Abstract
Background
Insufficient bone volume is a common problem encountered in the rehabilitation of the edentulous posterior maxillae with implant‐supported prostheses. Bone volume is limited by the presence of the maxillary sinus together with loss of alveolar bone height. Sinus lift procedures increase bone volume by augmenting the sinus cavity with autogenous bone or commercially available biomaterials, or both. This is an update of a Cochrane review first published in 2010.
Objectives
To assess the beneficial or harmful effects of bone augmentation compared to no augmentation when undertaking a sinus lift procedure. Secondly, to compare the benefits and harms of different maxillary sinus lift techniques for dental implant rehabilitation.
Search methods
We searched the Cochrane Oral Health Group's Trials Register (to 17 January 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 12), MEDLINE via OVID (1946 to 17 January 2014) and EMBASE via OVID (1980 to 17 January 2014). There were no language or date restrictions on the searches of the electronic databases.
Selection criteria
Randomised controlled trials (RCTs) of different techniques and materials for augmenting the maxillary sinus for rehabilitation with dental implants that report the outcome of implant success or failure at least to four months after initial loading.
Data collection and analysis
Screening of eligible studies, assessment of the risk of bias of the trials, and data extraction were conducted independently and in duplicate. Authors were contacted for any missing information. Results were expressed using fixed‐effect models as there were either less than four studies or we used Peto odds ratios (ORs) for dichotomous data when there were zero cells in either the treatment or control or both arms and the number of trials was small. The statistical unit of the analysis was the patient.
Main results
Eighteen RCTs out of 64 potentially eligible study reports met the inclusion criteria. They compared undertaking a sinus lift with not doing so, and the use of different sinus lift techniques. There were 650 patients providing data for the outcomes evaluated. Five studies were assessed as low risk of bias, 11 were assessed as high risk of bias, and in two the risk was unclear.
Sinus lift versus no sinus lift Four trials of moderate quality (three trials at low and one at high risk of bias) with 102 participants evaluated short implants (5 to 8.5 mm long) as an alternative to sinus lift in bone with residual height between 4 and 9 mm. One year after loading there was insufficient evidence to claim differences between the two procedures for prosthesis failure (OR (Peto) 0.37, 95% confidence interval (CI) 0.05 to 2.68; three trials) or implant failure (OR (Peto) 0.44, 95% CI 0.10 to 1.99; four trials). There was however an increase in complications at treated sites when undertaking the sinus lift (OR (Peto) 4.77, 95% CI 1.79 to 12.71, P value = 0.002; four trials).
Different sinus lift techniques Fourteen trials with 548 participants compared different sinus lift techniques. Only three comparisons included more than one trial (two trials for each). These were bone graft versus no bone graft, autogenous bone versus bone substitute, bone graft with or without platelet‐rich plasma (PRP). There was insufficient evidence to claim a benefit for any of these techniques for the primary outcomes of prosthesis and implant failure. For the other reported outcomes, in a single study at high risk of bias, only bone gain was greater for the bone graft site than the site without a graft six months after augmentation, however this was not significant at 18 or 30 months.
The other comparisons with single studies were rotary versus piezosurgery to open a lateral sinus window, two different bone substitutes, use or not of a membrane to seal the lateral window, one‐ versus two‐stage lateral sinus lift, two‐stage granular bone versus one‐stage autogenous bone blocks, and crestal versus lateral sinus lift; two trials compared three different crestal sinus lifting techniques: rotatory versus hand malleting (patients preferred rotatory instruments over hand malleting) and hand versus electric malleting. There was no evidence of a benefit for any sinus lift procedure compared to any other for the primary outcomes prosthesis or implant failure.
Authors' conclusions
There is moderate quality evidence which is insufficient to determine whether sinus lift procedures in bone with residual height between 4 and 9 mm are more or less successful than placing short implants (5 to 8.5 mm) in reducing prosthesis or implant failure up to one year after loading. However, there are more complications at sites treated with sinus lift procedures. Many trials compared different sinus lift procedures and none of these indicated that one procedure reduced prosthetic or implant failures when compared to the other. Based on low quality evidence, patients may prefer rotary instruments over hand malleting for crestal sinus lift.
Keywords: Humans; Alveolar Ridge Augmentation; Alveolar Ridge Augmentation/methods; Dental Implantation, Endosseous; Dental Implantation, Endosseous/methods; Dental Restoration Failure; Jaw, Edentulous, Partially; Jaw, Edentulous, Partially/rehabilitation; Maxillary Sinus; Maxillary Sinus/surgery; Randomized Controlled Trials as Topic; Sinus Floor Augmentation; Sinus Floor Augmentation/methods
Plain language summary
Interventions for replacing missing teeth: increasing bone thickness at the base of the natural sinus cavity above the upper jaw (maxillary sinus) to augment the maxillary sinus to enable implants
Review question
This review, carried out by the Cochrane Oral Health Group, seeks to determine whether and when it is necessary to increase the thickness of the bone layer at the base of the natural sinus cavity (maxillary sinus) that lies above the upper jaw in order to successfully insert dental implants onto which artificial teeth will be anchored. Also, to find the most effective techniques for doing this.
Background
Missing teeth may cause problems with eating and speaking, and affect how someone looks. Traditionally they have been replaced by loose false teeth (dentures) or bridges fixed between other teeth. Dental implants offer an alternative way of replacing teeth. Implants look like screws; they are made from materials such as titanium, which can fuse with the bone they are placed in (osseointegration) offering a stable base for artificial teeth to be fixed to. However, there needs to be enough depth of bone to successfully insert the implants. Bone thickness towards the back of the upper jaw can sometimes be too thin because of the natural sinus cavity (maxillary sinus) that lies above it. The cavity can also sometimes become larger following tooth loss.
Where the bone is too thin, there are a number of techniques that are used to create a thicker layer of bone at the base of the sinus cavity which are generally known as 'sinus lift' procedures. These methods involve using either bone taken from the patient (autogenous bone) or other materials known as biomaterials, a combination of the two, or sometimes simply using a blood clot as a base for the body to naturally form additional bone.
An alternative to a sinus lift is to use, where possible, short implants (4 to 8.5 mm long).
Study characteristics
The evidence on which this review is based is correct as of 17 January 2014. Eighteen trials with 650 participants were included. Four of the trials, with a total of 102 participants, compared implant‐supported prostheses using a sinus lift with prostheses on short implants (5 to 8.5 mm long) without sinus lift. The remaining 14 trials with a total of 548 participants compared different sinus lift techniques.
Key results
There is not enough evidence to show whether sinus lift techniques are more or less successful in reducing the number of failures of dental prostheses (artificial teeth) or dental implants when compared to simply using short implants, up to one year after loading.
However, there is limited evidence that there are fewer complications when short implants are used without surgical lifts. Complications include sinusitis, infection and bleeding, and when bone grafts are taken from the patient complications can also include nerve injury, problems with walking and infection.
Quality of the evidence
The quality of the evidence for whether or not to use a sinus lift procedure was moderate. The evidence for the 14 comparisons of different sinus lift procedures was based on a maximum of two comparisons for each comparison and was low.
Summary of findings
Summary of findings for the main comparison. Long implants with augmentation versus short implants without augmentation for replacing missing teeth.
| Long implants with augmentation versus short implants without augmentation for replacing missing teeth: augmentation procedures of the maxillary sinus | ||||||
| Patient or population: patients with replacing missing teeth Settings: general and specialist dental practice Intervention: sinus lift versus no sinus lift | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short implants | Long implants with sinus lift | |||||
| Prosthesis failures subjective assessment Follow‐up: median 1 year | Study population | OR 0.37 (0.05 to 2.68) | 109 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 55 per 1000 | 21 per 1000 (3 to 134) | |||||
| Moderate | ||||||
| 50 per 1000 | 19 per 1000 (3 to 124) | |||||
| Implant failures Follow‐up: median 1 year | Study population | OR 0.44 (0.1 to 1.99) | 137 (4 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 71 per 1000 | 33 per 1000 (8 to 133) | |||||
| Moderate | ||||||
| 50 per 1000 | 23 per 1000 (5 to 95) | |||||
| Complications Follow‐up: median 1 year | Study population | OR 4.77 (1.79 to 12.71) | 137 (4 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 43 per 1000 | 176 per 1000 (74 to 363) | |||||
| Moderate | ||||||
| 50 per 1000 | 201 per 1000 (86 to 401) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: Peto odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
1 Downgraded for imprecision (small number of events) 2 Downgraded for inconsistency (statistical heterogeneity present P value = 0.04, I2= 64%)
Summary of findings 2. Different sinus lift procedures.
| Comparison between different sinus lift procedures | |
|
Patient or population: patients with insufficient bone below maxillary sinus Settings: dental practice Intervention: sinus lift procedure Comparison: sinus lift procedure | |
| Outcomes | Comments |
|
Prosthesis failures (at 5 months to 5 years) |
Data for prosthetic failures were present for 5 comparisons (all only including 1 small study). There is insufficient evidence to conclude that 1 sinus lift procedure leads to fewer prosthetic failures than another. The comparisons for this outcome were: bone graft versus no bone graft, 2 different bone substitutes, 1‐ versus 2‐stage lateral sinus lift, 2‐stage granular bone versus 1‐stage autogenous bone blocks, and crestal versus lateral sinus lift |
|
Implant failures (at 5 months to 5 years) |
Data for implant failures were present for 8 comparisons (including 1 or 2 small studies). There is insufficient evidence to conclude that 1 sinus lift procedure leads to fewer implant failures than another. The comparisons for this outcome were: bone graft versus no bone graft, autogenous bone versus bone substitute, bone graft with or without platelet‐rich plasma (PRP), 2 different bone substitutes, 1‐ versus 2‐stage lateral sinus lift, 2‐stage granular bone versus 1‐stage autogenous bone blocks, crestal versus lateral sinus lift, and hand versus electric malleting for crestal sinus lifting |
Background
Description of the condition
Missing teeth may result in a functional and aesthetic deficit and have traditionally been replaced with dentures or fixed prostheses. Dental implants offer an alternative; they are inserted into the jawbones and used to support dental prostheses. Dental implants rely on the maintenance of a direct structural and functional connection between living bone and the implant surface. This is termed osseointegration and was first described by Brånemark (Brånemark 1977). Osseointegration has undoubtedly been one of the most significant scientific breakthroughs in dentistry over the past 50 years.
Insufficient bone volume is a common problem encountered in the rehabilitation of the edentulous posterior maxilla with implant‐supported prostheses. The bone available for implant placement may be limited by the presence of the maxillary sinus together with loss of alveolar bone height. Bone volume may be increased by augmentation. Commonly the sinus cavity is augmented with autogenous bone or biomaterials, or both. Procedures are variously described in the literature as sinus lift, sinus augmentation, sinus floor elevation or augmentation of atrophic maxillary sinus.
Implant placement may be combined with sinus augmentation as a 'one‐stage' technique. Alternatively sinus augmentation may be carried out some time prior to implant placement as a 'two‐stage' technique, which requires an additional surgical episode.
Description of the intervention
Techniques of sinus augmentation (sinus lift)
Boyne described the surgical technique of retrograde sinus augmentation, where in some cases blade implants were placed (Boyne 1980). The technique required a window to be prepared in the vestibular wall of the sinus, and the sinus epithelium was elevated to create a space into which particulate bone from the iliac crest was placed and allowed to heal for about six months or more before placing the implants.
Tatum described five tissue incisions (crestal, palatal, split thickness palatal, vertical and horizontal vestibular), three types of bone access (crestal, buccal wall and Le Forte I), and the use of autogenous bone, allograft and alloplast. In addition, Tatum described sinus augmentation and implant placement as a one‐stage and a two‐stage technique (Tatum 1986). The technique, known as a lateral window sinus lift, is widely used today and is considered reliable, particularly when autogenous bone is used (Wallace 2003; Del Fabbro 2004).
Summers described a less invasive one‐stage technique for sinus floor elevation with simultaneous implant placement, called the osteotome sinus floor elevation. Summers considered it necessary to have at least 6 mm of residual bone to ensure primary stability of the implant. Concave tipped osteotomes of increasing diameter applied via a crestal approach advanced a mass of bone beyond the level of the original sinus floor, elevating the sinus epithelium. Summers combined this procedure with the addition of a bone graft material (Summers 1994). For cases with less than 6 mm residual bone height, Summers proposed a two‐stage approach. A bone plug is defined with a trephine and displaced superiorly with the use of a broad osteotome. Hydrostatic pressure elevates the mucosal lining of the sinus. The resultant osteotomy is filled with a bone graft material and the implant placed after a period of healing (Summers 1995).
Cosci modified the crestal approach technique utilising an atraumatic lifting drill to reduce the risk of perforation of the mucosa lining of the sinus and using a one‐stage technique with as little as 3 mm of residual bone (Cosci 2000). Bone can be collected with a trephine directly from the osteotomy site, to be used as grafting material; a bone substitute can be used, or the implant tip can hold up the sinus membrane to work as a natural barrier for bone regeneration. While the crestal approach is less invasive, and is a one‐stage technique, there are some disadvantages associated with it. The amount of bone which can be gained using a crestal approach is usually less than that obtained with the lateral window technique, and a minimum of 3 mm crestal bone height is generally recommended to stabilize the implant at placement (Cosci 2000).
In order to obtain simultaneous vertical bone augmentation with a sinus lift procedure, Cannizzaro proposed a technique that is a combination of a sinus lift and an onlay graft. Implants are placed in the ulna and bone blocks containing the implants are retrieved with a trephine, inserted into the sinus via a crestal approach, and left protruding occlusally for some millimetres in order to obtain simultaneous vertical bone gain (Cannizzaro 2007).
Materials used in sinus lift procedures
Autogenous bone has long been considered the gold standard (Palmer 2000). Intra‐oral donor sites (chin and ramus) are convenient but yield limited volume. Extra‐oral donor sites (iliac crest, tibia, ulna, rib and calvarium) increase surgical complexity and are associated with significant (and under‐reported) morbidity and scarring. Therefore, alternative grafting materials (bone substitutes) have been developed.
Allografts consist of 'same species' tissue. Cadaveric bone is harvested and various techniques (freeze drying and irradiation) reduce antigenicity. The grafts are then sterilised and supplied by specially licensed tissue banks.
Xenografts consist of 'different species' tissue. Bovine, swine and equine bone predominate. Complete or partial thermo‐chemical removal of the organic component eventually creates a mineral scaffold with residual collagen, depending on the preparation procedures used (anorganic).
Alloplasts are synthetic bone substitutes. There are many types classified in terms of porosity as dense, macro‐porous, micro‐porous, and either crystalline or amorphous. The structure influences performance. Some examples are beta tri‐calcium phosphate, bio‐active glass, calcium sulphate, etc.
All these graft materials can be delivered in various convenient forms such as bone particles (eventually in streaky gels) or large blocks, can be mixed with autogenous bone, and can be very stable over time or are highly resorbable, depending on their physical characteristics.
Urist discovered that cell‐free, decalcified bone implanted into extra‐skeletal sites stimulated new bone formation (Urist 1965). The biologically active molecules that are responsible belong to the growth factor B family and are called bone morphogenetic proteins (BMPs) (Valentin‐Opran 2002). A number have been discovered and include growth factors, platelet‐rich plasma (PRP) and other molecules. Their use requires a delivery system that mimics the physical properties and release kinetics of bone.
Some authors have proposed sinus augmentation without the use of a graft material, with coagulated blood acting as a scaffold for bone formation. Lundgren proposed maintaining a space by suturing the sinus lining to the lateral wall (Lundgren 2004). The implant apex may be used to support the sinus membrane (Nedir 2006; Hatano 2007; Thor 2007; Sohn 2008; Gabbert 2009; Pjetursson 2009). Some bone regeneration does occur as a result of this procedure though the actual clinical benefit is in doubt since this method was not evaluated against appropriate control procedures.
Alternative techniques to sinus lift
There are some alternative techniques to sinus augmentation that may be possible. Onlay bone grafts may be used for horizontal or vertical augmentation. These procedures are evaluated in another Cochrane systematic review (Esposito 2009).
Implants can also be placed with an angulated direction in order to avoid the maxillary sinus (Aparicio 2001), or even placed trans‐sinus (Maló 2013). These implants are called 'tilted' or 'angulated' implants and they can only be used when anatomical conditions permit.
Zygomatic implants offer an alternative to sinus augmentation. Long implants pass through the sinus (Brånemark 2004) or laterally to the sinus into the zygomatic process and can also be loaded immediately (Davò 2013). Zygomatic implants are evaluated in another Cochrane review (Esposito 2013).
Another interesting and simple alternative to sinus lift procedures is the use of short implants (4 to 8 mm long). Current ongoing research is focused on evaluating short implants placed without augmentation, offering the opportunity of a less complex, cheaper and faster alternative to augmentation. There are few randomised controlled trials evaluating the efficacy of short implants both in upper and lower jaws (Cannizzaro 2009; Felice 2009a; Esposito 2011; Felice 2011; Esposito 2012; Felice 2012).
A review of longitudinal studies suggested a failure rate of approximately 10% for implants 7 mm long (das Neves 2006). However, the design of the studies on which this estimate is based suggests that this figure should be viewed with caution as it may represent a gross underestimation. Nevertheless, these figures suggest that shorter implants may have a poorer prognosis than longer ones. Since it is commonly believed that shorter implants (8 mm or less) have a poorer prognosis than longer implants, clinicians place longer implants if bone allows. When bone height is 4 to 8 mm, clinicians must decide whether to augment or place short implants. It is possible that improved implant surface modifications and designs, together with improved surgical techniques, may shift the balance in favour of short implants when the alternative is a more complex augmentation procedure. No reliable evidence of the superiority of currently available surface modifications or designs has been documented so far (Esposito 2007).
Why it is important to do this review
Insufficient bone volumes are a common problem encountered when replacing missing teeth in the maxilla with implant‐supported prostheses. Bone volumes are limited by the presence of the maxillary sinuses together with loss of alveolar bone height. If effective, sinus lift procedures will increase bone volume by augmenting the sinus cavity with autogenous bone or commercially available biomaterials, or both. This will allow patients who cannot be rehabilitated with conventional implants due to insufficient bone volumes to receive fixed implant‐supported prostheses, improving their quality of life. However, it is still unclear what the minimal bone heights are under which a sinus lift procedure will improve the prognosis of implant‐supported prostheses, and there is the risk that augmenting sinuses that do not require it would increase morbidity with no actual benefits for the patients. This is an update of a Cochrane review first published in 2010 (Esposito 2010) that originates from a previous larger review evaluating all types of augmentation procedures for dental implant placement (Coulthard 2003; Esposito 2006a; Esposito 2008).
Objectives
To assess the beneficial or harmful effects of bone augmentation compared to no augmentation when undertaking a sinus lift procedure. Secondly, to compare the benefits and harms of different maxillary sinus lift techniques for dental implant rehabilitation.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) including split‐mouth studies.
Types of participants
Patients with missing teeth and an atrophic posterior maxilla who may require augmentation of the maxillary sinus prior to or at placement of dental implants.
Types of interventions
Any bone augmentation technique, active agent (such as bone morphogenetic proteins, platelet‐rich plasma, stem cells) or biomaterials used together with osseointegrated, root‐formed dental implants. When comparing sinus lift procedures with no augmentation procedures, implants can have different dimensions (for instance be shorter and wider), can be placed in an angulated direction, and can be trans‐sinus. The use of zygomatic implants is evaluated in another Cochrane review (Esposito 2013).
For trials to be considered in this review, implants had to be placed and the success or failure of the implant therapy had to be reported at least at the endpoint of four months after initial loading of the implant‐supported prostheses. The following time points were considered: between four months to one year, three and five years after loading.
Types of outcome measures
Primary outcomes
Prosthesis failure: planned prosthesis that could not be placed due to implant failure(s), loss of the prosthesis secondary to implant failure(s), and any replacement of prosthesis.
Implant failure: implant mobility and removal of stable implants dictated by progressive marginal bone loss or infection (biological failures) and any mechanical complication such us implant fractures or platform deformations rendering the implant not usable (mechanical failure). Biological failures were grouped as early (failure to establish osseointegration) and late failures (failure to maintain the established osseointegration). Failures that occurred before prosthesis placement were considered early failures. Implant mobility could be assessed manually or with instruments such as the Periotest (Siemens AG, Benshein, Germany) or resonance frequency (Osstell, Integration Diagnostics, Göteborg, Sweden).
Secondary outcomes
Augmentation procedure failure: failure of the augmentation procedure, not affecting the success of the implant.
Complications at treated sites (e.g. sinusitis, infection, haemorrhage, etc.) including, when appropriate, complications at bone donor sites (e.g. nerve injury, gait disturbance, infection, etc.).
Patient satisfaction.
Patient preference (only in split‐mouth trials).
Bone gain expressed in millimetres or as a percentage.
Duration of the treatment time starting from the first intervention to the functional loading of the implants.
Treatment costs.
Trials evaluating only histological outcomes were not considered in this review.
Search methods for identification of studies
For the identification of studies to be included or considered for this review, detailed search strategies were developed for each database searched. These were based on the search strategy developed for MEDLINE (OVID) but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free text terms and was run with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2009 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Higgins 2011). Details of the MEDLINE search are provided in Appendix 1.
Searched databases
We searched the following electronic databases:
the Cochrane Oral Health Group's Trials Register (17 January 2014) (Appendix 2);
the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 12) (Appendix 3);
MEDLINE via OVID (1946 to 17 January 2014) (Appendix 1);
EMBASE via OVID (1980 to 17 January 2014) (Appendix 4).
We did not place any restrictions on language or date of publication when searching the electronic databases.
Unpublished studies
We wrote to all the authors of the identified RCTs, checked the bibliographies of all identified RCTs and relevant review articles, and used personal contacts in an attempt to identify unpublished or ongoing RCTs. In the first version of this review we also wrote to more than 55 oral implant manufacturers and we requested information on trials through an Internet discussion group (implantology@yahoogroups.com), however we discontinued these approaches due to poor yield.
Handsearching
Only handsearching done as part of the Cochrane Worldwide Handsearching Programme and uploaded to CENTRAL was included (see the Cochrane Masterlist for details of journal issues searched to date).
Data collection and analysis
Selection of studies
The titles and abstracts (when available) of all reports identified through the electronic searches were scanned independently by two review authors. For studies appearing to meet the inclusion criteria, or for which there were insufficient data in the title and abstract to make a clear decision, the full report was obtained. The full reports obtained from all the electronic and other methods of searching were assessed independently by two review authors to establish whether the studies met the inclusion criteria or not. Disagreements were resolved by discussion. Where resolution was not possible, a third review author was consulted. All studies meeting the inclusion criteria then underwent risk of bias assessment and data extraction. Studies rejected at this or subsequent stages were recorded in the 'Characteristics of excluded studies' table and the reasons for exclusion were recorded.
Data extraction and management
Data were extracted independently by two review authors using specially designed data extraction forms. The data extraction forms were piloted on several papers and modified as required before use. Any disagreement was discussed and a third review author or the Cochrane Oral Health Group consulted where necessary. All authors were contacted for clarification of details or missing information. Data were excluded until further clarification was available if agreement could not be reached.
For each trial the following data were recorded.
Year of publication, country of origin and source of study funding.
Details of the participants including demographic characteristics, source of recruitment and criteria for inclusion.
Details of the type of intervention.
Details of the outcomes reported, including method of assessment, and time intervals.
Assessment of risk of bias in included studies
An assessment of the risk of bias in the included studies was undertaken following the recommendations as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011). Two review authors independently and in duplicate assessed the risk of bias of all included studies. In the case that the paper to be assessed had one or more review authors in the authors list, it was independently evaluated only by those review authors not involved in the trial and by Philip Riley from the Cochrane Oral Health Group editorial base. Any disagreement was discussed and where necessary a third review author was consulted to achieve consensus. Authors were contacted directly for clarification.
A specific tool was adopted for assessing risk of bias in each included study. This comprised a description and a judgement for each entry in a risk of bias table, where each entry addressed a specific feature of the study:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding (of outcome assessor) (detection bias);
incomplete outcome data addressed (attrition bias);
free of selective reporting (reporting bias);
free of other bias.
The judgement for each entry involved an assessment of: low risk of bias, high risk of bias, or unclear indicating either lack of information or uncertainty over the potential for bias.
After taking into account the additional information provided by the authors of the trials, the overall risk of bias in included studies was assessed. Studies were grouped into the following categories. We assumed that the risk of bias was the same for all outcomes and each study was assessed as follows.
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
Further quality assessment was carried out to assess sample size calculations, definitions of exclusion and inclusion criteria, and comparability of control and test groups at entry.
Measures of treatment effect
For dichotomous outcomes, the estimate of effect of an intervention was expressed as odds ratios (ORs) together with 95% confidence intervals (CIs). For continuous outcomes, mean differences (MDs) and standard deviations were used to summarise the data for each group with 95% CIs. Appropriate data were extracted from the split‐mouth studies (Lesaffre 2009) and the generic inverse variance method was used to enter the data into Review Manager (RevMan).
Unit of analysis issues
In parallel group studies the statistical unit was the patient and not the augmentation procedure or the implants. In split‐mouth studies the augmentation procedures or the prostheses within each pair were the unit of analysis (Lesaffre 2009).
Dealing with missing data
All authors were contacted to retrieve missing data from trials. Methods for estimating missing standard deviations, in section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), were used.
Assessment of heterogeneity
The significance of any variations in the estimates of the treatment effects from the different trials was to be assessed by means of Cochran's test for heterogeneity, and heterogeneity would have been considered significant if P value < 0.1. The I2 statistic, which describes the percentage total variation across studies that is due to heterogeneity rather than chance, was used to quantify heterogeneity. with I2 over 50% being considered moderate to high heterogeneity.
Assessment of reporting biases
If there had been sufficient numbers of trials (more than 10) in any meta‐analysis, publication bias would have been assessed according to funnel plot asymmetry (Egger 1997) as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If asymmetry was identified we would have examined possible causes.
Data synthesis
Meta‐analysis was undertaken where studies of similar comparisons reported the same outcome measures. ORs were combined for dichotomous data, and MDs were to be combined for continuous data, using random‐effects models provided there were more than three studies in the meta‐analysis. When there were up to three studies in the meta‐analyses they were combined using fixed‐effect models. Peto ORs, from fixed‐effect models, were used when there were zero events in the control or treatment arms, or both. Data from split‐mouth studies were to be combined with data from parallel group trials by the method outlined by Elbourne (Elbourne 2002), using the generic inverse variance method in RevMan.
Subgroup analysis and investigation of heterogeneity
Clinical heterogeneity was to be assessed by examining the types of participants and interventions for all outcomes in each study. It was decided not to formulate any hypotheses to be investigated for subgroup analyses since no significant meta‐analysis was expected. However, this may be done in future updates of this review.
Sensitivity analysis
It was planned to undertake sensitivity analyses to examine the effect of the study overall risk of bias assessments on the overall estimates of effect by removing from the analyses studies at unclear and high risk of bias. In addition, the effect on the review's findings of including unpublished literature was also to be examined. There were too few trials to undertake these analyses.
Presentation of main results
We produced summary of findings tables for the main outcomes of this review using GRADEpro software. We assessed the quality of the body of evidence by considering the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates, the risk of publication bias and the magnitude of the effect. We categorised the quality of the body of evidence for each of the primary outcomes as high, moderate, low or very low.
Results
Description of studies
Results of the search
The search for this review was part of a wider search for all eligible trials for the series of Cochrane reviews on dental implants. This search is conducted every six months and has so far included about 8700 records.
Included studies
SeeCharacteristics of included studies table.
Eighteen trials were identified to be included in the review (Wannfors 2000; Hallman 2002; Raghoebar 2005; Cannizzaro 2009; Felice 2009a; Felice 2009b; Torres 2009; Checchi 2010; Felice 2011; Crespi 2012: Esposito 2012; Felice 2012; Lindgren 2012; Felice 2013; Merli 2013; Rickert 2013; Si 2013; Torres 2013).
Characteristics of the trial setting and investigators
Of the 18 included trials, 10 were conducted in Italy (Cannizzaro 2009; Felice 2009a; Felice 2009b; Checchi 2010; Felice 2011; Crespi 2012; Esposito 2012; Felice 2012; Felice 2013; Merli 2013), three in Sweden (Wannfors 2000; Hallman 2002; Lindgren 2012), two in the Netherlands (Raghoebar 2005; Rickert 2013), two in Spain (Torres 2009; Torres 2013), and one in China (Si 2013).
Seven trials had a parallel group study design (Wannfors 2000; Cannizzaro 2009; Felice 2011; Felice 2012; Felice 2013; Merli 2013; Si 2013), nine trials had a split‐mouth design (Hallman 2002; Raghoebar 2005; Felice 2009a; Felice 2009b; Checchi 2010; Crespi 2012; Esposito 2012; Lindgren 2012; Rickert 2013), one trial had a mixed split‐month and parallel group design (Torres 2009) but only data from its split‐mouth portion could be used in the present review, and another trial had two components: 106 patients were treated according to a parallel group study design and five according to a split‐mouth design (Torres 2013), but only data from its parallel group component could be used in the present review.
For 11 trials it was declared that support was received from industry directly involved in the product being tested, also in the form of free material (Hallman 2002; Raghoebar 2005; Felice 2009a; Felice 2009b; Checchi 2010; Felice 2011; Esposito 2012; Felice 2012; Lindgren 2012; Felice 2013; Si 2013). The authors of seven trials declared that no support was received from commercial parties whose products were being tested in the trials (Wannfors 2000; Cannizzaro 2009; Torres 2009; Crespi 2012; Merli 2013; Rickert 2013; Torres 2013).
Eight trials were conducted at university or specialist dental clinics (Wannfors 2000; Hallman 2002; Raghoebar 2005; Felice 2011; Crespi 2012; Lindgren 2012; Rickert 2013; Si 2013), six trials in private practices (Cannizzaro 2009; Felice 2009a; Torres 2009; Checchi 2010; Merli 2013; Torres 2013), and four multicentre trials both in private practices and hospitals (Felice 2009b; Esposito 2012; Felice 2012; Felice 2013).
Inclusion and exclusion criteria
For more details see the Characteristics of included studies table.
Main inclusion criteria
Severely resorbed maxillae (classes V‐VI according to Cawood 1991) with maxillary sinuses having less than 5 mm in height of residual alveolar bone with reduced stability and retention of upper dentures (Raghoebar 2005; Rickert 2013).
Less than 5 mm in residual alveolar bone height in the floor of the edentulous sinus (Hallman 2002; Lindgren 2012).
1 to 3 mm in residual alveolar bone height in the floor of the edentulous sinus (Felice 2013; Merli 2013).
1 to 5 mm in residual alveolar bone height in the floor of the edentulous sinus (Felice 2009b).
1 to 7 mm in residual alveolar bone height in the floor of the edentulous sinus (Torres 2009; Torres 2013).
2 to 7 mm in residual alveolar bone height in the floor of the edentulous sinus (Wannfors 2000).
2 to 8 mm in residual alveolar bone height in the floor of the edentulous sinus (Si 2013).
3 to 6 mm in residual alveolar bone height in the floor of the edentulous sinus (Cannizzaro 2009).
4 to 6 mm in residual alveolar bone height in the floor of the edentulous sinus (Felice 2009a; Felice 2012).
4 to 7 mm in residual alveolar bone height in the floor of the edentulous sinus (Checchi 2010).
5 to 7 mm in residual alveolar bone height in the floor of the edentulous sinus (Esposito 2012).
5 to 9 mm in residual alveolar bone height at implant sites of severely resorbed edentulous maxillas (Felice 2011).
Insufficient alveolar bone height in the floor of the edentulous sinus (baseline alveolar bone height: 6.71 mm + 1.55 for hand mallet and 6.54 mm + 1.67 for the electric mallet group) (Crespi 2012).
Main exclusion criteria
Bone metabolic diseases (Wannfors 2000; Merli 2013; Torres 2013).
Medications which could interfere with bone metabolism (i.e. corticosteroids, bisphosphonate, etc.) (Wannfors 2000; Cannizzaro 2009; Felice 2009a; Felice 2009b; Checchi 2010; Felice 2011; Esposito 2012; Felice 2012; Felice 2013; Torres 2013).
Sinusitis (Wannfors 2000; Cannizzaro 2009; Felice 2009a; Felice 2009b; Checchi 2010; Felice 2011; Crespi 2012; Esposito 2012; Felice 2012; Felice 2013; Si 2013).
History of maxillary sinusitis or sinus surgery (Torres 2009; Crespi 2012; Torres 2013).
History of reconstructive, pre‐prosthetic surgery or previous oral implantology (Raghoebar 2005; Rickert 2013; Si 2013).
Edentulous period less than one year (Raghoebar 2005; Rickert 2013).
Severe systemic disease (ASA III and IV) (Torres 2009; Torres 2013).
Smoking (Crespi 2012).
Smoking more than 10 cigarettes per day (Lindgren 2012).
Smoking more than 20 cigarettes per day (Merli 2013).
None specified (Hallman 2002).
Sample size
An a priori calculation for the sample size was reported in eight trials (Cannizzaro 2009; Felice 2009a; Checchi 2010; Felice 2011; Esposito 2012; Merli 2013; Si 2013; Torres 2013); however in four trials (Cannizzaro 2009; Felice 2011; Si 2013; Torres 2013) the number of included patients did not reach the calculated sample size and in one trial it was based on inappropriate numbers (Merli 2013).
Baseline comparability between treatment groups
No apparent major baseline differences (Wannfors 2000; Raghoebar 2005; Felice 2009b; Torres 2009; Checchi 2010; Esposito 2012; Felice 2012; Lindgren 2012; Merli 2013; Si 2013).
Unclear whether major baseline differences existed (Hallman 2002; Crespi 2012; Rickert 2013; Torres 2013).
The following major baseline differences existed:
more large diameter implants were placed in the sites treated with 8 mm long implants and crestal sinus lift (Cannizzaro 2009);
short 6 mm diameter implants were compared to longer implants with a 4 mm diameter (Felice 2009a);
more females and more implants were placed in the augmented group (Felice 2011), however the latter difference was obvious since the resorbed maxillas were grafted and therefore there was more available bone to place more and longer implants;
more implants placed in the one‐stage group (55 versus 47); more prostheses in the two‐stage group connected to implants placed in non‐augmented bone (12 versus 6); more 3.8 mm small diameter implants used in the one‐stage group (33 versus 20) (Felice 2013).
Characteristics of the interventions
The following comparisons were made.
1. Long implants in augmented sinuses versus short implants without augmentation (four trials with 102 participants)
Four trials evaluating sinus lift versus short implants (Felice 2009a; Felice 2011; Esposito 2012; Felice 2012).
2. Comparing different sinus lift procedures (14 trials with 548 participants)
One trial compared the use of rotary instruments or piezosurgery for opening a lateral window into the sinus (Rickert 2013).
Two trials compared sinus lift with and without bone grafting (Felice 2009b; Si 2013).
Two trials compared sinus list with autogenous bone versus bone substitutes (Hallman 2002; Merli 2013).
One trial compared different bone substitutes (Lindgren 2012).
Two trials evaluated the additional use of platelet‐rich plasma (PRP) to bone grafts (Raghoebar 2005; Torres 2009).
One trial evaluated the use of a resorbable barrier to seal the lateral window (Torres 2013).
One trial compared one‐stage versus two‐stage augmentation procedures (Felice 2013).
One trial compared one‐stage autogenous bone block versus two‐stage lateral window sinus lift (Wannfors 2000).
One trial compared lateral versus crestal sinus lift (Cannizzaro 2009).
Two trials compared different crestal sinus lift procedures (Checchi 2010; Crespi 2012).
Characteristics of outcome measures
Prosthesis failure: (Wannfors 2000; Hallman 2002; Raghoebar 2005; Cannizzaro 2009; Felice 2009a; Felice 2009b; Torres 2009; Checchi 2010; Felice 2011; Crespi 2012; Esposito 2012; Felice 2012; Lindgren 2012; Felice 2013; Merli 2013; Rickert 2013; Si 2013, Torres 2013).
Implant failure by individual implant stability assessment with removed prostheses (with the exception of single implants): (Wannfors 2000; Hallman 2002; Raghoebar 2005; Cannizzaro 2009; Felice 2009a; Felice 2009b; Torres 2009; Checchi 2010; Felice 2011; Crespi 2012; Esposito 2012; Felice 2012; Lindgren 2012; Felice 2013; Merli 2013; Rickert 2013; Si 2013, Torres 2013).
Augmentation procedure failure: (Wannfors 2000; Hallman 2002; Raghoebar 2005; Cannizzaro 2009; Felice 2009a; Felice 2009b; Torres 2009; Checchi 2010; Felice 2011; Crespi 2012; Esposito 2012; Felice 2012; Lindgren 2012; Felice 2013; Merli 2013; Rickert 2013; Si 2013; Torres 2013). In one trial (Si 2013), in the case of maxillary sinus membrane perforation patients were excluded and we classified these patients as failures of the augmentation procedures and for the rest of the rehabilitation.
Complications: (Hallman 2002; Raghoebar 2005; Cannizzaro 2009; Felice 2009a; Felice 2009b; Torres 2009; Checchi 2010; Felice 2011; Crespi 2012; Esposito 2012; Felice 2012; Felice 2013; Merli 2013; Rickert 2013). Two trials reported only selected complications: perforation of the sinus membrane (Wannfors 2000; Torres 2013).
Patient satisfaction: (Felice 2011).
Patient preference (only in split‐mouth trials): (Felice 2009a; Felice 2009b; Checchi 2010; Esposito 2012; Felice 2012). Data for one trial (Felice 2009a) were reported, however they might have been biased because of the study design. All augmentation procedures were performed first and, after four months, test and control implants were placed bilaterally in the same surgical session. The potential advantage of having the prostheses on the short implants loaded four months earlier was lost with this study design.
Bone gain expressed in millimetres or as a percentage: (Felice 2009b; Crespi 2012; Si 2013).
Duration of the treatment period starting from the first intervention to the functional loading of the implants: all trials.
Treatment costs: no trials. However, this outcome measure was indirectly extrapolated by us for all trials.
Duration of follow‐up (including unpublished data kindly provided by the investigators)
Five‐month post‐loading (Felice 2011).
Six‐month post‐loading (Merli 2013).
One‐year post‐loading (Hallman 2002; Felice 2009a; Felice 2009b; Esposito 2012; Felice 2012; Felice 2013; Rickert 2013; Torres 2013).
Nineteen‐month post‐loading (Crespi 2012).
Two‐year post‐loading (Raghoebar 2005; Torres 2009).
Two‐year and half post‐loading (Si 2013).
Three‐year post‐loading (Wannfors 2000; Checchi 2010; Lindgren 2012).
Five‐year post‐loading (Cannizzaro 2009).
Excluded studies
Forty‐five studies had to be excluded for various reasons such as: they reported only histological outcomes without presenting any implant‐related outcomes (Barone 2005; Kassolis 2005; Steigmann 2005; Froum 2006; Suba 2006; Consolo 2007; Aimetti 2008; Cordaro 2008; Froum 2008; Hallman 2008; Canullo 2009; Choi 2009; Crespi 2009; Kim 2009; Pikdöken 2011; Kühl 2012; Barone 2013; Corinaldesi 2013; Froum 2013; Kühl 2013; Payer 2013; Testori 2013; Yilmaz 2013); too short follow‐up (Szabó 2005; Barone 2008; Schaaf 2008; Bettega 2009; Badr 2010; Kock 2010; Bensaha 2011; Borges 2011; Sammartino 2011; Hermund 2012; Kühl 2012; Trombelli 2012; Froum 2013; Gassling 2013; Khairy 2013; Silvestri 2013); problems with study design and data reporting (Froum 1998; Tawil 2001; Boyne 2005; Triplett 2009; Wagner 2012); data presented for only four out of 16 treated patients (Aimetti 2008); not an RCT (Mangano 2007; Ghanaati 2014).
Risk of bias in included studies
Allocation
Random sequence generation
We assessed 14 studies as at low risk of bias for this domain, with four being assessed as unclear.
Allocation concealment
When assessing the information presented in the articles, allocation concealment was scored as adequate for 13 trials (Cannizzaro 2009; Felice 2009a; Felice 2009b; Torres 2009; Checchi 2010; Felice 2011; Esposito 2012; Felice 2012; Lindgren 2012; Felice 2013; Merli 2013; Si 2013; Torres 2013) and unclear for five trials (Wannfors 2000; Hallman 2002; Raghoebar 2005; Crespi 2012; Rickert 2013). When evaluating authors' replies one trial was judged to be properly concealed (Hallman 2002) and four trials remained unclear (Wannfors 2000; Raghoebar 2005; Crespi 2012; Rickert 2013). In Lindgren 2012, although the randomisation was unclear the envelope was opened after the sinus membrane was elevated.
Therefore, the overall risk of selection bias was low in 14 studies and unclear in four studies.
Blinding
Blinding was not feasible in all of the included studies. Based on the evaluation of the trial reports and responses from authors, outcome assessment was scored as blinded for 14 trials (Raghoebar 2005; Cannizzaro 2009; Felice 2009a; Felice 2009b; Torres 2009; Checchi 2010; Felice 2011; Crespi 2012; Esposito 2012; Felice 2012; Felice 2013; Rickert 2013; Si 2013; Torres 2013); not blinded for four trials (Wannfors 2000; Hallman 2002; Lindgren 2012; Merli 2013) although an independent assessor was used (Merli 2013). When reading the original articles blinding was unclear for five trials (Wannfors 2000; Hallman 2002; Torres 2009; Crespi 2012; Lindgren 2012) and one trial was not blinded (Merli 2013). When evaluating authors' replies, the outcome assessors of two trials were judged to be blinded (Torres 2009; Crespi 2012) and not blinded for three trials (Wannfors 2000; Hallman 2002; Lindgren 2012).
Incomplete outcome data
When assessing the information presented in the articles, information on drop‐outs was clearly presented in all trials with four exceptions (Torres 2009; Crespi 2012; Rickert 2013; Torres 2013) where the authors supplied the missing information. In another trial, in the case of maxillary sinus membrane perforation patients were excluded (Si 2013).
Selective reporting
Six trials did not present or only appeared to present full data on complications (Wannfors 2000; Crespi 2012; Lindgren 2012; Rickert 2013; Si 2013; Torres 2013), but the authors of three trials provided the missing information (Crespi 2012; Lindgren 2012; Rickert 2013); the remaining three trials were judged to be at high risk of bias since they did not answer to our request for information. Data for some of the outcome measures were not presented in one trial (Crespi 2012) resulting in it also being judged at high risk of bias.
Other potential sources of bias
Other potential sources of bias that were detected were: differences in implant diameters between groups (Cannizzaro 2009; Felice 2009a); and additional buccal onlays performed making more difficult result interpretation since the role of the additional buccal onlays on the final outcome cannot be quantified (Raghoebar 2005; Rickert 2013). These were assessed as at unclear risk of bias unless they were included in the following list. The following four trials were considered at high risk because: they used a mixed split‐mouth and parallel group design (Torres 2009); patients always had augmentation procedure performed first and then had implant placement bilaterally, since this may have affected patient preference (Felice 2009a); eight of 15 patients were treated by the inventor of one of the techniques under evaluation (Checchi 2010); it was a split‐mouth study not taking pairing into account (Rickert 2013).
Overall risk of bias
Looking at the summary risk of bias for each trial in Figure 1, five trials were judged to be at low risk of bias (Felice 2009b; Felice 2011; Esposito 2012; Felice 2012; Felice 2013), 11 trials were judged at high risk of bias (Wannfors 2000; Hallman 2002; Felice 2009a; Torres 2009; Checchi 2010; Crespi 2012; Lindgren 2012; Merli 2013; Rickert 2013; Si 2013; Torres 2013), and two trials at unclear risk of bias (Raghoebar 2005; Cannizzaro 2009).
1.
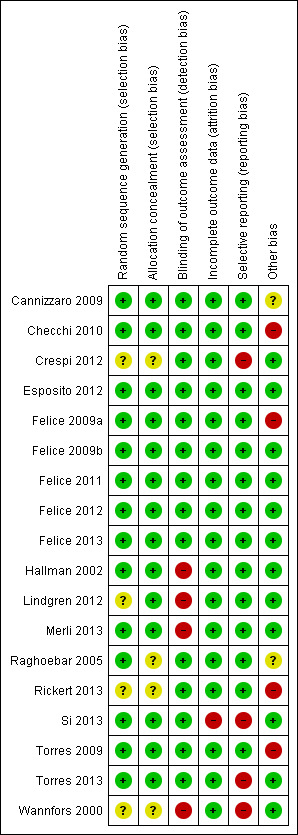
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
1. Long implants in augmented sinuses versus short implants without augmentation (four trials with 102 participants)
SeeTable 1.
Four trials (Felice 2009a; Felice 2011; Esposito 2012; Felice 2012) provided data for this comparison where short implants without augmentation were compared with long implants with augmentation. Three of these trials were assessed as at low risk of bias (Felice 2011; Esposito 2012; Felice 2012) and one at high risk of bias (Felice 2009a). Although two of these were split‐mouth studies, the prosthesis and implant failures, and complications were in different patients and so the data have been entered as though they were in parallel group studies for ease of analysis and interpretation.
Prosthesis, implant and augmentation failures at one year
The meta‐analyses for prosthesis failures and implant failures are shown in Analysis 1.1 and Analysis 1.2. There was no evidence of a difference in prosthesis or implant failures for the long implants with augmentation compared with the short implants, however the confidence intervals were wide. The odds ratio (OR) (Peto) for prosthesis failures was 0.37 (95% confidence interval (CI) 0.05 to 2.68, P value = 0.33) with no evidence of heterogeneity. The OR (Peto) for implant failures was 0.44 (95% CI 0.10 to 1.99, P value = 0.29) with no evidence of heterogeneity. One bilateral augmentation failure occurred in one trial only (Felice 2011).
1.1. Analysis.
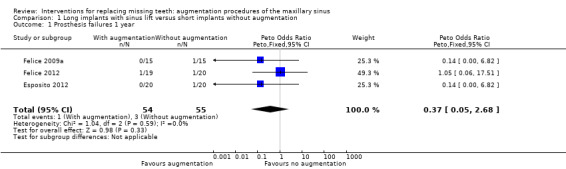
Comparison 1 Long implants with sinus lift versus short implants without augmentation, Outcome 1 Prosthesis failures 1 year.
1.2. Analysis.

Comparison 1 Long implants with sinus lift versus short implants without augmentation, Outcome 2 Implant failures 1 year.
Complications at one year
The meta‐analysis for complications at treated sites is shown in Analysis 1.3. There was some evidence of more complications with the sinus lift, with an OR (Peto) of 4.77 (95% CI 1.79 to 12.71, P value = 0.002), however there was heterogeneity between the studies (Chi2 P value = 0.04, I2 = 64%).
1.3. Analysis.
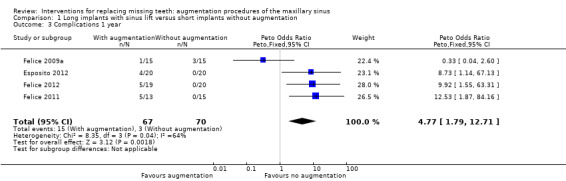
Comparison 1 Long implants with sinus lift versus short implants without augmentation, Outcome 3 Complications 1 year.
Patient preference
Patient preference could only be ascertained in two split‐mouth studies. In Felice 2009a all patients expressed no preference for any of the two procedures at four months post‐loading, judging both of them as acceptable, however this measurement was considered to be biased as previously described in the 'Characteristics of outcome measures'. In Esposito 2012, five months after loading 15 patients preferred short implants whereas five patients described both procedures as equally acceptable.
Costs and treatment time
There were additional costs associated with the long implants with augmentation group: in Felice 2009a this group required one additional surgical intervention for placing the implants (two‐stage procedure) plus there was the cost of the bone substitute with the barrier and four additional months to complete the treatment; in Esposito 2012 and Felice 2012 there was the additional cost of the bone substitute with the barrier; and in Felice 2011 there were the additional costs of three days of hospitalisation, the barrier, and four additional months required to rehabilitate the patients. Also more implants were placed in the long implant group with augmentation in one trial (Felice 2011).
2. Comparing different sinus lift procedures (14 trials with 548 participants)
These comparisons frequently involved only one trial, and the results for all outcomes are presented in Additional Table 3. Only results for more than one trial were shown in forest plots.
1. Comparison of different sinus lift procedures.
| Comparison | Outcome | Data | Effect estimate (95% CI) P value |
| Rotary instruments versus piezosurgery (Rickert 2013) Split‐mouth |
Prosthesis failures | N = 36; none | N/A |
| Implant failures | N = 36; none | N/A | |
| Augmentation procedure failure | N = 36; none | N/A | |
| Complications at augmented site | N = 36; both = 2, rotary only = 6, piezosurgery only = 6, neither = 22 | OR 1.00 (0.27 to 3.74) P = 1.00 | |
| Complications at donor site | N = 36; none | N/A | |
| With versus without bone graft (Felice 2009b) Split‐mouth |
Prosthesis failures | N = 9; none | N/A |
| Implant failures | N = 9; 1 failure for without | OR 3.35 (0.12 to 93.8) P = 0.48 | |
| Complications (1 year) | N =9; both = 1, without = 2, with = 1, none = 5 | OR 0.50 (0.01 to 9.60) P = 1.00 | |
| Bone gain at 6 months | N = 9 | MD 0.26 (‐0.91 to 1.43) P = 0.65 | |
| With versus without bone graft (Si 2013) Parallel group |
Prosthesis failures (1 year) | With bone versus without bone 2/22 versus 3/22 |
OR 0.63 (0.10 to 4.22) P = 0.64 |
| Implant failures (1 year) | 2/22 versus 3/22 | OR 0.63 (0.10 to 4.22) P = 0.64 | |
| Augmentation procedure failure (1 year) | 1/22 versus 2/22 | OR 0.48 (0.04 to 5.67) P = 0.56 | |
| Complications (1 year) | Not reported | N/A | |
| Bone gain at 6 months | Graft N = 21 5.66 (SD 0.99) versus no graft N = 20 2.06 (1.01) | MD 3.60 (2.99 to 4.21) P < 0.001 | |
| Bone gain at 18 months | Graft N = 20 3.02 (SD 0.48) versus no graft N = 19 3.12 (0.70) | MD ‐0.10 (‐0.47 to 0.27) P = 0.60 | |
| Bone gain at 30 months | Graft N = 20 3.17 (SD 1.95) versus no graft N = 19 3.07 (1.68) | MD 0.10 (‐1.04 to 1.24) P = 0.86 | |
| Autogenous bone versus bone substitute (Hallman 2002) Split‐mouth |
Implant failures (abutment connection) | N = 11; 5/11 autogenous bone versus 2/11 80% Bio‐Oss. Assume not bilateral | OR 3.75 (0.54 to 26.04) P = 0.18 |
| Complications | N = 11; none | N/A | |
| Autogenous bone versus bone substitute (Merli 2013) Parallel group |
Implant failures | 2/20 versus 0/20 | OR 5.54 (0.25 to 123.08) P = 0.28 |
| Complications | 2/20 versus 1/20 | OR 2.11 (0.18 to 25.35) P = 0.56 | |
| Autogenous bone versus bone substitute (Lindgren 2012) Split‐mouth |
Prosthesis failures | 0/11 versus 0/11 | N/A |
| Implant failures | 1/11 versus 1/11 | OR 1.00 (0.05 to 18.30) P = 1.00 | |
| Augmentation procedure failure | 0/11 versus 0/11 | N/A | |
| Complications at augmented site | N = 11; both = 0, Bio‐Oss only = 1, different bone substitute only = 0, neither = 10 | OR 3.29 (0.12 to 89.8) P= 0.48 | |
| Autogenous bone ± PRP (Raghoebar 2005) Split‐mouth |
Prosthetic failures | N = 5; none | N/A |
| Implant failures | N = 5; 1 failure in PRP | OR 3.67 (0.12 to 113.73) P = 0.46 | |
| Complications | N =5; 1 occurred in non‐PRP | OR 0.27 (0.01 to 8.46) P = 0.46 | |
| Autogenous bone or Bio‐Oss ± PRP (Torres 2009) Split‐mouth |
Implant failures | N = 57; both = 0, PRP only = 1, no PRP only = 2, neither = 54 | OR 0.50 (0.01 to 17.42) P = 0.71 |
| Complications | N = 57; both = 0, PRP only = 3, no PRP only = 2, neither = 52 | OR 1.49 (0.15 to 15.07) P = 1.00 | |
| Partial graft loss |
N = 57; both = 0, PRP only = 2, no PRP only = 3, neither = 52 | OR 1.5 (0.17 to 17.96) P = 1.00 (Stata exact OR) |
|
| Membrane versus no membrane to seal the lateral window (Torres 2013) Parallel group |
Prosthetic failures | 9/51 versus 4/53 | OR 2.63 (0.75 to 9.14) P = 0.13 |
| Implant failures | 9/51 versus 4/53 | OR 2.63 (0.75 to 9.14) P = 0.13 | |
| 1‐stage versus 2‐stage (Felice 2013) Parallel group |
Prosthesis failures | 1‐stage versus 2‐stage 0/28 versus 1/30 |
0.35 (0.01 to 6.83) P = 0.52 |
| Implant failures (before loading) | 3/28 versus 1/30 |
OR 3.48 (0.34 to 35.61) P = 0.29 | |
| Complications | 2/28 versus 1/30 | OR 2.23 (0.19 to 26.06) P = 0.52 | |
| 1‐stage block versus 2‐stage particulate bone (Wannfors 2000) Parallel group |
Prosthetic failures | 1/20 versus 1/20 | OR 1.00 (0.06 to 17.18) P = 1.00 |
| Implant failures | 8/20 versus 6/20 | OR 1.56 (0.42 to 5.76) P = 0.51 | |
| Complications | 9/20 versus 10/20 | OR 0.82 (0.24 to 2.84) P = 0.75 | |
| Crestal versus lateral sinus lift (Cannizzaro 2009) Parallel group |
Prosthetic failures | 1/20 versus 2/20 | OR 0.47 (0.04 to 5.69) P = 0.56 |
| Implant failures | 1/20 versus 3/20 | OR 0.30 (0.03 to 3.15) P = 0.31 | |
| Graft failures | 0/20 versus 2/20 | OR 0.18 (0.01 to 4.01) P = 0.28 | |
| Complications at treated and donor sites (1 year) | 1/20 versus 4/20 | OR 0.21 (0.02 to 2.08) P = 0.18 | |
| Partial graft loss |
2/20 versus 3/20 | OR 0.63 (0.09 to 4.24) P = 0.63 | |
| Mallet versus rotary (Checchi 2010) Split‐mouth |
Prosthetic failures | N = 12; none | N/A |
| Implant failures | N = 12; none | N/A | |
| Complications | N = 12; 5/12 versus 1/12 | OR 7.86 (0.75 to 82.13) P = 0.09 | |
| Preference 5 months after loading | N = 15; 13 preferred rotary technique | Binomial test P = 0.007 | |
| Hand mallet versus electric mallet (Crespi 2012) Parallel group Followed for 19 months after loading |
Prosthesis failures | Hand mallet versus electric mallet 0/40 versus 0/40 |
N/A |
| Implant failures (before loading) | 1/40 versus 1/40 |
OR 1.00 (0.06 to 16.56) P = 1.00 | |
| Augmentation failures | 0/40 versus 0/40 | N/A | |
| Complications at augmented site | 3/40 versus 0/40 | OR 3.16 (0.31 to 31.78) P = 0.33 | |
| Bone gain | N = 40 4.17 (SD 1.70) versus N = 40 4.07 (SD 1.03) | MD 0.10 (‐0.52 to 0.72) P = 0.75 |
Data on patients who dropped out removed from the table. CI ‐ confidence interval; MD ‐ mean difference; OR ‐ odds ratio; SD ‐ standard deviation.
Rotary instruments versus piezosurgery to open a lateral window in the maxillary sinus (one trial with 36 participants)
One trial at high risk of bias (Rickert 2013) with 36 patients undertook this comparison, at one year after loading. The following outcomes were reported and the data are given in Additional Table 3: prosthesis failure, implant failure, augmentation procedure failure, complications at augmented site and complications at donor site. None of these were significant.
Sinus lift with and without bone graft (two trials with 55 participants)
Two trials, one at low and one at high risk of bias (Felice 2009b; Si 2013), compared granular anorganic bovine bone (Bio‐Oss) with a resorbable rigid barrier (Inion) (Felice 2009b) or no bone (Si 2013) at one year. The following outcomes were reported and the data are given in Additional Table 3: prosthesis failure, implant failure, complications, augmentation procedure failure (one year), and bone gain (6, 18, 30 months after augmentation). Both studies provided data for bone gain at six months. This was significant for Si 2013 (P value < 0.001), with graft sites gaining more bone, but not for Felice 2009b. This observed gain in bone in Si 2013 was not apparent at 18 or 30 months.
The meta‐analysis of these two trials (Felice 2009b; Si 2013) for implant failures at one year is given in Analysis 2.1. There was no evidence to suggest that more implant failures occurred in sinus lift with or without a bone graft (OR 0.52, 95% CI 0.10 to 2.82).
2.1. Analysis.

Comparison 2 Different sinus lift procedures, Outcome 1 Bone versus no bone graft.
There was heterogeneity between the studies so it was probably not advisable to pool the data for bone gain at six months (Analysis 2.2).
2.2. Analysis.
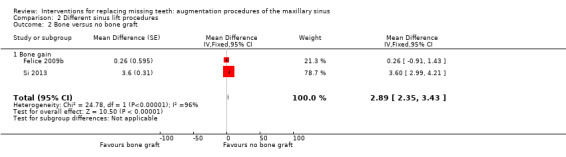
Comparison 2 Different sinus lift procedures, Outcome 2 Bone versus no bone graft.
Autogenous bone versus bone substitutes (two trials with 51 participants)
Two trials that were both at high risk of bias (Hallman 2002; Merli 2013) provided data for this comparison. One trial (Hallman 2002) compared autogenous bone versus 80% anorganic bovine bone (Bio‐Oss) and 20% autogenous bone in 11 patients. The other trial (Merli 2013) compared autogenous bone harvested from the mandibular ramus versus anorganic bovine bone in 40 patients. Both trials reported implant failures and complications (none for Hallman 2002), and the data are shown in Additional Table 3 and a forest plot (Analysis 2.3). No statistically significant difference was found for implant failures (OR 4.20, 95% CI 0.81 to 21.79). For both trials the additional cost was that of the bone substitute.
2.3. Analysis.
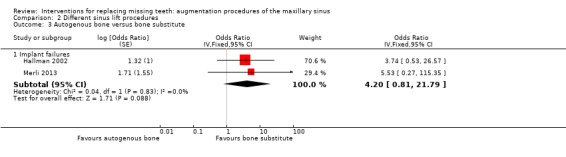
Comparison 2 Different sinus lift procedures, Outcome 3 Autogenous bone versus bone substitute.
Comparing different bone substitutes (one trial with 11 participants)
One trial at high risk of bias (Lindgren 2012) and with 11 patients undertook this comparison at one year after loading. The following outcomes were reported and the data are given in Additional Table 3: prosthesis failure, implant failure, augmentation procedure failure, and complications at augmented site. None of these were significant.
Grafts with or without platelet‐rich plasma (PRP) (two trials with 62 participants)
Two split‐mouth trials provided data for this comparison. One trial (Raghoebar 2005) with five patients was at unclear risk of bias and compared a two‐stage sinus lift with autogenous bone together with buccal onlay grafts that were harvested from the iliac crest, one side with PRP and the other without. The other trial (Torres 2009) with 57 patients was at high risk of bias and compared one‐ or two‐stage sinus lift procedures using a lateral window technique and 100% granular Bio‐Oss with or without PRP. Both trials provided useable data on implant failures and complications and forest plots are shown (Analysis 2.4). There were no statistically significant differences between groups who received PRP and those who did not for implant failures and complications. For both trials the difference in cost and treatment time was due to the use of PRP.
2.4. Analysis.
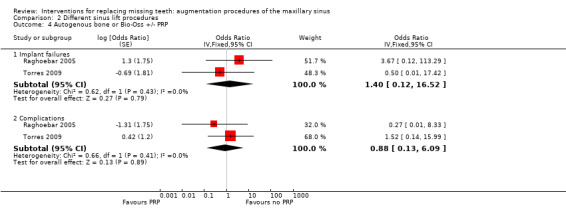
Comparison 2 Different sinus lift procedures, Outcome 4 Autogenous bone or Bio‐Oss +/‐ PRP.
Use or not of a resorbable barrier to seal the lateral window (one trial with 106 participants)
One trial at high risk of bias (Torres 2013) and with 106 patients undertook this comparison at one year after loading. The following outcomes were reported and the data are given in Additional Table 3: prosthesis failure and implant failure. None of these were significant.
One‐stage versus two‐stage augmentation procedures (one trial with 60 participants)
One trial at low risk of bias (Felice 2013) and with 60 patients undertook this comparison at one year after loading. The following outcomes were reported and the data are given in Additional Table 3: prosthesis failure, implant failure, and complications. None of these were significant.
One‐stage blocks versus two‐stage sinus lift with autogenous granular bone (one trial with 40 participants)
One trial at high risk of bias (Wannfors 2000) and with 40 patients undertook this comparison at three years after loading. The following outcomes were reported and the data are given in Additional Table 3: prosthesis failure, implant failure, and complications. None of these were significant.
Comparing lateral versus crestal sinus lift (one trial with 40 participants)
One trial at unclear risk of bias (Cannizzaro 2009) and with 40 patients undertook this comparison at five years after loading. The following outcomes were reported and the data are given in Additional Table 3: prosthesis failure, implant failure, graft failure, complications, and partial graft loss. There were no statistically significant differences. There was an additional cost of the bone substitute in the group with the lateral approach but treatment duration was the same.
Comparing different crestal sinus lift procedures (two trials with 55 participants)
(a) Osteotomes with mallet versus sites prepared with rotary instruments
One trial at high risk of bias compared sinus lifting procedures performed bilaterally and in the same surgical session: the Summers' technique (using osteotomes and a mallet) and the Cosci's technique (sites prepared only with rotary instruments), in 15 patients (Checchi 2010). The following outcomes were reported and the data are given in Additional Table 3: prosthetic failure, implant failure, and complications at three years after loading. There were no failures in either group, and no statistically significant differences for these outcomes. Five months after loading (one year after surgery) 13 out of 15 patients preferred the side treated with the Cosci technique (P value < 0.007). Treatment duration and costs were the same.
(b) One‐stage crestal sinus lifting procedures: osteotomes with a hand mallet versus an electric mallet without placing any bone grafts
One trial at high risk of bias (Crespi 2012) and with 40 patients per group undertook this comparison at three years. The following outcomes were reported and the data are given in Additional Table 3: prosthesis failure, implant failure, augmentation failure, complications at augmented site, and bone gain. None of these were statistically significant.
Discussion
Summary of main results
Four trials evaluated whether sinus lift procedures are indicated in patients having a residual crestal height between 4 to 9 mm. There was moderate quality evidence, which was insufficient to determine whether procedures with or without using a sinus lift led to more prosthesis or implant failures. However, there were more complications at the treated sites when using the sinus lift procedures.
Several trials compared different sinus lift procedures. Data for prosthetic failure were available for five comparisons but with only one small trial in each. There was insufficient evidence to conclude that one sinus lift procedure had more or fewer prosthetic failures than the other. Eight comparisons, including one or two small studies, presented data on implant failures. There was insufficient evidence to conclude that one sinus lift procedure led to more implant failures than the other.
Given the lack of evidence to support one sinus lift procedure over another, clinicians should use their clinical judgement and take patient preference into account when choosing between procedures.
Overall completeness and applicability of evidence
Most of the augmentation procedures evaluated in these trials were performed by experienced clinicians, therefore caution is recommended when extrapolating the results of the present review to other clinical settings, such as general practice. There were insufficient studies comparing the same interventions to enable robust conclusions to be made via meta‐analysis.
Quality of the evidence
Four trials (102 participants) provided data for the first objective of the review, to compare interventions with and without a sinus lift procedure. These trials provided moderate quality evidence for prosthesis and implant failures, which was downgraded for imprecision.
Sample sizes were relatively small with only eight trials (Cannizzaro 2009; Felice 2009a; Checchi 2010; Felice 2011; Esposito 2012; Merli 2013; Si 2013; Torres 2013) reporting a sample size calculation and several of them were definitively underpowered to detect a clinically significant difference.
Potential biases in the review process
There were no events in some of the trial arms for some outcomes and we therefore used Peto odds ratios to undertake the meta‐analysis. This may lead to conservative estimates of the effect size.
Although two of these were split‐mouth studies, the prosthesis and implant failures, and complications were in different patients and so the data have been entered as though they are from parallel group studies for ease of analysis and interpretation. This is unlikely to have produced biased estimates.
Eleven of the 18 trials received funding from manufacturers of the interventions, however there was no evidence that this caused any bias in the assessment of the trials. This is not included in the risk of bias assessment in accord with Cochrane policy.
Agreements and disagreements with other studies or reviews
Several 'systematic' reviews have been published on the outcomes of sinus lifting procedures (Tong 1998; Wallace 2003; Del Fabbro 2004; Emmerich 2005; Aghaloo 2007; Pjetursson 2008; Tan 2008; Nkenke 2009; Del Fabbro 2013) however, since these findings were not based on the most reliable clinical studies, direct comparisons with our findings could be misleading and difficult to interpret.
Authors' conclusions
Implications for practice.
There is moderate quality evidence which is insufficient to determine whether sinus lift procedures in bone with residual height between 4 and 9 mm are more or less successful than placing short implants (5 to 8.5 mm) for reducing prosthesis or implant failure. However, there are more complications at sites treated with the sinus lift procedure up to one year after loading. Many trials compared different sinus lift procedures and none of these indicated that one procedure reduced prosthetic or implant failures when compared to the other. Based on low quality evidence, patients may prefer rotary instruments over hand malleting for crestal sinus lift.
Implications for research.
In order to understand when sinus lift procedures are needed, and which are the most effective sinus lift techniques, larger, well designed trials are needed. Such trials should include long‐term follow‐up and be reported according to the Consolidated Standards of Reporting Trials (CONSORT) guidelines (Moher 2001). It is difficult to provide clear indications with respect to which sinus lift procedures should be evaluated first however, once the clinical situations are established in which these procedures are actually needed, priority should be given to those interventions that are simpler, less invasive, involve less risk of complications, and reach their goals within the shortest timeframe. Research efforts should be concentrated on a few important clinical questions, using larger sample sizes. This might be obtained through collaborative efforts among various research groups. Among the identified research priorities is the evaluation of whether and when sinus lift procedures are required, whether and when one‐stage lifting via a crestal approach can replace the more invasive lateral window procedures, and whether bone grafts are needed and, if needed, whether bone substitutes can be used for replacing autogenous bone in augmenting severely atrophic maxillary sinuses. Trials should focus on clinically important outcomes, such as implant failure and complication rates, rather than histological or surrogate outcomes such as histomorphometry or bone height.
What's new
| Date | Event | Description |
|---|---|---|
| 12 August 2014 | Amended | Minor edits. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 3, 2010
| Date | Event | Description |
|---|---|---|
| 7 May 2014 | New citation required and conclusions have changed | Review update including 8 new studies bringing the total to 18 included studies. The methods have been updated and the risk of bias done for all included studies. |
| 4 March 2014 | New search has been performed | Search updated to January 2014. |
| 17 March 2010 | Amended | Minor edits. |
Acknowledgements
We wish to thank all those people who helped us with the final preparation of the current review, and in particular: Anne Littlewood (Cochrane Oral Health Group) and Sylvia Bickley for their assistance with literature searching; Luisa Fernandez Mauleffinch and Philip Riley (Cochrane Oral Health Group) for their help with the preparation of this review; Paul Coulthard, Maria Gabriella Grusovin, Dimitrious Karasoulos, Stella Kwan, Alissa Rami, Jonathan Rees as a co‐author of one previous version of this review; and Lars Andersson, Filippo Cangini, Mats Hallman, Björn Johansson, Adriano Piattelli, Gerry Raghoebar, Heidrun Schaaf, Andreas Thor and Jesus Torres for kindly providing us with additional information on their trials. We would also like to thank the following referees: Stephen Chen, Matteo Chiapasco, Christer Dahlin, Mats Hallman, Jayne Harrison, Jan Hirsch, Anne Littlewood, Ian Needleman, Michele Nieri, Gerry Raghoebar, Heidrun Schaaf, Bill Shaw, Jesus Torres and Philip Riley.
Appendices
Appendix 1. MEDLINE (OVID) search strategy
1. exp Dental Implants/ 2. exp Dental Implantation/ or dental implantation 3. exp Dental Prosthesis, Implant‐Supported/ 4. ((osseointegrated adj implant$) and (dental or oral)) 5. dental implant$ 6. (implant$ adj5 dent$) 7. (((overdenture$ or crown$ or bridge$ or prosthesis or restoration$) adj5 (Dental or oral)) and implant$) 8. "implant supported dental prosthesis" 9. ("blade implant$" and (dental or oral)) 10. ((endosseous adj5 implant$) and (dental or oral)) 11. ((dental or oral) adj5 implant$) 12. OR/1‐11
The above search was run with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2009 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011):
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 2. Cochrane Oral Health Group's Trials Register search strategy
Updated searches were undertaken using the Cochrane Register of Studies and the search strategy below from January 2013:
#1 ("dental implant*" or "oral implant*" or "implant support*" or "endosseous implant*" or "blade implant*") AND (INREGISTER) #2 ((implant* and (oral or dental))) AND (INREGISTER) #3 ("subperiosteal implant*") AND (INREGISTER) #4 ((implant* AND overdenture*)) AND (INREGISTER) #5 (((overdenture* OR crown* OR bridge* OR prosthesis OR prostheses OR restoration*) AND ("dental implant*" OR "Oral implant" OR (zygoma* AND implant*)))) AND (INREGISTER) #6 (#1 or #2 or #3 or #4 or #5) AND (INREGISTER)
Previous searches of the Register were undertaken using the Procite software and the search strategy below:
(dental‐implants OR "dental implant*" OR "oral implant*" OR dental‐implantation OR dental‐prosthesis‐implant‐supported OR "implant supported" OR "implant supported prosthesis" OR dental‐implantation‐endosseous‐endodontic OR "endosseous implant*" OR blade‐implantation OR "blade implant*" OR (implant* AND (oral OR dental)) or dental‐implantation‐subperiosteal OR "subperiosteal implant" OR (implant* AND overdenture*) OR ((overdenture* OR crown* OR bridge* OR prosthesis OR prostheses OR restoration*) AND ("dental implant*" OR "Oral implant" OR (zygoma* AND implant*))))
Appendix 3. CENTRAL search strategy
#1 DENTAL IMPLANTS explode all trees (MeSH) #2 DENTAL IMPLANTATION explode all trees (MeSH) #3 DENTAL PROSTHESIS IMPLANT‐SUPPORTED single term (MeSH) #4 ((osseointegrat* near implant*) and (dental* or oral*)) #5 (dental next implant*) #6 (implant* near dent*) #7 dental‐implant* #8 ((overdenture* near dental*) and implant*) #9 ((overdenture* near oral*) and implant*) #10 ((crown* near dental*) and implant*) #11 ((crown* near oral*) and implant*) #12 ((bridge* near dental*) and implant*) #13 ((bridge* near oral*) and implant*) #14 ((prosthesis near dental*) and implant*) #15 ((prosthesis near oral*) and implant*) #16 ((prostheses near dental*) and implant*) #17 ((prostheses near oral*) and implant*) #18 ((restoration* near dental*) and implant*) #19 ((restoration* near oral*) and implant*) #20 (implant next supported next dental next prosthesis) #21 (blade next implant*) #22 ((endosseous near implant*) and dental) #23 ((endosseous near implant*) and oral*) #24 ((dental* near implant*) or (oral* near implant*)) #25 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24)
Appendix 4. EMBASE search strategy
1. tooth implantation/ 2. ((implant‐supported or implant$) adj support$).mp. 3. ((osseointegrated adj implant$) and (dental or oral)).mp. 4. ((dental implant$ or dental‐implant or implant$) adj (dent$ or oral or tooth)).mp. 5. (((overdenture$ or crown$ or bridge$ or prosthesis or prostheses or restoration$) adj5 (dental or oral)) and implant$).mp. 6. "implant supported dental prosthesis".mp. 7. ("blade implant$" and (dental or oral or tooth or teeth)).mp. 8. ((endosseous adj5 implant$) and (dental or oral or tooth or teeth)).mp. 9. ((dental or oral or tooth or teeth) and implant$).mp. 10. or/1‐9
The EMBASE subject search was run with the following sensitive search for controlled trials in EMBASE via OVID: 1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Data and analyses
Comparison 1. Long implants with sinus lift versus short implants without augmentation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prosthesis failures 1 year | 3 | 109 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.37 [0.05, 2.68] |
| 2 Implant failures 1 year | 4 | 137 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.10, 1.99] |
| 3 Complications 1 year | 4 | 137 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.77 [1.79, 12.71] |
Comparison 2. Different sinus lift procedures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bone versus no bone graft | 2 | Odds Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Implant failures (1 year) | 2 | Odds Ratio (Fixed, 95% CI) | 0.52 [0.10, 2.82] | |
| 2 Bone versus no bone graft | 2 | Mean Difference (Fixed, 95% CI) | 2.89 [2.35, 3.43] | |
| 2.1 Bone gain | 2 | Mean Difference (Fixed, 95% CI) | 2.89 [2.35, 3.43] | |
| 3 Autogenous bone versus bone substitute | 2 | Odds Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Implant failures | 2 | Odds Ratio (Fixed, 95% CI) | 4.20 [0.81, 21.79] | |
| 4 Autogenous bone or Bio‐Oss +/‐ PRP | 2 | Odds Ratio (Fixed, 95% CI) | Subtotals only | |
| 4.1 Implant failures | 2 | Odds Ratio (Fixed, 95% CI) | 1.40 [0.12, 16.52] | |
| 4.2 Complications | 2 | Odds Ratio (Fixed, 95% CI) | 0.88 [0.13, 6.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cannizzaro 2009.
| Methods | Randomised trial of parallel group design, 5‐year post‐loading follow‐up. 1 drop‐out from the lateral window sinus lift group who died of cancer just before the 3‐year post‐loading follow‐up | |
| Participants | Patients having 3 to 6 mm of alveolar bone at the floor of the sinus. Adults treated at a private dental practice, Pavia, Italy. Exclusion criteria were: general contraindications to implant surgery, subjected to irradiation in the head and neck area less than 1 year ago, treated or under treatment with intravenous amino‐bisphosphonates, poor oral hygiene and motivation, uncontrolled diabetes, pregnant or lactating, substance abusers, psychiatric problems or unrealistic expectations, lack of opposite occluding dentition/prosthesis in the area intended for implant placement, acute infection in the area intended for implant placement. 20 patients were treated in each group | |
| Interventions | 1‐stage sinus lift using 1 to 3 8 mm long implants placed in simultaneously crestally augmented sinus with autogenous particulate bone, harvested from the implant site, versus 1 to 3 10 mm or longer implants placed in simultaneously augmented sinuses using the lateral approach with a mixture of 50% particulate autogenous bone from the tuberosity area and 50% Bio‐Oss. A modified 'Cosci technique' was used to crestally augment the sinus. In brief implant sites were prepared with a 2.5 mm trephine drill up to about 1 mm of the sinus cortical wall, to collect autogenous bone, and with a 3.1 mm diameter atraumatic lifting drill. Resorbable barriers (Biomend Extend, Sulzer Dental Inc., Carlsbad, CA, USA) were used to seal the lateral windows. All the augmentation procedures were performed under local anaesthesia. All implants were left to heal submerged for 45 days and were functionally loaded within 1 week after abutment connection. All implants were tapered Screw‐Vent MP‐1 HA Dual Transition Selective Surface implants (Zimmer Dental, Carlsbad, CA, USA) inserted in under prepared osteotomy sites with a torque of at least 35 N/cm | |
| Outcomes | Prosthesis and implant failures, complications at the augmented and donor sites, and peri‐implant marginal bone levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Article: "A computer generated restricted randomisation list was created" |
| Allocation concealment (selection bias) | Low risk | Article: "Only one of the investigators (Marco Esposito), not involved in the selection and treatment of the patients, was aware of the randomization sequence and could have access to the randomization list stored in his pass‐word protected portable computer. The randomized codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially after eligible patients were anaesthetised, therefore, treatment allocation was concealed to the investigators in charge of enrolling and treating the patients" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Article: "One dentist (Giuseppe Fontana) not involved in the treatment of the patients made all the clinical assessments without knowing group allocation, therefore outcome assessor was blinded, however Bio‐Oss augmented sites could be identified on radiographs because they appeared more radio‐opaque and implants were longer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, no drop‐outs |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Unclear risk | The 8 mm implants were in general of larger diameter than the longer implants, therefore it cannot be ruled out that also the implant diameter played a role in the clinical outcome |
Checchi 2010.
| Methods | Randomised trial of split‐mouth design, 1 year post‐loading follow‐up. 3 withdrawals between years 1 and 3 | |
| Participants | Patients having 5 to 7 mm of alveolar bone height with a thickness of 5 mm or more below the sinus. Adults treated in 2 private practices in Bologna, Italy. Exclusion criteria were: general contraindications to implant surgery, subjected to irradiation in the head and neck area, treated or under treatment with intravenous amino‐bisphosphonates, poor oral hygiene and motivation, untreated periodontal disease, uncontrolled diabetes, pregnant or lactating, substance abusers, psychiatric problems or unrealistic expectations, lack of opposite occluding dentition/prosthesis in the area intended for implant placement, acute or chronic infection/inflammation in the area intended for implant placement, patients participating in other trials, if the present protocol could not be properly followed, referred only for implant placement. 15 patients were treated and 12 patients were followed up to 3 years after loading | |
| Interventions | 1‐stage crestal sinus lift according to the Summers' technique using dedicated osteotomes of increasing diameters and a mallet versus the Cosci's technique using dedicated rotatory instruments with increasing working lengths and finally a special drill with a non‐cutting head (atraumatic lifting drill). Particulate cancellous human allograft (Puros, Zimmer Dental, Carlsbad, Ca, USA) was used. Augmentation procedures were performed under local anaesthesia and 1 or 2 8 to 10 mm long TSV Screw‐Vent tapered implants with internal connection and MTX microtextured titanium surface (Zimmer Dental) were placed and submerged for 6 months. Provisional screw‐retained reinforced resin prostheses were replaced after 4 months by definitive provisionally cemented metal‐ceramic prostheses | |
| Outcomes | Prosthesis, implant and augmentation failures, complications, patient preference 1 and 5 months after augmentation, peri‐implant marginal bone level changes, operator preference, duration of the sinus lift procedures | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Article: "A computer generated restricted randomisation list was created.Only one of the investigators (Marco Esposito), not involved in the selection and treatment of the patients, was aware of the randomisation sequence and could have access to the randomisation list stored in his pass‐word protected portable computer" |
| Allocation concealment (selection bias) | Low risk | Article: "The randomised codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially after flap elevation, therefore, treatment allocation was concealed to the investigators in charge of enrolling and treating the patients" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Article: "Follow‐ups were conducted by an independent blind outcome assessor (Gerardo Pellegrino)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, no drop‐outs |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | High risk | 8 out of 15 patients were treated by Dr Cosci who was the inventor of the Cosci's technique |
Crespi 2012.
| Methods | Randomised trial of parallel group design, 19‐month post‐loading follow‐up. No withdrawals | |
| Participants | Patients having an unspecified "inadequate" alveolar bone height below the sinus and bone quality of type 3 or 4. Adults treated at the Department of Dentristy, San Raffaele Hospital, Milan, Italy. Exclusion criteria were: smoking, any chronic systemic disease and acute or chronic sinus problems, coagulation disorders, sing of acute infection around the alveolar bone at the site, alcohol and drug abuse. 40 patients were treated in each group | |
| Interventions | 1‐stage crestal sinus lift with osteotomes and hand mallet versus electric mallet (Magnetic Mallet MetaErgonomica, Turbigo (MI), Italy). Augmentation procedures were performed under local anaesthesia, no grafting material was used and Outlink tapered implants (Sweden & Martina, Due Carrare (PD), Italy), with external hexagon connection, titanium plasma‐spayed surface and 0.8 mm of polished neck, were left to heal submerged for 3 months. Provisional prostheses were replaced after 2 months by definitive screw‐retained metal‐ceramic prostheses | |
| Outcomes | Prosthesis, implant and augmentation failures, complications, patient preference, vertical bone gain and maintenance over time, peri‐implant marginal bone level changes, probing pocket depths, pain, modified plaque index and modified marginal bleeding index | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Article: "...group assignment was performed by lots in closed envelopes" Author's reply: "Each patient was assigned to control or test group by lots in closed envelopes" |
| Allocation concealment (selection bias) | Unclear risk | Article: "...group assignment was performed by lots in closed envelopes" Author's reply: "Each patient was assigned to control or test group by lots in closed envelopes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Article: "A blinded radiologist measured the changes in marginal bone height over time" Authors' reply: "In measuring all clinical and radiographic parameters, the assessors were blinded anyhow" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention regarding drop‐outs in the text Authors' reply: "No drop‐out occurred" |
| Selective reporting (reporting bias) | High risk | Complication data apparently not fully reported; for some other outcomes (PD) data not provided by study group, for other outcomes (modified plaque index and modified bleeding index) data not provided at all Authors' reply: "No, we didn't find other complications" |
| Other bias | Low risk | None apparent |
Esposito 2012.
| Methods | Randomised trial of split‐mouth design, 1 year post‐loading follow‐up. No withdrawals | |
| Participants | Patients having 5 to 7 mm of alveolar bone height with a thickness of 6 mm or more below the sinus. Adults treated in dental hospitals/university clinics and private practices in Bologna, Roma and Chieti, Italy. Exclusion criteria were: general contraindications to implant surgery, patients irradiated in the head and neck area, immunosuppressed or immunocompromised patients, patients who took or are taking bisphosphonates intravenously, patients with untreated periodontitis, poor oral hygiene and motivation, uncontrolled diabetes, pregnancy or lactation, addiction to alcohol or drugs, psychiatric problems, lack of opposite occluding dentition in the area intended for implant placement, patients with an acute or chronic infection inflammation in the area intended for implant placement. 20 patients were treated and followed up to 1 year after loading | |
| Interventions |
Comparison 1: short implants without augmentation versus long implants with augmentation 1 to 3 6 mm long implants of 4 mm in diameter versus 1 to 3 10 mm or longer implants of 4 mm in diameter placed in 1‐stage laterally augmented sinuses with 100% cortical porcine‐derived collagenated bone (Gen‐Os, OsteoBiol, Tecnoss Dental, Pianezza, TO, Italy) with their lateral windows sealed with a resorbable collagen membrane from equine pericardium (Evolution, Fine 30 x 30 mm, OsteoBiol) simultaneously to implant placement. Augmentation procedures were performed under local anaesthesia and implants were left to heal submerged for 4 months. Southern implants (Irene, South Africa), with external hexagon connection, were used. Provisional screw‐retained reinforced resin prostheses were replaced after 4 months by definitive screw‐retained or provisionally cemented metal‐ceramic prostheses |
|
| Outcomes | Prosthesis and implant failures, complications, peri‐implant marginal bone levels and patient preference at 5 months post‐loading | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Article: "A computer generated restricted random list was created" |
| Allocation concealment (selection bias) | Low risk | Article: "Only one of the investigators (ME), not involved in the selection and treatment of the patients, was aware of the random sequence and could have access to the random list stored in his pass‐word protected portable computer. The information on how to treat site number 1 was enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially before giving anaesthesia and surgeons should have treated both sites in the same surgical session, starting from the intervention allocated to site number 1. Therefore, treatment allocation was concealed to the investigators in charge of enrolling and treating the patients" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Article: "Two dentists (Dr Gerardo Pellegrino and Valeria Corvino) not involved in the treatment of the patients performed all clinical measurements without knowing group allocation..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, no drop outs |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Low risk | None apparent |
Felice 2009a.
| Methods | Randomised trial of split‐mouth design, 1 year post‐loading follow‐up. No withdrawals | |
| Participants | Patients having 4 to 6 mm of alveolar height with a thickness of 8 mm or more below the sinus. Adults treated in dental hospitals/university clinics in Bologna, Roma and Chieti, Italy. Exclusion criteria were: general contraindications to implant surgery, patients irradiated in the head and neck area, immunosuppressed or immunocompromised patients, patients who took or are taking bisphosphonates intravenously, patients with untreated periodontitis, poor oral hygiene and motivation, uncontrolled diabetes, pregnancy or lactation, addiction to alcohol or drugs, psychiatric problems, lack of opposite occluding dentition in the area intended for implant placement, patients with an acute or chronic infection inflammation in the area intended for implant placement. 15 patients were treated and all patients were followed up to 1 year after loading, therefore there were no drop‐outs | |
| Interventions |
Comparison 1: short implants without augmentation versus long implants with augmentation 1 to 3 5 mm long implants of 6 mm in diameter versus 1 to 3 10 mm or longer implants of 4 mm in diameter placed in 2‐stage laterally augmented sinus with 100% bovine anorganic bone (Bio‐Oss, Geistlich Pharmaceutical, Wolhusen, Switzerland) with their lateral windows sealed with a resorbable collagen membrane (OsseoGuard, Biomet 3i, Palm Beach, FL, USA) 4 months before. All augmentation procedures were performed under local anaesthesia. All implants were left to left to heal submerged for 4 months. Rescue implants (MegaGen Implant Co. Lld., Gyeongbuk, South Korea) as short implants and EZ Plus (MegaGen) as long implants, all with internal connection were used. Implant site preparation was also different since a 5 mm diameter trephine was used initially to prepare the osteotomy sites for Rescue implants. Provisional screw‐retained reinforced resin prostheses were replaced after 4 months by definitive screw‐retained metal‐ceramic prostheses |
|
| Outcomes | Prosthesis and implant failures, complications, peri‐implant marginal bone levels, and patient preference | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Article: "A computer generated restricted randomisation list was created" |
| Allocation concealment (selection bias) | Low risk | Article: "Only one of the investigators (ME), not involved in the selection and treatment of the patients, was aware of the randomisation sequence and could have access to the randomisation list stored in his pass‐word protected portable computer. The randomised codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes indicating which site to augment were opened sequentially just prior to the augmentation procedure. Therefore, treatment allocation was concealed to the investigators in charge of enrolling and treating the patients" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Article: "One dentist (GP) not involved in the treatment of the patients performed all clinical and radiographic assessments without knowing group allocation, therefore the outcome assessor was blinded, however the Bio‐Oss augmented sites could be identified both clinically when testing implant stability because of the different diameters and on radiographs because they appeared more radio‐opaque and implants were different" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, no drop‐outs |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | High risk | Implants of small diameter (4 mm) and of different design were inserted with a different surgical technique in the augmented group instead of the originally planned identical but longer implants Patients had always the augmentation procedure performed first and then had implant placement bilaterally. This may have affected patient preference since patient could not experience the benefit of having the prosthesis 4 months earlier |
Felice 2009b.
| Methods | Randomised trial of split‐mouth design, 1 year post‐loading follow‐up. 1 patient, who had a complication, dropped out before prosthetic loading | |
| Participants | Patients having 1 to 4 mm of alveolar bone at the floor of the sinus. Adults treated in dental hospitals/university clinics in Bologna, Roma and Chieti, Italy. Exclusion criteria were: general contraindications to implant surgery, patients irradiated in the head and neck area, immunosuppressed or immunocompromised patients, patients who took or are taking bisphosphonates intravenously, patients with untreated periodontitis, poor oral hygiene and motivation, uncontrolled diabetes, pregnancy or lactation, addiction to alcohol or drugs, psychiatric problems, lack of opposite occluding dentition in the area intended for implant placement, patients with an acute or chronic infection inflammation in the area intended for implant placement. 10 patients were treated | |
| Interventions | 2‐stage sinus lift with lateral window approach using either a synthetic resorbable barrier (Inion, GTR Biodegradable Membrane System, Tampere, Finland) to keep the sinus membrane or 100% granular Bio‐Oss. Inion barriers were used to seal the lateral windows. Inion barriers are made of a synthetic co‐polymer (trimethylene carbonate l‐lactide polyglycolide) that needs to be softened in a plasticising solution, allowing the membrane to be cut and mould to fit exactly the space. The barrier then harden in the new position maintaining the new shape and the space. This material should biodegrade in situ after 8‐12 weeks. All augmentation procedures were performed under local anaesthesia. After 6 months, 1 to 3 implants were placed per side and submerged for 4 months. Implants were Way (Geass, Pozzuolo del Friuli (UD), Italy) with a laser treated surface and internal connection. Provisional screw‐retained reinforced resin prostheses were replaced after 4 months by definitive screw‐retained metal‐ceramic prostheses. 1 patient was excluded after perforation | |
| Outcomes | Prosthesis and implant failures, complications, amount of vertically augmented bone (mm), peri‐implant marginal bone levels, patient and clinician preference. Time necessary to complete the augmentation procedure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Article: "A computer generated restricted randomisation list was created" |
| Allocation concealment (selection bias) | Low risk | Article: "Only one of the investigators (Marco Esposito), not involved in the selection and treatment of the patients, was aware of the randomization sequence and could have access to the randomization list stored in his pass‐word protected portable computer. The randomized codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially after the sinus lining epithelium of site number 1 was lifted, therefore, treatment allocation was concealed to the investigators in charge of enrolling and treating the patients" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Article: "One dentist (Gerardo Pellegrino), not involved in the treatment of the patients, made all the clinical assessments without knowing group allocation, therefore outcome assessor was blinded, however Bio‐Oss augmented sites could be identified on radiographs because they appeared more radio‐opaque while Inion treated sites appeared rather radiolucent" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, 1 drop‐out after perforation |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Low risk | None apparent |
Felice 2011.
| Methods | Randomised trial of parallel group design, 5‐month post‐loading follow‐up. No withdrawals | |
| Participants | Patients with fully edentulous maxillas having residual bone heights of 5 to 9 mm with a thickness of 5 mm at the implant sites. Adults treated in dental hospitals/university clinics in Bologna, Roma, Italy. Exclusion criteria were: general contraindications to implant surgery, patients irradiated in the head and neck area, immunosuppressed or immunocompromised patients, patients who took or are taking bisphosphonates intravenously, patients with untreated periodontitis, poor oral hygiene and motivation, uncontrolled diabetes, pregnancy or lactation, addiction to alcohol or drugs, psychiatric problems, severe intermaxillary discrepancies, patients with an acute or chronic infection inflammation in the area intended for implant placement, lack of opposite occluding dentition/prosthesis. 28 patients were treated, 15 with short implants and 13 with long implants after augmentation. Patients were followed up to 5 months after loading and no patients dropped out | |
| Interventions |
Comparison 1: short implants without augmentation versus long implants with augmentation 4 to 8 5 to 8.5 mm long implants versus 4 to 8 11.5 mm or longer implants placed in sinuses and maxillae of totally edentulous patients augmented with particulated autogenous bone from the iliac crest via lateral windows and, if necessary with onlay blocks 4 months prior to implant placement. The maxillary windows and the bone blocks were covered with 30 x 40 mm synthetic resorbable barriers (Inion GTR Biodegradable Membrane System, Tampere, Finland). Augmentation procedures were performed under general anaesthesia. Implants were left to heal submerged for 4 months. ExFeel and Rescue implants (MegaGen Implant Co. Lld., Gyeongbuk, South Korea) with external hexagon connections were used. Either overdentures or fixed provisional screw‐retained reinforced resin prostheses were placed. The latter were replaced after 4 months by fixed definitive screw‐retained metal‐resin prostheses |
|
| Outcomes | Prosthesis and implant failures, complications, peri‐implant marginal bone levels, patient satisfaction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Article: "A computer generated restricted randomisation list was created" |
| Allocation concealment (selection bias) | Low risk | Article: "Only one of the investigators (ME), not involved in the selection and treatment of the patients, was aware of the randomisation sequence and could have access to the randomisation list stored in his pass‐word protected portable computer.The randomised codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially after eligible patients signed the informed consent form to be enrolled in the trial. Therefore, treatment allocation was concealed to the investigators in charge of enrolling and treating the patients" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Article: "One dentist (ES) not involved in the treatment of the patients performed all clinical assessments without knowing group allocation, therefore the outcome assessor was blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, 1 drop‐out |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Low risk | None apparent |
Felice 2012.
| Methods | Randomised trial of parallel group design, 1 year post‐loading follow‐up. 1 patient, from the maxillary augmented group, dropped out before the 1 year follow‐up because she did not want to continue the follow‐up at the dental practice | |
| Participants | Patients having 4 to 6 mm of alveolar bone height with a thickness of 6 mm or more below the sinus. Adults treated in dental hospitals/university clinics and private practices in Bologna, Roma and Chieti, Italy. Exclusion criteria were: general contraindications to implant surgery, patients irradiated in the head and neck area, immunosuppressed or immunocompromised patients, patients who took or are taking bisphosphonates intravenously, patients with untreated periodontitis, poor oral hygiene and motivation, uncontrolled diabetes, pregnancy or lactation, addiction to alcohol or drugs, psychiatric problems, lack of opposite occluding dentition in the area intended for implant placement, patients with an acute or chronic infection inflammation in the area intended for implant placement. 20 patients were treated in each group | |
| Interventions |
Comparison 1: short implants without augmentation versus long implants with augmentation 1 to 3 5 mm long implants of 5 mm in diameter versus 1 to 3 10 mm or longer implants of 5 mm in diameter placed in 1‐stage laterally augmented sinuses using a 1 cc sterile syringe of a sticky paste of made of 600‐1000 micron pre‐hydrated collagenated cortico‐cancellous bone granules of porcine origin, mixed with OsteoBiol Gel 0 (mp3, OsteoBiol, Tecnoss Dental, Pianezza, TO, Italy) with their lateral windows sealed with a resorbable collagen membrane from equine pericardium (Evolution, Fine 30 x 30 mm, OsteoBiol) simultaneously to implant placement. Augmentation procedures were performed under local anaesthesia and implants were left to heal submerged for 4 months. ExFeel implants (MegaGen Implant Co. Lld., Gyeongbuk, South Korea), with external hexagon connection and a nanostructured calcium‐incorporated titanium surface (Xpeed), were used. Provisional screw‐retained reinforced resin prostheses were replaced after 4 months by definitive screw‐retained or provisionally cemented metal‐ceramic prostheses |
|
| Outcomes | Prosthesis and implant failures, complications, and peri‐implant marginal bone levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Article: "A computer generated restricted random list was created" |
| Allocation concealment (selection bias) | Low risk | Article: "Only one of the investigators (Marco Esposito), not involved in the selection and treatment of the patients, was aware of the random sequence and could have access to the random list stored in his pass‐word protected portable computer. The information on how to treat each patient was enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially after the patients signed the informed consent accepting to participate into the trial. Therefore, treatment allocation was concealed to the investigators in charge of enrolling and treating the patients" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Article: "Two dentists (Dr Laura Piana and Dr Daniele Panetta) not involved in the treatment of the patients performed all clinical measurements without knowing group allocation...." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, 1 drop‐out |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Low risk | None apparent |
Felice 2013.
| Methods | Randomised trial of parallel group design, 1 year post‐loading follow‐up. 2 patients dropped out from the 1‐stage group just after augmentation: 1 never come back for unknown reasons and the other because of financial reasons | |
| Participants | Patients having 1 to 3 mm of alveolar bone height with a thickness of 5 mm or more below the sinus. Adults treated in dental hospitals/university clinics and private practices in Bologna, Roma and Chieti, Italy. Exclusion criteria were: general contraindications to implant surgery, subjected to irradiation in the head and neck area, immunosuppressed or immunocompromised, treated or under treatment with intravenous amino‐bisphosphonates, untreated periodontitis, poor oral hygiene and motivation, uncontrolled diabetes, pregnant or nursing, substance abusers, psychiatric problems or unrealistic expectations, lack of opposite occluding dentition/prosthesis in the area intended for implant placement, acute chronic infection/inflammation (sinusitis) in the area intended for implant placement, patients participating in other trials, if the present protocol could not be properly followed, referred only for implant placement or unable to attend a 5‐year follow‐up 30 patients were treated in each group, followed up to 1 year after loading |
|
| Interventions | 1‐stage with simultaneous implant placement versus a 2‐stage lateral window sinus lift procedure with implant placement delayed by 4 months using a bone substitute (Bio‐Oss, Geistlich Pharmaceutical, Wolhusen, Switzerland). All patients were treated under local anaesthesia and the lateral sinus windows were sealed with resorbable collagen barriers (Bio‐Gide, Geistlich). 1 to 3, 11 to 15 mm long, tapered Way Milano implants (Geass, Pozzuolo del Friuli, UD, Italy) with microtextured surface treated with laser (Synthegra) and conical internal hexagonal connection were submerged for 4 months, loaded with reinforced provisional prostheses which were replaced, after 4 months, by definitive metal‐ceramic or zirconia restorations, rigidly joining the implants by being either provisionally cemented or screw‐retained | |
| Outcomes | Augmentation, prosthesis and implant failures, complications, and peri‐implant marginal bone level changes on periapical radiographs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Article: "3 computer generated restricted randomisation lists were created. Only 1 of the investigators (Marco Esposito), not involved in the selection and treatment of the patients, was aware of the random sequence and could have access to the randomisation lists stored in his pass‐word protected portable computer" |
| Allocation concealment (selection bias) | Low risk | Article: "The random codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially after the sinus lining epithelium of was lifted, therefore, treatment allocation was concealed to the investigators in charge of enrolling and treating the patients" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Article: "One dentist (Gerardo Pellegrino), not involved in the treatment of the patients, made all clinical assessments without knowing group allocation, therefore outcome assessor was blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, 2 drop‐outs |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Low risk | None apparent |
Hallman 2002.
| Methods | Randomised, split‐mouth study, 1 year post‐loading follow‐up. No withdrawals | |
| Participants | Patients having less than 5 mm of alveolar bone in the floor of the sinus. Adults treated at the Gävla Hospital, Gävla, Sweden. No specific exclusion criteria were given (unhealthy, systemic or local contraindications such as undergoing radiation therapy). 11 patients were treated in the split‐mouth study and 10 in the preference group | |
| Interventions | 2‐stage sinus lift with autogenous particulate bone from the mandibular ramus versus 2‐stage sinus lift with a mixture of 80% of bovine anorganic bone (Bio‐Oss) and 20% of particulate bone from the mandibular ramus left to heal for 6 months. A fibrin glue (Tisseel Duo Quick, Immuno, Wien, Austria) was added to the grafts after thrombin (Thrombin, Immuno). Procedures were performed under local anaesthesia and oral sedation. All implants were turned titanium self tapping (Nobel Biocare, Göteborg, Sweden): Mark II type implants were used in the former 2 groups and Mark III in the latter. All patients were rehabilitated with screw‐retained metal‐ceramic fixed prostheses A third group (not part of this review) was composed by patients who refused to provide autogenous bone but accepted the treatment with a 2‐stage sinus lift with 100% Bio‐Oss |
|
| Outcomes | Prosthesis and implant failures, complications at augmented and donor sites; histomorphometry | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Article: No information presented Author's reply: "The randomization was done by a third party as a lottery. In an envelope there were 12 papers with either 6 possibilities for the mixture to be on the left side or the right side. Each patient was allotted by picking up one paper which said mixture on the left or right side" |
| Allocation concealment (selection bias) | Low risk | Article: No information presented Author's reply: "The surgeon knew the outcome of the surgery at the time of surgery just before making the incision" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Article: No information presented Author's reply: "The study was not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, no drop‐outs |
| Selective reporting (reporting bias) | Low risk | All planned outcomes apparently reported |
| Other bias | Low risk | None apparent |
Lindgren 2012.
| Methods | Randomised, split‐mouth study, 3‐year post‐loading follow‐up. No withdrawals | |
| Participants | Completelly or partially edentulous patients in the maxilla with less than 5 mm of residual bone in the floor of the maxillary sinus and a crest width of at least 4 mm. Adults treated at the department of Oral and Maxillofacial Surgery, Public Health Service, Gävle, Sweden. Patients were excluded if they any severe disease or smoked more than 10 cigarettes/day. 11 patients were treated | |
| Interventions | 2‐stage lateral window sinus lift with granular bone substitutes: anorganic bovine bone (Bio‐Oss®, Geistlich, Biomaterials, Wolhusen, Switzerland, particle diameters 0.25 to 1 mm) versus synthetic biphasic calcium phosphate consisting of 60% hydroxyapatite (HA) and 40% tricalcium phosphate (TCP) (Bone‐Ceramic, Straumann® Basel, Switzerland, particle diameters 0.5 to 1 mm) left to heal for 8 months in a split‐mouth trial. Lateral windows were sealed with resorbable collagen barriers (BioGide®, Geistlich). Implants were inserted into the healed graft of each side and were left to heal for an additional 4 months. All the augmentation procedures were performed under local anaesthesia. All implants were non‐submerge SLActive surface (Straumann®) which were rehabilitated with cross‐arch or partial fixed implant‐supported prostheses | |
| Outcomes | Prosthesis, implant and augmentation failures, complications (selectively reported), radiographic stability of bone height gain on panoramic radiographs, histomorphometry, peri‐implant marginal bone level changes on peri‐apical radiographs, plaque index (PLI), bleeding on probing (BPI), sulcus bleeding index (SBI), and probing pocket depth (PPD) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Article: No information presented Unclear reply to letter |
| Allocation concealment (selection bias) | Low risk | Article: "The randomization envelope was opened after the sinus membrane was elevated" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Article: No information presented Authors' answer: "Outcome assessors not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐out |
| Selective reporting (reporting bias) | Low risk | Full data of complications not provided Authors's provided the missing information |
| Other bias | Low risk | Appropriate analysis for split‐mouth study. None apparent |
Merli 2013.
| Methods | Randomised trial of parallel group design, 6‐month post‐loading follow‐up. 1 drop‐out from the autogenous bone group because patient moved to another country | |
| Participants | Partially edentulous patients in the maxilla with 1 to 3 mm of residual bone in the floor of the maxillary sinus. Adults treated in a private practice in Rimini, Italy. Patients were excluded if there were general contraindications to implant surgery, irradiated in the head and neck area, poor oral hygiene (full mouth plaque score and full mouth bleeding score ≥ 20%) and lack of motivation, uncontrolled diabetes, metabolic disease and drugs affecting bone remodeling, pregnancy and lactating period, substance abusers, smoking more than 20 cigarettes per day. 20 patients were treated in each group | |
| Interventions | 1‐stage lateral window sinus lift with particulated autogenous bone harvested from the mandibular ramus versus granules of anorganic bovine bone (Bio‐Oss). The windows were sealed with resorbable collagen barriers (Bio‐Gide). Implants were left to heal submerged for 8 months. All the augmentation procedures were performed under conscious sedation and local anaesthesia. All implants were titanium Nobel Speedy Groovy or MK IV implants (Nobel Biocare AG, Kloten, Switzerland) with oxidised surfaces (TiUnite) and were rehabilitated with provisional implant‐supported prostheses replaced, after 6 months, by metal‐ceramic screwed partial fixed prostheses | |
| Outcomes | Prosthesis, implant and augmentation failures, complications, radiographic stability of bone height gain and peri‐implant marginal bone level changes on periapical radiographs, chair‐time from local anaesthesia administration to last suture placement, post‐operative pain with a visual analogue scale and analgesic intake for 6 post‐operative days | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Article: "An investigator (LF), not involved in the selection and treatment of the patients, randomly assigned participants following simple randomization procedures (computerised random numbers) to 1 of 2 treatment groups" |
| Allocation concealment (selection bias) | Low risk | Article: "The randomised codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially only after the sinus membrane was elevated. Therefore, treatment allocation was concealed to the investigator in charge of enrolling and treating the patients included in the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Article: "While both the patients and the surgeon were aware of the allocated arm, the radiographic outcome examiner (GM) was unaware of the aim of the study and blinded to group allocation although Bio‐Oss is usually more radiopaque than bone and can be recognized on radiographs. Implant failure and complications were assessed by an independent examiner (Moscatelli M.), who was not blinded to the intervention. Some complications, such as perforation of the sinus membrane, could occur during the surgical phase. The clinical examiner was present during surgery to register any complications, hence he was aware of the treatment administered" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, 1 drop‐out |
| Selective reporting (reporting bias) | Low risk | All planned outcomes apparently reported |
| Other bias | Low risk | None apparent |
Raghoebar 2005.
| Methods | Randomised, split‐mouth study, 2‐year post‐loading follow‐up. No withdrawals | |
| Participants | Patients with severely resorbed fully edentulous maxillae and reduced stability and retention of the upper dentures. Adults treated at the University Hospital Groningen, the Netherlands. Patients were excluded if were edentulous for a period less than 1 year, history of irradiation in the head and neck area, history of reconstructive pre‐prosthetic surgery or previous implant surgery. 5 patients were treated | |
| Interventions | 2‐stage lateral window sinus lift with autogenous blocks and particulate bone together with buccal onlays monocortico‐cancellous bone grafts, to reconstruct the width of the maxilla, fixed with titanium screws harvested from the iliac crest with or without PRP left to heal for 3 months in a split‐mouth trial. Barriers were not used. PRP was made using the Platelet Concentration Collection System kit (PCCS kit, 3i Implant Innovations Inc. Palm Beach Gardens, FL, USA). 54 ml of blood were mixed with 6 ml of anticoagulant (citrate dextrose) and processed with the platelet concentration system. To promote the release of growth factors from the platelets, 10% calcium chloride solution and the patient's serum, as a source of autologous thrombin, were added before actual reconstruction of the defect with the bone graft. The resulting gel was mixed with the bone graft and some gel was applied at the closure of the wound at the side treated with PRP. 3 implants were inserted into the healed graft of each side and were left to heal for an additional 6 months. All the augmentation procedures were performed under general anaesthesia. Surgical templates were used to optimise implant insertion. All implants were turned titanium self tapping (Nobel Biocare, Göteborg, Sweden) and were rehabilitated with 2 implant‐supported prostheses | |
| Outcomes | Prosthesis, implant and graft failures, complications and histomorphometric evaluation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Article: No information presented Author's reply: "Randomisation by lot" |
| Allocation concealment (selection bias) | Unclear risk | Article: No information presented Author's reply failed to clarify the issue |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Article: "The investigators were blinded for both the clinical and laboratory investigations with regard to the PRP‐treated side" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, no drop‐outs |
| Selective reporting (reporting bias) | Low risk | All planned outcomes apparently reported |
| Other bias | Unclear risk | In all patients additional buccal onlays were performed meaning that these patients might have not been the ideal candidates for the hypothesis tested. It is therefore more difficult to interpret the results since the role of the additional buccal onlays on the final outcome cannot be quantified |
Rickert 2013.
| Methods | Randomised, split‐mouth study, 1 year post‐loading follow‐up. No withdrawals | |
| Participants | Patients with severely resorbed fully edentulous maxillae and reduced stability and retention of the upper dentures. Adults treated at the University Hospital Groningen, the Netherlands. Patients were excluded if were edentulous for a period less than 1 year, history of irradiation in the head and neck area, history of reconstructive pre‐prosthetic surgery or previous implant surgery, pathology in the maxillary sinuses. 36 patients were treated | |
| Interventions | 2‐stage lateral window sinus lift with autogenous particulate bone together with buccal onlays monocortico‐cancellous bone blocks, to increase the width of the maxilla, fixed with titanium screws, harvested from the iliac crest left to heal for 3 to 4 months. The bilateral windows were opened following randomisation using rotative instruments of piezosurgery (Piezosurgery, Mectron Medical Technology Spa, Carasco, Genoa, Italy). In addition patients' sides were randomly allocated to received or not resorbable collagen membranes (Bio‐Gide®, Geistlich Pharma AG, Wolhusen, Switzerland) over the grafts. All augmentation procedures were performed under general anaesthesia. 4 to 6 non‐submerged 1‐piece titanium implants (ITI®, Dental Implant System,Institut Straumann, Waldenburg, Switzerland) were inserted and left to heal for an additional 3 months. Patients were rehabilitated with implant‐supported overdentures | |
| Outcomes | Prosthesis, implant and graft failures, complications, time required to open the lateral window, changes over times of the maxillary bone width | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information in the text and no reply to answer |
| Allocation concealment (selection bias) | Unclear risk | Article: "Randomly, by envelopes, one side was treated with conventional rotative instruments .... and the other side with piezosurgery" Authors' reply: "We used envelopes with the operative procedures to randomize. The surgeon had to draw a envelope and had to treat the patient according to the procedure within that envelope" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Article: "Clinically, all patients were evaluated according to a standardized protocol 1, 3, 6, and 12 weeks after surgery by a clinical research not knowing which procedure had been performed at a particular site" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the article bone dehiscence at implant placement were not described by study group and it was unclear whether all complications were reported. The authors clarified the data after request of information |
| Selective reporting (reporting bias) | Low risk | Drop‐outs, if any, were not specified in the article but the authors replied that no drop‐outs occurred |
| Other bias | High risk | Split‐mouth study and pairing of data not taken into account |
Si 2013.
| Methods | Randomised trial of parallel group design, 2‐year and half post‐loading follow‐up. 1 drop‐out from the augmentation group because of death and 3 patients excluded because of sinus epithelium perforation, 1 from the augmented group and 2 from the non‐augmented group. These latter 3 patients were accounted for as failures | |
| Participants | Patients having 2 to 8 mm of bone height below the maxillary sinus. Adult treated at the Department of Oral and Maxillofacial Implantology, Shanghai 9th People's Hospital, Shanghai Jiaotong University, China. Exclusion criteria were uncontrolled diabetes mellitus or other systemic disorders, untreated periodontal disease, endodontic lesions or other oral disorders, heavy smokers (> 10 cigarettes per day), rhinitis or sinusitis, insufficient residual bone quality to achieve implant stability, and previous implant installation or bone grafting at the surgical site. 45 patients were treated, 23 in the augmented group and 22 in the non‐augmented group | |
| Interventions | 1‐stage crestal sinus lift procedure with osteotomes with or without granular Bio‐Oss. Implants left to heal for 6 months. Straumann SLA (Waldenburg, Switzerland) implants were used | |
| Outcomes | Prosthesis, implant and augmentation failures, radiographic bone gain and peri‐implant marginal bone levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Article: "Patients eligible for this study were assigned to two groups using the random numbers table (Complete randomization) by an assistant" |
| Allocation concealment (selection bias) | Low risk | Article: "The assignment was concealed from the clinical operators until the sealed, numbered envelopes were opened before OSFE application during implant surgery" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Article: "The outcome examiners and the patients were kept blinded to the assignment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Article: "Any patient with membrane perforation was excluded from this study" 3 patients were actually excluded and the outcome of the therapy remains unknown, but we counted them as failures |
| Selective reporting (reporting bias) | High risk | Data on complications not presented and no reply to letter |
| Other bias | Low risk | None apparent |
Torres 2009.
| Methods | Randomised hybrid study design combining patients with a split‐mouth study design with patients treated according to a quasi‐random parallel group design with follow‐up to 2 years after loading. No withdrawals | |
| Participants | Patients having less than 7 mm of alveolar bone at the floor of the sinus. Adults treated at private clinic in Madrid, Spain. Exclusion criteria were severe systemic diseases (ASA score 3 or more), a previous history of chronic sinusitis. 57 patients were treated | |
| Interventions | 1‐ or 2‐stage sinus lift procedures using a lateral window technique and 100% granular Bio‐Oss with or without PRP, left to heal for 6 months. Patients having up to 4 mm of residual bone height were augmented first and implant were placed after 6 months whereas patients with residual bone more than 4 mm up to 7 mm received implants during the sinus lift procedures. Implants were left to heal unloaded for 6 months. 10 to 20 cc of venous blood were collected 30 minutes prior to the surgery and mixed with a 3.8% sodium citrate solution at a 5/1 ratio, achieving anticoagulation through calcium binding. The blood was then centrifuged into 3 and separated into 3 layers: red blood cells (RBCs), PRP and poor plasma. Flow cytometry was used for platelet counting. Platelets counts were 2.97 + 0.7‐fold over peripheral blood. PRP was activated with 30% CaCl2 solution and a PRP gel was obtained and mixed with Bio‐Oss. The entire bone of the buccal window was removed, and after the sinus was filled with the bone substitute no barrier was used to seal the windows. Patients were instructed not to wear their upper dentures for 2‐3 weeks after surgery. Osseotite (Biomet 3I, Palm Beach, FL, USA) implants were used | |
| Outcomes | Prosthetic and implant failures, complications, and histomorphometric evaluation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Article: "..randomized sequence was performed by a computerized random number generated using GraphPadQuickCalc software (GraphPad Software Inc., La Joya, Ca, USA), including the concealment of the allocation schedule until the assignment was done" |
| Allocation concealment (selection bias) | Low risk | Article: "Patients included in the inter‐patient clinical trial were allocated by a blinded assistant into two groups: the first was to be treated with ABB alone, and the second with ABB + PRP" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Article: "The surgeon was blinded to the graft material applied to each patient before graft implantation. An assistant handled PRP‐ABB or the ABB group after the surgeon had already accessed the sinus and elevated membrane. The histologist was blinded to the samples' groups throughout the histomorphometric analysis" Author's reply: "Implant stability was assessed manually with removed prostheses and mobile implants were considered as failures and this evaluation was done by a prosthodontist who was not aware of study groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We could only evaluate those data kindly provided by the authors |
| Selective reporting (reporting bias) | Low risk | All planned outcomes apparently reported |
| Other bias | High risk | A mixed split‐mouth and parallel group design was used: patients requiring augmentation at bilateral sinuses were randomised in a split‐mouth study design whereas those requiring unilateral sinus lift sinus were alternated in a quasi‐random study design. We have not included data from the quasi‐random study |
Torres 2013.
| Methods | Randomised study design having 106 patients treated according to a parallel group study design and 5 patients according to a split‐mouth study design (not considered in the present review) with 1 year follow‐up after loading. 2 withdrawals from the membrane group because moved to another city | |
| Participants | Patients having less than 7 mm of alveolar bone at the floor of the sinus. Adults treated at private clinic in Madrid, Spain. Exclusion criteria were patients with severe systemic disease (American Society of Anaesthesiology III or IV), previous history of chronic sinusitis, pregnant, diseases affecting bone, such as osteomalacia, Paget's disease, vitamin D deficiency, hyperthyroidism, cancer (excluding non‐melanoma skin cancer), alcoholism, those on corticosteroids, antiepileptic drugs, bisphosphonates, who had a perforation of the Schneiderian membrane. 106 patients treated, 55 without membranes and 51 with membranes | |
| Interventions | The sinus floor augmentation was made using as graft material (Bio‐Oss; Geistlich Pharmaceutical AG, Wolhusen, Switzerland) via a lateral window. No membranes were used to cover the antrostomy defect in the experimental sites while in the control sites a resorbable porcine derived‐collagen membrane was placed (Bio‐ Gide; GeitlishPharma, Wolhusen, Switzerland). The membrane was extended 2–3 mm beyond the antrostomy borders and stabilized with tacks. When residual bone height was ≥ 4 mm, implants were placed simultaneously to sinus augmentation, otherwise, delayed placements were conducted 6 months after graft surgery. Implants were surgically exposed 6 months after placement, and restored with a fixed implant‐supported prosthesis or bar for retention of a removable prosthesis | |
| Outcomes | Prosthetic and implant failures, selected complications, and selected histomorphometric evaluation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Article: "Patients or sites were allocated to intervention groups in a randomized sequence using a computer generated random number (GraphPad Software Inc., La Joya, CA, USA)" |
| Allocation concealment (selection bias) | Low risk | Article: "All surgeries were performed by the same surgeon, who was blinded to group allocation until the last step of the surgery (closure of antrostomy defect)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Article: "Evaluations were performed by the same prosthodontist who was blinded to group allocation throughout the restorative treatment. Patients were also blinded to group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors informed as that 2 patients from the membrane group moved to another town |
| Selective reporting (reporting bias) | High risk | Full data of complications not provided. Patients with perforations of the sinus epithelium the apparently were excluded from the study |
| Other bias | Low risk | None apparent |
Wannfors 2000.
| Methods | Randomised trial of parallel group design, 3‐year post‐loading follow‐up. No withdrawals at 3 years though 3 patients in the 1‐stage group refused consent to remove the prostheses for testing implant stability | |
| Participants | Edentulous patients with more than 2 mm but less than 7 mm of residual bone under the maxillary sinuses. Adults treated under general anaesthesia at the Karolinska Hospital, Stockholm, Sweden. Patients were included if they were edentulous in the upper jaw. Patients were excluded if they were older than 80 years, had pathologies in the maxillary sinus, had bone diseases or took medications known to effect bone metabolism (i.e. corticosteroids and bisphosphonates). 40 patients enrolled, 20 in each group | |
| Interventions | 1‐stage sinus lift with monocortical iliac bone blocks fixed usually with 2 implants left to heal for 6 months versus 2‐stage sinus lift with particulate bone from the iliac crest left to heal for 6 months and then usually 2 implants were inserted into the healed graft and were left to heal for an additional 6 months. All implants were titanium self tapping (Brånemark System, Nobel Biocare) | |
| Outcomes | Prosthesis failures, implant failures and marginal bone level changes on intraoral radiographs taken with a paralleling technique at abutment connection, 1 and 3 years. Intraoperative sinus membrane perforations | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Article: "He (patient) was allotted to one of the two treatments according to a previously designed scheme by a third person" Author's reply: "The randomization was performed by a third person without any beforehand contact with the patients" |
| Allocation concealment (selection bias) | Unclear risk | Article: No information presented Author's reply failed to clarify the issue |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Article: No information presented Author's reply: "The outcome assessor had knowledge of the randomized group, however not when assessing the x‐ray data" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals at 3 years |
| Selective reporting (reporting bias) | High risk | Full data of complications not provided |
| Other bias | Low risk | None apparent |
PRP ‐ platelet‐rich plasma
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aimetti 2008 | The article presented data from 4 patients treated following a split‐mouth design. Authors informed us that they actually treated 16 patients. We are unable to present the data for the remaining 12 patients |
| Badr 2010 | Insufficient follow‐up time (up to abutment connection), implants were not put even in function |
| Barone 2005 | No clinical outcome measures related to implant treatment |
| Barone 2008 | Insufficient follow‐up time with no clinical outcome measures related to implant treatment |
| Barone 2013 | No clinical outcome measures related to implant treatment |
| Bensaha 2011 | Insufficient follow‐up time (up to implant placement), implants were not even put in function |
| Bettega 2009 | Insufficient follow‐up time (up to abutment connection), implants were not even put in function |
| Borges 2011 | Insufficient follow‐up time (up to abutment connection), implants were not even put in function |
| Boyne 2005 | Described as RCT, unclear number of patients, unequal number of patients in the treatment groups. No reply to letter |
| Canullo 2009 | No clinical outcome measures related to implant treatment |
| Choi 2009 | No clinical outcome measures related to implant treatment |
| Consolo 2007 | No clinical outcome measures related to implant treatment |
| Cordaro 2008 | No clinical outcome measures related to implant treatment |
| Corinaldesi 2013 | No clinical outcome measures related to implant treatment |
| Crespi 2009 | No clinical outcome measures related to implant treatment |
| Froum 1998 | Described as RCT, unclear number of patients and tested interventions which seem to be much more than 8, unequal number of patients in the treatment groups. No reply to letter |
| Froum 2006 | No clinical outcome measures related to implant treatment |
| Froum 2008 | No clinical outcome measures related to implant treatment |
| Froum 2013 | No clinical outcomes useful for the review and too short follow‐up (study terminated before implant placement) |
| Gassling 2013 | Insufficient follow‐up time (up to abutment connection), implants were not put even in function |
| Ghanaati 2014 | Trial is a CCT, not an RCT |
| Hallman 2008 | No clinical outcome measures related to implant treatment |
| Hermund 2012 | Insufficient follow‐up time (up to abutment connection), implants were not even put in function |
| Kassolis 2005 | No clinical outcome measures related to implant treatment |
| Khairy 2013 | Insufficient follow‐up time (up to abutment connection), implants were not put even in function |
| Kim 2009 | No clinical outcome measures related to implant treatment |
| Kock 2010 | Insufficient follow‐up time (possibly up to initial prosthetic loading) |
| Kühl 2012 | No clinical outcomes useful for the review and too short follow‐up (study terminated before implant placement) |
| Kühl 2013 | No clinical outcome measures related to implant treatment |
| Mangano 2007 | The authors informed us that the trial was not an RCT but a CCT |
| Payer 2013 | No clinical outcome measures related to implant treatment |
| Pikdöken 2011 | No clinical outcome measures related to implant treatment |
| Sammartino 2011 | Insufficient follow‐up time (up to a couple of days after implant placements), implants were not even put in function |
| Schaaf 2008 | Insufficient follow‐up time (up to abutment connection), implants were not even put in function |
| Silvestri 2013 | Insufficient follow‐up time (up to abutment connection), implants were not even put in function |
| Steigmann 2005 | No clinical outcome measures related to implant treatment |
| Suba 2006 | No clinical outcome measures related to implant treatment |
| Szabó 2005 | Insufficient follow‐up time (up to abutment connection), implants were not even put in function |
| Tawil 2001 | Inappropriate study design, neither parallel group nor split‐mouth |
| Testori 2013 | No clinical outcome measures related to implant treatment |
| Triplett 2009 | Unclear how many patients were randomised to each group, data very confused and we were unable to retrieve sufficient data from the original publication. No reply to letter |
| Trombelli 2012 | Insufficient follow‐up time (up to 6 months after implant placement), implants were possibly loaded for a very short period |
| Wagner 2012 | Data presented in a way we could not use. No reply to letter |
| Yilmaz 2013 | No clinical outcome measures related to implant treatment |
CCT ‐ controlled clinical trial; RCT ‐ randomised controlled trial
Differences between protocol and review
This review originates from a previous larger review evaluating all types of augmentation procedures for dental implant placement (Esposito 2008). The minimal follow‐up for trials to be included has been moved from abutment connection to four months post‐loading.
Contributions of authors
Conceiving, designing and co‐ordinating the review (Marco Esposito (ME)). Screening search results and retrieved papers against inclusion criteria (ME, Helen Worthington (HW)). Writing to authors for additional information (ME). Appraising quality (ME, Pietro Felice (PF), HW). Data extraction (ME, HW, PF). Analysis and interpretation of the data (ME, HW). Writing the review (ME, HW). Performing previous work that was the foundation of the current study (ME, HW).
Sources of support
Internal sources
School of Dentistry, The University of Manchester, UK.
-
MAHSC, UK.
The Cochrane Oral Health Group is supported by the Manchester Academic Health Sciences Centre (MAHSC) and the NIHR Manchester Biomedical Research Centre.
External sources
-
National Institute for Health Research (NIHR), UK.
CRG funding acknowledgement: The NIHR is the largest single funder of the Cochrane Oral Health Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
-
Cochrane Oral Health Group Global Alliance, UK.
All reviews in the Cochrane Oral Health Group are supported by Global Alliance member organisations (British Association of Oral Surgeons, UK; British Orthodontic Society, UK; British Society of Paediatric Dentistry, UK; British Society of Periodontology, UK; Canadian Dental Hygienists Association, Canada; Mayo Clinic, USA; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and Royal College of Surgeons of Edinburgh, UK) providing funding for the editorial process (http://ohg.cochrane.org/).
Declarations of interest
Marco Esposito and Pietro Felice are among the authors of eight of the included trials, however, they were not involved in the quality assessment of these trials. Helen V Worthington: no interests to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Cannizzaro 2009 {published data only}
- Cannizzaro G, Felice P, Leone M, Viola P, Esposito M. Early loading of hydroxyapatite coated implants in the atrophic posterior maxilla: lateral sinus lift with autogenous bone and Bio‐Oss versus crestal mini‐sinus lift and 8 mm implants. A randomized controlled clinical trial. European Journal of Oral Implantology 2009;2:25‐38. [PubMed] [Google Scholar]
- Cannizzaro G, Felice P, Minciarelli AF, Leone M, Viola P, Esposito M. Early implant loading in the atrophic posterior maxilla: 1‐stage lateral versus crestal sinus lift and 8 mm hydroxyapatite‐coated implants. A 5‐year randomised controlled trial. European Journal of Oral Implantology 2013;6:13‐25. [PubMed] [Google Scholar]
Checchi 2010 {published and unpublished data}
- Checchi L, Felice P, Soardi Antonini E, Cosci F, Pellegrino G, Esposito M. Crestal sinus lift for implant rehabilitation: a randomised clinical trial comparing the Cosci and the Summers techniques. A preliminary report on complications and patient preference. European Journal of Oral Implantology 2010;3:231‐2. [PubMed] [Google Scholar]
- Esposito M, Cannizzaro G, Barausse C, Cosci F, Soardi E, Felice P. Cosci versus Summers technique for crestal sinus lift: 3‐year results from a randomised controlled trial. European Journal of Oral Implantology 2014;7:in press. [PubMed] [Google Scholar]
Crespi 2012 {published data only}
- Crespi R, Caparré P, Gherlone E. Sinus floor elevation by osteotome: hand mallet versus electric mallet. A prospective clinical study. International Journal of Oral and Maxillofacial Implants 2012;27:1144‐50. [PubMed] [Google Scholar]
Esposito 2012 {published and unpublished data}
- Esposito M, Cannizzaro G, Soardi E, Pistilli R, Piattelli M, Corvino V, et al. Posterior atrophic jaws rehabilitated with prostheses supported by 6 mm short 4 mm wide implants or by longer implants in augmented bone. Preliminary results from a pilot randomised controlled trial. European Journal of Oral Implantology 2012;5:19‐33. [PubMed] [Google Scholar]
- Pistilli R, Felice P, Cannizzaro G, Piattelli M, Corvino M, Barausse C, et al. Posterior atrophic jaws rehabilitated with prostheses supported by 6 mm short 4 mm wide implants or by longer implants in augmented bone. One‐year post‐loading results from a pilot randomised controlled trial. European Journal of Oral Implantology 2013;6(4):359‐72. [PubMed] [Google Scholar]
Felice 2009a {published data only}
- Esposito M, Pellegrino G, Pistilli R, Felice P. Rehabilitation of posterior atrophic edentulous jaws: prostheses supported by 5 mm short implants or by longer implants in augmented bone? One‐year results from a pilot randomised clinical trial. European Journal of Oral Implantology 2011;4:21‐30. [PubMed] [Google Scholar]
- Felice P, Checchi V, Pistilli R, Scarano A, Pellegrino G, Esposito M. Bone augmentation versus 5 mm long dental implants in posterior atrophic jaws. Four‐month post‐loading results from a randomized controlled clinical trial. European Journal of Oral Implantology 2009;2:267‐81. [PubMed] [Google Scholar]
Felice 2009b {published data only}
- Esposito M, Piattelli M, Pistilli R, Pellegrino G, Felice P. Sinus lift with guided bone regeneration or anorganic bovine bone: 1‐year post‐loading results of a pilot randomised clinical trial. European Journal of Oral Implantology 2010;3:297‐305. [PubMed] [Google Scholar]
- Felice P, Esposito M, Scarano A, Pistilli R, Lizio G, Checchi L. A comparison of two techniques to augment maxillary sinus with the lateral approach: no grafting procedure vs anorganic bone placement. Preliminary histological and clinical outcomes of a randomized controlled clinical trial. Clinical Oral Implants Research 2009;20:973 (Abs No 261). [Google Scholar]
- Felice P, Scarano A, Pistilli R, Checchi L, Piattelli M, Pellegrino G, et al. A comparison of two techniques to augment maxillary sinuses using the lateral window approach: rigid synthetic resorbable barriers versus anorganic bovine bone. Five‐month post‐loading clinical and histological results of a pilot randomized controlled clinical trial. European Journal of Oral Implantology 2009;2:293‐306. [PubMed] [Google Scholar]
Felice 2011 {published data only}
- Felice P, Soardi E, Pellegrino G, Pistilli R, Marchetti C, Gessaroli M, et al. Treatment of the atrophic edentulous maxilla: short implants versus bone augmentation for placing longer implants. Five‐month post‐loading results of a pilot randomised controlled trial. European Journal of Oral implantology 2011;4:191‐202. [PubMed] [Google Scholar]
Felice 2012 {published and unpublished data}
- Felice P, Pistilli R, Piattelli M, Soardi E, Corvino V, Esposito M. Posterior atrophic jaws rehabilitated with prostheses supported by 5x5 mm implants with a novel nanostructured calcium‐incorporated titanium surface or by longer implants in augmented bone. Preliminary results from a randomised controlled trial. European Journal of Oral Implantology 2012;5:149‐61. [PubMed] [Google Scholar]
- Pistilli R, Felice P, Piattelli M, Gessaroli M, Soardi E, Baruasse C, et al. Posterior atrophic jaws rehabilitated with prostheses supported by 5x5 mm implants with a novel nanostructured calcium‐incorporated titanium surface or by longer implants in augmented bone. One‐year results from a randomised controlled trial. European Journal of Oral Implantology 2013;6(4):343‐57. [PubMed] [Google Scholar]
Felice 2013 {published and unpublished data}
- Felice P, Pistilli R, Piattelli M, Soardi E, Barausse C, Corvino V, et al. 1‐stage versus 2‐stage lateral sinus lift procedures: 1‐year post‐loading results of a multicenter randomised controlled trial. European Journal of Oral Implantology 2014;7:in press. [PubMed] [Google Scholar]
- Felice P, Pistilli R, Piattelli M, Soardi E, Pellegrino G, Corvino V, et al. 1‐stage versus 2‐stage lateral maxillary sinus lift procedures: 4‐month post‐loading results of a multicenter randomised controlled trial. European Journal of Oral Implantology 2013;6:153‐65. [PubMed] [Google Scholar]
Hallman 2002 {published and unpublished data}
- Hallman M, Sennerby L, Lundgren S. A clinical and histologic evaluation of implant integration in the posterior maxilla after sinus floor augmentation with autogenous bone, bovine hydroxyapatite, or a 20:80 mixture. International Journal of Oral and Maxillofacial Implants 2002;17(5):635‐43. [PubMed] [Google Scholar]
Lindgren 2012 {published and unpublished data}
- Lindgren C, Mordenfeld A, Hallman M. A prospective 1‐year clinical and radiographic study of implants placed after maxillary sinus floor augmentation with synthetic biphasic calcium phosphate or deproteinized bovine bone. Clinical Implant Dentistry and Related Research, 2012;14:41‐50. [DOI] [PubMed] [Google Scholar]
- Lindgren C, Mordenfeld A, Johansson CB, Hallman M. A three‐year clinical follow‐up of implants placed in two different biomaterials used for sinus augmentation. International Journal of Oral and Maxillofacial Implants 2013;27:1151‐62. [PubMed] [Google Scholar]
- Lindgren C, Sennerby L, Mordenfeld A, Hallman M. Clinical histology of microimplants placed in two different biomaterials. International Journal of Oral and Maxillofacial Implants 2009;24:1093‐100. [PubMed] [Google Scholar]
Merli 2013 {published data only}
- Merli M, Moscatelli M, Mariotti G, Rotundo R, Nieri M. Autogenous bone versus deproteinised bovine bone matrix in 1‐stage lateral sinus floor elevation in the severely atrophied maxilla: a randomised controlled trial. European Journal of Oral Implantology 2013;6:27‐37. [PubMed] [Google Scholar]
Raghoebar 2005 {published and unpublished data}
- Raghoebar GM, Schortinghuis J, Liem RS, Ruben JL, Wal JE, Vissink A. Does platelet‐rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor?. Clinical Oral Implants Research 2005;16(3):349‐56. [DOI] [PubMed] [Google Scholar]
Rickert 2013 {published and unpublished data}
- Rickert D, Vissink A, Huddleston Slater JJR, Meijer HJA, Raghoebar GM. Comparison between conventional and piezoelectric surgical tools for maxillary sinus floor elevation. A randomized controlled clinical trial. Clinical Implant Dentistry and Related Research 2013;15:297‐302. [DOI] [PubMed] [Google Scholar]
Si 2013 {published data only}
- Si M‐S, Zhuang L‐F, Gu Y‐X, Mo J‐J, Qiao S‐C, Lai H‐C. Osteotome sinus floor elevation with or without grafting: a 3‐year randomized controlled clinical trial. Journal of Clinical Periodontology 2013;40:396–403. [DOI] [PubMed] [Google Scholar]
Torres 2009 {published and unpublished data}
- Torres J, Tamimi F, Martinez PP, Alkhraisat MH, Linares R, Hernandez G, et al. Effect of platelet‐rich plasma on sinus lifting: a randomized‐controlled clinical trial. Journal of Periodontology 2009;36:677‐87. [DOI] [PubMed] [Google Scholar]
Torres 2013 {published and unpublished data}
- Torres Garcia‐Denche J, Wu X, Martinez P‐P, Eimar H, Ikbal DJ‐A, Hernandez G, et al. Membranes over the lateral window in sinus augmentation procedures: a two‐arm and split‐mouth randomized clinical trials. Journal of Clinical Periodontology 2013;40:1043–51. [DOI] [PubMed] [Google Scholar]
Wannfors 2000 {published and unpublished data}
- Johansson B. Bone Grafts and Dental Implants in the Reconstruction of the Severely Atrophied, Edentulous Maxilla. Uppsala: Uppsala University, 2001. [Google Scholar]
- Johansson B. Bone grafts. In manuscript 2005.
- Wannfors K, Johansson B, Hallman M, Strandkvist T. A prospective randomized study of 1‐ and 2‐stage sinus inlay bone grafts: 1‐year follow‐up. International Journal of Oral and Maxillofacial Implants 2000;15(5):625‐32. [PubMed] [Google Scholar]
References to studies excluded from this review
Aimetti 2008 {published and unpublished data}
- Aimetti M, Romano F, Dellavia C, Paoli S. Sinus grafting using autogenous bone and platelet‐rich plasma: histologic outcomes in humans. The International Journal of Periodontics and Restorative Dentistry 2008;28:585‐91. [PubMed] [Google Scholar]
Badr 2010 {published data only}
- Badr M, Coulthard P, Alissa R, Oliver R. The efficacy of platelet‐rich plasma in grafted maxillae. A randomized clinical trial. European Journal of Oral Implantology 2010;3:233‐44. [PubMed] [Google Scholar]
Barone 2005 {published data only}
- Barone A, Crespi R, Nicoli N, Fini M, Giardino R, Covani U. Maxillary sinus augmentation: histologic and histomorphometric analysis. International Journal of Oral and Maxillofacial Implants 2005;20:519‐25. [PubMed] [Google Scholar]
Barone 2008 {published data only}
- Barone A, Santini S, Marconcini S, Giacomelli L, Gherlone E, Covani U. Osteotomy and membrane elevation during the maxillary sinus augmentation procedure. A comparative study: piezoelectric device vs conventional rotative instruments. Clinical Oral Implants Research 2008;19:511‐5. [DOI] [PubMed] [Google Scholar]
Barone 2013 {published data only}
- Barone A, Ricci M, Grassi RF, Nannmark U, Quaranta A, Covani U. A 6‐month histological analysis on maxillary sinus augmentation with and without use of collagen membranes over the osteotomy window: randomized clinical trial. Clinical Oral Implants Research 2013;26:1‐4. [DOI] [PubMed] [Google Scholar]
Bensaha 2011 {published data only}
- Bensaha T. Evaluation of the capability of anew water lift system to reduce the risk of Schneiderian membrane perforation during sinus elevation. International Journal of Oral Maxillofacial Surgery 2011;40:815–20. [DOI] [PubMed] [Google Scholar]
Bettega 2009 {published data only}
- Bettega G, Brun JP, Boutonnat J, Cracowski JL, Quesada JL, Hegelhofer H, et al. Autologous platelet concentrates for bone graft enhancement in sinus lift procedure. Transfusion 2009;49:779‐85. [DOI] [PubMed] [Google Scholar]
- Bettega G, Brun JP, Cracowski JL, Vérain A, Raphael B. Use of autologous platelet concentrates during pre‐implantation maxillary reconstruction. Revue de Stomatologie et de Chirurgie Maxillofaciale 2005;106(3):189‐91. [DOI] [PubMed] [Google Scholar]
Borges 2011 {published data only}
- Borges FL, Dias RO, Piattelli A, Onuma T, Gouveia Cardoso LA, Salomo M, et al. Simultaneous sinus membrane elevation and dental implant placement without bone graft: a 6‐month follow‐up study. Journal of Periodontology 2011;82:403‐12. [DOI] [PubMed] [Google Scholar]
Boyne 2005 {published data only}
- Boyne PJ, Lilly LC, Marx RE, Moy PK, Nevins M, Spagnoli DB, et al. De novo bone induction by recombinant human bone morphogenetic protein‐2 (rh BMP‐2) in maxillary sinus floor augmentation. Journal of Oral and Maxillofacial Surgery 2005;63(12):1693‐707. [DOI] [PubMed] [Google Scholar]
Canullo 2009 {published data only}
- Canullo L, Dellavia C. Sinus lift using a nanocrystalline hydroxyapatite silica gel in severely resorbed maxillae: histological preliminary study. Clinical Implant Dentistry and Related Research 2009;11: Supplement 1:e7‐e13. [DOI] [PubMed] [Google Scholar]
Choi 2009 {published data only}
- Choi KS, Kan JY, Boyne PJ, Goodacre CJ, Lozada JL, Rungcharassaeng K. The effects of resorbable membrane on human maxillary sinus graft: a pilot study. International Journal of Oral and Maxillofacial Implants 2009;24:73‐80. [PubMed] [Google Scholar]
Consolo 2007 {published data only}
- Consolo U, Zaffe D, Bertoldi C, Ceccherelli G. Platelet‐rich plasma activity on maxillary sinus floor augmentation by autologous bone. Clinical Oral Implants Research 2007;18(2):252‐62. [DOI] [PubMed] [Google Scholar]
Cordaro 2008 {published data only}
- Cordaro L, Bosshardt DD, Palattella P, Rao W, Serino G, Chiapasco M. Maxillary sinus grafting with Bio‐Oss or Straumann Bone Ceramic: histomorphometric results from a randomized controlled multicenter clinical trial. Clinical Oral Implants Research 2008;19:796‐803. [DOI] [PubMed] [Google Scholar]
Corinaldesi 2013 {published data only}
- Corinaldesi G, Piersanti L, Piattelli A, Iezzi G, Pieri F, Marchetti C. Augmentation of the floor of the maxillary sinus with recombinant human bone morphogenetic protein‐7: a pilot radiological and histological study in humans. British Journal of Oral and Maxillofacial Surgery 2013;3:247‐52. [DOI] [PubMed] [Google Scholar]
Crespi 2009 {published data only}
- Crespi R, Mariani E, Benasciutti E, Capparé P, Cenci S, Gherlone E. Magnesium‐enriched hydroxyapatite versus autologous bone in maxillary sinus grafting: combining histomorphometry with osteoblast gene expression profiles ex vivo. Journal of Periodontology 2009;80:586‐93. [DOI] [PubMed] [Google Scholar]
Froum 1998 {published data only}
- Froum SJ, Tarnow DP, Wallace SS, Rohrer MD, Cho S‐C. Sinus floor elevation using anorganic bovine bone matrix (OsteoGraf/N) with and without autogenous bone: a clinical, histologic, radiographic, and histomorphometric analysis ‐ Part 2 of an ongoing prospective study. The International Journal of Periodontics and Restorative Dentistry 1998;18(6):529‐43. [PubMed] [Google Scholar]
- Tarnow DP, Wallace SS, Froum SJ, Rohrer MD, Cho SC. Histologic and clinical comparison of bilateral sinus floor elevations with and without barrier membrane placement in 12 patients: Part 3 of an ongoing prospective study. The International Journal of Periodontics and Restorative Dentistry 2000;20(2):117‐25. [PubMed] [Google Scholar]
Froum 2006 {published data only}
- Froum SJ, Wallace SS, Elian N, Cho SC, Tarnow DP. Comparison of mineralized cancellous bone allograft (Puros) and anorganic bovine bone matrix (Bio‐Oss) for sinus augmentation: histomorphometry at 26 to 32 weeks after grafting. The International Journal of Periodontics and Restorative Dentistry 2006;26(6):543‐51. [PubMed] [Google Scholar]
Froum 2008 {published data only}
- Froum SJ, Wallace SS, Cho SC, Elian N, Tarnow DP. Histomorphometric comparison of a biphasic bone ceramic to anorganic bovine bone for sinus augmentation: 6‐ to 8‐month postsurgical assessment of vital bone formation. A pilot study. The International Journal of Periodontics and Restorative Dentistry 2008;28:273‐81. [PubMed] [Google Scholar]
Froum 2013 {published data only}
- Froum SJ, Wallace S, Cho SC, Khouly I, Rosenberg E, Corby P, et al. Histomorphometric comparison of different concentrations of recombinant human bone morphogenetic protein with allogeneic bone compared to the use of 100% mineralized cancellous bone allograft in maxillary sinus grafting. The International Journal of Periodontics and Restorative Dentistry 2013;33(6):721‐30. [DOI] [PubMed] [Google Scholar]
Gassling 2013 {published data only}
- Gassling V, Purcz N, Braesen J‐H, Will M, Gierloff M, Behrens E, et al. Comparison of two different absorbable membranes for the coverage of lateral osteotomy sites in maxillary sinus augmentation: A preliminary study. Journal of Cranio‐Maxillo‐Facial Surgery 2013;41:76‐82. [DOI] [PubMed] [Google Scholar]
Ghanaati 2014 {published data only}
- Ghanaati S, Lorenz J, Obreja K, Choukroun J, Landes C, Sader RA. Nanocrystalline hydroxyapatite‐based material already contributes to implant stability after 3 months: a clinical and radiologic 3‐year follow‐up investigation. Journal of Oral Implantology 2014;40(1):103‐9. [DOI] [PubMed] [Google Scholar]
Hallman 2008 {published data only}
- Hallman M, Lindgren C, Sennerby L, Mordenfeld A, Strandkvist T. Clinical histology of SLA microimplants placed into different biomaterials. EAO conference, Warsaw. 2008. [PubMed]
Hermund 2012 {published data only}
- Hermund NU, Stavropoulos A, Donatsky O, Nielsen H, Clausen C, Reibel J, et al. Reimplantation of cultivated human bone cells from the posterior maxilla for sinus floor augmentation. Histological results from a randomized controlled clinical trial. Clinical Oral Implants Research 2012;23:1031–7. [DOI] [PubMed] [Google Scholar]
Kassolis 2005 {published data only}
- Kassolis JD, Reynolds MA. Evaluation of the adjunctive benefits of platelet‐rich plasma in subantral sinus augmentation. The Journal of Craniofacial Surgery 2005;16(2):280‐7. [DOI] [PubMed] [Google Scholar]
Khairy 2013 {published data only}
- Khairya NM, Shendya EE, Askara NA, El‐Rouby DH. Effect of platelet rich plasma on bone regeneration in maxillary sinus augmentation (randomized clinical trial). International Journal of Oral and Maxillofacial Surgery 2012;42:249–55. [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim YK, Yun PY, Kim SG, Lim SC. Analysis of the healing process in sinus bone grafting using various grafting materials. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2009;107:204‐11. [DOI] [PubMed] [Google Scholar]
Kock 2010 {published data only}
- Koch FP, Becker J, Terheyden H, Capsius B, Wagner W. A prospective, randomized pilot study on the safety and efficacy of recombinant human growth and differentiation factor‐5 coated onto b‐tricalcium phosphate for sinus lift augmentation. Clinical Oral Implants Research 2010;21:1301–8. [DOI] [PubMed] [Google Scholar]
- Stavropoulos A, Becker J, Capsius B, Ac¸il Y, Wagner W, Terheyden H. Histological evaluation of maxillary sinus floor augmentation with recombinant human growth and differentiation factor‐5‐coatedb‐tricalcium phosphate: results of a multicenter randomized clinical trial. Journal of Clinical Periodontology 2011;38:966–74. [DOI] [PubMed] [Google Scholar]
Kühl 2012 {published data only}
- Kühl S, Götz H, Brochhausen C, Jakse N, Filippi A, d'Hoedt B, et al. The influence of substitute materials on bone density after maxillary sinus augmentation: a microcomputed tomography study. International Journal of Oral and Maxillofacial Implants 2012;27(6):1541‐6. [PubMed] [Google Scholar]
Kühl 2013 {published data only}
- Kühl S, Payer M, Kirmeier R, Wildburger A, Acham S, Jakse N. The influence of particulated autogenous bone on the early volume stability of maxillary sinus grafts with biphasic calcium phosphate: a randomized clinical trial. Clinical Implant Dentistry and Related Research 2013 May 28 [Epub ahead of print]. [DOI: 10.1111/cid.12086] [DOI] [PubMed]
Mangano 2007 {published and unpublished data}
- Mangano C, Scarano A, Perrotti V, Iezzi G, Piattelli A. Maxillary sinus augmentation with a porous synthetic hydroxyapatite and bovine‐derived hydroxyapatite: a comparative clinical and histologic study. International Journal of Oral and Maxillofacial Implants 2007;22(6):980‐6. [PubMed] [Google Scholar]
Payer 2013 {published data only}
- Payer M, Lohberger B, Strunk D, Reich KM, Acham S, Jakse N. Effects of directly autotransplanted tibial bone marrow aspirates on bone regeneration and osseointegration of dental implants. Clinical Oral Implants Research 2013;23:1‐7. [DOI] [PubMed] [Google Scholar]
Pikdöken 2011 {published data only}
- Pikdöken L, Gürbüzer B, Küçükodacý Z, Urhan M, Barýs E, Tezulas E. Scintigraphic, histologic, and histomorphometric analyses of bovine bone mineral and autogenous bone mixture in sinus floor Augmentation: a randomized controlled trial ‐ results after 4 months of healing. Journal of Oral and Maxillofacial Surgery 2001;69:160‐9. [DOI] [PubMed] [Google Scholar]
Sammartino 2011 {published data only}
- Sammartino G, Mariniello M, Scaravilli MS. Benign paroxysmal positional vertigo following closed sinus floor elevation procedure: mallet osteotomes vs. screwable osteotomes. A triple blind randomized controlled trial. Clinical Oral implants Research 2011;22:669–72. [DOI] [PubMed] [Google Scholar]
Schaaf 2008 {published and unpublished data}
- Schaaf H, Streckbein P, Lendeckel S, Heidinger K, Görtz B, Bein G, et al. Topical use of platelet‐rich plasma to influence bone volume in maxillary augmentation: a prospective randomized trial. Vox Sanguinis 2008;94(1):64‐9. [DOI] [PubMed] [Google Scholar]
- Schaaf H, Streckbein P, Lendeckel S, Heidinger K, Rehmann P, Boedeker RH, et al. Sinus lift augmentation using autogenous bone grafts and platelet‐rich plasma: radiographic results. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 2008;106:673‐8. [DOI] [PubMed] [Google Scholar]
Silvestri 2013 {published data only}
- Silvestri M, Martegani P, D'Avenia F, Farneti M, Capri D, Paolantoni G, Landi L. Simultaneous sinus augmentation with implant placement: histomorphometric comparison of 2 different grafting materials. A multicenter double‐blind prospective randomized controlled clinical trial. International Journal of Oral and Maxillofacial Implants 2013;28:543‐9. [DOI] [PubMed] [Google Scholar]
Steigmann 2005 {published data only}
- Steigmann M, Garg AK. A comparative study of bilateral sinus lifts performed with platelet‐rich plasma alone versus alloplastic graft material reconstituted with blood. Implant Dentistry 2005;14(3):261‐6. [DOI] [PubMed] [Google Scholar]
Suba 2006 {published data only}
- Suba Z, Takács D, Matusovicz D, Fazekas A, Szabó G, Barabás J. Quantitative and qualitative comparison of the maxillary bone regeneration after beta‐tricalcium phosphate and autogenous bone implantation [A maxilla csontregeneraciojanak mennyisegi es minosegi osszehasonlitasa beta‐tricalcium phosphate es autolog csontbeultetes utan]. Fogorvosi Szemle 2006;99(1):21‐8. [PubMed] [Google Scholar]
- Suba Z, Takács D, Matusovits D, Barabás J, Fazekas A, Szabó G. Maxillary sinus floor grafting with beta‐tricalcium phosphate in humans: density and microarchitecture of the newly formed bone. Clinical Oral Implants Research 2006;17(1):102‐8. [DOI] [PubMed] [Google Scholar]
Szabó 2005 {published and unpublished data}
- Szabó G, Huys L, Coulthard P, Maiorana C, Garagiola U, Barabas J, et al. A prospective multicenter randomized clinical trial of autogenous bone versus beta‐tricalcium phosphate graft alone for bilateral sinus elevation: histologic and histomorphometric evaluation. International Journal of Oral and Maxillofacial Implants 2005;20(3):371‐81. [PubMed] [Google Scholar]
Tawil 2001 {published data only}
- Tawil G, Mawla M. Sinus floor elevation using a bovine bone mineral (Bio‐Oss) with or without the concomitant use of a bilayered collagen barrier (Bio‐Guide): a clinical report of immediate and delayed implant placement. International Journal of Oral and Maxillofacial Implants 2001;16(5):713‐21. [PubMed] [Google Scholar]
Testori 2013 {published data only}
- Testori T, Wallace SS, Trisi P, Capelli M, Zuffetti F, Fabbro M. Effect of xenograft (ABBM) particle size on vital bone formation following maxillary sinus augmentation: a multicenter, randomized, clinical, controlled histomorphometric trial. The International Journal of Periodontics and Restorative Dentistry 2013;23:467‐75. [DOI] [PubMed] [Google Scholar]
Triplett 2009 {published data only}
- Triplett RG, Nevins M, Marx RE, Spagnoli DB, Oates TW, Moy PK, et al. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein‐2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. Journal of Oral and Maxillofacial Surgery 2009;67:1947‐60. [DOI] [PubMed] [Google Scholar]
Trombelli 2012 {published data only}
- Trombelli L, Franceschetti G, Rizzi A, Minenna P, Minenna L, Farina R. Minimally invasive transcrestal sinus floor elevation with graft biomaterials. A randomized clinical trial. Clinical Oral Implant Research 2012;23:424–32. [DOI] [PubMed] [Google Scholar]
Wagner 2012 {published data only}
- Wagner W, Wiltfang J, Pistner H, Yildirim M, Ploder B, Chapman M, et al. Bone formation with a biphasic calcium phosphate combined with fibrin sealant in maxillary sinus floor elevation for delayed dental implant. Clinical Oral Implants Research 2012;23:1112‐7. [DOI] [PubMed] [Google Scholar]
Yilmaz 2013 {published data only}
- Yilmaz S, Ozkan Karaca E, Dirikan Ipci S, Cakar G, Kuru BE, Kullu S, et al. Radiographic and histologic evaluation of platelet‐rich plasma and bovine‐derived xenograft combination in bilateral sinus augmentation procedure. Platelets 2013;24:308‐15. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Rickert 2014 {published data only}
- Rickert D, Vissink A, Slot WJ, Sauerbier S, Meijer HJ, Raghoebar GM. Maxillary sinus floor elevation surgery with BioOss® mixed with a bone marrow concentrate or autogenous bone: test of principle on implant survival and clinical performance. International Journal of Oral and Maxillofacial Surgery 2014;43(2):243‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Aghaloo 2007
- Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement?. International Journal of Oral and Maxillofacial Implants 2007;22 Suppl:49‐70. [PubMed] [Google Scholar]
Aparicio 2001
- Aparicio C, Perales P, Rangert B. Tilted implants as an alternative to maxillary sinus grafting: a clinical, radiologic, and Periotest study. Clinical Implant Dentistry and Related Research 2001;3:39‐49. [DOI] [PubMed] [Google Scholar]
Boyne 1980
- Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. Journal of Oral Surgery 1980;38:613‐6. [PubMed] [Google Scholar]
Brånemark 1977
- Brånemark PI, Hansson BO, Adell R, Breine U, Lindstrom J, Hallen O, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10‐year period. Scandinavian Journal of Plastic and Reconstructive Surgery. Supplementum 1977;16:1‐132. [PubMed] [Google Scholar]
Brånemark 2004
- Brånemark PI, Gröndahl K, Öhrnell LO, Nilsson, P, Petruson B, Svensson B, et al. Zygoma fixture in the management of advanced atrophy of the maxilla: technique and long‐term results. Scandinavian Journal of Plastic and Reconstructive Surgery 2004;38(2):70‐85. [DOI] [PubMed] [Google Scholar]
Cannizzaro 2007
- Cannizzaro G, Leone M, Consolo U, Ferri V, Licitra G, Worthington H, et al. Augmentation of the posterior atrophic edentulous maxilla with implants placed in the ulna: a prospective single‐blind controlled clinical trial. International Journal of Oral and Maxillofacial Implants 2007;22:280‐8. [PubMed] [Google Scholar]
Cawood 1991
- Cawood JI, Howell RA. Reconstructive preprosthetic surgery. I. Anatomical considerations. International Journal of Oral and Maxillofacial Surgery 1991;20(2):75‐82. [DOI] [PubMed] [Google Scholar]
Cosci 2000
- Cosci F, Luccioli M. A new sinus lift technique in conjunction with placement of 265 implants: a 6‐year retrospective study. Implant Dentistry 2000;9:363‐8. [DOI] [PubMed] [Google Scholar]
das Neves 2006
- das Neves FD, Fones D, Bernardes SR, do Prado CJ, Neto AJ. Short implants ‐ an analysis of longitudinal studies. International Journal of Oral and Maxillofacial Implants 2006;21:86‐93. [PubMed] [Google Scholar]
Davò 2013
- Davó R, Pons O. Prostheses supported by four immediately loaded zygomatic implants: a 3‐year prospective study. European Journal of Oral Implantology 2013;6(3):263‐9. [PubMed] [Google Scholar]
Del Fabbro 2004
- Fabbro M, Testori T, Francetti L, Weinstein R. Systematic review of survival rates for implants placed in the grafted maxillary sinus. The International Journal of Periodontics and Restorative Dentistry 2004;24(6):565‐77. [PubMed] [Google Scholar]
Del Fabbro 2013
- Fabbro M, Wallace SS, Testori T. Long‐term implant survival in the grafted maxillary sinus: a systematic review. International Journal of Periodontics and Restorative Dentistry 2013;33:772‐83. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Emmerich 2005
- Emmerich D, Att W, Stappert C. Sinus floor elevation using osteotomes: a systematic review and meta‐analysis. Journal of Periodontology 2005;76(8):1237‐51. [DOI] [PubMed] [Google Scholar]
Esposito 2007
- Esposito M, Murray‐Curtis L, Grusovin MG, Coulthard P, Worthington HV. Interventions for replacing missing teeth: different types of dental implants. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003815.pub3] [DOI] [PubMed] [Google Scholar]
Esposito 2009
- Esposito M, Grusovin MG, Felice P, Karatzopoulos G, Worthington HV, Coulthard P. Interventions for replacing missing teeth: horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003607.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Esposito 2011
- Esposito M, Cannizzaro G, Soardi E, Pellegrino G, Pistilli R, Felice P. A 3‐year post‐loading report of a randomised controlled trial on the rehabilitation of posterior atrophic mandibles: short implants or longer implants in vertically augmented bone?. European Journal of Oral Implantology 2011;4:301‐11. [PubMed] [Google Scholar]
Esposito 2013
- Esposito M, Worthington HV. Interventions for replacing missing teeth: dental implants in zygomatic bone for the rehabilitation of the severely deficient edentulous maxilla. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD004151.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gabbert 2009
- Gabbert O, Koob A, Schmitter M, Rammelsberg P. Implants placed in combination with an internal sinus lift without graft material: an analysis of short‐term failure. Journal of Clinical Periodontology 2009;36:177‐83. [DOI] [PubMed] [Google Scholar]
Graves 1994
- Graves SL. The pterygoid plate implant: a solution for restoring the posterior maxilla. The International Journal of Periodontics and Restorative Dentistry 1994;14:513‐23. [PubMed] [Google Scholar]
Hatano 2007
- Hatano N, Sennerby L, Lundgren S. Maxillary sinus augmentation using sinus membrane elevation and peripheral venous blood for implant‐supported rehabilitation of the atrophic posterior maxilla: case series. Clinical Implant Dentistry and Related Research 2007;9:150‐5. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lesaffre 2009
- Lesaffre E, Philstrom B, Needleman I, Worthington H. The design and analysis of split‐mouth studies: What statisticians and clinicians should know. Statistics in Medicine 2009;28(28):3470‐82. [DOI] [PubMed] [Google Scholar]
Lundgren 2004
- Lundgren S, Andersson S, Gualini F, Sennerby L. Bone reformation with sinus membrane elevation: a new surgical technique for maxillary sinus floor augmentation. Clinical Implant Dentistry and Related Research 2004;6:165‐73. [PubMed] [Google Scholar]
Maló 2013
- Maló P, Araújo Nobre M, Lopes A. Immediate loading of “All‐on‐4” maxillary prostheses using trans‐sinus tilted implants without sinus bone grafting: A retrospective study reporting the 3‐year outcome. European Journal of Oral Implantology 2013;6(3):273‐83. [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. [PubMed] [Google Scholar]
Nedir 2006
- Nedir R, Bischof M, Vazquez L, Szmukler‐Moncler S, Bernard J P. Osteotome sinus floor elevation without grafting material: a 1‐year prospective pilot study with ITI implants. Clinical Oral Implants Research 2006;17:679‐86. [DOI] [PubMed] [Google Scholar]
Nkenke 2009
- Nkenke E, Stelzle F. Clinical outcomes of sinus floor augmentation for implant placement using autogenous bone or bone substitutes: a systematic review. Clinical Oral implants Research 2009;20 Suppl 4:124‐33. [DOI] [PubMed] [Google Scholar]
Palmer 2000
- Palmer P, Palmer R. Implant surgery to overcome anatomical difficulties. In: Palmer R editor(s). A Clinical Guide to Implants in Dentistry. London: British Dental Association, 2000:57‐65. [Google Scholar]
Pjetursson 2008
- Pjetursson BE, Tan WC, Zwahlen M, Lang NP. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. Journal of Clinical Periodontology 2008;35(8 Suppl):216‐40. [DOI] [PubMed] [Google Scholar]
Pjetursson 2009
- Pjetursson BE, Ignjatovic D, Matuliene G, Brägger U, Schmidlin K, Lang NP. Transalveolar maxillary sinus floor elevation using osteotomes with or without grafting material. Part II: Radiographic tissue remodeling. Clinical Oral Implants Research 2009;20:677‐83. [DOI] [PubMed] [Google Scholar]
Sohn 2008
- Sohn DS, Lee JS, Ahn MR, Shin HI. New bone formation in the maxillary sinus without bone grafts. Implant Dentistry 2008;17:321‐31. [DOI] [PubMed] [Google Scholar]
Summers 1994
- Summers RB. A new concept in maxillary implant surgery: the osteotome technique. Compendium of Continuing Education in Dentistry 1994;15:152‐8. [PubMed] [Google Scholar]
Summers 1995
- Summers RB. The osteotome technique: Part 4 ‐ Future site development. Compendium of Continuing Education in Dentistry 1995;16:1080‐92. [PubMed] [Google Scholar]
Tan 2008
- Tan WC, Lang NP, Zwahlen M, Pjetursson BE. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. Part II: transalveolar technique. Journal of Clinical Periodontology 2008;35(8 Suppl):241‐54. [DOI] [PubMed] [Google Scholar]
Tatum 1986
- Tatum H. Maxillary and sinus implant reconstructions. Dental Clinics of North America 1986;30(2):207‐29. [PubMed] [Google Scholar]
Thor 2007
- Thor A, Sennerby L, Hirsch JM, Rasmusson L. Bone formation at the maxillary sinus floor following simultaneous elevation of the mucosal lining and implant installation without graft material: an evaluation of 20 patients treated with 44 Astra Tech implants. Journal of Oral and Maxillofacial Surgery 2007;65(7 Suppl 1):64‐72. [DOI] [PubMed] [Google Scholar]
Tong 1998
- Tong DC, Rioux K, Drangsholt M, Beirne OR. A review of survival rates for implants placed in grafted maxillary sinuses using meta‐analysis. International Journal of Oral and Maxillofacial Implants 1998;13(2):175‐82. [PubMed] [Google Scholar]
Urist 1965
- Urist MR. Bone: formation by autoinduction. Science 1965;150(3698):893‐9. [DOI] [PubMed] [Google Scholar]
Valentin‐Opran 2002
- Valentin‐Opran A, Wozney J, Csimma C, Lilly L, Riedel GE. Clinical evaluation of recombinant human bone morphogenic protein‐2. Clinical Orthopaedics and Related Research 2002;395:110‐20. [DOI] [PubMed] [Google Scholar]
Wallace 2003
- Wallace SS, Froum SJ. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Annals of Periodontology 2003;8(1):328‐43. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Coulthard 2003
- Coulthard P, Esposito M, Jokstad A, Worthington HV. Interventions for replacing missing teeth: bone augmentation techniques for dental implant treatment. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003607] [DOI] [PubMed] [Google Scholar]
Esposito 2006a
- Esposito M, Grusovin MG, Worthington HV, Coulthard P. Interventions for replacing missing teeth: bone augmentation techniques for dental implant treatment. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD003607.pub2] [DOI] [PubMed] [Google Scholar]
Esposito 2006b
- Esposito M, Grusovin MG, Coulthard P, Worthington HV. The efficacy of various bone augmentation procedures for dental implants: a Cochrane systematic review of randomized controlled clinical trials. The International Journal of Oral and Maxillofacial Implants 2006;21(5):696‐710. [PubMed] [Google Scholar]
Esposito 2008
- Esposito M, Grusovin MG, Kwan S, Worthington HW, Coulthard P. Interventions for replacing missing teeth: bone augmentation techniques for dental implant treatment. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD003607.pub3] [DOI] [PubMed] [Google Scholar]
Esposito 2010
- Esposito M, Grusovin MG, Rees J, Karasoulos D, Felice P, Alissa R, et al. Interventions for replacing missing teeth: augmentation procedures of the maxillary sinus. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD008397] [DOI] [PubMed] [Google Scholar]
Esposito 2010b
- Esposito M, Grusovin MG, Rees J, Karasoulos D, Felice P, Alissa R, et al. Effectiveness of sinus lift procedures for dental implant rehabilitation: a Cochrane systematic review. European Journal of Oral Implantology 2010;3:7‐26. [PubMed] [Google Scholar]


