Abdou A, Puzenat E, et al. Observational case series on Adalimumab-induced paradoxical hidradenitis suppurativa. J Dermatol Treat. 2016;27(3):251–253. doi: 10.3109/09546634.2015.1094179 [DOI] [PubMed] [Google Scholar]
- 34.Martora F, Fabbrocini G, Marasca C, Battista T, Megna M. Paradoxical hidradenitis suppurativa induced by Adalimumab biosimilar successfully treated with guselkumab in a patient with psoriasis. Comment on ‘Paradoxical hidradenitis suppurativa due to anti-interleukin-1 agents for mevalonate kinase deficiency successfully treated with the addition of ustekinumab’. Clin Experimental Dermatol. 2023;48(6):701–703. doi: 10.1093/ced/llad082 [DOI] [PubMed] [Google Scholar]
- 35.Martora F, Marasca C, Fabbrocini G, Ruggiero A. Strategies adopted in a southern Italian referral centre to reduce Adalimumab discontinuation: comment on “Can we increase the drug survival time of biologic therapies in hidradenitis suppurativa?”. Clin Experimental Dermatol. 2022;47(10):1864–1865. doi: 10.1111/ced.15291 [DOI] [PubMed] [Google Scholar]
- 36.Dudink K, Bouwman K, Chen Y, et al. Guselkumab for hidradenitis suppurativa: a Phase II, open-label, mode-of-action study. Br J Dermatol. 2023;188(5):601–609. doi: 10.1093/bjd/ljad010 [DOI] [PubMed] [Google Scholar]
- 37.Melgosa Ramos FJ, García Ruiz R, MateuPuchades A, AlfagemeRoldán F. Guselkumab effectiveness, and posology in patients with moderate to severe hidradenitis suppurativa: a retrospective bicentric experience. Dermatologic Therapy. 2022;35(7):e15558. doi: 10.1111/dth.15558 [DOI] [PubMed] [Google Scholar]
- 38.Kimball AB, Podda M, Alavi A, et al. Guselkumab for the treatment of patients with moderate-to-severe hidradenitis suppurativa: a Phase 2 randomized study. J Eur Acad Dermatol Venereol. 2023;37(10):2098–2108. doi: 10.1111/jdv.19252 [DOI] [PubMed] [Google Scholar]
- 39.Martora F, Scalvenzi M, Battista T, et al. Guselkumab, risankizumab, and tildrakizumab in the management of hidradenitis suppurativa: a review of existing trials and real-life data. Clin Cosmet Invest Dermatol. 2023;16:2525–2536. doi: 10.2147/CCID.S418748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimball AB, Prens EP, Passeron T, et al. Efficacy and safety of risankizumab for the treatment of hidradenitis suppurativa: a phase 2, randomized, placebo-controlled trial. Dermatol Ther. 2023;13(5):1099–1111. doi: 10.1007/s13555-023-00913-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martora F, Scalvenzi M, Ruggiero A, Potestio L, Battista T, Megna M. Hidradenitis suppurativa and JAK inhibitors: a review of the published literature. Medicina. 2023;59(4):801. doi: 10.3390/medicina59040801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zouboulis CC, Frew JW, Giamarellos-Bourboulis EJ, et al. Target molecules for future hidradenitis suppurativa treatment. Exper Dermatol. 2021;30(Suppl 1):8–17. doi: 10.1111/exd.14338 [DOI] [PubMed] [Google Scholar]
- 43.Hwang J, Rick J, Hsiao J, Shi VY. A review of IL-36: an emerging therapeutic target for inflammatory dermatoses. J Dermatol Treat. 2022;33(6):2711–2722. doi: 10.1080/09546634.2022.2067819 [DOI] [PubMed] [Google Scholar]
- 44.Megna M, Camela E, Battista T, et al. Efficacy and safety of biologics and small molecules for psoriasis in pediatric and geriatric populations. Part II: focus on elderly patients. Expert Opin Drug Saf. 2023;22(1):43–58. doi: 10.1080/14740338.2023.2173171 [DOI] [PubMed] [Google Scholar]
- 45.Megna M, Camela E, Battista T, et al. Efficacy and safety of biologics and small molecules for psoriasis in pediatric and geriatric populations. Part I: focus on pediatric patients. Expert Opin Drug Saf. 2023;22(1):25–41. doi: 10.1080/14740338.2023.2173170 [DOI] [PubMed] [Google Scholar]
- 46.Ruggiero A, Potestio L, Cacciapuoti S, et al. Tildrakizumab for the treatment of moderate to severe psoriasis: results from a single center preliminary real-life study. Dermatologic Therapy. 2022;35(12):e15941. doi: 10.1111/dth.15941 [DOI] [PubMed] [Google Scholar]
- 47.Camela E, Potestio L, Fabbrocini G, Pallotta S, Megna M. The holistic approach to psoriasis patients with comorbidities: the role of investigational drugs. Expert Opin Invest Drugs. 2023;32(6):537–552. doi: 10.1080/13543784.2023.2219387 [DOI] [PubMed] [Google Scholar]
- 48.Fukaura R, Akiyama M. Targeting IL-36 in Inflammatory Skin Diseases. BioDrugs. 2023;37(3):279–293. doi: 10.1007/s40259-023-00587-5 [DOI] [PubMed] [Google Scholar]
- 49.Alavi A, Hamzavi I, Brown K, et al. Janus kinase 1 inhibitor INCB054707 for patients with moderate-to-severe hidradenitis suppurativa: results from two Phase II studies. Br J Dermatol. 2022;186(5):803–813. doi: 10.1111/bjd.20969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martora F, Picone V, Fabbrocini G, Marasca C. Hidradenitis suppurativa flares following COVID-19 vaccination: a case series. JAAD Case Rep. 2022;23:42–45. doi: 10.1016/j.jdcr.2022.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martora F, Picone V, Fornaro L, Fabbrocini G, Marasca C. Can COVID-19 cause atypical forms of pityriasis rosea refractory to conventional therapies? J Med Virol. 2022;94(4):1292–1293. doi: 10.1002/jmv.27535 [DOI] [PubMed] [Google Scholar]
- 52.Martora F, Fabbrocini G, Marasca C. Pityriasis rosea after Moderna mRNA-1273 vaccine: a case series. Dermatologic Therapy. 2022;35(2):e15225. doi: 10.1111/dth.15225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Picone V, Martora F, Fabbrocini G, Marano L. ”Covid arm”: abnormal side effect after Moderna COVID-19 vaccine. Dermatologic Therapy. 2022;35(1):e15197. doi: 10.1111/dth.15197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jenkins T, Isaac J, Edwards A, Okoye GA. Hidradenitis Suppurativa. Dermatol Clin. 2023;41(3):471–479. doi: 10.1016/j.det.2023.02.001 [DOI] [PubMed] [Google Scholar]
- 55.Bukvić Mokos Z, Marinović B. Hidradenitis suppurativa: I. Clin Dermatol. 2023;41(5):549–550. doi: 10.1016/j.clindermatol.2023.08.017 [DOI] [PubMed] [Google Scholar]
- 56.van Straalen KR, Prens EP, Gudjonsson JE. Insights into hidradenitis suppurativa. J Allergy Clin Immunol. 2022;149(4):1150–1161. doi: 10.1016/j.jaci.2022.02.003 [DOI] [PubMed] [Google Scholar]
- 57.Choi E, Ooi XT, Chandran NS. Hidradenitis suppurativa in pediatric patients. J Am Acad Dermatol. 2022;86(1):140–147. doi: 10.1016/j.jaad.2020.08.045 [DOI] [PubMed] [Google Scholar]
- 58.Hasan SB, Harris C, Collier F. Hidradenitis suppurativa. BMJ. 2022;379:e068383. doi: 10.1136/bmj-2021-068383 [DOI] [PubMed] [Google Scholar]