Abstract
Polyunsaturated fatty acids (PUFAs) have been extensively studied for their health benefits because they can be oxidized by lipoxygenases to form bioactive oxylipins. In this study, we investigated the impact of double bond placement on the kinetic properties and product profiles of human platelet 12-lipoxygenase (h12-LOX), human reticulocyte 15-lipoxygenase-1 (h15-LOX-1), and human endothelial 15-lipoxygenase-2 (h15-LOX-2) by using 22-carbon (C22) fatty acid substrates with differing double bond content. With respect to kcat/KM values, the loss of Δ4 and Δ19 led to an 18-fold loss of kinetic activity for h12-LOX, no change in kinetic capability for h15-LOX-1, but a 24-fold loss for h15-LOX-2 for both C22–FAs. With respect to the product profiles, h12-LOX produced mainly 14-oxylipins. For h15-LOX-1, the 14-oxylipin production increased with the loss of either Δ4 and Δ19, however, the 17-oxylipin became the major species upon loss of both Δ4 and Δ19. h15-LOX-2 produced mostly the 17-oxylipin products throughout the fatty acid series. This study also investigated the effects of various 17-oxylipins on platelet activation. The results revealed that both 17(S)-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-DHA (17-HDHA) and 17-hydroxy-4Z,7Z,10Z,13Z,15E-DPAn6 (17-HDPAn6) demonstrated anti-aggregation properties with thrombin or collagen stimulation. 17-hydroxy-7Z,10Z,13Z,15E,19Z-DPAn3 (17-HDPAn3) exhibited agonistic properties, and 17-hydroxy-7Z,10Z,13Z,15E-DTA (17-HDTA) showed biphasic effects, inhibiting collagen-induced aggregation at lower concentrationsbut promoting aggregation at higher concentrations. Both 17-hydroxy-13Z,15E,19Z-DTrA (17-HDTrA), and 17-hydroxy-13Z,15E-DDiA (17-HDDiA) induced platelet aggregation. In summary, the number and placement of the double bonds affect platelet activation, with the general trend being that more double bonds generally inhibit aggregation, while less double bonds promote aggregation. These findings provide insights into the potential role of specific fatty acids and their metabolizing LOX isozymes with respect to cardiovascular health.
1. Introduction
Omega-3 fatty acids are a group of essential fatty acids (FA) that are important for preventing hypertriglyceridemia [1], hypertension [2], inflammation [3,4], arrhythmia [5,6], atherosclerosis [7,8], and thrombosis [9,10]. cis-4,7,10,13,16,19-docosahexaenoic acid (DHA) is a long-chain polyunsaturated fatty acid with six double bonds (22:6) that is found in high concentrations in salmon, mackerel, and tuna. DHA plays a role in preventing atherothrombosis, which is the formation of blood clots within arteries [11-13]. Docosapentaenoic acid (DPA), another essential FA, is a 22-carbon long polyunsaturated fatty acid with five double bonds (22:5) [14,15], which can be both an omega-3 (DPAn3) and an omega-6 fatty acid (DPAn6) (15). These two DPA fatty acids have been shown to be important in the prevention of atherosclerosis [16,17]. DPAn3 affects colorectal cancer [18], chronic inflammatory diseases [19,20], and myocardial infarct [21], while DPAn6 affects stroke [22]. In addition, higher levels of cis-7,10,13,16,19-docosapentaenoic acid (DPAn3) have been shown to be associated with improved cognitive function, specifically memory [23-25]. One other essential FA, cis-7,10,13,16-docosatetraenoic acid (DTA), is a 22-carbon long polyunsaturated fatty acid with four double bonds (22:4), it composes 17.5% of all fatty acids found in cerebral grey matter [26,27], has been shown to have an inverse association with fetal growth [28], and is positively associated with primary open-angle glaucoma, a disease that leads to vision loss and blindness [29-33].
The biological benefits of these fatty acids can be attributed to the fact that they can be oxidized into oxylipins by lipoxygenases (LOXs). Lipoxygenases are a family of enzymes that catalyze the oxidation of polyunsaturated fatty acids (PUFAs) to form a variety of biologically active lipid mediators, including leukotrienes and lipoxins [34-39]. There is a wide spectrum of PUFA substrates that can be catalyzed by lipoxygenase isozymes in the human body [40-42]. A key chemical property of LOX substrates is the presence of the 1,4-pentadiene moiety, with its position relative to either the carboxylate or methyl end of the fatty acids dictating which LOX isozyme is a competent catalyst [43-45]. Arachidonic acid (AA) is the canonical substrate of LOX isozymes due to its high levels in the human body and thus the LOX isozymes are named according to their positional specificity with AA [46-50].
With respect to the biological role of LOX isozymes, platelet activation is highly dependent on LOX products, as seen by the varied aggregation response with the addition of oxylipins [22,51-58]. The 12-oxylipin products of AA and eicosapentaenoic acid (EPA, C20:5) do not inhibit platelet aggregation, while the dihomo-γ-linolenic acid (DGLA, C20:3) product does inhibit aggregation, indicating the positional importance of double bonds with respect to 12-oxylipin activity [52,53]. Interestingly, the 15-oxylipins of all three of these C20 fatty acids inhibit platelet aggregation, indicating that both the number of double bonds and the position of the oxidation are critical to the selectivity of receptor binding [51]. This hypothesis is extended to DHA (C22:6), where its 11-, 14-, and 17-oxylipins are all potent aggregation inhibitors, with their potency increasing with the loss of a double bond at either end of the C22-oxylipin (i.e., DPAn3 and DPAn6 oxylipins versus DHA oxylipins) [53,56,59,60]. These data highlight the biological importance of double bond location, length, and oxidation position of oxylipins with respect to platelet activation.
In the current work, the kinetics, product profile, and platelet activity of a family of C22–FAs against h12-LOX, h15-LOX-1, and h15-LOX-2 were performed. This C22 family of fatty acids includes DHA (22:6), DPAn3 (22:5), cis-4,7,10,13,16-docosapentaenoic acid (DPAn6) (22:5), DTA (22:4), cis-13,16,19-docosatrienoic acid (DTrA) (22:3), and cis-13,16-docosadienoic acid (DDiA) (22:2) (Fig. 1). By quantifying the catalytic efficiency of these C22–FAs and examining the distribution of oxylipins, their biological availability was determined. Finally, the platelet activity of these oxylipins was determined, establishing the effect of double bond positioning on platelet aggregation.
Fig. 1.
C22–FAs used in this study.
2. Experimental procedures
2.1. Chemicals
Fatty acids used in this study were purchased from Nu Chek Prep, Inc. (MN, USA) and the oxylipins isolated in house (vide infra). Soybean Lipoxygenase-1 (SLO-1) was purchased from Cayman Chemicals. All other solvents and chemicals were reagent grade or better and were used as purchased without further purification.
2.2. Expression and purification of 12LOX, h15-LOX-1, and h15-LOX-2
Recombinant expression and purification of his-tagged h12-LOX (Uniprot entry P18054), h15-LOX-1 (Uniprot entry P16050) and h15-LOX-2 (Uniprot entry O15296) were performed using nickel-affinity chromatography, as previously described [61,62]. The purity of h12LOX, h15-LOX-1 and h15-LOX-2 were assessed by SDS gel to be greater than 85%. The metal content was assessed on a Finnigan inductively-coupled plasma-mass spectrometer (ICP-MS), via comparison with iron standard solution and applied to the kinetic parameters. Cobalt-EDTA was used as an internal standard.
2.3. Product determination and isolation
h15-LOX-1 (0.125 μM, pH 7.5), h12-LOX (0.300 μM, pH 8.0), h15-LOX-2 (0.5 μM, pH 7.5) were reacted in 2 mL of 25 mM HEPES at room temperature and ambient oxygen. The enzymatic turnover was monitored by absorbance at 234 nm, with 20 μM fatty acid reacted until 20% turnover occurred (DHA, DPAn3, DPAn6, DTA, DTrA and DDiA). The reactions were quenched with 1% glacial acetic acid and extracted three times with dichloromethane (DCM). The products were then reduced with trimethyl phosphite and evaporated under a stream of nitrogen gas. The reaction products were reconstituted in methanol and analyzed via liquid chromatography-tandem mass spectrometry (LC-MS/MS). Chromatographic separation was performed using a C18 column (Phenomenex Kinetex, 4 μm, 150 × 2.0 mm). Mobile phase solvent A consisted of 99.9% water and 0.1% formic acid, and solvent B consisted of 99.9% acetonitrile and 0.1% formic acid. Analysis was carried out over 60 min using isocratic 50:50 A:B for 0–30 min followed by a gradient from 50:50 A:B to 75:25 A:B from 30 to 60 min. The chromatography system was coupled to a Thermo-Electron LTQ LC-MS/MS instrument for mass analysis. All analyses were performed in negative ionization mode at the normal resolution setting. MS/MS was performed in a targeted manner with a mass list containing the following m/z ratios containing the following m/z ratios of 343.2 ± 0.5 (HDHAs), 345.2 ± 0.5 (HDPAs), 347.2 ± 0.5 (HDTAs), 349.2 ± 0.5 (HDTrAs), 351.2 ± 0.5 (HDDiAs) were used (see Supporting Information, Figs. S1-S5). Matching retention times, UV spectra, and fragmentation patterns for four common fragments were used to identify products without standards. To identify the known standards, at least three common fragments were used to identify the products (listed in the supporting information). DHA fragmentations used to identify our oxylipins were from previous literature [54,63]. The commercially obtained standards that were used were: d8-12-HETE, 17-HDHA, 14-HDHA, 11-HDHA, and 12-HETE. It should be noted that the enzyme products coeluted with the known standards, so this fact along with the catalytic nature of LOX isozymes allowed us to assume the products were of the S-configuration.
For product isolation for platelet activity, the same protocol was used except for the high-performance liquid chromatography (HPLC) purification method and amounts of enzyme and fatty acid used. The products were purified isocratically via HPLC on a Higgins Haisil Semi-preparative (5 μm, 250 mm × 10 mm) C18 column with 45:55 acetonitrile: water, and 0.1% acetic acid. Oxylipin production reactions required at least 2 h to complete. 17-HDHA was synthesized from DHA (50 μM) and h15-LOX-1. 17-DPAn3 was synthesized by SLO-1 and DPAn3 (50 μM), and 17-DPAn6 was synthesized by SLO-1 with DPAn6 (50 μM). 17-HDTA and 14-HDTA were synthesized by reaction of DTA (50 μM) with h15-LOX-1 and h12-LOX respectively. 17-HDTrA was synthesized by reaction of DTrA (50 μM) with SLO-1. 17-HDDiA were synthesized by reaction of DDiA (50 μM) with SLO-1.
2.4. Steady-state kinetics
h12-LOX, h15-LOX-1 and h15-LOX-2 reactions were performed at 22 °C in a 1 cm2 quartz cuvette containing 2 mL of 25 mM HEPES (pH 7.5) with substrates (DHA, DPAn3, DPAn6, DTA, DTrA, and DDiA). All substrate concentrations varied from 0.25 to 25 μM. Concentrations of all substrates were determined by measuring the amount of oxylipins produced from complete reaction with SLO-1. Concentrations of oxylipins were determined by measuring the absorbance at 234 nm. Reactions were initiated by the addition of h12-LOX (130 nM), h15-LOX-1 (300 nM) and h15-LOX-2 (325 nM). Product formation was determined by the increase in absorbance at 234 nm for oxylipin products (ε234nm = 25,000 M−1 cm−1) with a PerkinElmer Lambda 45 UV/Vis spectrophotometer. KaleidaGraph (Synergy) was used to fit initial rates (at less than 10% turnover), to the Michaelis-Menten equation for the calculation of kinetic parameters.
2.5. Oxylipin titration in human platelet aggregometry
All research involving human subjects was carried out in accordance with the Declaration of Helsinki and approved by the University of Michigan Institutional Review Board (approval number: HUM00100677). Written informed consent was obtained prior to blood collection. Washed platelets were isolated from human whole blood via serial centrifugation and resuspended at a concentration of 3.0 × 108 platelets per milliliter in Tyrode’s buffer (10 mM HEPES, 12 mM NaHCO3, 127 mM NaCl, 5 mM KCl, 0.5 mM NaH2PO4, 1 mM MgCl2, and 5 mM glucose), as previously published [56,64,65]. Platelets (250 μl at 3.0 × 108 platelets per milliliter) were dispensed into glass cuvettes and oxylipin at the indicated concentrations (0–40 μM) was added to the platelet solution. The aggregation response to each oxylipin was measured in a Chrono-log Model 700D lumi-aggregometer for 10 min at 37 °C. Oxylipins that did not induce platelet aggregation were subsequently added to 250 μl of platelets and incubated for 10 min at 37 °C to determine if the oxylipin had an inhibitory effect. Oxylipin-treated platelets were stimulated with an EC80 concentration of either collagen (Chrono-log) or thrombin receptor agonist under stirring conditions (1100 rpm) at 37 °C. Platelet aggregation was recorded for 10 min. Bar graphs are shown in the following figures (vide infra), however, aggregation dose responses are shown as Figs. S6-S9 in the supporting information.
3. Results and discussion
3.1. h12-LOX kinetics and product profile of fatty acids
The kcat values for h12-LOX with DHA, DPAn3, DPAn6, and DTA at 22 °C compare to that of AA (Table 1) [66]. They were 13 ± 0.4 s−1, 7.1 ± 0.5 s−1, 11 ± 0.9 s−1, and 0.40 ± 0.05 s−1, respectively; however, DTrA and DDiA were not substrates (Table 1). The kcat/KM for h12-LOX with DHA, DPAn3, DPAn6, and DTA at 22 °C was 7.4 ± 0.3 μM−1 s−1, 4.0 ± 0.3 μM−1 s−1, 5.3 ± 1 μM−1 s−1, and 0.30 ± 0.03 μM−1 s−1, respectively (Table 1). These data indicate that the loss of either Δ4 or Δ19 double bonds of DHA (i.e., DPAn3 and DPAn6) does not significantly affect the kcat or kcat/KM values. However, losing both Δ4 and Δ19 (i.e., DTA) causes more than a 10-fold decrease in the kcat and kcat/KM values and the loss of additional double bonds (i.e., DTrA and DDiA) leads to complete loss of catalysis. The latter results can be easily explained due to the lack of a 1,4-diene in the appropriate position relative to the active site iron for DTrA and DDiA, however, the 10-fold loss of activity for DTA relative to DPAn3 and DPAn6 is less clear. The loss of both Δ4 and Δ19 would make DTA more flexible and more hydrophobic due to the loss of double bonds, which could affect its positioning in the active site. Another possible explanation is the loss of an aromatic interaction. Previously, it was observed that F414 of h12-LOX pi-pi stacked with Δ11 of AA [66], therefore it is possible that there is an aromatic residue which could pi-pi stack with both Δ4 and Δ19, leading to a loss of catalytic activity for DTA. For example, a loss of activity relative to the number of double bonds was previously observed where the kcat value for mead acid (20:3) was ten-fold less than that found for AA (20:4), illustrating how the loss of a double bond could have drastic effects to LOX activity [66]. It should be noted that the kcat and kcat/KM values of DHA, DPAn3, DPAn6 with h12-LOX correlated well with our previous work [51,56,63,66,67].
Table 1.
Kinetics values of h12-LOX with various C22–FAs. NA = no activity.a previously published kinetic values (Aleem et al., 2019).
| kcat (s−1) | kcat/KM (μM−1 s−1) | KM (μM) | |
|---|---|---|---|
| AAa | 28 (1) | 14 (1) | 2 (0.2) |
| DHA | 13 (0.4) | 7.4 (0.3) | 1.7 (0.3) |
| DPAn3 | 7.1 (0.5) | 4.0 (0.3) | 1.8 (0.4) |
| DPAn6 | 11 (0.9) | 5.3 (1) | 2.2 (0.2) |
| DTA | 0.40 (0.05) | 0.30 (0.03) | 1.1 (0.09) |
| DTrA | NA | NA | NA |
| DDiA | NA | NA | NA |
With respect to product formation, h12-LOX with DHA and DPAn6 produced mostly the 14-oxylipin products, with the 14-oxylipin: 11-oxylipin ratios being 90:10 and 100:0, respectively (Table 2), consistent with previous work [55,58,64]. There is an increase in promiscuity in product formation with DPAn3 and DTA, with 17-oxylipins, 14-oxylipins, and 11-oxylipins being made in the ratios of 4:75:21 and 11:76:12, respectively. Nevertheless, h12-LOX still produces mainly the 14-oxylipin product for all the C22–FAs which had activity. Considering that an increase of the 17-oxylipin relative to the 14-oxylipin occurs when the substrate enters the active site less deeply, and the 11-oxylipin occurs when the fatty acid enters more deeply, it appears that loss of either Δ4 or both Δ4 and Δ19 leads to less precise oxidation. This is consistent with the substrate not being held as tightly in the active site, possibly due to an increase in flexibility/hydrophobicity and/or a loss of pi-pi stacking (vide supra) [51,56,63,66-70]. It is interesting to note that this hypothesis for product formation parallels the lowered rates observed for DTA but not DPAn3. This could indicate that the product ratios are more sensitive to enzyme/substrate interactions, indicating that the double bond at Δ4 is critical for product profiling, while the loss of both Δ4 and Δ19 is more critical for kinetic efficiency. We are currently investigating specific active site amino acid interactions with Δ4 and Δ19 through mutagenesis in order to tease out the specific interactions.
Table 2.
Product distribution of h12-LOX with various fatty acids. n/d = not detected, NA = no activity.
| 17-product | 14-product | 11-product | |
|---|---|---|---|
| DHA | n/d | 90 ± 1 | 10 ± 1 |
| DPAn6 | n/d | 100 ± 1 | n/d |
| DPAn3 | 4 ± 1 | 75 ± 1 | 21 ± 0.5 |
| DTA | 11 ± 0.2 | 76 ± 3 | 12 ± 1 |
| DTrA | NA | NA | NA |
| DDiA | NA | NA | NA |
3.2. h15-LOX-1 kinetics and product profile of fatty acids
The kcat values for h15-LOX-1 with DHA, DPAn3, DPAn6, DTA, DTrA, and DDiA at 22 °C compare to that of AA (Table 3) [71]. They were 1.6 ± 0.1 s−1, 1.0 ± 0.1 s−1, 1.7 ± 0.2 s−1, and 2.5 ± 0.04 s−1, 1.6 ± 0.06 s−1, and 3.3 ± 0.04 s−1 respectively. The kcat/KM with DHA, DPAn3, DPAn6, DTA, DTrA, and DDiA at 22 °C was 0.49 ± 0.03 μM−1 s−1, 0.72 ± 0.1 μM−1 s−1, 0.47 ± 0.1 μM−1 s−1, and 0.35 ± 0.01 μM−1 s−1, 0.37 ± 0.02 μM−1 s−1, and 0.81 ± 0.06 μM−1 s−1, respectively (Table 3). Surprisingly, there were no significant changes in the kcat or kcat/KM values with the loss of multiple double bonds in the C22-FA scaffold, indicating that the degree and/or placement of the double bonds does not affect h15-LOX-1 kinetics appreciably. This is consistent with past literature results which reported that the loss of the Δ5 double bond in AA did not affect the kinetic values for h5-LOX-1 [51]. It should be noted that the kcat and kcat/KM values of DHA with h15-LOX-1 correlated well with our previous work [51,56,63,66,67].
Table 3.
Kinetic values of h15-LOX-1 with various C22–FAs.a previously published kinetic values (Wecksler et al., 2008).
| kcat (s−1) | kcat/KM (μM−1 s−1) | KM (μM) | |
|---|---|---|---|
| AAa | 5.3 (0.5) | 2.0 (0.2) | 2.7 (0.3) |
| DHA | 1.6 (0.1) | 0.49 (0.03) | 3.3 (0.7) |
| DPAn3 | 1.0 (0.1) | 0.72 (0.1) | 1.4 (0.5) |
| DPAn6 | 1.7 (0.2) | 0.47 (0.1) | 3.7 (0.3) |
| DTA | 2.5 (0.04) | 0.35 (0.01) | 7.2 (0.1) |
| DTrA | 1.6 (0.06) | 0.37 (0.02) | 4.3 (0.2) |
| DDiA | 3.3 (0.04) | 0.81 (0.06) | 4.1 (0.2) |
With respect to product profiling, h15-LOX-1 reacts with DHA, DPAn6, and DPAn3 producing both the 14-oxylipin and 17-oxylipin products, with a preference for the 14-oxylipin products with DPAn3 and DPAn6 (Table 4). With DHA as the substrate, the ratio of 17HDHA:14HDHA:11HDHA was 48:45:7, which is consistent with previous work [51,56,63,66,67]. With DPAn6 as the substrate, the ratio of 17-HDPAn6:14-HDPAn6 was 37:63, with no 11-product being made. With DPAn3 as the substrate, the ratio of 17-HDPAn3:14-HDPAn3 was 23:77. However, with DTA as the substrate, the product preference shifts to the 17-oxylipin product, with the ratio of 17-HDTA:14-HD-TA:11-HDTA being 68:23:5. For DTrA and DDiA, the ratio of 17-HDTrA:13-HDTrA is 82:18 and 86:14, respectively. The 17-oxylipin was the majority product because the 14-oxylipin is not catalytically competent due the position of the 1,4-diene relative to the methyl end of the substrate.
Table 4.
Product distribution of h15-LOX-1 with various C22–FAs. n/d = not detected.
| 17 -product | 14 -product | 13-product | 11-product | |
|---|---|---|---|---|
| DHA | 48 ± 3 | 45 ± 2 | n/d | 7 ± 0.5 |
| DPAn6 | 37 ± 3 | 63 ± 3 | n/d | n/d |
| DPAn3 | 23 ± 3 | 77 ± 3 | n/d | n/d |
| DTA | 68 ± 2 | 23 ± 1 | n/d | 5 ± 0.4 |
| DTrA | 82 ± 1 | n/d | 18 ± 1 | n/d |
| DDiA | 86 ± 2 | n/d | 14 ± 2 | n/d |
Analyzing the kinetic and product data for h15-LOX-1, it is observed that the number and placement of double bonds do not affect enzymatic rates significantly, but the product profile is affected. When both the Δ4 and Δ19 double bonds are removed, there is a novel shift in the product specificity that has not been reported previously. For DHA, DPAn6, and DPAn3, the 14-oxylipin is the majority product, but for DTA, the 17-oxylipin is the majority product, indicating that DTA, slides less deep into the cavity site of h15-LOX-1 than DHA, DPAn6, and DPAn3, thus making more 17-oxylipins. Since DTA is both more flexible and more hydrophobic than either DPAn3 or DPAn6, this effect could be due to a general binding mode change. Another explanation could be that there is a specific residue interacting with the double bonds of DTA, pulling it further into the active site. However, considering that the effect is only observed when both Δ4 and Δ19 are removed, it appears there is not one interaction that is important, but rather that there is a synergistic effect between the two double bonds and the active site residues. Previously in the literature, a pi-pi interaction between the Δ11 or Δ14 double bond of AA and F414 was observed, but the Δ11 and Δ14 double bonds are in close proximity so in theory, F414 could interact with both double bonds [69,72]. The distance between Δ4 and Δ19 double bonds of DHA is approximately 4.5 Å, greater than that between Δ11 and Δ14 and thus one critical amino acid interaction seems unlikely. Nevertheless, H425 is within 5 Å of F414, suggesting the possibility that H425 could be at least partially responsible for the observed kinetic effects. We are currently beginning a mutagenesis campaign to probe the role of H425 and other active site residues that affect C22 substrate catalysis. With respect to DTrA and DDiA, 17-oxylipin is the major product because 14-oxylipin is not catalytically feasible. Due to improper double bond positioning relative to the active site iron, both fatty acids appear to flip and enter the active site in the opposite orientation. This reverse orientation could produce the 13-oxylipin from DTrA and DDiA, similar to that seen for h5-LOX catalysis [54,55,71,72], It is also possible that this change in product profile could also be due to improper substrate positioning in the active site, but this is less likely based on previous results (vide supra).
3.3. h15-LOX-2 kinetics and product profile of fatty acids
The kcat values for h15-LOX-2 with DHA, DPAn3, DPAn6, DTA, DTrA, and DDiA at 22 °C compare to that of AA (Table 5) [73]. They were 3.0 ± 0.1 s−1, 1.5 ± 0.07 s−1, 2.0 ± 0.1 s−1, and 0.13 ± 0.3 s−1, 0.029 ± 0.08 s−1, and 0.014 ± 0.02 s−1, respectively. The kcat/KM with DHA, DPAn3, DPAn6, DTA, DTrA, and DDiA at 22 ◦C was 0.78 ± 0.07 μM−1 s−1, 0.38 ± 0.08, 0.76 ± 0.2 μM−1 s−1, and 0.032 ± 0.004 μM−1 s−1, 0.022 ± 0.003 μM−1 s−1, and 0.045 ± 0.005 μM−1 s−1, respectively (Table 5). There is no significant difference in kcat or kcat/KM values with the loss of either Δ4 or Δ19 from DHA (i.e., DPAn3 and DPAn6). However, the loss of both Δ4 and Δ19, such as for DTA, DTrA and DDiA, leads to more than a greater than 10-fold difference in the kcat and kcat/KM for h15-LOX-2. As previously proposed for h12-LOX (vide supra), this loss of reactivity could be due to an increase in flexibility/hydrophobicity of the fatty acid, or a loss of a specific interaction with an active site residue, such as pi-pi stacking [70,72,74].
Table 5.
Kinetic values of h15-LOX-2 with various C22–FAs.a previously published kinetic values (Joshi et al., 2013).
| kcat (s−1) | kcat/KM (μM−1 s−1) | KM (μM) | |
|---|---|---|---|
| AAa | 1.5 (0.1) | 0.40 (0.05) | 3.8 (0.4) |
| DHA | 3.0 (0.1) | 0.78 (0.07) | 3.9 (0.3) |
| DPAn3 | 1.5 (0.07) | 0.38 (0.08) | 3.8 (0.4) |
| DPAn6 | 2.0 (0.1) | 0.76 (0.2) | 2.6 (0.5) |
| DTA | 0.13 (0.3) | 0.032 (0.004) | 4.0 (0.4) |
| DTrA | 0.029 (0.08) | 0.022 (0.003) | 1.3 (0.2) |
| DDiA | 0.014 (0.02) | 0.045 (0.005) | 0.31 (0.03) |
With all C22–FAs, h15-LOX-2 produced mostly the 17-oxylipins, consistent with its high level of specificity (Table 6). With DHA, DPAn6, DPAn3, and DTA, the ratios between the 17-product: 14-product were 94:6, 96:4, 92:8, and 100:0, respectively. An exception to this trend is the production of the 13-product with DTrA and DDiA, with the ratio of 17-HDDiA:13-HDDiA being 88:12 and 37:63, respectively. As discussed previously for h15-LOX-1 (vide supra), the production of the 14-oxylipin is not possible due to the positions of the double bonds relative to the terminal methyl, and thus 13-oxylipin from h15-LOX-2 and DTrA and DDiA is most likely due to the fatty acid entering the active site carboxylic acid end first presumably making the R-product, as seen for h5-LOX [75].
Table 6.
Product distribution of h15-LOX-2 with various C22–FAs. n/d = not detected.
| 17-product | 14 -product | 13-product | |
|---|---|---|---|
| DHA | 94 ± 1 | 6 ± 1 | n/d |
| DPAn6 | 96 ± 1 | 4 ± 1 | n/d |
| DPAn3 | 92 ± 4 | 8 ± 4 | n/d |
| DTA | 100 ± 1 | n/d | n/d |
| DTrA | 88 ± 1 | n/d | 12 ± 1 |
| DDiA | 37 ± 5 | n/d | 63 ± 5 |
Excluding the production of the 13-oxylipin, the number of double bonds does not affect the product profile for h15-LOX-2 substantially, which could be due to a lack of pi-pi stacking. Previous literature has shown that F414 is involved in pi-pi stacking with the substrate for h12-LOX and h15-LOX-1, but this residue does not exist in the active site of h15-LOX-2 [70,75], which could explain the lack of dependence on double bond content for product production.
3.4. Platelet aggregation effect of C22-oxylipins with variation of oxidation position and double bond number/placement
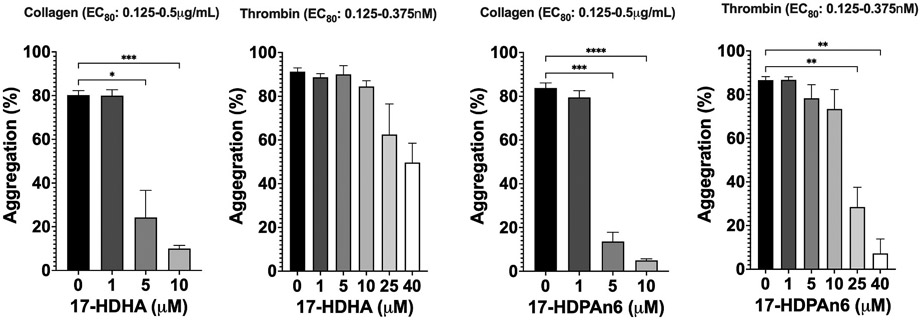
The C22-oxylipins were assessed for their ability to regulate platelet aggregation, either through a stimulatory role, an inhibitory role, or a biphasic capacity. Previously, it was demonstrated that 17-HDHA was a potent anti-aggregation effector molecule [53,59,76]. In the current investigation, this family of 17-oxylipins with 22 carbons was investigated further, with 17-HDPAn3, 17-HDPAn6, 17-HDTA, 17-HDTrA, and 17-HDDiA being assessed. In the present investigation, 17-HDHA demonstrated inhibition of collagen-stimulated platelets at relatively low concentrations (24±10% aggregation at 5 μM), while higher concentrations of 17-HDHA were required to inhibit thrombin-stimulated platelets (63±10% aggregation at 25 μM) (Fig. 2). 17-DPAn6 had comparable potency with 14±4% aggregation at 5 μM when stimulated with collagen and 29±9% at 25 μM when stimulated with thrombin (Fig. 2). Interestingly, 17-DPAn3 showed no inhibitory affects but did have agonistic properties with 26±10% aggregation at 5 μM with no platelet stimulation. 17-HDDiA and 17-HDTrA were also agonists with 55±10% and 84±2% at 5 μM, respectively (Fig. 3). 17-HDTA is the unusual oxylipin of this family of 17-oxylipins. At 0.5 μM of 17-HDTA, 20±10% platelet aggregation was observed with collagen added, making it a more potent inhibitor than either 17-HDHA or 17-HDPAn6. However, at higher concentrations, 17-HDTA was an agonist. At 5 μM of 17-HDTA, 41±10% aggregation was observed with no platelet stimulation (Fig. 4).
Fig. 2.
Inhibition of platelet aggregation with 17-HDHA and 17-HDPAn6.
Fig. 3.
Agonistic effect of platelet aggregation with 17-HDPAn3, 17-HDTrA and 17-HDDiA.
Fig. 4.
Biphasic effect of platelet aggregation with 17-HDTA.
In general, these data indicate that as the 17-oxylipin loses double bond content, it converts from a platelet activation inhibitor to a platelet activation agonist (Table 7). The transition point appears to be between 17-HDPAn3 and 17-HDPAn6, with loss of Δ19 maintaining the inhibitory properties of the 17-HDPAn6, but loss of Δ4 leads to agnostic properties (i.e., 17-HDPAn3). Interestingly, 17-HDTA, which has lost both Δ4 and Δ19, is both an inhibitor and an agonist. It is tempting to speculate that Δ4 and Δ19 on the 17-oxylipin regulate two distinct receptor pathways. The mechanism of action of these 17-oxylipins is currently being investigated to better understand these intriguing results.
Table 7.
Effects of oxylipins on platelet aggregation.
| Oxylipin | Inhibition Effect (Thrombin Stimulation) | Inhibition Effect Collagen Stimulation | Agonistic Effect No Stimulation |
|---|---|---|---|
| 17-HDHA | 63 ± 10% @ 25 μM | 24 ± 10% @ 5 μM | None |
| 17-DPAn6 | 29 ± 9% @ 25 μM | 14 ± 4% @ 5 μM | None |
| 17-DPAn3 | None | None | 26 ± 10% @ 5 μM |
| 17-HDTA | None | 20 ± 10% @ 0.5 μM | 41 ± 10% @ 5 μM |
| 17-HDTrA | None | None | 84 ± 2% @ 5 μM |
| 17-HDDiA | None | None | 55 ± 10% @ 5 μM |
| 14-HDTA | 49 ± 10% @ 10 μM | 4.8 ± 1% @ 5 μM | None |
With respect to the 14-oxylipins, it has previously been observed that 14-HDHA is a potent aggregation inhibitor with 20% aggregation at 5 μM with collagen stimulation [56]. 14-HDPAn6 was also shown to be a potent inhibitor, with 5% at 1 μM when stimulated with collagen and 10% at 1 μM, when stimulated with thrombin [22]. Considering the similar potency between the 14-oxylipins and the 17-oxylipins, the platelet aggregation effect was assessed for 14-HDTA to determine if the same transition to agonist as seen for 17-HDTA would also be observed for 14-HDTA. Contrary to the data for 17-HDTA, 14-HDTA was only an inhibitor, with 4.8±1% aggregation at 5 μM when stimulated with collagen and 49±10% at 10 μM when stimulated with thrombin (Fig. 5). These data indicate that the 14-oxylipins do not follow a similar platelet effect mechanism as do the 17-oxylipins and thus, the change in location of oxidation affects their mechanism of actions. This result is like that seen for the 12- and 15-oxylipins derived from AA, where 12-HETE induces pro-thrombotic platelet activity, while 15-HETE induces anti-thrombotic activity [51,53]. It should be noted that due to the lack of activity of h12-LOX against DTrA and DDiA, the isolation of 14-oxylipin products from these fatty acids was not achieved.
Fig. 5.
Inhibition of platelet aggregation with 14-HDTA.
4. Conclusion
In summary, the kinetic rates and product preference of each of the three LOX isozymes, h12-LOX, h15-LOX-1, and h15-LOX-2, is generally not affected if either Δ4 or Δ19 is removed from DHA (i.e., DPAn3 or DPAn6). However, if both Δ4 and Δ19 are removed, the rate for both h12-LOX and h15-LOX-2 is reduced more than 10-fold, suggesting that the change in carbon hybridization increases the flexibility and hydrophobicity of DTA, which leads to a less-productive ES complex. For h15-LOX-2, the rate and product distribution are affected even further with additional loss of double bonds (i.e., DTrA and DDiA), indicating the ES complex becomes even more un-productive with additional flexibility and hydrophobicity. In contrast, the kinetic values for h15-LOX-1 do not change appreciably with a decrease in the number of double bonds, indicating an ES complex that is less dependent on flexibility and hydrophobicity than that for h12-LOX and h15-LOX-2. Interestingly, while the kinetics of h15-LOX-1 are not affected by the double bond content of the substrate, the product profile is affected. As the C22–FAs have fewer double bonds, more of the 17-oxylipin is produced indicating a reduced penetration of the substrate into the active site. It is unclear why these C22–FAs have reduced active site penetration as their flexibility and hydrophobicity are increased. It is possible that the loss of a specific pi-pi interaction with an active site residue is lost, lowering the energetics of active site insertion. We are currently investigating if a specific active site residue interacts with the fatty acids and accounts for these data through mutagenesis.
With respect to the effect the 17-oxylipins had on platelet activity, the number of double bonds on the C22-FA-derived oxylipins had substantial effects, on both inhibition and aggregation. 17-HDHA and 17-HDPAn6 exhibited inhibitory effects on platelet aggregation, with the effect being larger with collagen activation. However, as the number of double bonds decreases, the effect of the 17-oxylipin switches to agonistic. The transition occurs with 17-DPAn3, which is purely agonistic, and 17-HDTA, which is mixed inhibitor/agnostic, with the key chemical feature being the loss of Δ4. Surprisingly, the 14-oxylipin of DTA, 14-HDTA, has a different effect on platelet aggregation. While 14-HDTA inhibits platelet aggregation with both thrombin and collagen, 17-HDTA is inhibitory at low concentrations but agonistic at high concentrations. These findings highlight the complexity of platelet regulation by oxylipins and may have implications for their biological significance. For example, since h15-LOX-1 reacts equally well with each of these four C22–FAs, but their 17-oxylipin effects on platelet aggregation are orthogonal to each other, the micro-distribution of these C22–FAs in one’s diet could have distinct consequences with regards to platelet activity and hence cardiovascular indications. In addition, although platelets do not contain 15-LOX isozymes, the oxylipins produced by 15-LOXs are highly potent biomolecules towards platelet activation, suggesting that other cells, such as leukocytes may influence platelet function significantly. We are currently investigating the inhibitory and agonistic mechanism of action of these oxylipins to determine the biochemical requirements for these distinct platelet effects.
Supplementary Material
Funding
NIH: GM131835 (MH), NIH: GM140223 (LS), AHA: 23PRE1019986 (LS).
Abbreviations
- LOX
lipoxygenase
- h12-LOX
human platelet 12S-lipoxygenase
- h15-LOX-1
human reticulocyte 15-lipoxygenase-1
- h15-LOX-2
human epithelial 15-lipoxygenase-2
- r15-LOX-1 or r12/15-LOX or rALOX15
rabbit 15S-LOX-1
- COX
cyclooxygenase
- WT h12-LOX
wild-type human platelet 12S-lipoxygenase
- SLO-1
soybean lipoxygense-1
- ML355
h12-LOX specific inhibitor
- NSAIDs
nonsteroidal anti-inflammatory drugs
- coxibs
COX-2 selective inhibitors
- ICP-MS
inductively coupled plasma mass spectroscopy
- SEC
size exclusion chromatography
- BSA
bovine serum albumin
- DCM
dichloromethane
- EDTA
Ethylenediaminetetraacetic acid
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- PDB
Protein Data Bank
- AA
arachidonic acid
- 12(S)-HpETE
12(S)-hydroperoxyeicosatetraenoic acid
- 12(S)-HETE
12(S)-hydroxyeicosatetraenoic acid
- 12(S)-HETrE
12(S)-hydroxyeicosatrienoic acid
- d8-12HETE
12(S)-Hydroxyeicosatetraenoic Acid-d8
- C22–FAs
22 carbon long fatty acids
- DHA
cis-4,7,10,13,16,19-docosahexaenoic acid
- 11-HDHA
11(S)-hydroxy-4Z,7Z,9E,13Z,16Z,19Z-DHA
- 14-HDHA
14(S)-hydroxy-4Z,7Z,10Z,12E,16Z,19Z-DHA
- 17S-HDHA
17(S)-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-DHA
- 20-HDHA
20-hydroxy-4Z,7Z,10Z,13Z,16Z,18E-DHA
- DPAn3
cis-7,10,13,16,19-docosapentaenoic acid
- 11-HDPAn3
11-hydroxy-7Z,9E,13Z,16Z,19Z-DPAn3
- 14-HDPAn3
14-hydroxy-7Z,10Z,12E,16Z,19Z-DPAn3
- 17-HDPAn3
17-hydroxy-7Z,10Z,13Z,15E, 19Z -DPAn3
- DPAn6
cis-4,7,10,13,16-docosapentaenoic acid
- 11-HDPAn6
11-hydroxy-4Z,7Z,9E,13Z,16Z-DPAn6
- 14-HDPAn6
14-hydroxy-4Z,7Z,10Z,12E, 16Z – DPAn6
- 17-HDPAn6
17-hydroxy-4Z,7Z,10Z,13Z,15E-DPAn6
- DTA
cis-7,10,13,16-docosatetraenoic acid
- 11-HDTA
11-hydroxy-7Z,9E,13Z,16Z-DTA
- 14-HDTA
14-hydroxy-7Z,10Z,12E,16Z-DTA
- 17-HDTA
17-hydroxy-7Z,10Z,13Z,15E-DTA
- DTrA
cis-13,16,19-docosatrienoic acid
- 13-HDTrA
13-hydroxy-14E,16Z,19Z-DTrA
- 17-HDTrA
17-hydroxy-13Z,15E,19Z-DTrA
- DDiA
cis-13,16-docosadienoic acid
- 13-HDDiA
13-hydroxy-14E,16Z-HDDiA
- 17-HDDiA
17-hydroxy-13Z,15E-DDiA
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.abb.2023.109742.
Data availability
No data was used for the research described in the article.
References
- [1].Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK, Jacobson TA, Engler MB, Miller M, Robinson JG, Blum CB, Rodriguez-Leyva D, de Ferranti SD, Welty FK, Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American heart association, Circulation 140 (2019) e673–e691. [DOI] [PubMed] [Google Scholar]
- [2].Cabo J, Alonso R, Mata P, Omega-3 fatty acids and blood pressure, Br. J. Nutr 107 (Suppl 2) (2012) S195–S200. [DOI] [PubMed] [Google Scholar]
- [3].Simopoulos AP, Omega-3 fatty acids in inflammation and autoimmune diseases, J. Am. Coll. Nutr 21 (2002) 495–505. [DOI] [PubMed] [Google Scholar]
- [4].Calder PC, Omega-3 fatty acids and inflammatory processes: from molecules to man, Biochem. Soc. Trans 45 (2017) 1105–1115. [DOI] [PubMed] [Google Scholar]
- [5].Cheng JW, Santoni F, Omega-3 fatty acid: a role in the management of cardiac arrhythmias? J. Alternative Compl. Med 14 (2008) 965–974. [DOI] [PubMed] [Google Scholar]
- [6].Curfman G, Omega-3 fatty acids and atrial fibrillation, JAMA 325 (2021) 1063. [DOI] [PubMed] [Google Scholar]
- [7].Alfaddagh A, Kapoor K, Dardari ZA, Bhatt DL, Budoff MJ, Nasir K, Miller M, Welty FK, Miedema MD, Shapiro MD, Tsai MY, Blumenthal RS, Blaha MJ, Omega-3 fatty acids, subclinical atherosclerosis, and cardiovascular events: implications for primary prevention, Atherosclerosis 353 (2022) 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Barry AR, Dixon DL, Omega-3 fatty acids for the prevention of atherosclerotic cardiovascular disease, Pharmacotherapy 41 (2021) 1056–1065. [DOI] [PubMed] [Google Scholar]
- [9].Zheng X, Jia R, Li Y, Liu T, Wang Z, Omega-3 fatty acids reduce post operative risk of deep vein thrombosis and pulmonary embolism after surgery for elderly patients with proximal femoral fractures: a randomized placebo-controlled, double-blind clinical trial, Int. Orthop 44 (2020) 2089–2093. [DOI] [PubMed] [Google Scholar]
- [10].McEwen BJ, Morel-Kopp MC, Tofler GH, Ward CM, The effect of omega-3 polyunsaturated fatty acids on fibrin and thrombin generation in healthy subjects and subjects with cardiovascular disease, Semin. Thromb. Hemost 41 (2015) 315–322. [DOI] [PubMed] [Google Scholar]
- [11].Horrocks LA, Yeo YK, Health benefits of docosahexaenoic acid (DHA), Pharmacol. Res 40 (1999) 211–225. [DOI] [PubMed] [Google Scholar]
- [12].Calder PC, Docosahexaenoic acid, Ann. Nutr. Metab 69 (Suppl 1) (2016) 7–21. [DOI] [PubMed] [Google Scholar]
- [13].Li J, Pora BLR, Dong K, Hasjim J, Health benefits of docosahexaenoic acid and its bioavailability: a review, Food Sci. Nutr 9 (2021) 5229–5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Miller E, Kaur G, Larsen A, Loh SP, Linderborg K, Weisinger HS, Turchini GM, Cameron-Smith D, Sinclair AJ, A short-term n-3 DPA supplementation study in humans, Eur. J. Nutr 52 (2013) 895–904. [DOI] [PubMed] [Google Scholar]
- [15].Drouin G, Rioux V, Legrand P, The n-3 docosapentaenoic acid (DPA): a new player in the n-3 long chain polyunsaturated fatty acid family, Biochimie 159 (2019) 36–48. [DOI] [PubMed] [Google Scholar]
- [16].Chen J, Jiang Y, Liang Y, Tian X, Peng C, Ma KY, Liu J, Huang Y, Chen ZY, DPA n-3, DPA n-6 and DHA improve lipoprotein profiles and aortic function in hamsters fed a high cholesterol diet, Atherosclerosis 221 (2012) 397–404. [DOI] [PubMed] [Google Scholar]
- [17].de Oliveira Otto MC, Wu JH, Baylin A, Vaidya D, Rich SS, Tsai MY, Jacobs DR Jr., Mozaffarian D, Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis, J. Am. Heart Assoc 2 (2013), e000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Morin C, Rousseau É, Fortin S, Anti-proliferative effects of a new docosapentaenoic acid monoacylglyceride in colorectal carcinoma cells, Prostaglandins Leukot. Essent. Fatty Acids 89 (2013) 203–213. [DOI] [PubMed] [Google Scholar]
- [19].Skulas-Ray AC, Flock MR, Richter CK, Harris WS, West SG, Kris-Etherton PM, Red blood cell docosapentaenoic acid (DPA n-3) is inversely associated with triglycerides and C-reactive protein (CRP) in healthy adults and dose-dependently increases following n-3 fatty acid supplementation, Nutrients 7 (2015) 6390–6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gobbetti T, Dalli J, Colas RA, Federici Canova D, Aursnes M, Bonnet D, Alric L, Vergnolle N, Deraison C, Hansen TV, Serhan CN, Perretti M, Protectin D1(n-3 DPA) and resolvin D5(n-3 DPA) are effectors of intestinal protection, Proc. Natl. Acad. Sci. U. S. A 114 (2017) 3963–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sun Q, Ma J, Campos H, Rexrode KM, Albert CM, Mozaffarian D, Hu FB, Blood concentrations of individual long-chain n-3 fatty acids and risk of nonfatal myocardial infarction, Am. J. Clin. Nutr 88 (2008) 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yeung J, Adili R, Yamaguchi A, Freedman CJ, Chen A, Shami R, Das A, Holman TR, Holinstat M, Omega-6 DPA and its 12-lipoxygenase-oxidized lipids regulate platelet reactivity in a nongenomic PPARα-dependent manner, Blood Adv. 4 (2020) 4522–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dyall SC, Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA, Front. Aging Neurosci 7 (2015) 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lim SY, Hoshiba J, Salem N Jr., An extraordinary degree of structural specificity is required in neural phospholipids for optimal brain function: n-6 docosapentaenoic acid substitution for docosahexaenoic acid leads to a loss in spatial task performance, J. Neurochem 95 (2005) 848–857. [DOI] [PubMed] [Google Scholar]
- [25].Welty FK, Omega-3 fatty acids and cognitive function, Curr. Opin. Lipidol 34 (2023) 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Böckmann KA, von Stumpff A, Bernhard W, Shunova A, Minarski M, Frische B, Warmann S, Schleicher E, Poets CF, Franz AR, Fatty acid composition of adipose tissue at term indicates deficiency of arachidonic and docosahexaenoic acid and excessive linoleic acid supply in preterm infants, Eur. J. Nutr 60 (2021) 861–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gorusupudi A, Chang FY, Nelson K, Hageman GS, Bernstein PS, n-3 PUFA supplementation alters retinal very-long-chain-PUFA levels and ratios in diabetic animal models, Mol. Nutr. Food Res 63 (2019), e1801058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li LJ, Wu J, Chen Z, Weir NL, Tsai MY, Albert P, Zhang C, Plasma phospholipid polyunsaturated fatty acids composition in early pregnancy and fetal growth trajectories throughout pregnancy: findings from the US fetal growth studies-singletons cohort, EBioMedicine 82 (2022), 104180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen Y, Qiu X, Yang J, Comparing the in vitro antitumor, antioxidant and anti-inflammatory activities between two new very long chain polyunsaturated fatty acids, docosadienoic acid (DDA) and docosatrienoic acid (DTA), and docosahexaenoic acid (DHA), Nutr. Cancer 73 (2021) 1697–1707. [DOI] [PubMed] [Google Scholar]
- [30].Robinson LE, Mazurak VC, N-3 polyunsaturated fatty acids: relationship to inflammation in healthy adults and adults exhibiting features of metabolic syndrome, Lipids 48 (2013) 319–332. [DOI] [PubMed] [Google Scholar]
- [31].Saito H, Ioka H, Lipids and fatty acids of sea hares Aplysia kurodai and Aplysia juliana: high levels of icosapentaenoic and n-3 docosapentaenoic acids, J. Oleo Sci 68 (2019) 1199–1213. [DOI] [PubMed] [Google Scholar]
- [32].AbuGhazaleh AA, Holmes LD, Jacobson BN, Kalscheur KF, Short communication: eicosatrienoic acid and docosatrienoic acid do not promote vaccenic acid accumulation in mixed ruminal cultures, J. Dairy Sci 89 (2006) 4336–4339. [DOI] [PubMed] [Google Scholar]
- [33].Innis SM, Vaghri Z, King DJ, n-6 Docosapentaenoic acid is not a predictor of low docosahexaenoic acid status in Canadian preschool children, Am. J. Clin. Nutr 80 (2004) 768–773. [DOI] [PubMed] [Google Scholar]
- [34].Kretzer C, Jordan PM, Bilancia R, Rossi A, Gür Maz T, Banoglu E, Schubert US, Werz O, Shifting the biosynthesis of leukotrienes toward specialized pro-resolving mediators by the 5-lipoxygenase-activating protein (FLAP) antagonist BRP-201, J. Inflamm. Res 15 (2022) 911–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schebb NH, Kühn H, Kahnt AS, Rund KM, O’Donnell VB, Flamand N, Peters-Golden M, Jakobsson PJ, Weylandt KH, Rohwer N, Murphy RC, Geisslinger G, FitzGerald GA, Hanson J, Dahlgren C, Alnouri MW, Offermanns S, Steinhilber D, Formation, signaling and occurrence of specialized pro-resolving lipid mediators-what is the evidence so far? Front. Pharmacol 13 (2022), 838782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gilbert NC, Newcomer ME, Werz O, Untangling the web of 5-lipoxygenase-derived products from a molecular and structural perspective: the battle between pro- and anti-inflammatory lipid mediators, Biochem. Pharmacol 193 (2021), 114759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lehmann C, Homann J, Ball AK, Blöcher R, Kleinschmidt TK, Basavarajappa D, Angioni C, Ferreirós N, Häfner AK, Rådmark O, Proschak E, Haeggström JZ, Geisslinger G, Parnham MJ, Steinhilber D, Kahnt AS, Lipoxin and resolvin biosynthesis is dependent on 5-lipoxygenase activating protein, Faseb. J 29 (2015) 5029–5043. [DOI] [PubMed] [Google Scholar]
- [38].Mashima R, Okuyama T, The role of lipoxygenases in pathophysiology; new insights and future perspectives, Redox Biol. 6 (2015) 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pidgeon GP, Lysaght J, Krishnamoorthy S, Reynolds JV, O’Byrne K, Nie D, Honn KV, Lipoxygenase metabolism: roles in tumor progression and survival, Cancer Metastasis Rev. 26 (2007) 503–524. [DOI] [PubMed] [Google Scholar]
- [40].Zeng M, Cao H, Fast quantification of short chain fatty acids and ketone bodies by liquid chromatography-tandem mass spectrometry after facile derivatization coupled with liquid-liquid extraction, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci 1083 (2018) 137–145. [DOI] [PubMed] [Google Scholar]
- [41].Di Pasquale MG, The essentials of essential fatty acids, J. Diet. Suppl 6 (2009) 143–161. [DOI] [PubMed] [Google Scholar]
- [42].Srivastava LM, Chapter 19 - seed germination, mobilization of food reserves, and seed dormancy, in: Srivastava LM (Ed.), Plant Growth and Development, Academic Press, San Diego, 2002, pp. 447–471. [Google Scholar]
- [43].Prigge ST, Boyington JC, Faig M, Doctor KS, Gaffney BJ, Amzel LM, Structure and mechanism of lipoxygenases, Biochimie 79 (1997) 629–636. [DOI] [PubMed] [Google Scholar]
- [44].Andreou A, Feussner I, Lipoxygenases - structure and reaction mechanism, Phytochemistry 70 (2009) 1504–1510. [DOI] [PubMed] [Google Scholar]
- [45].Nelson MJ, Seitz SP, The structure and function of lipoxygenase, Curr. Opin. Struct. Biol 4 (1994) 878–884. [DOI] [PubMed] [Google Scholar]
- [46].Tran M, Signorelli RL, Yamaguchi A, Chen E, Holinstat M, Iavarone AT, Offenbacher AR, Holman T, Biochemical and hydrogen-deuterium exchange studies of the single nucleotide polymorphism Y649C in human platelet 12-lipoxygenase linked to a bleeding disorder, Arch. Biochem. Biophys 733 (2023), 109472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Armstrong M, van Hoorebeke C, Horn T, Deschamps J, Freedman JC, Kalyanaraman C, Jacobson MP, Holman T, Human 15-LOX-1 active site mutations alter inhibitor binding and decrease potency, Bioorg. Med. Chem 24 (2016) 5380–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tallima H, El Ridi R, Arachidonic acid: physiological roles and potential health benefits - a review, J. Adv. Res 11 (2018) 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hanna VS, Hafez EAA, Synopsis of arachidonic acid metabolism: a review, J. Adv. Res 11 (2018) 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang B, Wu L, Chen J, Dong L, Chen C, Wen Z, Hu J, Fleming I, Wang DW, Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets, Signal Transduct. Targeted Ther 6 (2021) 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yamaguchi A, van Hoorebeke C, Tourdot BE, Perry SC, Lee G, Rhoads N, Rickenberg A, Green AR, Sorrentino J, Yeung J, Freedman JC, Holman TR, Holinstat M, Fatty acids negatively regulate platelet function through formation of noncanonical 15-lipoxygenase-derived eicosanoids, Pharmacol Res Perspect 11 (2023), e01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yeung J, Tourdot BE, Adili R, Green AR, Freedman CJ, Fernandez-Perez P, Yu J, Holman TR, Holinstat M, 12(S)-HETrE, a 12-lipoxygenase oxylipin of dihomo-γ-linolenic acid, inhibits thrombosis via gαs signaling in platelets, Arterioscler. Thromb. Vasc. Biol 36 (2016) 2068–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yeung J, Hawley M, Holinstat M, The expansive role of oxylipins on platelet biology, J. Mol. Med. (Berl.) 95 (2017) 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Perry SC, van Hoorebeke C, Sorrentino J, Bautista L, Akinkugbe O, Conrad WS, Rutz N, Holman TR, Structural basis for altered positional specificity of 15-lipoxygenase-1 with 5S-HETE and 7S-HDHA and the implications for the biosynthesis of resolvin E4, Arch. Biochem. Biophys 727 (2022), 109317. [DOI] [PubMed] [Google Scholar]
- [55].Tsai WC, Kalyanaraman C, Yamaguchi A, Holinstat M, Jacobson MP, Holman TR, In vitro biosynthetic pathway investigations of neuroprotectin D1 (NPD1) and protectin DX (PDX) by human 12-lipoxygenase, 15-lipoxygenase-1, and 15-lipoxygenase-2, Biochemistry 60 (2021) 1741–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yamaguchi A, Stanger L, Freedman CJ, Standley M, Hoang T, Adili R, Tsai WC, van Hoorebeke C, Holman TR, Holinstat M, DHA 12-LOX-derived oxylipins regulate platelet activation and thrombus formation through a PKA-dependent signaling pathway, J. Thromb. Haemostasis 19 (2021) 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Perry SC, Horn T, Tourdot BE, Yamaguchi A, Kalyanaraman C, Conrad WS, Akinkugbe O, Holinstat M, Jacobson MP, Holman TR, Role of human 15-lipoxygenase-2 in the biosynthesis of the lipoxin intermediate, 5S,15S-diHpETE, implicated with the altered positional specificity of human 15-lipoxygenase-1, Biochemistry 59 (2020) 4118–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Freedman C, Tran A, Tourdot BE, Kalyanaraman C, Perry S, Holinstat M, Jacobson MP, Holman TR, Biosynthesis of the maresin intermediate, 13S,14S-Epoxy-DHA, by human 15-lipoxygenase and 12-lipoxygenase and its regulation through negative allosteric modulators, Biochemistry 59 (2020) 1832–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fredman G, Van Dyke TE, Serhan CN, Resolvin E1 regulates adenosine diphosphate activation of human platelets, Arterioscler. Thromb. Vasc. Biol 30 (2010) 2005–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Vijil C, Hermansson C, Jeppsson A, Bergstrom G, Hulten LM, Arachidonate 15-lipoxygenase enzyme products increase platelet aggregation and thrombin generation, PLoS One 9 (2014), e88546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Vasquez-Martinez Y, Ohri RV, Kenyon V, Holman TR, Sepúlveda-Boza S, Structure-activity relationship studies of flavonoids as potent inhibitors of human platelet 12-hLO, reticulocyte 15-hLO-1, and prostate epithelial 15-hLO-2, Bioorg. Med. Chem 15 (2007) 7408–7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Robinson SJ, Hoobler EK, Riener M, Loveridge ST, Tenney K, Valeriote FA, Holman TR, Crews P, Using enzyme assays to evaluate the structure and bioactivity of sponge-derived meroterpenes, J. Nat. Prod 72 (2009) 1857–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Perry SC, Kalyanaraman C, Tourdot BE, Conrad WS, Akinkugbe O, Freedman JC, Holinstat M, Jacobson MP, Holman TR, 15-Lipoxygenase-1 biosynthesis of 7S,14S-diHDHA implicates 15-lipoxygenase-2 in biosynthesis of resolvin D5, J. Lipid Res 61 (2020) 1087–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ikei KN, Yeung J, Apopa PL, Ceja J, Vesci J, Holman TR, Holinstat M, Investigations of human platelet-type 12-lipoxygenase: role of lipoxygenase products in platelet activation, J. Lipid Res 53 (2012) 2546–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Tourdot BE, Conaway S, Niisuke K, Edelstein LC, Bray PF, Holinstat M, Mechanism of race-dependent platelet activation through the protease-activated receptor-4 and Gq signaling axis, Arterioscler. Thromb. Vasc. Biol 34 (2014) 2644–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Aleem AM, Tsai WC, Tena J, Alvarez G, Deschamps J, Kalyanaraman C, Jacobson MP, Holman T, Probing the electrostatic and steric requirements for substrate binding in human platelet-type 12-lipoxygenase, Biochemistry 58 (2019) 848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tsai WC, Gilbert NC, Ohler A, Armstrong M, Perry S, Kalyanaraman C, Yasgar A, Rai G, Simeonov A, Jadhav A, Standley M, Lee HW, Crews P, Iavarone AT, Jacobson MP, Neau DB, Offenbacher AR, Newcomer M, Holman TR, Kinetic and structural investigations of novel inhibitors of human epithelial 15-lipoxygenase-2, Bioorg. Med. Chem 46 (2021), 116349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gilbert NC, Bartlett SG, Waight MT, Neau DB, Boeglin WE, Brash AR, Newcomer ME, The structure of human 5-lipoxygenase, Science 331 (2011) 217–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gilbert NC, Gerstmeier J, Schexnaydre EE, Börner F, Garscha U, Neau DB, Werz O, Newcomer ME, Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products, Nat. Chem. Biol 16 (2020) 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kobe MJ, Neau DB, Mitchell CE, Bartlett SG, Newcomer ME, The structure of human 15-lipoxygenase-2 with a substrate mimic, J. Biol. Chem 289 (2014) 8562–8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wecksler AT, Kenyon V, Deschamps JD, Holman TR, Substrate specificity changes for human reticulocyte and epithelial 15-lipoxygenases reveal allosteric product regulation, Biochemistry 47 (2008) 7364–7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gan QF, Browner MF, Sloane DL, Sigal E, Defining the arachidonic acid binding site of human 15-lipoxygenase. Molecular modeling and mutagenesis, J. Biol. Chem 271 (1996) 25412–25418. [DOI] [PubMed] [Google Scholar]
- [73].Joshi N, Hoobler EK, Perry S, Diaz G, Fox B, Holman TR, Kinetic and structural investigations into the allosteric and pH effect on the substrate specificity of human epithelial 15-lipoxygenase-2, Biochemistry 52 (2013) 8026–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Droege KD, Keithly ME, Sanders CR, Armstrong RN, Thompson MK, Structural dynamics of 15-lipoxygenase-2 via hydrogen-deuterium exchange, Biochemistry 56 (2017) 5065–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Newcomer ME, Brash AR, The structural basis for specificity in lipoxygenase catalysis, Protein Sci. 24 (2015) 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ramon S, Baker SF, Sahler JM, Kim N, Feldsott EA, Serhan CN, Martínez-Sobrido L, Topham DJ, Phipps RP, The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant? J. Immunol 193 (2014) 6031–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.