Abstract
Sarcopenia is a condition marked by progressive loss of skeletal muscle mass and function while frailty is a multidimensional concept characterized by diminished physiological reserve and increased vulnerability to stressors. Both of these were previously considered as related to aging and shown to impact the quality of life and carry prognostic significance. Emerging data show that both sarcopenia and frailty carry similar relevance in chronic illness. Inflammatory bowel disease (IBD) is characterized by chronic inflammation of the gastrointestinal tract and malnourishment, both of which contribute to the development of sarcopenia by increasing protein breakdown and reducing protein synthesis. The coexistence of frailty further compounds the clinical complexity of IBD patients. Published evidence suggests a bidirectional association with IBD contributing to muscle wasting, while the resultant sarcopenia and frailty could further exacerbate the disease course. Sarcopenia and frailty are independently associated with adverse outcomes, including hospitalizations, increased surgical interventions, and surgical complications. As therapeutic strategies for IBD evolve, understanding the nuanced relationship between inflammatory bowel disease, sarcopenia, and frailty is crucial for devising holistic management. Comprehensive care should encompass not only disease‐modifying therapies but also interventions targeting frailty and sarcopenia, as they have been shown to have a significant impact not only on the disease course but also on the quality of life. Future research could focus on further elucidating underlying mechanisms, simple screening strategies, and developing targeted interventions to improve the overall quality of life for individuals grappling with the complex interplay of IBD, sarcopenia, and frailty.
Keywords: Crohn's disease muscle strength, frailty, inflammatory bowel disease, sarcopenia, ulcerative colitis
Frailty and sarcopenia in IBD: Frailty is multifaceted and is measured predominantly across four domains,i.e., cognitive, psychological, social, and physical. Sarcopenia is a unidimensional concept defined by reduced grip strength along with reduced musclemass.
Introduction
Both sarcopenia and frailty emerged as important concepts in the elderly population because of their significant implications on health and quality of life. These concepts have led to the development of assessments, tools, and interventions aimed at preventing or managing age‐related muscle loss and enhancing the overall health and resilience of frail elderly people. Healthcare professionals use this concept to identify individuals at high risk and to tailor care that addresses the specific needs and challenges faced by the elderly population. 1 Increasingly, the relevance of sarcopenia and frailty is being explored in the realm of chronic diseases as emerging data showed the impact of sarcopenia and frailty on the outcomes of the disease course and quality of life. 2 , 3 , 4 Lately in the field of gastroenterology, sarcopenia and frailty have been shown to impact the disease course, and outcomes of treatment including post‐transplant outcomes in patients with cirrhosis. 5 , 6 In this article, we review the conceptual framework of sarcopenia and frailty, their prevalence in patients with inflammatory bowel disease (IBD), the relationship between these conditions and disease activity, and their impact on outcomes and response to IBD‐related therapies.
Frailty
The frailty construct has several domains, including physical, cognitive, psychosocial, and emotional. Fried's group was the first to give an operational definition of frailty in 2001 based on the analysis of data from a large cohort, the Cardiovascular Health study group. 7 However, Fried's phenotype was mostly confined to the physical domain of frailty. Rockwood later proposed a cumulative deficit model and devised the frailty index that included an assessment of cognitive impairment and psychosocial factors. The frailty index classifies frailty into mild, moderate, and severe phenotypes. 8
Frailty, like sarcopenia, is a manifestation of cellular senescence. It is a result of age‐related mitochondrial dysfunction and progressive accumulation of reactive oxygen species leading to a rise in inflammatory mediators in blood and tissues (inflammaging) and increased cellular apoptosis. However, unlike sarcopenia, frailty is not singularly focused on myopenia and the resulting muscle dysfunction. Changes at the cellular level manifest across various organ systems (musculoskeletal, nervous, vascular, immune, and endocrine systems) leading to the frail phenotype. 9 The result is a state of decreased reserve and function across these systems, leading to increased vulnerability to stressors and adverse outcomes after exposure to stressors. 10
Frailty, although associated with aging, is not sine qua non of aging. Long‐standing inflammatory conditions are associated with or known to cause frailty, irrespective of age. 11 , 12 , 13 , 14 It is also important to recognize that frailty may be reversible with the correction of underlying stressors, nutrition support, and strength training. 15
Defining frailty
There is no accepted standard definition for frailty. Theoretically, physical frailty is defined as “A clinical syndrome with a distinct phenotype associated with a decreased reserve and high vulnerability to stressors and with risk of adverse outcomes including mortality.” 9 However, the operational definition of frailty as per Fried's group is dependent on satisfying at least three of the five characters mentioned in Table 1. 7 On the other hand, Rockwood et al. described an all‐encompassing frailty index that measures 92 symptoms. The frailty index is calculated as the fraction of deficits present from the overall (if a person has 24 symptoms, then his FI would be 24/92, i.e., 0.26). 8 The overview of the concept of frailty and sarcopenia is shown in Figure 1.
Table 1.
Criteria for Pre‐frail and Frail individuals as per Fried group
| S.NO | Parameter | Criteria | |
|---|---|---|---|
| 1 | Weight loss | Unintentional weight loss of 4.5 kg over the last 12 months | |
| 2 | Weakness | Reduced grip strength (adjusted for gender and BMI) † | |
| Men | Cutoff for grip strength (Kg) | ||
| BMI ≤24 | ≤29 | ||
| BMI 24.1–26 | ≤30 | ||
| BMI 26.1–28 | ≤30 | ||
| BMI >28 | ≤32 | ||
| Women | |||
| BMI ≤23 | ≤17 | ||
| BMI 23.1–26 | ≤17.3 | ||
| BMI 26.1–29 | ≤18 | ||
| BMI >29 | ≤21 | ||
| 3 | Slowness | Walk time for 4 m or 15 feet at usual pace (adjusted for gender and standing height) ‡ | |
| Men | Cutoff for Time to Walk 15 feet | ||
| Height ≤ 173 cm | ≥7 s | ||
| Height > 173 cm | ≥6 s | ||
| Women | |||
| Height ≤ 159 cm | ≥7 s | ||
| Height > 159 cm | ≥6 s | ||
| 4 | Exhaustion | Self‐reported | |
| 5 | Physical activity | Assessed by the WHO‐Global physical activity questionnaire or a short version of the Minnesota leisure time physical activity questionnaire (adjusted for sex) † | |
| Sex | Physical activity per week (Kcal) | ||
| Men | <383 | ||
| Women | <270 | ||
|
Score 0: Robust Score 1–2: Pre‐Frail Score ≥ 3: Frail | |||
Figure 1.
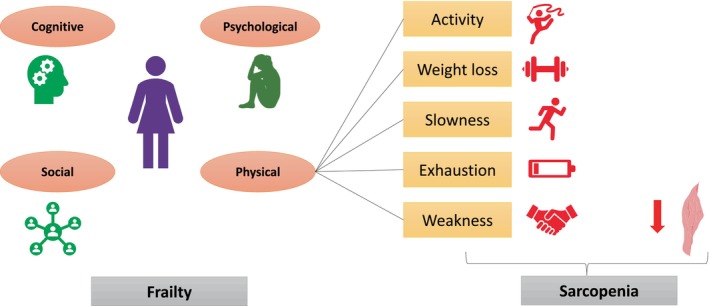
Overview of the concept of frailty and sarcopenia. Frailty is multifaceted and is measured predominantly across four domains, i.e., cognitive, psychological, social, and physical. The physical frailty domain is calculated across several variables like activity (assessed through physical activity scale), unintentional weight loss (>4.5 kg loss in 1 year), slowness (>7 s to travel 15 feet), exhaustion (self‐reported; at least 3 days a week), and weakness (reduced grip strength). Sarcopenia on the other hand is a measure of reduced grip strength along with reduced muscle mass, which is a part of one of the frailty domains.
Identifying frailty
There are broadly two methods to assess frailty: one is through questionnaires, and the other is through objective tests. Available tools to identify frailty use either of these approaches independently or a composite of these. Comprehensive geriatric assessment (CGA) is considered the gold standard of all available tools to detect frailty. It assesses frailty across seven domains, that is, functional, comorbidities, polypharmacy, nutritional, cognitive, psychosocial, and geriatric syndromes in the elderly, and is a sensitive tool to detect various degrees of frailty in the elderly. 17 CGA, however, is very elaborate and difficult to administer in a routine clinical setting. Hence, several screening tools are available, like PRISMA‐7, the Clinical Frailty Scale, and the FRAIL scale, which identify patients at risk and select them for detailed evaluation with CGA. CGA has a role not only in detailed assessment but also in making a comprehensive interventional plan and for follow‐up. There are no specific recommendations for timing and methods of screening frailty in IBD patients. Present studies have employed different tools to measure frailty in IBD patients. From the available tools, Timed Up and Go test (TUG test) and Indicator of Relative Need (IoRN) can be employed easily in a clinical setting for screening of frailty. Several different tools used for the measurement and the setting for the usage of these tools are summarized in Table 2.
Table 2.
Summary of commonly employed tools used for the screening and assessment of frailty
| S.No | Setting | Tools | Comments |
|---|---|---|---|
| 1 | Whole population screening | e‐Frailty Index |
Time taking. Requires population data. Requires expertise in data analysis. |
| 2 | Community based screening | Timed Up and Go test (TUG test) |
Can be done by non‐clinicians. Easy to use. Can be used in both community and acute care setting |
| Indicator of relative need (IoRN) |
Clinician administered. Easy to use. Can be used in both community, primary and acute care setting. |
||
| 3 | Self‐assessment tools | Frail non‐Disabled questionnaire (FiND) |
Self‐administered questionnaire Can be done at both community level and primary care centers |
| Prisma 7 | |||
| 4 | Predictors of individual risk of decline | Acutely presenting older patient (APOP) |
Questionnaire Used in acute clinical setting. Easy to use |
| Identification of seniors at risk (ISAR) | |||
| 5 | Severity of frailty | Clinical frailty scale |
Acute care setting Administered by Geriatrician |
| Frailty phenotype |
Both primary care and acute care setting Administered by clinician. Easy to use |
Frailty in IBD
Prevalence of frailty in IBD
A meta‐analysis of 1 495 695 patients with IBD showed the pooled prevalence of frailty at 18% (95% CI: 12%–24%). However, there was considerable heterogeneity in the studies (I 2: 99.9%) and the scales that were used to measure frailty were different in the studies. 18 In a prospective study by Salvatori et al., reporting on a cohort of 386 IBD patients, the prevalence of pre‐frailty and frailty in IBD patients was 17% and 28%, respectively, as assessed by Fried's phenotype. However, in the age group of ≥60 years, the prevalence of frailty rose to 24.2% and there was no significant difference in the prevalence of frailty between UC and CD. They also have shown that disease activity, steroid usage, and extraintestinal manifestations were significantly higher in frail individuals and multivariate analysis showed that disease activity was an independent predictor of frailty. 19
Impact of frailty on IBD
Disease activity
Among UC and CD, in the retrospective study by Kochar et al., CD patients had a higher prevalence of frailty (60%). They noted that there was no difference in the levels of inflammatory markers among the frail and non‐frail IBD patients. 20 In a retrospective analysis of 2978 IBD patients, where frailty was defined based on frailty index (score > 0.27), patients with more than five flares were higher in the frail group (35.6% vs. 27.5%); however, this finding was not found to be significant on regression analysis. 21 In a prospective cohort study by Salvatori et al., in frail IBD individuals, defined as per Fried's criteria, active disease was higher in the individuals who maintained a frail phenotype (73%) as compared with those with pre‐frail (34%) and fit phenotypes (17%, P:0.012). Multivariate analysis found that frailty was associated with clinically active disease (OR:0.1, 95% CI: 0.01–0.6), extraintestinal manifestations (OR:0.1, 95% CI: 0.02–0.8), and is inversely related to biological use (OR:21.7, 95% CI: 3.4–263). This suggests that therapy with biologics may have a role in the improvement of frailty status. 22 Another prospective cohort study in elderly IBD patients measured frailty by geriatric deficit assessment and found a direct relationship between the number of geriatric deficits with clinical (OR:2.192, 95% CI: 1.284–3.743), biochemical disease activity (OR:3.358, 95% CI: 1.936–5.825), and fecal calprotectin levels (OR: 2.721, 95% CI: 1.376–5.379). Interestingly, it was noted that the CD patients had higher geriatric deficits (OR: 1.799, 95% CI: 1.179–2.743). 23
IBD therapy
Regarding the requirement of surgical intervention in frail IBD patients, there is heterogeneity in the literature. Kochar et al. noted that a history of IBD‐related surgery was higher in frail (32%) than fit (11%) IBD patients (P < 0.01), a similar finding noted in the study by Singh et al., where frail patients had higher rates of abdominal surgery than non‐frail patients (14.4% vs. 9.6%). 20 , 24 Contrary to these findings, another retrospective analysis by Kochar et al., using a different frailty scale to diagnose frailty, found that there was no significant difference in the prior IBD surgery among the frail and non‐frail group (11% vs. 9%, P:0.41). 21 However, Qian et al. have noted that frailty was independently associated with a lower risk of surgery (HR: 0.8, 95% CI: 0.66–0.91) and frail patients had a lower incidence of disease flare as compared with fit IBD patients (14.8% vs. 30.9%, P < 0.01). 21
Anti‐TNF therapy was shown to improve frailty scores with a mean overall improvement from 19.83 to 11.8 (P:<0.001), with 45% of frail patients showing improvement in scores to >50% and a complete improvement seen in 12% of cases. Response to therapy was significantly associated with lower post‐treatment frailty (OR:0.24, 95% CI: 0.16–0.34). 25 Surprisingly, Singh et al., from their retrospective analysis of 5987 IBD patients initiated on biologicals, found that the risk of serious infections was higher in frail patients who were on vedolizumab (OR:1.69, 95% CI: 1.03–2.79) but not on anti‐TNF α therapy (OR:1.03, 95% CI: 0.83–1.27). 24 A similar finding was noted by Asscher et al. in IBD patients on biologicals, where the risk of infections was independently associated with comorbidities, as measured by the Charlson comorbidity index (CCI), in patients on vedolizumab (OR:1.387, 95% CI: 1.022–1.883) and ustekinumab (OR:1.621, 95% CI: 1.034–2.541). 26 However, Kochar et al. have noted that pretreatment frail individuals who were initiated on anti‐TNF therapy and immunomodulators are at increased risk of infections compared with non‐frail individuals even after adjusting for all the confounders like age and steroid use (OR:2.05, 95% CI: 1.07–3.93; OR:1.81, 95% CI: 1.22–2.70 respectively). Infection‐related hospitalization between frail and non‐frail groups was significantly higher in patients initiated on immunomodulators (OR:2.08, 95% CI: 1.33–3.26) but not on biologicals (OR:1.51, 95% CI: 0.62–3.66). 27 It is unclear whether the risk of infection is related to the frail status or the underlying disease activity and the therapies.
Post‐operative outcomes
In a retrospective analysis of 9023 patients of Crohn's disease who underwent bowel resection, frailty (simplified frailty index ≥2) was a better predictor of postoperative (post‐op) outcomes than age (OR‐2.59, 95% CI: 1.84–3.63). They also noted that frailty was associated with the need for emergency surgery (sFI ≥2–13%, P:0.01) and malnutrition (sFI≥2–48.9%, P:0.01). 28 In a retrospective analysis of 943 UC cases who underwent colectomy, frailty was present in 19.5% of cases and was an independent predictor of overall morbidity (aOR:25.5, P < 0.001) and serious morbidity (aOR:66.8, P < 0.001). Frail patients also had a higher incidence of post‐op complications including septic (aOR:31.26, P:0.006), cardiopulmonary (aOR:216.3, P < 0.001) and Clavien class IV complications (aOR:204.9, P < 0.001). 29 However, in another study by Cohan et al., in UC patients undergoing colectomy with IPAA, there was no difference between frail and non‐frail patients in the mean number of complications (0.31 vs. 0.34, p: 0.36) and the length of hospital stay (7.4 vs. 7.7 days, P: 0.25). 30
Mortality
In a retrospective study from a nationwide database of 1 405 529 IBD patients (36% UC, 63% CD), the overall prevalence of frailty was 10.9%, and it progressively increased from 10.2% to 11.5% over 5 years (2010–2014). In this report, ICD‐9‐CM from Johns Hopkins clinical groups frailty was the e‐frailty (electronic) index employed to diagnose frailty. Frailty was an independent predictor of readmission risk (RR: 1.16 95% CI: 1.14–1.17) and mortality on readmission (RR: 1.12, 95% CI: 1.02–1.23). 31 Similar findings were noted in another retrospective analysis of 47 402 IBD admissions where frail IBD patients had higher readmission rates (HR: 1.21, 95% CI: 1.17–1.25), in‐hospital mortality (HR: 1.57, 95% CI: 1.34–1.83), and IBD‐related severe hospitalizations (HR: 1.22, 95% CI: 1.16–1.29). 32 In the analysis of 11 001 IBD cases, Kochar et al. noted that after adjusting for other confounders like age, duration of IBD, history of surgery, and immunosuppression use, the frail IBD cohort had significantly higher mortality compared with fit patients (OR: 2.90, 95% CI: 2.29–3.68). 20 Gondal et al., have also noted that the mortality rate was higher in IBD patients who were frail (defined as per FI) compared with non‐frail (OR:1.52, 95% CI: 1.07–2.16). 21 Studies relating to IBD and frailty are summarized in Table 3.
Table 3.
Studies on frailty in patients with inflammatory bowel disease
| Author and year | Study population | Number | Frailty measures | Prevalence of frailty | Outcome | OR/RR/HR and P value |
|---|---|---|---|---|---|---|
| Wolf et al., 2021 28 | CD with bowel resections | 9023 | Simplified frailty index (sFI) | sFI ≥1 in 1610 (17.8%) | Higher sFI (≥2) is associated with | |
| morbidity (OR) | 2.59 (P < 0.001) | |||||
| malnutrition | P < 0.01 | |||||
| emergency surgery | P < 0.01 | |||||
| Telemi BS et al., 2018 29 | UC with colectomy, frailty as a predictor (OR) | 943 | Modified frailty index (mFI) |
mFI of 0.09: 184 (19.5%) mFI ≥0.18: 121 (12.8%) |
Complications (OR) | |
| Septic | 31.26 (P:0.006) | |||||
| Cardiopulmonary | 216.3 (P < 0.001) | |||||
| Clavien class IV | 204.9 (P < 0.001) | |||||
|
Morbidity (OR) | ||||||
|
Overall |
25.5 (P < 0.001) | |||||
| serious | 66.8 (P < 0.001) | |||||
| Cohan J et al., 2015 30 | UC with colectomy and IPAA | 2493 | Frailty trait count | NA | Mean number of complications | 0.31 vs. 0.34, P: 0.36 |
| Length of hospital stay | 7.4 vs. 7.7 days, P: 0.25 | |||||
| Faye S et al. 31 | Admitted IBD |
UC—510 199 (36%) CD—888 981 (63%) |
ICD‐9‐CM codes from Johns Hopkins clinical groups frailty | 152 974 (10.9%) | Increased risk of readmission (RR) | 1.16 (1.14–1.17) |
| Increased risk of readmission (RR) mortality in CD | 1.32 (1.13–1.55) | |||||
| Qian S et al., 2021 32 | Admitted IBD | 47,402 | Hospital frailty risk score |
15 507 (32.7%) Medium frailty—14 207 High frailty—1480 |
IBD‐related severe hospitalizations (HR) | 1.22 (1.16–1.29) |
| Higher readmission rates (HR) | 1.21 (1.17–1.25) | |||||
| Higher inpatient mortality (HR) | 1.57 (1.34–1.83) | |||||
| Kochar B et al., 2020 20 |
Admitted IBD on predictors of frailty |
11 001 | Based on ICD‐10 codes | 675 (6%) |
≥1 comorbidities (CCI) |
17.31 (8.14–36.79) |
|
Hospitalization All cause IBD related |
7.67 (4.62–12.73) 2.29 (1.85–2.83) |
|||||
| Kochar B et al., 2022 25 | On response of frailty to anti‐TNF, predictors of post treatment frailty |
1210 CD: 71% UC: 29% |
Claims‐based frailty index (CFI) | 189 (15.6%) | Pretreatment frailty (OR) | 2.10 (1.35–3) |
| IBD‐related hospitalization (OR) | 1.63 (1.15–2.3) | |||||
| Singh S et al., 2020 24 | Risk of infections in patients on biologicals | 5987 IBD | Hospital frailty risk score | 2350 (39.3%) | Risk of serious infections (HR) | |
| Anti TNF α | 1.03 (0.83–1.27) | |||||
| Vedolizumab | 1.69 (1.03–2.79) | |||||
| Gondal et al., 2020 21 | Mortality and disease activity in IBD |
2978 IBD UC: 53.5% CD: 46% Indeterminate: 0.5% |
Frailty index (score > 0.27) | 953 (32%) | Mortality (OR) | 1.52 (1.07–2.16) |
| >5 IBD flares (OR) | 1.2 (0.99–1.45) | |||||
| Salvatori S et al., 2023 22 | Prospective cohort | 64 with IBD and frailty | Fried frailty score | Median 8‐month follow‐up | Extraintestinal manifestations (OR) | 0.1 (0.02–0.8) |
| Clinically active disease (OR) | 0.1 (0.01–0.6) | |||||
| Biological use (OR) | 21.7 (3.4–263) | |||||
| Asscher et al., 2022 23 | Prospective multicenter study on IBD factors associated with geriatric deficits |
405 IBD CD: 191 UC: 202 IBD‐UC: 12 |
Geriatric assessment |
Moderate deficits: 160 (39.5%) Severe deficits: 32 (7.9%) |
Crohn's disease (OR) | 1.799 (1.179–2.743) |
| Clinical disease activity (OR) | 2.192 (1.284–3.743) | |||||
| Fecal calprotectin (OR) | 2.721 (1.376–5.379) | |||||
| Previous all‐cause hospitalization (OR) | 1.994 (1.267–3.137) | |||||
| Kochar et al., 2020 27 | Retrospective study on patients being initiated on biologicals and immunomodulators | 1299 on anti‐TNF and 2676 on immunomodulator | Frailty risk score (ICD‐10) |
Anti‐TNF: 68 (5.2%) Immunomodulator: 212 (7.9%) |
Risk of infection on anti‐TNF (OR) | 2.05 (1.07–3.93) |
| Risk of infection on Immunomodulator (OR) | 1.81 (1.22–2.70) | |||||
Anti‐TNF, anti‐tumor necrosis factor; CD, Crohn's disease; CFI, Claims‐based frailty index; HR, hazard ratio; IBD, inflammatory bowel disease; IBD‐UC, inflammatory bowel disease‐unclassified; ICD, international classification of diseases; IPAA, ileal pouch‐anal anastomosis; mFI, modified frailty index; OR, odds ratio; RR, risk ratio; sFI, simplified frailty index, UC, ulcerative colitis.
IBD and aging
Back in 2010, epidemiological estimates showed that people of ≥65 years with IBD constituted 20% of the total IBD population. 33 Given the chronic nature of the condition and lack of curative therapies, it is estimated that the cohort of IBD with ≥65 years of age will increase to a third of the IBD cohort. 34 Aging is associated with multiple comorbidities, and people with IBD are no exception, resulting in polypharmacy adding to the pill burden in the elderly. This potentially leads to nonadherence to IBD treatment. 19 , 35 IBD also brings up a new spectrum of complications, especially in the elderly, associated with steroid use like cataracts and osteoporosis. The risk of infections and malignancies due to the use of immune suppressants, biologics, and small molecules may be higher in the elderly. 36 , 37 Elderly with IBD have a significantly increased risk of postoperative complications (OR in CD: 1.4 and UC: 1.74), mortality (OR in CD:11.6 and UC:4.39), infections (OR in CD: 1.11 and UC: 1.52), and embolic events (OR in CD: 1.68 and UC: 1.35). 38 Long‐term follow‐up after ileal pouch‐anal anastomosis in patients with UC showed that elderly patients have significantly higher incontinence rates (young: 25.25%, elderly: 44.91, P:<0.001), stool frequency (mean bowel movements per day in young: 5.55, elderly: 6.79, P:<0.001), and are at increased risk of dehydration and electrolyte loss (young: 23.6%, elderly: 60%, P:<0.001). 39 Kochar et al., in their retrospective study, have noted that the prevalence of frailty increased as age progressed in IBD patients. Overall, 4% of IBD patients were frail who were in their 20s and almost one fourth of IBD patients were frail in their 90s. 20 Reduction in the ratio of T3/T4 has been identified as an independent marker of frailty in the elderly. 40 An extrapolation of this, Bertani et al., in their prospective study, included patients of elderly IBD, who were initiated on biologicals and the ratio of T3/T4 was used as a response predictor. They found that the baseline T3/T4 ratio was proportionate to the mucosal healing rates regardless of disease type and biological use. 41
Sarcopenia
Sarcopenia deals with only the physical component of frailty but has been very well studied. Sarcopenia results from manifestations of a progressive (age‐related) decline in muscle mass. The term sarcopenia, first used by Rosenberg in 1989, was not recognized as a separate disease entity until ICD‐9. 42 , 43 It had no specific definition, evaluation, or diagnostic criteria and management aspects were uncertain. In the years 2010 and 2011, three consensus papers were released defining sarcopenia and diagnosing sarcopenia. Later ICD‐10 recognized sarcopenia as a separate pathological entity (M62.84) 44 , 45 , 46 . 47 Sarcopenia can be defined as a “progressive and generalized skeletal muscle disorder with significant reduction in skeletal muscle mass associated with low muscle strength and low physical performance.”. 48
Mechanism of sarcopenia
Muscle mass is maintained by a tightly regulated balance between protein synthesis and breakdown. Stimulation of muscle protein synthesis is by activation of the mTOR pathway. When insulin‐like growth factor 1 (IGF‐1) or insulin binds to IGF‐1 receptors (a tyrosine kinase receptor) on the myocytes, it results in a chain of reactions leading to the phosphorylation of Akt, a cellular kinase. 49 Phosphorylated Akt, in turn, phosphorylates and activates mTOR complex 1 (mTORC1), activated mTORC1 stimulates protein synthesis by activating ribosomal protein kinase, which is p70‐S6 kinase 1, and it also inhibits proteolysis by inhibiting autophagy‐activating kinases ULK1/2. 50 The FoxO (Forkhead box O) family of transcription factors that have a role in promoting autophagy and maintenance of the ubiquitin‐proteasome system causing cellular apoptosis, Akt phosphorylates members of the FoxO family, which leads to their inhibition. 51 Akt also enhances mitochondrial respiration by activating ATP citrate lyase (ACL) leading to increased ATP production, which is essential for protein synthesis in muscle. In addition, fibroblast growth factor 19 (FGF‐19), secreted by enterocytes in response to bile acids, directly stimulates cytoplasmic protein synthesis via activation of the extracellular signal‐regulated kinases (ERK). 52
Protein breakdown is predominantly mediated by two pathways, that is, the ubiquitin‐proteasome system and the autophagy‐lysosomal system. Aging is associated with ‘Somatopause’, a phenomenon seen due to reduced GH secretion and thereby reduced circulating IGF‐1 levels. IGF‐1 is a major hormone required for maintaining muscle protein synthesis. 53 , 54 , 55 , 56 Thus, the interplay of all these factors is associated with a physiological reduction in muscle mass as age progresses. In patients with IBD, it was shown that the total levels of Akt were similar in both CD patients and matched healthy controls in the muscle biopsy specimens; however, the phosphorylated Akt (activated) levels were significantly lower in those with CD compared with matched healthy controls. 57 The mechanism of muscle protein synthesis and breakdown operating in patients with IBD is shown in Figure 2.
Figure 2.
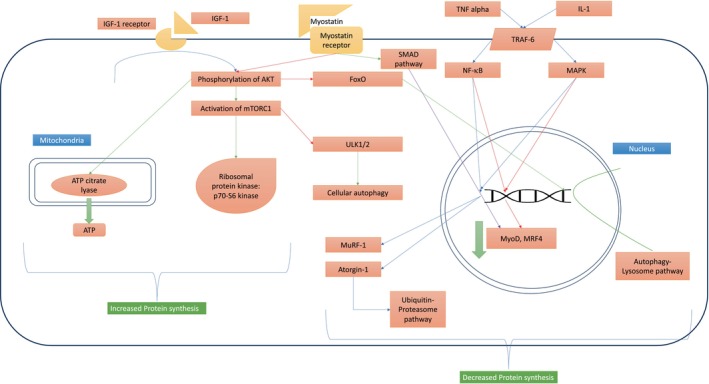
Mechanism of muscle protein synthesis and breakdown operating in patients with IBD. Muscle protein synthesis is stimulated by binding IGF‐1 to its receptor leading to phosphorylation of Akt. Activated Akt activates mTORC‐1 and inhibits FoxO and ULK1/2, both of which are involved in protein degradation. Activated mTORC‐1 stimulates ribosomal protein kinases resulting in synthesis of proteins, and this is supported by an increase in ATP production in mitochondria by activated ATP citrate lyase, providing the energy required for protein synthesis. Protein breakdown is predominantly mediated by two pathways, that is, the ubiquitin‐proteasome system and the autophagy‐lysosomal system. Absence of IGF‐1 stimulation releases FoxO from its cytoplasmic captivity, which translocate into nucleus and stimulate the autophagy‐lysosome pathway. Inflammatory mediators (IL‐1 and TNF alpha) activate the ubiquitin‐proteasome pathway via the Nf‐κB and MAPK pathways, which reduce the expression of myogenic genes and stimulate the production of MuRF‐1 and Atorgin‐1 proteins, both are ubiquitin ligases that are involved in the third step (E3) of ubiquitination. Myostatin receptor via Smad pathway directly reduce the expression of myogenic genes and indirectly by inhibiting the phosphorylation of Akt. In the absence of Akt activity, mTORC‐1 complex is dormant, thus ULK1/2 remains uninhibited, leading to increased cellular autophagy. ATP: Adenosine triphosphate, FoxO: Forkhead box O, IGF‐1: insulin‐like growth factor 1. IGF‐1R: insulin‐like growth factor 1 receptor, IL‐1: Interleukin 1, MAPK: mitogen‐activated protein kinase, MRF4: myogenic regulatory factor 4, mTORC‐1: mTOR complex 1, MuRF‐1: muscle ring finger protein‐1, MyoD: myoblast determination protein 1, NF‐κB: nuclear factor‐κB, TNF alpha: Tumor necrosis factor alpha, TRAF‐6: TNF receptor‐associated factor 6, ULK1/2: Unc‐51‐like autophagy‐activating kinases 1 and 2.
Mechanisms of sarcopenia in IBD
Reduced IGF‐1 levels
Circulating IGF‐1 and IGF‐1 binding protein‐3 (IGFBP‐3) are significantly lower in patients with IBD. 58 , 59 Therapy with infliximab and corticosteroids normalizes the circulating total IGF‐1 and IGFBP‐3 levels. 60 , 61 Thus, the reduction of IGF‐1 levels in patients with active IBD reduces muscle mass by favoring protein catabolism.
Chronic inflammation
Patients with active IBD have increased circulating levels of proinflammatory cytokines like TNF‐α and IL‐6. 62 These act via mitogen‐activated protein kinase (MAPK) and nuclear factor‐κB (NF‐κB) pathways involved in the transcription of muscle ring finger protein‐1 (MuRF‐1) and Atrogin‐1 leading to proteasome‐mediated degradation of ubiquitinated proteins. 63 , 64 , 65 IL‐6 via the JAK–STAT pathway stimulates the expression of MuRF‐1, Atorgin‐1, and myostatin and increases the expression of proteasomal subunits cathepsin B and L, leading to protein degradation. 66 , 67 Myostatin (TGF‐β family), produced from the skeletal muscle in response to IL‐6, reduces the expression of myogenic genes via the Smad pathway and inhibits the phosphorylation and activation of Akt. Myostatin also activates the MAPK pathway, leading to increased expression of MuRF‐1 and Atorgin‐1. 68 Through these mechanisms, inflammation reduces myogenesis and increases protein breakdown.
Malnutrition
Malnutrition in IBD is multifactorial, encompassing factors such as inflammation‐related anorexia, pain from strictures, reduced intestinal absorptive surface due to multiple intestinal resections and inflamed mucosa, dietary restrictions, bacterial overgrowth. It has similar incidence in both UC and CD. 69 , 70 Studies from the elderly cohort have shown that malnutrition is associated with severe sarcopenia and nutrition intervention and physical activity improve sarcopenia in the elderly. 71 , 72 Vitamin D plays an important role in the maintenance of musculoskeletal health, involved in the upregulation of IGF‐2 and follistatin genes and the downregulation of the myostatin gene. 73 The prevalence of vitamin D deficiency is high in patients with UC and CD, with the latest study showing a prevalence rate of 65% in CD and 55% in UC. 74 Improvement in bone and muscle parameters with vitamin D supplementation was noted in pediatric IBD patients. 75
Defining sarcopenia
The European Working Group on Sarcopenia in Older People (EWGSOP) has defined sarcopenia as the reduction in muscle mass along with a reduction in muscle strength or performance. Pre‐sarcopenia refers to a reduction in muscle mass alone while severe sarcopenia was defined if all three are present. 44 Later in 2018, the working group noted that muscle strength rather than mass had a greater correlation with all‐cause morbidity and mortality. In the 2018 operational definition by EWGSOP, sarcopenia was defined as a reduction in muscle strength and mass or quality. Probable sarcopenia was defined as a reduction in muscle strength and severe sarcopenia was defined as the presence of sarcopenia along with reduced physical performance. 76 The Asian working group on sarcopenia (AWGS) definition of sarcopenia is more on the lines of the older EWGSOP definition. They have defined possible sarcopenia as either a reduction in muscle strength or physical performance; sarcopenia was defined as the presence of reduced muscle mass along with either reduced muscle strength or physical performance and severe sarcopenia as the presence of all three parameters. 77 Different consensus definitions of sarcopenia are summarized in Table 4. 45 , 46 , 78 , 79 , 80 , 81
Table 4.
Various definitions of sarcopenia
| S.no | Consensus Group | Year | Sarcopenia definition | |
|---|---|---|---|---|
| 1 | Special interest group 45 | 2010 | Low muscle mass and low gait speed | |
| 2 | European working group on sarcopenia in older people (EWGSOP) 44 | 2010 | Pre‐sarcopenia | Reduced muscle mass |
| Sarcopenia | Reduced muscle mass along with either reduction in muscle strength or performance | |||
| Severe sarcopenia | Reduction in muscle mass, muscle strength and performance | |||
| 3 | International working group on sarcopenia 46 | 2011 | Low muscle mass and muscle function | |
| 4 | Society on sarcopenia, cachexia and wasting disorders 78 | 2011 | Low muscle mass with limited mobility (walking speed ≤1 m/s or <400 m distance covered during a 6‐min walk) | |
| 5 | Asian working group on sarcopenia (AWGS) 79 | 2014 | Low Muscle mass along with either low handgrip strength or low gait speed | |
| 6 | Foundation for the national institutes of health sarcopenia project 80 | 2014 | Low muscle mass and low grip strength | |
| 7 | European working group on sarcopenia in older people (EWGSOP) 76 | 2019 | Probable sarcopenia | Low muscle strength |
| Sarcopenia | Low muscle strength with reduced muscle quality or quantity | |||
| Severe sarcopenia | Sarcopenia with reduced physical performance | |||
| 8 | Sarcopenia definitions and outcomes consortium 81 | 2020 | Presence of both Weakness (reduced grip strength) and slowness (reduced physical performance) | |
| 9 | Asian working group on sarcopenia (AWGS) 77 | 2020 | Possible sarcopenia | Reduced muscle strength or physical performance |
| Sarcopenia | Low muscle mass along with either reduced muscle strength or physical performance | |||
| Severe sarcopenia | Low muscle mass along with either reduced muscle strength and reduced physical performance | |||
Assessment of sarcopenia
Sarcopenia is assessed at three levels. First, screening was conducted on susceptible individuals to detect the presence of sarcopenia by assessing muscle strength, and then individuals with reduced muscle strength are assessed for reduced muscle mass and physical performance. Reduced muscle mass with optimal physical performance is categorized as sarcopenia. The presence of both reduced mass and physical performance is categorized as severe sarcopenia. Normal cutoff for grip strength and muscle mass varies between races and sexes and respective societies have given their cutoffs based on local studies, as mentioned in Table 5.
Assessing muscle strength: It is typically assessed by performing grip strength. It is performed with a handheld dynamometer, and the recommended device is the Jamar dynamometer. Hand grip correlates moderately with other body compartments strength and is relatively easy to perform, such as in those with hand deformities, knee flexor and extensor isometric torque can be measured. 79 Another easy way to assess muscle strength is through a chair stand test, where a patient is asked to stand up from a standard chair without support and arms flexed; the time taken for the subject to perform it five times is then recorded. 82
Assessing muscle mass: Muscle mass of appendicular skeletal muscle or a total body skeletal mass can be assessed directly through DEXA scan, computed tomography (CT), and magnetic resonance imaging (MRI), and indirectly through bioelectrical impedance analysis. Both CT and MRI are considered as gold standard in noninvasive assessment of muscle quantity. Taller individuals would have larger mass when compared with shorter individuals, hence muscle mass is adjusted according to either height, weight, or BMI. Cross‐sectional area at the L3 level correlates well with total skeletal mass and provides a window for assessing myopenia in IBD patients especially undergoing CT or MRI scans for other reasons. 83 Ultrasound is now emerging as a cheaper, alternative, and an accurate modality for assessing muscle mass of quadriceps femoris. 84
Assessing physical performance: Gait speed is an accurate and easy measure of physical performance and has been shown to predict the adverse outcomes related to sarcopenia. 85 The time taken to walk for 4 meters is measured and gait speed is calculated; a speed of ≤0.8 m/s is considered as a marker of poor physical performance; this assessment can be done in a clinical setting and is recommended by EWGSOP2. A composite score of gait speed, chair stand test, and balance test forms a short physical performance battery (SPPB); a score of ≤8 out of 12 is suggestive of poor performance; however, it takes more than 10 min to administer SBBP. Other tests like Timed Up and Go (TUG) and 400‐meter walk tests can also be performed to assess physical performance. 76
Table 5.
Cutoffs for the diagnosis of sarcopenia
| S.NO | Working Group | Parameter | Cutoff in Male | Cutoff in Female |
|---|---|---|---|---|
| 1 | European working group on sarcopenia in older people (EWGSOP) | Grip strength | <27 kg | <16 kg |
| Muscle mass | <7 kg/m2 | <5.5 kg/m2 | ||
| 2 | Asian working group on sarcopenia (AWGS) | Grip strength | <28 kg | <18 kg |
| Muscle mass | ||||
| DEXA | <7 kg/m2 | <5.4 kg/m2 | ||
| Bioelectrical impedance analysis | <7 kg/m2 | <5.7 kg/m2 | ||
| 3 | South Asian Working Action Group on Sarcopenia (SAWG‐SARCO) | Grip strength | <27.5 kg | 18 kg |
| Muscle mass | <7 kg/m2 | <5.7 kg/m2 | ||
Prevalence of sarcopenia in IBD
Bryant et al. studied the prevalence of sarcopenia in IBD, diagnosed based on a reduction in muscle mass and grip strength. They noted that myopia was present in 21% and sarcopenia in 12% of cases, with no significant difference among patients with CD and UC (12% and 14%, respectively). 86 Another study by the same group in 2018 showed that the prevalence of sarcopenia in patients with IBD progressed over time from 9% to 15% over 2 years. 87 Another group from Italy studied the prevalence of sarcopenia in which muscle strength was assessed as a degree of asthenia measured through a visual analogue scale and found a higher rate of sarcopenia prevalence at 28%. 88 In 2021, ünal et al. studied the prevalence of sarcopenia, diagnosed based on the 2019 operational definition of EWGSOP, and found that 31.3% had myopenia while only 7.6% had sarcopenia, and severe sarcopenia was found in 2.6% of patients. 89 In another study in China where sarcopenia was diagnosed based on the AWGS definition, 56.3% had pre‐sarcopenia, 30% had sarcopenia, and 8.1% had severe sarcopenia. 90 In a study from India on 114 patients with UC, the prevalence of sarcopenia and severe sarcopenia was 21.9% and 12.2%. 91 Data from the systematic review of sarcopenia in IBD showed that the overall prevalence of pre‐sarcopenia was 34%, myopenia was 42%, and sarcopenia was 17%, with no significant difference between UC and CD and similar occurrences in both the genders. 92 Studies on the prevalence of sarcopenia in IBD are summarized in Table 6 while those reporting myopenia are depicted in Table 7.
Table 6.
Studies reporting on prevalence of sarcopenia in inflammatory bowel disease as per revised definition (myopenia and grip strength)
| S.no | Author | Year | Country | Number | Parameters measured | Prevalence |
|---|---|---|---|---|---|---|
| 1 | Bryant et al. 82 | 2015 | Australia | 137 | Muscle strength: Grip strength | 12% |
| Muscle mass: DEXA (ASMI) | ||||||
| 2 | Bryant et al. 83 | 2018 | Australia |
110 CD‐79 UC‐28 |
Muscle strength: Grip strength | 15% |
| Muscle mass: DEXA (ASMI) | ||||||
| 3 | Pizzoferrato et al. 84 | 2019 | Italy |
127 CD‐69 UC‐58 |
Muscle strength: Degree of asthenia based on VAS of 1–100 | 28% |
| Muscle mass: DEXA and Bioelectrical impedance analysis | ||||||
| 4 | Ünal et al. 85 | 2021 | Turkey |
344 CD‐122 UC‐222 |
Muscle strength: Grip strength | 7.6% |
| Muscle mass: Bioelectrical impedance analysis | ||||||
| 5 | Liu et al. 86 | 2022 | China |
110 CD‐25 UC‐85 |
Muscle strength: Grip strength | 30% |
| Muscle mass: Bioelectrical impedance analysis | ||||||
| 6 | Bharath et al. 91 | 2023 | India | 114 (UC) | Muscle strength: Grip strength | 21.9% |
| Muscle mass: DEXA (ASMI) |
ASMI, appendicular skeletal muscle index; CD, Crohn's disease; DEXA, dual energy X‐ray absorptiometry; UC, ulcerative colitis: VAS, visual analogue scale.
Table 7.
Studies reporting on myopenia in IBD from Asia
| S.No | Author | Year | Country | Number | Parameter used to measure muscle mass | Prevalence | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Zhang T et al. 93 | 2017 | China |
204 CD‐105 UC‐99 |
L3 skeletal muscle index on CT scan † | 25.5% | Myopenia is associated with higher disease activity and poor clinical outcomes |
| 2 | Bamba S et al. 94 | 2017 | Japan |
72 CD‐43 UC‐29 |
L3 skeletal muscle index on CT scan † | 42% | Myopenia predicts intestinal resection |
| 3 | Zhang T et al. 95 | 2017 | China | 114 (CD) | L3 skeletal muscle index on CT scan † | 61.4% | Patients undergoing bowel resection with myopenia had higher post‐operative complications |
| 4 | Bamba S et al. 96 | 2020 | Japan |
187 CD‐99 UC‐88 |
L3 skeletal muscle index on CT scan † | 34.7% | Myopenia is associated with prolonged hospital stay |
| 5 | Kang MK et al. 97 | 2020 | South Korea | 443 (UC and CD) | Psoas muscle index on CT ‡ | 34.9% | Sarcopenia was an independent risk factor for NAFLD in patients with IBD |
| 6 | Kurban M et al. 98 | 2020 | China | 47 (CD) | Bioelectrical impedance analysis | 66% | Body composition analysis is more accurate for muscle mass assessment than nutrition assessment scales |
| 7 | Lee CH et al. 99 | 2020 | South Korea | 79 (CD) | L3 skeletal muscle index on CT scan † | 51% | Myopenia correlated with CRP levels |
| 8 | Ge Xi et al. 100 | 2021 | China | 233 (ASUC) | L3 skeletal muscle index on CT scan † | 50.2% | Myopenia associated with failure of response to IV steroid, higher colectomy rates and postoperative complications |
| 9 | Boparai et al. 101 | 2021 | India | 44 (CD) | L3 skeletal muscle index on CT scan † | 43% | Combination of myopenia and high visceral fat was associated with poor outcomes in CD |
| 10 | Ge Xi et al. 102 | 2022 | China | 254 (ASUC) | L3 skeletal muscle index on CT scan † | 50% | Sarcopenia is an independent predictor for rescue therapy and colectomy |
| 11 | Nam K et al. 103 | 2023 | South Korea |
1027 CD‐854 UC‐173 |
L3 skeletal muscle index on CT scan † | 56.8% | Myopenia is associated with perianal CD |
L3 skeletal muscle index: Total cross‐sectional area of the skeletal muscles at L3 level divided by height (in meters) squared.
Psoas muscle index: Total psoas cross‐sectional area at L3 level divided by height (in meters) squared.
ASUC, acute severe ulcerative colitis; CD, Crohn's disease; NAFLD, non‐alcoholic fatty liver disease; UC, ulcerative colitis.
Nutrition status and sarcopenia in IBD
Sarcopenia is often thought to be associated with malnourishment, which is true in the elderly cohort, but studies in IBD show varying results. 71 A study on nutritional status and body composition of a cohort of 88 CD patients revealed that malnourishment is prevalent (21.6%), but a greater number of patients were overweight and obese (32.8%). Among these, most of the patients with myopenia either had a normal BMI (49%) or were overweight and obese (15.7%). 104 A similar finding was noted in a cohort of IBD patients who were initiated on anti‐TNF therapy: 41.5% of patients with myopenia had normal BMI and 19.5% were considered to be overweight or obese. 105 A study of body composition in patients with CD suggests that BMI has only an intermediate correlation with skeletal muscle index (SMI) (r was between 0.5 and 0.7). 106 In a cross‐sectional study where sarcopenia was measured as reduced muscle mass and muscle strength, the prevalence of normal BMI among patients with sarcopenia was as high as 76%. 86
Relation to disease activity
A longitudinal analysis of 44 patients with UC showed that myopenia had a positive correlation with the Mayo score and myopenia was an independent risk factor for a higher Mayo score in patients with UC (OR‐8.49, 95% CI: 1.80–40.1, P = 0.007). Patients with UC and myopenia also had higher colectomy rates (P = 0.003) and the myopenia improved significantly after colectomy (P = 0.033). 107 A retrospective study measured adverse outcomes, a composite of death or need for surgery, in patients with CD who underwent CT and found out that although nonsignificant the adverse event rate was higher numerically in patients with myopenia than those without (52% vs. 37.4%). 106 Further analysis showed that malnutrition had no significant effect on adverse outcomes (composite of surgery, hospitalization, abscess formation) but patients with myopenia had a significantly higher rate of adverse outcomes compared with those without myopenia (surgery: 63.3% vs. 27.8%, P = 0.001; hospitalizations: 61.2% vs. 36.1%, P = 0.022; abscesses: 51.0% vs. 16.7%, P = 0.001); myopenia and visceral obesity but not BMI were independent predictors for the need of surgery. 104 In contrast to the above findings, a study of myopenia (measured on CT at L3 level) in relation to disease activity (CDAI score) in patients with CD showed no significant difference in the prevalence of sarcopenia across varied disease activity (47.4%—moderately active, 42.1%—mildly active, and 10.5%—remission, P = 0.228). However, they have noted that the disease course was more aggressive in those with myopenia (31.6% vs. 16%, P = 0.06) and a higher requirement of surgery in patients with myopenia (31.6% vs. 4%, P = 0.01). A combination of myopenia and visceral fat to subcutaneous fat ratio of <0.88 was a better predictor of the need for surgery than sarcopenia alone. 93 In a cross‐sectional study of CD patients, body composition was measured on CT, and although subcutaneous and visceral adiposity along with skeletal muscle index (SMI) were associated with moderate to severely active endoscopic disease, SMI performs the best in diagnosing disease activity with an AUC value of 0.865. 101 Similar findings were noted in a follow‐up study of CD patients where authors noted that SMI had a negative correlation with disease activity and lower SMI was associated with severe disease (P = 0.001) and the SMI at the last follow‐up was the only significant predictor of remission in patients with CD (OR‐1.21, 95% CI: 1.03–1.42, P = 0.021). 108 Sarcopenia as a risk factor for poor outcomes was first shown in a prospective study of 110 IBD patients; after a 90‐day follow‐up period, sarcopenia was found to be associated with higher rates of surgery (OR = 6.651, 95% CI: 2.333–18.959, P < 0.001), re‐hospitalization (OR‐6.344, 95% CI: 2.874–14.003, P < 0.001) and death (P = 0.003); similar trend was seen in those with pre‐sarcopenia when compared with controls. 90 Among patients with ulcerative colitis, disease severity was measured with the Mayo score. The prevalence of sarcopenia (13%) and severe sarcopenia (47.2%) was significantly higher in those with mild to moderately active and severe disease than in those who were in remission (6.3%) (P < 0.001). 91
Impact on management
Acute severe colitis
In a retrospective analysis of the outcomes in patients with acute severe colitis (ASUC) sarcopenia (defined as a reduction in muscle mass at the level of L3 vertebrae) (OR: 3.130, 95% CI: 1.609–6.087, P = 0.001) along with disease extent, Mayo score and hemoglobin were risk factors for IV steroid failure. Sarcopenia remained to be an independent predictor of second‐line therapy failure (OR: 3.401, 95% CI: 1.104–10.479, P = 0.033). In patients who underwent colectomy, the rate of post‐op complications was significantly higher in those with sarcopenia compared with those without sarcopenia (58.6% vs. 33.3% respectively, P = 0.037); sarcopenia alone was an independent risk factor for post‐op complications (OR: 4.157, 95% CI: 1.364–12.667, P = 0.012) in ASUC patients who underwent colectomy. 109 Another larger retrospective study of 254 patients with ASUC similarly assessed muscle mass on CT. It showed that patients with myopenia had a significantly higher need for rescue therapy (40.9% vs. 23.6%, P = 0.003) and a need for surgery (22.0% vs. 7.1%, P = 0.001). Still, the same did not stand true for those who were malnourished and well nourished; myopenia rather than BMI was an important determinant of the above outcomes and myopenia remained an independent risk factor for rescue therapy (OR: 4.079, 95% CI: 2.245–7.412, P < 0.001) and colectomy (OR: 1.985, 95% CI: 1.028–3.834, P = 0.041). 100
Response to therapy
In a retrospective analysis of IBD patients, patients on Anti TNF therapy were analyzed after a median period of 24 months; those with skeletal muscle area less than the median value of the study group had greater rates of therapy failure than those with muscle area greater than the median value (61.7% vs. 27.6%, P = 0.014). 102 Another 5‐year follow‐up study of IBD patients initiated on Anti TNF noted that skeletal muscle index (measured on CT) noted that SMI was an independent risk factor for secondary failure (HR: 2.15, 95% CI: 1.04–4.44, P = 0.039) and bowel resection (HR: 4.19, 95% CI: 1.01–17.3, P = 0.048). 110
Surgery
In a retrospective study in patients with CD, myopenia (assessed on CT at L3) was found to be a risk factor for bowel resection on multivariate analysis in patients with CD, and in the post‐op follow‐up period, myopenia improved. 111 Another multicenter retrospective study of 187 IBD patients (CD‐99, UC‐89) showed that low psoas index (cross‐sectional area of psoas at L3/height in m2) on CT was a significant risk factor for both intestinal resection (OR: 0.754, 95% CI: 0.578–0.972, P = 0.033) and prolonged hospital stay (OR: 0.662, 95% CI: 0.480–0.883, P = 0.004) in both UC and CD cases. 94 Data from a single‐center retrospective study in patients undergoing either elective or emergent surgery revealed that myosteatosis (muscle quality) rather than myopenia was associated with longer hospital stays (median duration: 13 vs. 10.5 days). On multivariate analysis, myosteatosis was an independent risk factor for readmission (OR: 4.803, 95% CI: 1.053–21.889, P = 0.043). 96 Another 5‐year follow‐up study on muscle quality, assessed on MRI, in patients with CD, showed that patients with lower muscle quality (lower intensity on MRI) had a significantly shorter resection‐free period than those with higher muscle intensity on MRI (P = 0.037). 112 This suggests that muscle quality is an important parameter that needs to be taken into consideration and not muscle mass alone. This was addressed in the latest EWGSOP statement where they suggested a reduction in either muscle mass or quality for the diagnosis of sarcopenia. 76
Role of IBD therapy on sarcopenia
In a retrospective study of patients admitted with ASUC, biological use was higher in patients without myopenia than in those with myopenia (7.9% vs. 0.8%, P = 0.006). 100 In another retrospective study of patients with CD, the median SMI values were significantly higher in those who were on Anti TNF therapy than those who were not (P = 0.01). 113 In a longitudinal cohort study on patients initiated on anti‐TNF therapy, after a median follow‐up of 5 years, there was a significant improvement in all the muscle parameters (skeletal muscle index; P < 0.001, skeletal muscle area; P < 0.001, psoas muscle area, P = 0.03) measured on CT. 114 In another retrospective study where the prevalence of myopenia was studied in patients who underwent surgery for IBD (surgical cohort) and who were initiated on biologics (medical cohort), after a follow‐up period of 1 year, the prevalence of myopenia was higher in the surgical cohort when compared with the medical cohort (32% vs. 16%, P < 0.02). 115 The impact of biological use in IBD was also prospectively studied in patients with CD, where the assessment of muscle strength and mass was done at baseline and 25 weeks later and found that there was a significant improvement in both muscle strength (P = 0.002) and muscle mass (P = 0.01), and this gain was independent of steroid usage. 116
Pediatric IBD
A study on the impact of Anti TNF therapy in pediatric IBD found that the skeletal muscle mass (as measured on Bioimpedance) at the end of induction and at the end of 6 months of therapy, the difference in skeletal muscle mass scores between the groups with and without sarcopenia had significantly reduced. However, there was no significant gain in muscle mass in the group who had no sarcopenia at the baseline. 117 Even in the pediatric group, there was a poor correlation between BMI and muscle mass, as shown in a cross‐sectional study of 101 pediatric IBD cases; the psoas area index (average psoas area divided by body surface area) was significantly lower in children with IBD than those of controls (P < 0.001) and had a poor correlation with the BMI (r = 0.019). In the same study, they found out that PAI is associated with radiologically severe disease (P = 0.03) and is an important risk factor for the initiation of biological therapy (HR: 12.1, 95% CI: 1.4–104, P = 0.023) and disease exacerbation (HR: 8.9, 95% CI:1.5–53.1, P = 0.016). 118
Interventions in IBD for sarcopenia
Nutrient supplementation
The recommended daily protein intake in IBD patients is 1 mg/kg/day for those who are in remission and 1.2–1.5 gm/kg/day for those with active disease. 119 These recommendations are not specific for those with sarcopenia but for IBD in general. There is still a lack of data on the effect of protein supplementation on muscle health in IBD patients. However, evidence from the meta‐analysis of studies in the elderly had shown that protein supplementation along with physical activity had been shown to improve sarcopenia and frailty in the elderly. 72 Hence adequate protein consumption is of paramount importance for IBD patients, especially with sarcopenia. Additionally, data from pediatric IBD patients showed that daily supplementation of 2000 IU of cholecalciferol for a median period of 13.8 months showed improvement of both BMD and muscle power as compared with baseline. 75
Exercise
In a pilot study in patients with Crohn's disease with quiescent or mild disease activity endurance and muscle training for 3 months had been shown to improve muscle strength and quality of life without improvement in disease activity. 120 In patients with childhood‐onset IBD, physical exercise for 12 months showed that there was improvement in bone mineral density (BMD) and body composition as compared with the sedentary controls. 121 Similar outcomes were seen in pediatric IBD patients in remission, where moderate to vigorous physical activity had a significant positive correlation with BMD and lean body mass. 122 In an RCT of Crohn's disease patients who were randomized to either a resistance and impact training program or a sedentary life for 6 months, at the end of the study period patients who were in the exercise program had significantly higher BMD and muscle function than control. 123
In addition to these measures, as discussed above, controlling the underlying disease activity would lead to an improvement in muscle mass.
Summary and recommendations
Frailty and sarcopenia are important measures for adverse outcomes in the elderly. Growing evidence in the field of IBD has shown that irrespective of age, frailty and sarcopenia have poor outcomes on several aspects of the disease including activity, response to therapy, post‐op outcomes, and overall mortality. Also, measuring overall well‐being is an important outcome in several interventions related to IBD, of which sarcopenia and frailty are important contributors. However, the relationship between frailty, sarcopenia, and IBD remains poorly studied. The available data suggest that both frailty and sarcopenia are prevalent in those with active disease. From the existing evidence, we can logically conclude that the existence of frailty and sarcopenia in individuals with IBD is associated with poorer outcomes. Hence this should trigger us to identify at‐risk individuals with IBD and screen them with appropriate tools. However, there are no specific recommendations on how to screen, when to screen, how frequently to screen, and how to intervene. Emerging data suggest that in individuals with IBD who were found to have sarcopenia or frailty, an inverted pyramid approach may be considered. The use of the most effective therapies (biologicals) being offered upfront could positively impact sarcopenia. There are still several lacunae in the available evidence as to the role of steroids in frail or sarcopenia with IBD, as steroids are known to positively modify the disease activity and yet negatively affect the musculoskeletal system as a result worsening both frailty and sarcopenia. There is no good grade of evidence to back up interventions targeting sarcopenia and frailty resulting in improvement of the disease outcomes, hence it cannot be recommended. But in total, the approach is to be wholesome, clinicians should be aware of the negative influences of sarcopenia and frailty on disease activity and outcomes and have an active lookout for it. It can be done opportunistically in patients who have undergone abdominal CT scans for the assessment of IBD or identifying the at‐risk individuals with simple parameters or tests like handgrip strength or timed up‐and‐go tests. The role of aggressive management of active disease and interventions aimed at improving the muscle strength in such individuals should be actively studied as prospective data backing such approach are lacking.
Acknowledgements
None.
Declaration of conflict of interest: None.
Author contribution: Pardhu B Neelam: Literature search and initial draft and final approval. Alka Sharma: Literature review and manuscript revisions. Vishal Sharma: Conception, critical revision, editing, and final approval.
References
- 1. Sampaio RA, Sewo Sampaio PY, Capelo LP, Uchida MC, Arai H. Sarcopenia and frailty: the role of physical activity for better aging. Front. Public Health. 2023; 11: 1303223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bone AE, Hepgul N, Kon S, Maddocks M. Sarcopenia and frailty in chronic respiratory disease. Chron. Respir. Dis. 2017; 14: 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Musso CG, Jauregui JR, Macías Núñez JF. Frailty phenotype and chronic kidney disease: a review of the literature. Int. Urol. Nephrol. 2015; 47: 1801–1807. [DOI] [PubMed] [Google Scholar]
- 4. Kinugasa Y, Yamamoto K. The challenge of frailty and sarcopenia in heart failure with preserved ejection fraction. Heart. 2017; 103: 184–189. [DOI] [PubMed] [Google Scholar]
- 5. Wang S, Whitlock R, Xu C et al. Frailty is associated with increased risk of cirrhosis disease progression and death. Hepatology. 2022; 75: 600–609. [DOI] [PubMed] [Google Scholar]
- 6. Kumar VV, Kothakota SR, Nair AK et al. Impact of sarcopenia on post‐liver transplant morbidity and mortality in cirrhotic patients. Indian J. Gastroenterol. 2022; 41: 440–445. [DOI] [PubMed] [Google Scholar]
- 7. Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. Ser. A. 2001; 56: M146–M157. [DOI] [PubMed] [Google Scholar]
- 8. Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J. Am. Geriatr. Soc. 2004; 52: 1929–1933. [DOI] [PubMed] [Google Scholar]
- 9. Fried LP, Cohen AA, Xue QL, Walston J, Bandeen‐Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat. Aging. 2021; 1: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng MH, Chang SF. Frailty as a risk factor for falls among community dwelling people: evidence from a meta‐analysis. J. Nurs. Scholarsh. 2017; 49: 529–536. [DOI] [PubMed] [Google Scholar]
- 11. Lorenz EC, Kennedy CC, Rule AD, LeBrasseur NK, Kirkland JL, Hickson LJ. Frailty in CKD and Transplantation. Kidney Int. Rep. 2021; 6: 2270–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vitale C, Jankowska E, Hill L et al. Heart Failure Association of the European Society of Cardiology position paper on frailty in patients with heart failure. Eur. J. Heart Fail. 2019; 21: 1299–1305. [DOI] [PubMed] [Google Scholar]
- 13. Katz PP, Andrews J, Yazdany J, Schmajuk G, Trupin L, Yelin E. Is frailty a relevant concept in SLE? Lupus Sci Med. 2017; 4: e000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tandon P, Montano‐Loza AJ, Lai JC, Dasarathy S, Merli M. Sarcopenia and frailty in decompensated cirrhosis. J. Hepatol. 2021; 75: S147–S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Travers J, Romero‐Ortuno R, Bailey J, Cooney MT. Delaying and reversing frailty: a systematic review of primary care interventions. Br. J. Gen. Pract. 2019; 69: e61–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tell GS, Fried LP, Lind B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann. Epidemiol. 1993; 3: 358–366. [DOI] [PubMed] [Google Scholar]
- 17. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013; 381: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang X, Xiao M, Jiang B et al. Prevalence of frailty among patients with inflammatory bowel disease and its association with clinical outcomes: a systematic review and meta‐analysis. BMC Gastroenterol. 2022; 22: 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salvatori S, Marafini I, Venuto C et al. Frail phenotype in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2023; 29: 1555–62. [DOI] [PubMed] [Google Scholar]
- 20. Kochar B, Cai W, Cagan A, Ananthakrishnan AN. Frailty is independently associated with mortality in 11 001 patients with inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2020; 52: 311–318. [DOI] [PubMed] [Google Scholar]
- 21. Gondal A, Rehman M, Farooq U, Talluri S, Georgetson MJ, Ghimire S. S0671 The association of frailty with mortality and relapse frequency in inflammatory bowel disease. Off. J. Am. Coll. Gastroenterol. ACG. 2020; 115: S337. [Google Scholar]
- 22. Salvatori S, Marafini I, Franchin M et al. Reversibility of frail phenotype in patients with inflammatory bowel diseases. J. Clin. Med. 2023; 12: 2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asscher VER, Waars SN, van der Meulen‐de Jong AE, et al. Deficits in geriatric assessment associate with disease activity and burden in older patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2022; 20: e1006–e1021. [DOI] [PubMed] [Google Scholar]
- 24. Singh S, Heien HC, Sangaralingham L et al. Frailty and risk of serious infections in biologic‐treated patients with inflammatory bowel diseases. Inflamm. Bowel Dis. 2021; 27: 1626–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kochar BD, Cai W, Ananthakrishnan AN. Inflammatory bowel disease patients who respond to treatment with anti‐tumor necrosis factor agents demonstrate improvement in pre‐treatment frailty. Dig. Dis. Sci. 2022; 67: 622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Asscher VER, Biemans VBC, Pierik MJ et al. Comorbidity, not patient age, is associated with impaired safety outcomes in vedolizumab‐ and ustekinumab‐treated patients with inflammatory bowel disease—a prospective multicentre cohort study. Aliment. Pharmacol. Ther. 2020; 52: 1366–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kochar B, Cai W, Cagan A, Ananthakrishnan AN. Pretreatment frailty is independently associated with increased risk of infections after immunosuppression in patients with inflammatory bowel diseases. Gastroenterology. 2020; 158: 2104–2111.e2. [DOI] [PubMed] [Google Scholar]
- 28. Wolf JH, Hassab T, D'Adamo CR, et al. Frailty is a stronger predictor than age for postoperative morbidity in Crohn's disease. Surgery 2021; 170: 1061–1065. [DOI] [PubMed] [Google Scholar]
- 29. Telemi E, Trofymenko O, Venkat R, Pandit V, Pandian TK, Nfonsam VN. Frailty predicts morbidity after colectomy for ulcerative colitis. Am. Surg. 2018; 84: 225–229. [PubMed] [Google Scholar]
- 30. Cohan JN, Bacchetti P, Varma MG, Finlayson E. Outcomes after ileoanal pouch surgery in frail and older adults. J. Surg. Res. 2015; 198: 327–333. [DOI] [PubMed] [Google Scholar]
- 31. Faye AS, Wen T, Soroush A et al. Increasing prevalence of frailty and its association with readmission and mortality among hospitalized patients with IBD. Dig. Dis. Sci. 2021; 66: 4178–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qian AS, Nguyen NH, Elia J, Ohno‐Machado L, Sandborn WJ, Singh S. Frailty is independently associated with mortality and readmission in hospitalized patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2021; 19: 2054–2063.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burisch J, Pedersen N, Čuković‐Čavka S et al. East‐West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO‐EpiCom inception cohort. Gut. 2014; 63: 588–597. [DOI] [PubMed] [Google Scholar]
- 34. Coward S, Clement F, Benchimol EI et al. Past and future burden of inflammatory bowel diseases based on modeling of population‐based data. Gastroenterology. 2019; 156: 1345–1353.e4. [DOI] [PubMed] [Google Scholar]
- 35. Mesonero F, Fernández C, Sánchez‐Rodríguez E et al. Polypharmacy in patients with inflammatory bowel disease: prevalence and outcomes in a single‐center series. J. Clin. Gastroenterol. 2022; 56: e189–e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Román ALS, Muñoz F. Comorbidity in inflammatory bowel disease. World J. Gastroenterol. 2011; 17: 2723–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mishra S, Jena A, Kakadiya R, Sharma V, Ahuja V. Positioning of tofacitinib in treatment of ulcerative colitis: a global perspective. Expert Rev. Gastroenterol. Hepatol. 2022; 16: 737–752. [DOI] [PubMed] [Google Scholar]
- 38. Bollegala N, Jackson TD, Nguyen GC. Increased postoperative mortality and complications among elderly patients with inflammatory bowel diseases: an analysis of the national surgical quality improvement program cohort. Clin. Gastroenterol. Hepatol. 2016; 14: 1274–1281. [DOI] [PubMed] [Google Scholar]
- 39. Ramage L, Qiu S, Georgiou P, Tekkis P, Tan E. Functional outcomes following ileal pouch‐anal anastomosis (IPAA) in older patients: a systematic review. Int. J. Colorectal Dis. 2016; 31: 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pasqualetti G, Calsolaro V, Bernardini S et al. Degree of peripheral thyroxin deiodination, frailty, and long‐term survival in hospitalized older patients. J. Clin. Endocrinol. Metab. 2018; 103: 1867–1876. [DOI] [PubMed] [Google Scholar]
- 41. Bertani L, Tricò D, Pugliese D et al. Serum triiodothyronine‐to‐thyroxine (T3/T4) ratio predicts therapeutic outcome to biological therapies in elderly IBD patients. Aliment. Pharmacol. Ther. 2021; 53: 273–280. [DOI] [PubMed] [Google Scholar]
- 42. Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014; 11: 177–180. [PMC free article] [PubMed] [Google Scholar]
- 43. ICD . ICD‐9‐CM—International Classification of Diseases, Ninth Revision, Clinical Modification [Internet]. 2021. U.S. Department of Health and Human Services; Washington, DC. Cited 2022 Dec 21. Available from: https://www.cdc.gov/nchs/icd/icd9cm.htm [Google Scholar]
- 44. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Muscaritoli M, Anker SD, Argilés J et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia‐anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010; 29: 154–159. [DOI] [PubMed] [Google Scholar]
- 46. Fielding RA, Vellas B, Evans WJ et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011; 12: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. ICD . ICD‐10—International Classification of Diseases, Tenth Revision [Internet]. 2021. Cited 2022 Dec 21. Available from: https://www.cdc.gov/nchs/icd/icd10.htm.
- 48. Delmonico MJ, Harris TB, Lee JS et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007; 55: 769–774. [DOI] [PubMed] [Google Scholar]
- 49. Ward CW, Garrett TPJ, Lou M, et al. The structure of the type 1 insulin‐like growth factor receptor. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000–2013. Cited 2023 Sep 26. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6216/. [Google Scholar]
- 50. Frost RA, Lang CH. mTOR signaling in skeletal muscle during sepsis and inflammation: Where does it all go wrong? Physiology (Bethesda). 2011; 26: 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen K, Gao P, Li Z et al. Forkhead Box O signaling pathway in skeletal muscle atrophy. Am. J. Pathol. 2022; 192: 1648–1657. [DOI] [PubMed] [Google Scholar]
- 52. Guthrie G, Vonderohe C, Burrin D. Fibroblast growth factor 15/19 expression, regulation, and function: An overview. Mol. Cell. Endocrinol. 2022; 548: 111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF‐1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013; 9: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao J, Brault JJ, Schild A et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007; 6: 472–483. [DOI] [PubMed] [Google Scholar]
- 55. Zachari M, Ganley IG. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017; 61: 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cui H, Kong Y, Zhang H. Oxidative Stress, Mitochondrial Dysfunction, and Aging. J Signal Transduct. 2012; 2012: 646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Langenberg DR, Gatta PD, Hill B, Zacharewicz E, Gibson PR, Russell AP. Delving into disability in Crohn's disease: Dysregulation of molecular pathways may explain skeletal muscle loss in Crohn's disease☆. Journal of Crohn's and Colitis. 2014; 8: 626–634. [DOI] [PubMed] [Google Scholar]
- 58. Katsanos KH, Tsatsoulis A, Christodoulou D, Challa A, Katsaraki A, Tsianos EV. Reduced serum insulin‐like growth factor‐1 (IGF‐1) and IGF‐binding protein‐3 levels in adults with inflammatory bowel disease. Growth Horm. IGF Res. 2001; 11: 364–367. [DOI] [PubMed] [Google Scholar]
- 59. Eivindson M, Nielsen JN, Grønbaek H, Flyvbjerg A, Hey H. The insulin‐like growth factor system and markers of inflammation in adult patients with inflammatory bowel disease. Horm. Res. 2005; 64: 9–15. [DOI] [PubMed] [Google Scholar]
- 60. Eivindson M, Grønbaek H, Skogstrand K et al. The insulin‐like growth factor (IGF) system and its relation to infliximab treatment in adult patients with Crohn's disease. Scand. J. Gastroenterol. 2007; 42: 464–470. [DOI] [PubMed] [Google Scholar]
- 61. Eivindson M, Grønbaek H, Flyvbjerg A, Frystyk J, Zimmermann‐Nielsen E, Dahlerup JF. The insulin‐like growth factor (IGF)‐system in active ulcerative colitis and Crohn's disease: relations to disease activity and corticosteroid treatment. Growth Horm. IGF Res. 2007; 17: 33–40. [DOI] [PubMed] [Google Scholar]
- 62. Mitsuyama K, Toyonaga A, Sasaki E et al. Soluble interleukin‐6 receptors in inflammatory bowel disease: relation to circulating interleukin‐6. Gut. 1995; 36: 45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gumucio JP, Mendias CL. Atrogin‐1, MuRF‐1, and sarcopenia. Endocrine. 2013; 43: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bakkar N, Wang J, Ladner KJ et al. IKK/NF‐κB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J. Cell Biol. 2008; 180: 787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang G, Li YP. p38β MAPK upregulates atrogin1/MAFbx by specific phosphorylation of C/EBPβ. Skelet. Muscle. 2012; 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Silva KAS, Dong J, Dong Y et al. Inhibition of Stat3 activation suppresses caspase‐3 and the ubiquitin‐proteasome system, leading to preservation of muscle mass in cancer cachexia. J. Biol. Chem. 2015; 290: 11177–11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yamaguchi T, Naruishi K, Arai H, Nishimura F, Takashiba S. IL‐6/sIL‐6R enhances cathepsin B and L production via caveolin‐1‐mediated JNK‐AP‐1 pathway in human gingival fibroblasts. J. Cell. Physiol. 2008; 217: 423–432. [DOI] [PubMed] [Google Scholar]
- 68. Elkina Y, von Haehling S, Anker SD, Springer J. The role of myostatin in muscle wasting: an overview. J. Cachexia. Sarcopenia Muscle. 2011; 2: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Singh A, Midha V, Mahajan R et al. Evaluation of nutritional characteristics reveals similar prevalence of malnutrition in patients with ulcerative colitis and Crohn's disease. Dig. Dis. Sci. 2022; 68: 580–595. [DOI] [PubMed] [Google Scholar]
- 70. Scaldaferri F, Pizzoferrato M, Lopetuso LR et al. Nutrition and IBD: malnutrition and/or sarcopenia? A practical guide. Gastroenterol. Res. Pract. 2017; 2017: 8646495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Verstraeten LMG, van Wijngaarden JP, Pacifico J, Reijnierse EM, Meskers CGM, Maier AB. Association between malnutrition and stages of sarcopenia in geriatric rehabilitation inpatients: RESORT. Clin. Nutr. 2021; 40: 4090–4096. [DOI] [PubMed] [Google Scholar]
- 72. Mareschal J, Genton L, Collet TH, Graf C. Nutritional intervention to prevent the functional decline in community‐dwelling older adults: a systematic review. Nutrients. 2020; 12: 2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Garcia LA, King KK, Ferrini MG, Norris KC, Artaza JN. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology. 2011; 152: 2976–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Janssen CE, Globig AM, Busse Grawitz A, Bettinger D, Hasselblatt P. Seasonal variability of vitamin D status in patients with inflammatory bowel disease—a retrospective cohort study. PloS One. 2019; 14: e0217238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hradsky O, Soucek O, Maratova K et al. Supplementation with 2000 IU of cholecalciferol is associated with improvement of trabecular bone mineral density and muscle power in pediatric patients with IBD. Inflamm. Bowel Dis. 2017; 23: 514–523. [DOI] [PubMed] [Google Scholar]
- 76. Cruz‐Jentoft AJ, Bahat G, Bauer J et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen LK, Woo J, Assantachai P et al. Asian Working Group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020; 21: 300–307.e2. [DOI] [PubMed] [Google Scholar]
- 78. Morley JE, Abbatecola AM, Argiles JM et al. Sarcopenia with limited mobility: an international consensus. J. Am. Med. Dir. Assoc. 2011; 12: 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen LK, Liu LK, Woo J et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014; 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 80. Studenski SA, Peters KW, Alley DE et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014; 69: 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bhasin S, Travison TG, Manini TM et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020; 68: 1410–1418. [DOI] [PubMed] [Google Scholar]
- 82. Francis P, Toomey C, Mc Cormack W, Lyons M, Jakeman P. Measurement of maximal isometric torque and muscle quality of the knee extensors and flexors in healthy 50‐ to 70‐year‐old women. Clin. Physiol. Funct. Imaging. 2017; 37: 448–455. [DOI] [PubMed] [Google Scholar]
- 83. Beaudart C, McCloskey E, Bruyère O et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016; 16: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Choudhary S, Wadhawan M, Dhawan S et al. Normative values of skeletal muscle indices for nutritional assessment and implications on definition of sarcopenia in Indian adult population. Indian J. Gastroenterol. 2022; 41: 69–76. [DOI] [PubMed] [Google Scholar]
- 85. Galindo Martín CA, Monares Zepeda E, Lescas Méndez OA. Bedside Ultrasound Measurement of Rectus Femoris: A Tutorial for the Nutrition Support Clinician. J. Nutr. Metab. 2017; 2017: 2767232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Abellan van Kan G, Rolland Y, Andrieu S et al. Gait speed at usual pace as a predictor of adverse outcomes in community‐dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging. 2009; 13: 881–889. [DOI] [PubMed] [Google Scholar]
- 87. Bryant RV, Ooi S, Schultz CG et al. Low muscle mass and sarcopenia: common and predictive of osteopenia in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015; 41: 895–906. [DOI] [PubMed] [Google Scholar]
- 88. Bryant RV, Schultz CG, Ooi S et al. Obesity in Inflammatory Bowel Disease: Gains in Adiposity despite High Prevalence of Myopenia and Osteopenia. Nutrients. 2018; 10: 1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pizzoferrato M, de Sire R, Ingravalle F et al. Characterization of sarcopenia in an IBD population attending an Italian Gastroenterology Tertiary Center. Nutrients. 2019; 11: 2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ünal NG, Oruç N, Tomey O, Ömer ÖA. Malnutrition and sarcopenia are prevalent among inflammatory bowel disease patients with clinical remission. Eur. J. Gastroenterol. Hepatol. 2021; 33: 1367–1375. [DOI] [PubMed] [Google Scholar]
- 91. Neelam PB, Pal R, Gupta P et al. Sarcopenia is common in ulcerative colitis and correlates with disease activity. Intest. Res. 2023; in press. 10.5217/ir.2023.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liu S, Ding X, Maggiore G et al. Sarcopenia is associated with poor clinical outcomes in patients with inflammatory bowel disease: a prospective cohort study. Ann. Transl. Med. 2022; 10: 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang T, Ding C, Xie T et al. Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy. Clin. Nutr. 2017; 36: 1586–1592. [DOI] [PubMed] [Google Scholar]
- 94. Bamba S, Sasaki M, Takaoka A et al. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn's disease. PLoS One. 2017; 12: e0180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhang T, Cao L, Cao T et al. Prevalence of sarcopenia and its impact on postoperative outcome in patients with Crohn's disease undergoing bowel resection. J. Parenter. Enteral Nutr. 2017; 41: 592–600. [DOI] [PubMed] [Google Scholar]
- 96. Bamba S, Inatomi O, Takahashi K et al. Assessment of body composition from CT images at the level of the third lumbar vertebra in inflammatory bowel disease. Inflamm. Bowel Dis. 2021; 27: 1435–1442. [DOI] [PubMed] [Google Scholar]
- 97. Kang MK, Kim KO, Kim MC, Park JG, Jang BI. Sarcopenia is a new risk factor of nonalcoholic fatty liver disease in patients with inflammatory bowel disease. Dig. Dis. 2020; 38: 507–514. [DOI] [PubMed] [Google Scholar]
- 98. Kurban M, Zeng N, Wang M et al. Role of human body composition analysis and malnutrition risk questionnaire in the assessment of nutritional status of patients with initially diagnosed Crohn's disease. Front. Med. 2020; 7: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lee CH, Yoon H, Oh DJ et al. The prevalence of sarcopenia and its effect on prognosis in patients with Crohn's disease. Intest. Res. 2020; 18: 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ge X, Jiang L, Yu W et al. The importance of sarcopenia as a prognostic predictor of the clinical course in acute severe ulcerative colitis patients. Dig. Liver Dis. 2021; 53: 965–971. [DOI] [PubMed] [Google Scholar]
- 101. Boparai G, Kedia S, Kandasamy D et al. Combination of sarcopenia and high visceral fat predict poor outcomes in patients with Crohn's disease. Eur. J. Clin. Nutr. 2021; 75: 1491–1498. [DOI] [PubMed] [Google Scholar]
- 102. Ge X, Xia J, Wu Y et al. Sarcopenia assessed by computed tomography is associated with colectomy in patients with acute severe ulcerative colitis. Eur. J. Clin. Nutr. 2022; 76: 410–418. [DOI] [PubMed] [Google Scholar]
- 103. Nam K, Lee JY, Ko Y et al. Impact of Sarcopenia on Clinical Course of Inflammatory Bowel Disease in Korea. Dig. Dis. Sci. 2023; 68: 2165–2179. [DOI] [PubMed] [Google Scholar]
- 104. Fatani H, Olaru A, Stevenson R et al. Systematic review of sarcopenia in inflammatory bowel disease. Clin. Nutr. 2023; 42: 1276–1291. [DOI] [PubMed] [Google Scholar]
- 105. Grillot J, D'Engremont C, Parmentier AL et al. Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn's disease. Clin. Nutr. 2020; 39: 3024–3030. [DOI] [PubMed] [Google Scholar]
- 106. Adams DW, Gurwara S, Silver HJ et al. Sarcopenia is common in overweight patients with inflammatory bowel disease and may predict need for surgery. Inflamm. Bowel Dis. 2017; 23: 1182–1186. [DOI] [PubMed] [Google Scholar]
- 107. Thiberge C, Charpentier C, Gillibert A et al. Lower subcutaneous or visceral adiposity assessed by abdominal computed tomography could predict adverse outcome in patients with Crohn's disease. J. Crohns Colitis. 2018; 12: 1429–1437. [DOI] [PubMed] [Google Scholar]
- 108. Tang W, Xie G, Li J et al. Body composition parameters correlate with the endoscopic severity in Crohn's disease patients treated with infliximab. Front. Nutr. 2023; 10: 1251448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lee JY, Kim KW, Ko Y et al. Serial changes in body composition and the association with disease activity during treatment in patients with Crohn's disease. Diagnostics (Basel). 2022; 12: 2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Holt DQ, Varma P, Strauss BJG, Rajadurai AS, Moore GT. Low muscle mass at initiation of anti‐TNF therapy for inflammatory bowel disease is associated with early treatment failure: a retrospective analysis. Eur. J. Clin. Nutr. 2017; 71: 773–777. [DOI] [PubMed] [Google Scholar]
- 111. Ando K, Uehara K, Sugiyama Y et al. Correlation among body composition parameters and long‐term outcomes in Crohn's disease after anti‐TNF therapy. Front. Nutr. 2022; 9: 765209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. O'Brien S, Kavanagh RG, Carey BW, Maher MM, O'Connor OJ, Andrews EJ. The impact of sarcopenia and myosteatosis on postoperative outcomes in patients with inflammatory bowel disease. Eur. Radiol. Exp. 2018; 2: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Spooren CEGM, Lodewick TM, Beelen EMJ et al. The reproducibility of skeletal muscle signal intensity on routine magnetic resonance imaging in Crohn's disease. J. Gastroenterol. Hepatol. 2020; 35: 1902–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang Y, Yao D, He Y, He Q, Li Y. Earlier anti‐TNF therapy reduces the risk of malnutrition associated with alterations in body composition in patients with Crohn's disease. Front. Nutr. 2023; 10: 1114758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Choi EJ, Baek DH, Lee HS, et al. The effect of biological agent on body composition in patients with Crohn's disease. BMC Gastroenterol. 2023; 23: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Campbell JP, Teigen L, Manski S et al. Sarcopenia is more prevalent among inflammatory bowel disease patients undergoing surgery and predicts progression to surgery among medically treated patients. Inflamm. Bowel Dis. 2022; 28: 1844–1850. [DOI] [PubMed] [Google Scholar]
- 117. Subramaniam K, Fallon K, Ruut T et al. Infliximab reverses inflammatory muscle wasting (sarcopenia) in Crohn's disease. Aliment. Pharmacol. Ther. 2015; 41: 419–428. [DOI] [PubMed] [Google Scholar]
- 118. Boros KK, Veres G, Cseprekál O et al. Body composition, physical activity, and quality of life in pediatric patients with inflammatory bowel disease on anti‐TNF therapy‐an observational follow‐up study. Eur. J. Clin. Nutr. 2023; 77: 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Forbes A, Escher J, Hébuterne X et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017; 36: 321–347. [DOI] [PubMed] [Google Scholar]
- 120. Seeger WA, Thieringer J, Esters P et al. Moderate endurance and muscle training is beneficial and safe in patients with quiescent or mildly active Crohn's disease. United Eur. Gastroenterol. J. 2020; 8: 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sigurdsson GV, Schmidt S, Mellström D et al. Physical exercise is associated with beneficial bone mineral density and body composition in young adults with childhood‐onset inflammatory bowel disease. Scand. J. Gastroenterol. 2021; 56: 699–707. [DOI] [PubMed] [Google Scholar]
- 122. Trivić I, Sila S, Batoš AT, Mišak Z, Kolaček S, Hojsak I. Moderate‐to‐vigorous physical activity is associated with higher bone mineral density in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2022; 74: 54–59. [DOI] [PubMed] [Google Scholar]
- 123. Jones K, Baker K, Speight RA, Thompson NP, Tew GA. Randomised clinical trial: combined impact and resistance training in adults with stable Crohn's disease. Aliment. Pharmacol. Ther. 2020; 52: 964–975. [DOI] [PubMed] [Google Scholar]


