Abstract
Background:
The recovery of independent walking is one of the major goals of stroke rehabilitation; however, due to the current acute inpatient rehabilitation care paradigm, the intensity of walking practice provided has been far below that recommended for motor recovery to occur. A quality improvement initiative was implemented to encourage the physical therapist (PT) to incorporate various robotic gait training devices as part of the standard allotted PT sessions to improve the intensity of gait training.
Materials and Methods:
After 6 months, a retrospective review was performed to assess the feasibility of the robotic-assisted gait training (RAGT) intervention in limited-ambulatory stroke patients and determine preliminary efficacy of the RAGT program by analyzing Functional Index Measure (FIM) motor gain and accelerometer-based daily step counts in patients who received the RAGT versus a group treated with conventional therapy.
Results:
About 30% of limited-ambulatory patients admitted to the stroke rehabilitation unit received consistent integrated RAGT without safety concerns. Compared to those who received conventional treatment, these patients showed greater mean FIM motor gain (32.30 versus 17.88) at discharge (P < 0.005) and higher number of step counts in PT sessions (P < 0.005). Age, gender, or admission FIM motor were not associated with FIM motor gain.
Conclusions:
Across a 6-month initial implementation period, RAGT was feasible and was associated with higher repetition of walking practice and also with improved FIM motor scores in limited-ambulatory individuals in an acute inpatient stroke rehabilitation program. However, the frequency of RAGT and the percentage of patients participating need to further improve. Some strategies to address these concerns were identified.
Keywords: Inpatient rehabilitation, quality improvement, robotics, stroke
Introduction
Although most stroke survivors recover at least limited walking function, more than half of patients participating in acute inpatient rehabilitation facility (IRF) are unable to walk independently in this early recovery phase.[1] The current inpatient rehabilitation care paradigm is based on both government requirements and internally created clinical protocols that are financially sustainable and support a minimum of 3 h of multidisciplinary therapy per day.[2] Although large amount of task-specific stepping activity is believed to promote neuroplasticity of the central nervous system and result in improving walking function,[3,4] the intensity of treatment currently provided during standard care is far below recommended levels for this to occur.[5]
Many challenges exist to improve the intensity of ambulation practice in the inpatient rehabilitation setting, particularly for severe stroke patients who demand a significant amount of physical assistance. The fast development of technology in rehabilitation engineering has led to a variety of gait training devices that hold promise to increase repetition and patient engagement in therapy.[6-8] Although the effectiveness of these devices in stroke recovery has been in debate in the chronic phase of recovery, the emerging evidence including a recently revised Cochrane review supports that nonambulatory patients benefit most from mechanically assisted walking when provided early resulting in more independent walking.[7,9-13]
An inpatient rehabilitation single-blind randomized pilot study was recently conducted demonstrating the feasibility of an additional hour of gait training provided outside of regulated PT sessions using the Lokomat compared to manually assisted training.[14] However, very few studies have reported the strategy and effectiveness of providing robotic-assisted gait training (RAGT) in IRF.
The goal of this quality improvement (QI) project was to improve the intensity of gait training for severely affected stroke patients with limited ambulation function in IRF. The implementation focused on increasing the utilization of RAGT during the allotted clinical time for physical therapy while monitoring step counts. After implementation, the devices’ utilization, compliance, patients’ safety, and their potential impact on Functional Index Measure (FIM)-M gain were reviewed retrospectively to assess the effectiveness and preliminary efficacy of this initiative.
Materials and Methods
To maximize the intensity of training provided to severely affected stroke patients in daily PT sessions, a QI task force was created in September 2017 for the planning process. This QI project utilized the “Plan, Do, Study, Act” (PDSA) cycle. The collaborative interdisciplinary locomotor committee was created including the administrators, physiatrists, and clinical champions. The core group met regularly to develop guidelines based on literature review and clinical experience. Training and educational resources included an online video, written materials, and hands-on training modules which were developed for all involved therapists. Different work groups met to focus on identifying barriers and facilitators of logistics implementation.
Implementation process
The implementation was initiated in March 2019 and completed at the end of October 2019. The targeted population were patients with ambulatory dysfunction secondary to primary diagnose of an acute stroke (ischemic, hemorrhage, or embolic) who were admitted to our 26-bed inpatient stroke rehabilitation during this period. Those whose initial walking status was identified as “low level” by the treating physical therapist (PT) were considered for inclusion. “Low level” was defined as walking <15 feet with any amount of assistance or requiring assist of more than 2 people or only using parallel bars for walking. We did not include patients who were nonambulatory prior to admission or patients who could not complete the acute inpatient rehabilitation program due to medical complications.
The physical therapy team was comprised PTs specializing in poststroke rehabilitation who have received extensive locomotor training. The six participating PTs have an average of 10 years of experience, and five are board certified in neurologic physical therapy.
During the implementation, the PTs were encouraged to integrate the robotic devices to facilitate gait training as soon after admission as feasible. Given the goal of maximizing walking practice during therapy, the use of robotic training within the standard allotted PT time was encouraged but not mandatory. Locomotor training devices within the institution included the overground devices SafeGait and Andago, the tethered robotic exoskeleton Lokomat, and the end effector robotic device G-EO. The choices of devices were not limited to one kind of device per patient, the clinical decisions with regard to how and when to integrate the robotics devices were primarily made by the individual therapist based on walking deficits of patients, other training needs and goals, and devices availabilities. Heart rate and oxygen saturation were monitored by pulse oximeter during the training time to help assess voluntary effort and safety.[15] The physiatrist provided guidance and management regarding medical stability and safety. An ankle accelerometer (StepWatch, Modus, LLC, Edmonds, WA, USA) was placed on the patient’s paretic ankle to monitor the stepping activity recorded throughout the day between 7:30 am and 5:00 pm on weekdays as soon as following admission.[16] The daily placement and removal of accelerometers were done by nursing staff and physical therapy staff. The individual step count data were stored electronically for retrospective review but were not provided in real time to patients or treating therapists as daily feedback.
At the end of implementation, the charts of patients admitted consecutively to the unit from March to October 2019 who met the desired criteria were reviewed. Patient information was de-identified, their training modality, step counts, and weekly FIM scores were collected and retrospectively analyzed. The exempt IRB from our institution has approved that no written informed consent from participants needed for the retrospective chart review study.
Outcome measures
Feasibility outcome
To assess the feasibility of RAGT in regular PT sessions, the proportion of patients with low walking function who received locomotor training and individual robotics devices utilization frequency were calculated. Patients who received robotics devices at least three times per week during the majority of (>2/3) their IRF stay were identified as the locomotor group. Any device-related adverse events including falls, skin breakdown, and hypotension events were reviewed. The barriers encountered in the process of training implementation were discussed among team members.
Preliminary efficacy
To assess for preliminary efficacy, both changes in FIM-M (calculated as discharge FIM-M score minus admission FIM-M score) and daily step counts were recorded. The minimal clinically important difference (MCID) for FIM-M score was identified as 17 points.[17] This was compared between those who received consistent locomotor training, as above, and those who received conventional care. Other potentially associated factors including demographic and clinical characteristics between the two groups were analyzed as well.
Statistical analysis
Baseline demographics and clinical characteristics were summarized using standard descriptive statistics and compared between those who received conventional training and the locomotor group using the Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables. Pearson correlation coefficient was calculated to evaluate the correlation between the FIM motor gain and the maximum number of steps. Coefficients between 0.5 and 0.7 were considered moderate correlations, while coefficients >0.7 were considered strong. Linear regression was used to assess the difference in FIM motor gain between the two groups, adjusted for maximum practice step counts. A prespecified significance level of 0.05 (two-sided) was used for all analyses. Statistical analyses were performed using STATA 15.0 (College Station, TX, USA).
Results
One-hundred and five electronic medical charts were retrospectively reviewed for patients admitted during the period of March to October 2019. A total of 37 files were identified as the “low level” walking group, who had been ambulatory prior. We also excluded those whose steps were unable to be recorded and tracked, which resulted in 33 patients for analysis.
Patient characteristics
Table 1 provides demographic data, clinical characteristics, and baseline measures at admission. All the patients were admitted to stroke unit within 3 weeks post stroke. Admission FIM-M scores indicated all the patients included presented initially with severe motor deficits.
Table 1:
Characteristics of participants
| Variables | Locomotor (n=10), n (%) | Convention (n=23), n (%) | P |
|---|---|---|---|
| Age (years) | |||
| Mean | 63.1+/−12.1 | 66.2+/−18.0 | 0.32 |
| Male | 6 (60.0) | 9 (39) | 0.45 |
| Race | 3 (30.0) | 13 (56.5) | 0.38 |
| Caucasian | 5 (50.0) | 8 (34.8) | |
| African-American | 2 (20.0) | 2 (8.7) | |
| Others time since stroke (days) | 3–21 | 3–21 | 0.46 |
| Affected side | |||
| Right | 3 (30.0) | 11 (47.8) | 0.44 |
| Left | 7 (70.0) | 12 (52.2) | |
| Stroke type | |||
| Infarct | 7 (70.0) | 14 (60.9) | 0.094 |
| Hemorrhage | 1 (10.0) | 3 (13.0) | |
| Embolic | 1 (10.0) | 6 (26.1) | |
| Subdural hematoma | 1 (10.0) | 0 | |
| Admission FIM (mean) | 22.6 (4.9) | 19.2 (6.8) |
FIM: Functional Index Measure
Feasibility outcomes
There were 10 patients (30%) found to utilize the locomotor devices consistently during the inpatient rehab stay and these were identified as the “locomotor group” for analysis. The Lokomat (seven patients used it consistently in PT sessions) and G-EO (three patients used it consistently in PT sessions) were identified as the most used locomotor training devices in our institution during the period of implementation. Andago and SafeGait which are body weight support overground training devices had a low frequency of utilization in this group of patients (<20% of PT sessions).
The patients’ demographic characteristics (age, sex, race) and their clinical characteristics were not significantly different between groups, neither their admission FIM motor [shown in Table 1]. No device-related adverse events were reported. Hypotension episodes reported in PT sessions were not directly related to locomotor usage.
Preliminary efficacy
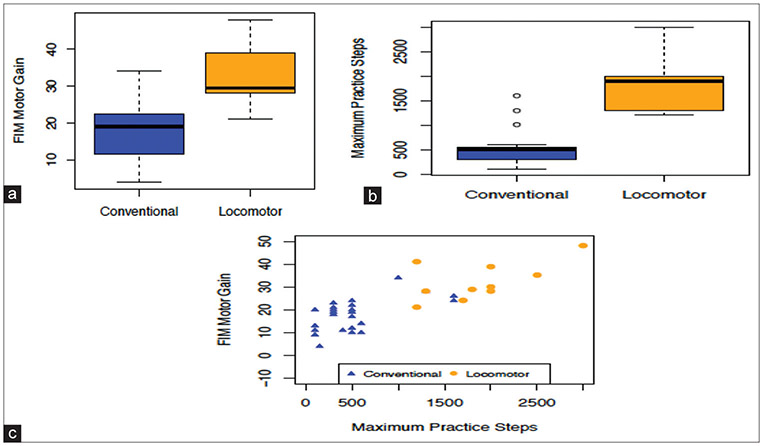
The impact of the locomotor program on the patients’ functional outcomes is summarized in Figure 1. The locomotor group showed greater FIM motor gain (n = 10, mean 32.30) at discharge compared to the conventional group (n = 23, mean 17.88) (P < 0.005) [Figure 1a], and the step counts between these groups were significantly different. The conventional care group performed an average of 527 steps/day, compared to 1870 steps/day for the locomotor group [Figure 1b, P < 0.005]. Furthermore, the number of steps performed in daily therapy showed a strong positive correlation (r = 0.8, P < 0.0001) to the FIM motor gain [Figure 1c]. To further probe the relationship among use of robotic devices, number of steps, and FIM motor gain, a regression analysis of motor gain on treatment (locomotor vs. conventional) adjusted for maximum steps was performed. When the number of steps is accounted , the effect of whether a device was used or not on FIM motor was no longer significant(p=0.23). The maximum steps remained strongly associated with motor gain (P = 0.0021).
Figure 1:
(a-c) Preliminary efficacy in Functional Index Measure-M change after implementation of robotic-assisted gait training and its association with step counts in low-level stroke patients
All of the patients (10/10) who received consistent robotic-assisted locomotor training reached MCID for FIM motor change, while only 60% (14/23) of patients who received mainly conventional training reached MCID at discharge.[17]
Discussion
Before implementation of this QI project, we collected 15 nonambulatory patients for baseline accelerometer data, we found that these patients (15/15) in our institution attained on average between 20 and 200 steps in daily PT sessions during inpatient rehab stay. This observation was similar to reports from other acute inpatient rehabilitation facilities.[5,18] The QI project primarily targeted to improve the intensity of gait training for low-level patients.
This retrospective review indicates that it is feasible to integrate robotic-assisted locomotor training into daily PT sessions for patients with limited walking ability after stroke. Findings suggest this intervention can be both effective and safe as there were no increased adverse effects reported. However, the percentage of participants and frequency of locomotor utilization in the program can be improved. Despite the great effort made for education and training prior to the program implementation, there are multiple barriers the treatment team encountered which contributed to a large proportion of patients not receiving consistent locomotor training by the end of the implementation. Support from management was beneficial, but the key for change of practice was driven by therapists. The barriers and facilitators identified from this process can help to guide future implementation. Therapists who participated in the project were interviewed to identify these barriers. Training or logistics were not felt to be significant obstacles. Due to patient medical status and physiological and behavioral tolerability, some of the clinicians struggled with their confidence to push patient intensity, difficulty prioritizing intensity of practice over traditional approaches, as well as having sufficient time to address other plans of care needs. The time required for accessing, setup, and donning is device dependent but required between 5 and 20 min of the therapy session. These barriers were consistent in most part with other studies.[19,20] Some team members reported difficulty with identifying the best robotic tool to meet their patients’ needs which is unique to the institution with a larger collection of devices and ongoing new acquisition. At a system level, the challenges included financial and time investments needed toward technology, education and training, and protocol optimization without allocated increased contribution from payers.
Based on our preliminary efficacy data, achievement of greater motor FIM gain at discharge from IRF using these devices is likely associated with the achievement of a higher amount of step repetition than conventional therapy with manual assist training [Figure 1]. The patients’ demographic and clinical characteristics were not found to be a driver for PT team’s treatment decision [Table 1] (all P > 0.05).
Based on the chart review results, Lokomat and GEO were identified as the most frequently used devices during this phase of implementation. Both of the devices provide dynamic body weight support, have the ability to assist with limb advancement, and can provide pelvic and upper trunk stability, all of which may be required to mobilize a low-level patient following acute stroke. Lokomat was observed to have a higher utilization frequency than G-EO, which may be attributable to its exoskeleton design to better support for patients with knee instability than end effectors devices. In this study, real-time feedback from accelerometers/activity monitors was not provided to the treatment team or patients as a part of the intervention. The intention was not to use the step counts to influence clinicians’ decision or patients’ performance.[21] However, in the next phase of implementation, how clinicians can utilize this real-time feedback to optimize the training will be explored as we observed the positive correlation of step counts and FIM-M outcomes. The accelerometers/activity monitors may provide valuable information with regard to the dosage of intervention in IRF setting.[3,4]
Limitations
The main limitation was the use of just one cycle of PDSA, resulting in small sample size and nonrandomized QI study. Another limitation was that the walking capacity outcomes (10-m walk test and 6-min walk test) were not obtained from the chart review consistently due to the limited ambulation capability of this group of patients. The heart rate and blood pressure were monitored closely before and after training for the safety consideration of patients, but the training intensity was not consistently guided by the targeted heart rate achieved as initially planned. As we pointed out, the results from this project may not be able to be generalized to other institutions due to clinicians’ experience and technology availabilities.
Future Direction
Based on what we have learned, we have seen that patients with stroke and lower levels of functional mobility benefit from robotic-assisted locomotor training in the earlier phases after injury. To our knowledge, there has been no work to date investigating the practicality of instituting a robotic training program primarily targeting this group of patients in an acute rehabilitation program. Our program intended to encourage clinicians to develop clinical reasoning to match various training tools to individual patients’ clinical characteristics which might have stronger potential to enhance outcomes in the future. This is an ongoing effort that our field needs to continue to develop and is relevant to nonrobotic intervention as well. The success of implementation of the RAGT in clinical practice would rely on perceived benefit and phased implementation.
In future efforts, the intervention protocol will be tailored to individual patient’s clinical recovery patterns and walking deficits, and we will utilize real-time quantification of intensity of intervention and robust data collection and analysis. The knowledge gained from continued implementation and study will further reduce gaps between robotics design, development, research trials, and clinical practice. More work needs to be done to develop a practical algorithm to guide the use of rehabilitation robotics devices in the real world, as well as provide feedback to the technology developers as to how to further enhance the robotics performance for improved clinical implementation and effectiveness in future.
Preliminary efficacy in FIM-M change after implementation of RAGT and its association with step counts in low-level stroke patients is shown in Figure 1
Financial support and sponsorship
This study was funded by the Einstein Society Innovative Program Grant.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Preston E, Ada L, Dean CM, Stanton R, Waddington G. What is the probability of patients who are nonambulatory after stroke regaining independent walking? A systematic review. Int J Stroke 2011;6:531–40. [DOI] [PubMed] [Google Scholar]
- 2.Miller LS, Forer SK. History and Efficacy of the “Three-Hour Rule”. PM R 2020;13:535–9. [DOI] [PubMed] [Google Scholar]
- 3.Hornby TG, Holleran CL, Leddy AL, Hennessy P, Leech KA, Connolly M, et al. Feasibility of focused stepping practice during inpatient rehabilitation poststroke and potential contributions to mobility outcomes. Neurorehabil Neural Repair 2015;29:923–32. [DOI] [PubMed] [Google Scholar]
- 4.Klassen TD, Dukelow SP, Bayley MT, Benavente O, Hill MD, Krassioukov A, et al. Higher doses improve walking recovery during stroke inpatient rehabilitation. Stroke 2020;51:2639–48. [DOI] [PubMed] [Google Scholar]
- 5.Lang CE, Macdonald JR, Reisman DS, Boyd L, Jacobson Kimberley T, Schindler-Ivens SM, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil 2009;90:1692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morone G, Paolucci S, Cherubini A, De Angelis D, Venturiero V, Coiro P, et al. Robot-assisted gait training for stroke patients: Current state of the art and perspectives of robotics. Neuropsychiatr Dis Treat 2017;13:1303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpe BT, Huerta PT, Zipse JL, Rykman A, Edwards D, Dipietro L, et al. Robotic devices as therapeutic and diagnostic tools for stroke recovery. Arch Neurol 2009;66:1086–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber LM, Stein J. The use of robots in stroke rehabilitation: A narrative review. NeuroRehabilitation 2018;43:99–110. [DOI] [PubMed] [Google Scholar]
- 9.Ada L, Dean CM, Vargas J, Ennis S. Mechanically assisted walking with body weight support results in more independent walking than assisted overground walking in non-ambulatory patients early after stroke: A systematic review. J Physiother 2010;56:153–61. [DOI] [PubMed] [Google Scholar]
- 10.Dean CM, Ada L, Bampton J, Morris ME, Katrak PH, Potts S. Treadmill walking with body weight support in subacute non-ambulatory stroke improves walking capacity more than overground walking: A randomised trial. J Physiother 2010;56:97–103. [DOI] [PubMed] [Google Scholar]
- 11.Moucheboeuf G, Griffier R, Gasq D, Glize B, Bouyer L, Dehail P, et al. Effects of robotic gait training after stroke: A meta-analysis. Ann Phys Rehabil Med 2020;63:518–34. [DOI] [PubMed] [Google Scholar]
- 12.Lo K, Stephenson M, Lockwood C. Effectiveness of robotic assisted rehabilitation for mobility and functional ability in adult stroke patients: A systematic review protocol. JBI Database System Rev Implement Rep 2017;15:39–48. [DOI] [PubMed] [Google Scholar]
- 13.Mehrholz J, Pohl M, Kugler J, Elsner B. Electromechanical-assisted training for walking after stroke: Update of the evidence. Stroke 2021;52:e153–4. [Google Scholar]
- 14.Cao N, Esquenazi A, Lee S, Charles A. Feasibility study of randomized supplemental therapeutic lower limb exercise in acute stroke rehabilitation. In: 2017 International Symposium on Wearable Robotics and Rehabilitation (WeRob); 2017. p. 1–1. [doi: 10.1109/WEROB.2017.8383878]. [DOI] [Google Scholar]
- 15.Holleran CL, Rodriguez KS, Echauz A, Leech KA, Hornby TG. Potential contributions of training intensity on locomotor performance in individuals with chronic stroke. J Neurol Phys Ther 2015;39:95–102. [DOI] [PubMed] [Google Scholar]
- 16.Prajapati SK, Gage WH, Brooks D, Black SE, McIlroy WE. A novel approach to ambulatory monitoring: investigation into the quantity and control of everyday walking in patients with subacute stroke. Neurorehabil Neural Repair 2011;25:6–14. [DOI] [PubMed] [Google Scholar]
- 17.Choon-Huat Koh G, Chen CH, Petrella R, Thind A. Rehabilitation impact indices and their independent predictors: A systematic review. BMJ 2013;3:e003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernhardt J, Thrift A, Donnan G. Inactive and alone physical activity within the first 14 days of acute stroke unit care. Stroke 2004;35:1005–9. [DOI] [PubMed] [Google Scholar]
- 19.Prout EC, Mansfield A, McIlroy WE, Brooks D. Physiotherapists’ perspectives on aerobic exercise early after stroke: A preliminary study. Physiother Theory Pract 2016;32:452–60. [DOI] [PubMed] [Google Scholar]
- 20.Connell LA, Klassen TK, Janssen J, Thetford C, Eng JJ. Delivering intensive rehabilitation in stroke: Factors influencing implementation. Phys Ther 2018;98:243–50. [DOI] [PubMed] [Google Scholar]
- 21.Mansfield A, Wong JS, Bryce J, Brunton K, Inness EL, Knorr S, et al. Use of accelerometer-based feedback of walking activity for appraising progress with walking-related goals in inpatient stroke rehabilitation: A randomized controlled trial. Neurorehabil Neural Repair 2015;29:847–57. [DOI] [PubMed] [Google Scholar]