Abstract
Introduction
The aim of this study was to report the nearly ubiquitous prevalence of melanocytic hyperplasia in benign pterygia/pingueculae and establish that the entity is insufficiently recognized.
Methods
This is a retrospective immunohistochemical pathology case series of 30 consecutive pterygia/pingueculae samples selected from an ophthalmic pathology database at a single institution. Histopathologic and immunohistochemistry analyses with anti-SOX-10 and anti-MART-1 antibodies were used for identifying melanocytes. The number of squamous cells intervening between melanocytes was determined.
Results
The frequency of dendritic melanocytes was found to meet the criteria for dendritic melanocytic hyperplasia in 29 of 30 pterygia/pingueculae samples using specific antibodies. Melanocytes were found in several patterns: diffuse (28%), multifocal (28%), and focal (44%). In each case, the melanocytes were distributed as single melanocytes at the base; clusters of melanocytes were seen in 17% of samples. There were an average of about two intervening epithelial cells between melanocytes at the base.
Conclusion
When diagnosed with immunohistochemistry, dendritic melanocytic hyperplasia is nearly ubiquitous in pterygia and pingueculae. Melanocytic hyperplasia may have a distribution that includes nests and single melanocytes above the basal layer, which can be confused with forms of primary acquired melanosis. It is important for pathologists to recognize these lesions as a distinct benign clinicopathologic entity.
Keywords: Melanocytic hyperplasia, Conjunctiva, Primary acquired melanosis
Introduction
Pterygia/pingueculae are among the most common ocular surface lesions of the bulbar conjunctiva and have been attributed to sun exposure. Jakobiec described dendritic melanocytic hyperplasia and related it to solar exposure [1], similar to the relationship proffered between ultraviolet light and conjunctival melanoma [2]. Therefore, one might expect a high prevalence of melanocytic hyperplasia in pterygia. Perra found S100 immunoreactivity in pterygia. This was attributed to presumed Langerhans cells. But no immunoreaction was observed for anti-HMB-45 or -MART-1 in either pterygia or in the “undiseased” conjunctival controls [3]. Neither the frequency nor distribution of the immunoreactivity was reported. Because these studies predated the use of highly sensitive and specific melanocytic markers and immunohistochemistry was not employed for all of the specimens, the prevalence of melanocytic hyperplasia was undoubtedly underestimated. Cellular antigens expressed by melanocytes such as SOX-10 (Sry-related HMG-box gene 10), MART-1 (melanoma antigen recognized by T cells 1 staining), and HMB-45 (human melanoma black-45) can be used to identify melanocytes and characterize their histologic pattern. The advantage of these antibodies includes an ability to appreciate characteristics of atypical architectures, to better classify lesions, and to help differentiate benign from premalignant conjunctival lesions. Furthermore, studies have compared the differences in several antibodies for melanocytic lesions. Antibodies for SOX-10 can reliably identify melanocytes including dendritic melanocytes [4]. Both SOX-10 and Melan-A (MART-1) antibodies showed increased sensitivity for melanocytes compared to those of HMB-45, but no significant difference was found between SOX-10 and Melan-A reactivity [5]. Dass and colleagues found Melan-A does not delineate clear cytoplasmic margins, which would make differentiation and counting of cells difficult [5]. Anti-SOX-10 appeared to be the most consistent and reliable antibody and was chosen for quantification of melanocytes in this study.
In our practice, cases have been frequently referred from pathologists, who were baffled by the apparent proliferation of melanocytes in conjunctival pterygia/pinguecula samples; the immunohistochemistry testing was triggered by the customary note on the requisition to rule out neoplasia. The goal of this study was to determine the frequency of melanocytic hyperplasia in pterygia and pingueculae and characterize any distinguishing patterns.
Materials and Methods
A retrospective case series was available for 30 consecutive pathology cases that were clinically diagnosed as pterygium or pinguecula. All samples originated from a de-identified database obtained in the ophthalmic pathology laboratory for an unrelated immunohistochemical validation study. All cases were obtained from archival tissue that would otherwise be discarded. The 30 cases were obtained from 29 eyes (two samples were obtained from the same eye). Cases with a history of a pigmented lesion were excluded. Clinical information was limited to age and gender of the patient and history at the time of presentation. A sample size of 30 was determined to be representative based on a pilot study of 10 cases in which anti-MART-1 had been performed for benign melanosis; all 10 cases showed concomitant dendritic melanocytic hyperplasia. The current study was reviewed by the Institutional Review Board and was determined to be exempt from formal review because there was no risk to the de-identified data set.
Hematoxylin-eosin (H&E)-stained slides for each of the samples were re-examined. Immunohistochemistry had been performed with anti-SOX-10 (prediluted RTU, Cell Marque, Rocklin, CA) and anti-MART-1 antibody (BioCare Medical, CM77C, Pacheco, CA) via an automated Leica Bond-Max Polymer Refine Detection Kit (Leica Biosystems, Buffalo Grove, IL), according to manufacturer instructions.
Immunohistochemistry for SOX-10 discretely identified melanocytic cells, and the number of melanocytes was counted. Dendritic melanocytic hyperplasia was defined according to Jakobiec’s criteria as clusters and/or single dendritic melanocytes with no more than six intervening squamous cells between melanocytes [1]. Intervening squamous cells between SOX-10 reactive cells were counted at the base of the epithelium from areas that were sectioned orthogonally (not tangentially). In the areas where there were no more than six intervening keratinocytes, the number of intervening epithelial cells between melanocytes was counted. The average number of intervening keratinocytes was calculated for each case. The mean (± standard deviation) of the case averages for all samples was calculated. The degree of clustering of melanocytes at the base was assessed. Each case was evaluated for melanocytes confined to the base as well as melanocytes above the base.
All cases were divided into categories of diffuse, multifocal, and focal patterns of staining. If the area of melanocytic cells extended for the majority of the biopsy, the process was considered diffuse. Cases in which areas of melanocytic hyperplasia were separated by seven or more intervening keratinocytes were considered multifocal. Cases in which the melanocytes were not separated by seven or more keratinocytes but did not involve the entire biopsy were considered focal.
Results
The 30 pterygia/pingueculae samples are shown (Table 1). The average age of the patients for all samples was 57.2 years. Nine of the 30 samples (30%) were from female patients. Five samples (17%) were clinically diagnosed as pingueculae, while the remaining 25 (83%) were pterygia.
Table 1.
Clinical and histopathology characteristics of 30 pterygium/pinguecula samples
| # | OD/OS | Clinical diagnosis | Mean # of intervening squamous cells at highest hpf | Clusters at base | Single cells in epithelium | Pigment in adjacent areas | Focal/diffuse/multifocal |
|---|---|---|---|---|---|---|---|
| 1 | OD | Pterygium | 1.8 | Yes | Yes | No | Focal |
| 2 | OD | Pterygium | 0.5 | Yes | Yes | Yes | Focal |
| 3 | OS | Pterygium | 3.0 | No | Yes | Yes | Diffuse |
| 4 | OS | Pterygium | 1.9 | Yes | Yes | Yes | Diffuse |
| 5 | OS | Pterygium | 2.9 | No | Yes | No | Focal |
| 6 | OD | Pterygium | 1.7 | Yes | Yes | No | Diffuse |
| 7 | OS | Pterygium | 0.8 | No | Yes | No | Focal |
| 8 | OD | Pterygium | 2.3 | No | No | No | Focal |
| 9 | OD | Pinguecula | 2.0 | No | Yes | No | Multifocal |
| 10 | OS | Pterygium | 2.2 | No | Yes | No | Focal |
| 11 | OD | Pterygium | 1.8 | No | Yes | No | Focal |
| 12 | OD | Pterygium | 2.2 | No | Yes | No | Multifocal |
| 13 | OS | Pterygium | 2.4 | No | Yes | Yes | Multifocal |
| 14 | OS | Pterygium | 4.1 | No | Yes | Yes | Focal |
| 15 | OD | Pterygium | 2.2 | No | Yes | No | Multifocal |
| 16 | OD | Pterygium | 2.0 | No | Yes | No | Multifocal |
| 17 | OD | Pterygium | 1.5 | No | Yes | No | Focal |
| 18 | OS | Pterygium | 1.4 | No | No | Yes | Focal |
| 19 | OS | Pterygium | 1.4 | No | Yes | No | Focal |
| 20 | OS | Pterygium | 1.3 | No | Yes | No | Diffuse |
| 21 | OD | Pterygium | 1.3 | No | Yes | No | Diffuse |
| 22 | OS | Pterygium | 1.8 | No | Yes | No | Diffuse |
| 23 | OS | Pterygium | 1.4 | No | Yes | No | Diffuse |
| 24 | OD | Pinguecula | 2.4 | No | Yes | No | Multifocal |
| 25 | OD | Pterygium | 1.8 | No | Yes | Yes | Focal |
| 26 | OD | Pterygium | 2.5 | No | Yes | No | Multifocal |
| 27 | OS | Pinguecula | 1.1 | Yes | Yes | No | Multifocal |
| 28 | OS | Pinguecula | 1.2 | No | Yes | No | Diffuse |
| 29 | OS | Pinguecula | N/A | N/A | N/A | N/A | N/A |
| 30 | OD | Pterygium | 2.4 | No | Yes | Yes | Focal |
OD, right eye; OS, left eye.
Immunohistochemistry with SOX-10 staining showed positive reactivity in 29 of 30 (97%) pterygia/pingueculae cases. The mean of the average number of intervening squamous cells in the regions of melanocytic hyperplasia was 1.9 ± 0.7 for all cases overall. Of the 29 samples, 5 (17%) showed at least 1 cluster of melanocytes at the basal layer. One sample showed clusters at the base only in pairs. All but two samples showed melanocytes above the basal layer of the epithelium (Table 1, case 8 and 18).
When categorized into diffuse, multifocal, or focal patterns of reactivity, 28% of samples showed a diffuse pattern, 28% multifocal, and 44% focal (Table 1). Four samples were considered extremely focal given there were less than 15 total cells involved. Spheroidal degeneration was noted in three samples. One sample showed separate benign melanosis that was not seen clinically (Table 1, Case 25).
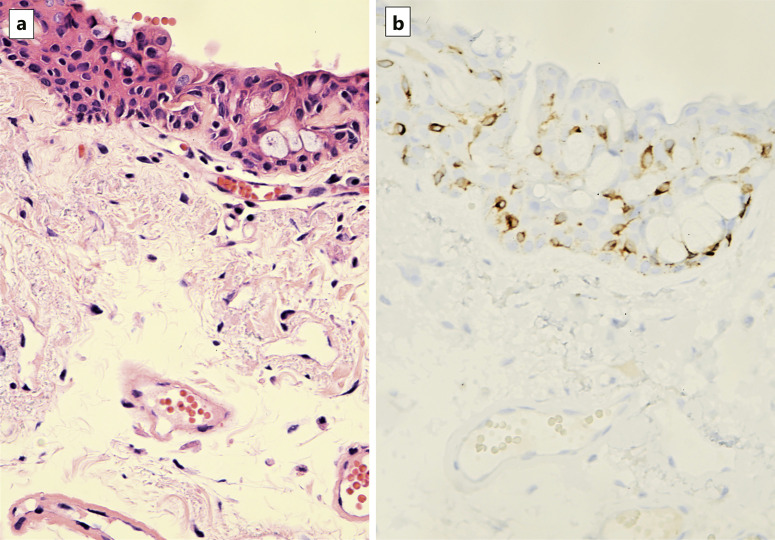
The features of dendritic melanocytic hyperplasia are shown (Fig. 1–5). Figure 1 exhibits the most common representation of melanocytic hyperplasia, illustrated by H&E (Fig. 1a) and SOX-10 staining (Fig. 1b). Melanocytes were subtle in H&E stains and identified by clearing of the cytoplasm and compact nuclei. With anti-SOX-10, melanocytes were easily visualized at the base with a few reactive cells higher in the epithelial layer. Figure 2 illustrates the less common finding of single melanocytic cells and a cluster of melanocytes at the base (Fig. 2a, b). On H&E stain, there is a vague hint of a cluster of nonpigmented cells at the base. The cluster becomes readily apparent with anti-SOX-10. Few cells were seen above the basal layer. In cases with nests of melanocytes, usually 1 or 2 nests were identified in each case. Figure 3 shows another case with nests of dendritic melanocytes at higher magnification with H&E, anti-MART-1, and anti-S0X-10. Fig. 4 illustrates multifocal and intraepithelial involvement of melanocytic hyperplasia. Melanocytic nuclei were visualized in red with SOX-10 scattered in all levels of the epithelium not just along the basement membrane. This case also showed faint coexistent pigment in the squamous cells adjacent to some melanocytes, which was clinically unrecognized. In addition, all melanocytes examined in the study appeared dendritic in nature. Compared to the corresponding H&E stain, the MART-1 stain highlights the dendritic pattern of melanocytes (Fig. 5a, b).
Fig. 1.
Immunohistochemical image of adjacent sections submitted as pterygium. Hematoxylin and eosin (H&E, a) slides with corresponding anti-SOX-10 (b) reveal a population of melanocytes spread diffusely across the epithelium. Goblet cells are present.
Fig. 2.
Adjacent sections show focally nested basal melanocytes in a pterygium sample are difficult to identify with H&E (a). b These nested melanocytes are highlighted by anti-SOX-10. There are also immunoreactive single cells dispersed in the epithelial layer. This represents a rare case where there are clusters of melanocytes in addition to dispersion of single melanocytes.
Fig. 3.
The panel shows nests and single nonpigmented cells with small dark nuclei and cytoplasmic clearing H&E (a). b The melanocytes are reactive to anti-SOX-10 and show dendritic cytoplasmic extensions highlighted with anti-MART-1 (c).
Fig. 4.
SOX-10 immunoreactivity reveals melanocytes spread across the epithelium beyond the basal layer in a pterygium specimen. Area with higher magnification outlined (top left). Single melanocytes are dispersed beyond the basal epithelial layer. This case also showed clinically unrecognized benign melanosis.
Fig. 5.
a Representative H&E of melanocytic hyperplasia in specimen submitted as pterygium. b Corresponding MART-1 (brown chromogen) emphasizes the elongated processes characteristic of dendritic melanocytes.
Discussion
The classification of conjunctival melanocytic lesions is controversial yet important for understanding the categorization of dendritic melanocytic hyperplasia for this study. Several schemes can be used to grade conjunctival melanocytic lesions. Primary acquired melanosis (PAM) was originally described by Folberg et al. [6] in two distinct forms: with and without atypia. PAM with atypia was associated with progression to melanoma in 46% of cases. In contrast, lesions without cytologic atypia had little progression to melanoma, although 1 case showed recurrence. Because patients with PAM without atypia were younger and these lesions were frequently found adjacent to PAM with atypia, it was hypothesized that non-atypical lesions were precursors of atypical ones. Clinically, both types of lesions appear flat and pigmented. In contrast, our cases of dendritic melanocytic hyperplasia were not pigmented. Damato and Coupland suggested that the term PAM be reserved for clinicians only, arguing the term refers to a lesion that is not histologically specific [7]. In 2016, Jakobiec recommended clinicians forego groupings of melanocytic proliferations with and without atypia with other forms of acquired melanosis to prevent overcalling benign lesions [8]. Folberg responded in a letter that there was no reason to further subdivide the lesions. He argued that new nomenclature can introduce confusion and have little impact on improving clinical outcomes [9]. In 2018, the WHO Classification of Tumors of the Eye simplified these lesions into three categories: low- and high-grade conjunctival melanocytic intraepithelial lesion (CMIL) and melanoma in situ [10]. Milman et al. then evaluated the categories. Low-grade CMIL includes PAM without or with mild atypia and conjunctival melanocytic intraepithelial neoplasia (C-MIN) scores 1 and 2, while high-grade CMIL includes PAM with moderate to severe atypia and C-MIN scores 3 to 5. Melanoma in situ includes PAM with severe atypia involving over 75% of the epithelium and C-MIN scores greater than 5 [11]. Melanocytic hyperplasia without atypia was considered equivalent to PAM without atypia, C-MIN 1, or low-grade CMIL. Inter-observer agreement for discriminating low- versus high-grade lesions ranged from 67 to 81% depending on the lesion and classification scheme used [11]. Discordance was greater for low-grade lesions and could in part be overcome with immunohistochemistry. On purely statistical grounds, a correlation above 70% is excellent for 2 independent variables [12]. But practically, this is a discordance of 30% for the diagnosis on the same slide among expert authors for low- versus high-grade lesions. Their study was based solely on H&E evaluation. This level of discrepancy raises concerns about the repeatability of diagnoses among experts, not to mention in the general community setting.
In this study, dendritic melanocytic hyperplasia would perhaps be considered by some in the foregoing classification as PAM without atypia or C-MIN-1. However, the lesions were not pigmented clinically, the clustering was extremely focal, and the nuclei were small. We would argue that this pattern, with increased number and density of melanocytes in pterygia without other findings, should not be misconstrued as evidence for potential recurrence or progression. Shields reports recurrence rates of 11% and up to 50% in PAM without and with atypia, respectively [13]. But these were pigmented lesions, both clinically and histologically. Melanocytic hyperplasia in pterygia appears nearly ubiquitous in our data, and pterygia have not been proven to progress to any form of PAM.
One must consider studies that relate pterygia to PAM without atypia. PAM with and without atypia has been touted to accompany 6% of nonpigmented pterygia samples (six out of 100, five without atypia and one with atypia) [14]. Perra and colleagues reported a similar rate of 8.75% (seven out of 80 pterygia samples, five without atypia and two with atypia) from a population in Equador [15]. Perhaps more relevant to our study, melanocytic hyperplasia was found in 32% (47 of 149 pterygia lesions) with a significant correlation to chronic inflammation [16]. None of these studies performed immunohistochemistry in each case and were reliant on recognizing the lesions first with H&E staining.
In our study, immunohistochemical analysis revealed melanocytic hyperplasia in all but one of the 30 pterygia/pingueculae samples (Table 1, Case #29). This was unexpected because none of the melanocytic lesions were clinically or histologically pigmented. The accompanying histologic benign melanosis in 1 case was separate from the melanocytic hyperplasia and not noted clinically. The higher prevalence of melanocytic hyperplasia seen in our study is likely due to the increased sensitivity with anti-SOX-10 antibodies. The subtle features of melanocytes on H&E stain indicate melanocytic cells may be easily overlooked without target antibodies.
The significance of a nearly ubiquitous prevalence of melanocytic hyperplasia in pterygia is the risk of over-diagnosis. Pathologists may order immunoreactive markers for melanocytes when, for example, there is no clear histopathologic diagnosis, history is inadequate, and/or the requisition bears the request to exclude malignancy. Clusters of melanocytes or a distribution of single melanocytes above the epithelium may be interpreted as worrisome for PAM or even melanoma. In these cases and especially those with nests of melanocytes, we do recommend close clinical surveillance. Immunohistochemistry has been recommended for study of some melanocytic lesions. The use of immunohistochemistry for Melan-A resulted in the reclassification of 33% of cases initially called melanoma in situ. After immunohistochemistry, the diagnoses were revised to invasive melanoma of the skin [17]. HMB-45 and p16 immunoreactivity were touted as successful parameters predictive of conjunctival melanoma in 61 conjunctival melanocytic lesions [18]. Milman and colleagues also argue immunohistochemical profiling may not be as beneficial for distinguishing low- from high-grade intraepithelial melanocytic proliferations [19]. In any case, the pathologist using immunohistochemistry in pingueculae and pterygia will need to be aware of dendritic melanocytic hyperplasia and its prevalence and pattern.
Our study has similarities to the ultrastructural study of Jakobiec for dendritic melanocytic hyperplasia. Arborizing cellular processes were observed in melanocytes between basal and suprabasilar keratinocytes [1]. Using cytoplasmic reactivity to anti-MART-1, the dendritic nature of the melanocytic hyperplasia is evident (Fig. 4a, b). The melanocytes are generally intermingled between keratinocytes and may extend above the basal layer. Different from Jakobiec, we observed that dendritic melanocytes may also be clustered in groups, albeit uncommonly. Furthermore, in a sample of benign epithelial melanosis of the conjunctiva, Jakobiec observed about five to six intervening keratinocytes between melanocytes. In our sample, we found a higher density of melanocytes with an average of 1.9 ± 0.7 keratinocytes between melanocytes. It is possible the increased density of melanocytes may be related to the increased sensitivity of anti-SOX-10 or to prolonged solar exposure in pterygia. In the skin, the number of melanocytes doubles with chronic sun exposure [20, 21].
This study and most cited herein lack control groups of normal bulbar conjunctivae. We accepted the definition of dendritic melanocytic hyperplasia according to Jakobiec’s original study, which provided presumed normal conjunctiva for comparison [1, 4]. Perra et al. [3] reported negative immunohistochemical reactivity for melanocytes in samples considered nondiseased conjunctiva. In identifying controls, it is essentially impossible to integrate ultraviolet light exposure over a lifetime. It is also difficult to obtain healthy conjunctival tissue from topographically and age-matched controls. The high prevalence of melanocytic hyperplasia in our study raises the possibility that we and others have underestimated the number of melanocytes in normal conjunctiva. For a review article, Bresler et al. [2] illustrated melanocytes in tissue that were designated as normal, melanosis, and PAM without atypia, albeit without clinical or histologic details of selection. Many of our cases showed only focal areas meeting Jakobiec’s criteria for melanocytic hyperplasia and may represent findings seen in most conjunctiva. Clarification of a normal distribution is needed. One may expect to find and we have anecdotally observed a high prevalence of melanocytic hyperplasia in other lesions associated with solar exposure such as dysplasia of squamous epithelium.
Because our samples were gathered from archival data, clinical follow-up data are not available. It is reasonable to assume the risk for malignancy in these lesions is low because the cases were routine pterygia/pingueculae that lacked cytologic atypia and melanocytic hyperplasia was nearly ubiquitous. Perhaps melanocytic hyperplasia can be considered a “normal finding” in pterygia/pingueculae. A study with prolonged follow-up would be useful.
Statement of Ethics
This study protocol was reviewed and determined to be exempt from formal review by the UCLA IRB Staff, approval number 22-001143. The study was granted an exemption from requiring written consent by UCLA IRB Staff, approval number 22-001143, as the source of the data was a de-identified dataset.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was supported by Edith and Lew Wasserman Professorship (Dr. Glasgow). There were no financial disclosures.
Author Contributions
Dr. Oh and Dr. Glasgow wrote and edited the paper. Dr. Glasgow designed the study and performed the microscopy, data collection, and photomicroscopy.
Funding Statement
This study was supported by Edith and Lew Wasserman Professorship (Dr. Glasgow). There were no financial disclosures.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1.Jakobiec FA. The ultrastructure of conjunctival melanocytic tumors. Trans Am Ophthalmol Soc. 1984;82:599–752. [PMC free article] [PubMed] [Google Scholar]
- 2.Bresler SC, Simon C, Shields CL, McHugh JB, Stagner AM, Patel RM. Conjunctival melanocytic lesions. Arch Pathol Lab Med. 2022 May;146(5):632–46. 10.5858/arpa.2021-0006-RA. [DOI] [PubMed] [Google Scholar]
- 3.Perra MT, Maxia C, Zucca I, Piras F, Sirigu P. Immunohistochemical study of human pterygium. Histol Histopathol. 2002;17(1):139–49. 10.14670/HH-17.139. [DOI] [PubMed] [Google Scholar]
- 4.Jakobiec FA, Colby K, Bajart AM, Saragas SJ, Moulin A. Immunohistochemical studies of atypical conjunctival melanocytic nevi. Arch Ophthalmol. 2009 Aug;127(8):970–80. 10.1001/archophthalmol.2009.171. [DOI] [PubMed] [Google Scholar]
- 5.Dass SE, Huizenga T, Farshchian M, Mehregan DR. Comparison of SOX-10, HMB-45, and melan-A in benign melanocytic lesions. Clin Cosmet Investig Dermatol. 2021 Oct;14:1419–25. 10.2147/CCID.S333376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folberg R, McLean IW. Primary acquired melanosis and melanoma of the conjunctiva: terminology, classification, and biologic behavior. Hum Pathol. 1986;17(7):652–4. 10.1016/s0046-8177(86)80175-7. [DOI] [PubMed] [Google Scholar]
- 7.Damato B, Coupland SE. Management of conjunctival melanoma. Expert Rev Anticancer Ther. 2009 Sep;9(9):1227–39. 10.1586/era.09.85. [DOI] [PubMed] [Google Scholar]
- 8.Jakobiec FA. Conjunctival primary acquired melanosis: is it time for a new terminology? Am J Ophthalmol. 2016 Feb;162:3–19.e1. 10.1016/j.ajo.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Folberg R. Naming the precursors of conjunctival melanoma. Am J Ophthalmol. 2016 Feb;162:1–2. 10.1016/j.ajo.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 10.Eberhart CG, Folberg R, Margo C, et al. Conjunctival melanocytic intraepithelial neoplasia. 4th ed. In: Grossniklaus HE, Eberhart CG, Kivela TT, editors. WHO classification of tumours of the eye. Lyon, France: International Agency for Research on Caner; 2018. p. 31–3. [Google Scholar]
- 11.Milman T, Eiger-Moscovich M, Henry RK, Folberg R, Coupland SE, Grossniklaus HE, et al. Validation of the newly proposed world health organization classification system for conjunctival melanocytic intraepithelial lesions: a comparison with the C-MIN and PAM classification schemes. Am J Ophthalmol. 2021 Mar;223:60–74. 10.1016/j.ajo.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J R Stat Soc. 1922;85(1):87–94. 10.2307/2340521. [DOI] [Google Scholar]
- 13.Shields JA, Shields CL, Mashayekhi A, Marr BP, Benavides R, Thangappan A, et al. Primary acquired melanosis of the conjunctiva: risks for progression to melanoma in 311 eyes. The 2006 lorenz E. Zimmerman lecture. Ophthalmology. 2008;115(3):511–9.e2. 10.1016/j.ophtha.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Chui J, Coroneo MT, Tat LT, Crouch R, Wakefield D, Di Girolamo N. Ophthalmic pterygium: a stem cell disorder with premalignant features. Am J Pathol. 2011;178(2):817–27. 10.1016/j.ajpath.2010.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perra MT, Colombari R, Maxia C, Zucca I, Piras F, Corbu A, et al. Finding of conjunctival melanocytic pigmented lesions within pterygium. Histopathology. 2006 Mar;48(4):387–93. 10.1111/j.1365-2559.2006.02346.x. [DOI] [PubMed] [Google Scholar]
- 16.Bergeron S, Ito H, Dossous YE, Burnier MN. Histopathological variability and concomitant lesions in pterygium in a large case series. J Ophthalmol. 2021;2021:6623794. 10.1155/2021/6623794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drabeni M, Lopez-Vilaró L, Barranco C, Trevisan G, Gallardo F, Pujol RM. Differences in tumor thickness between hematoxylin and eosin and Melan-A immunohistochemically stained primary cutaneous melanomas. Am J Dermatopathol. 2013 Feb;35(1):56–63. 10.1097/DAD.0b013e31825ba933. [DOI] [PubMed] [Google Scholar]
- 18.Milman T, Zhang Q, Ang SM, Elder D, Ida CM, Salomao DR, et al. Conjunctival nevi and melanoma: multiparametric immunohistochemical analysis, including p16, SOX10, HMB45, and Ki-67. Hum Pathol. 2020 Sep;103:107–19. 10.1016/j.humpath.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Milman T, Zhang Q, Ang SM, Elder D, Lally SE, Shields JA, et al. Immunohistochemical profiling of conjunctival melanocytic intraepithelial lesions, including SOX10, HMB45, Ki67, and P16. Am J Ophthalmol. 2021 Feb;222:148–56. 10.1016/j.ajo.2020.09.033. [DOI] [PubMed] [Google Scholar]
- 20.Quevedo WC, Szabó G, Virks J, Sinesi SJ. Melanocyte populations in UV-irradiated human skin. J Invest Dermatol. 1965;45(4):295–8. 10.1038/jid.1965.131. [DOI] [PubMed] [Google Scholar]
- 21.Gilchrest BA, Blog FB, Szabo G. Effects of aging and chronic sun exposure on melanocytes in human skin. J Invest Dermatol. 1979;73(2):141–3. 10.1111/1523-1747.ep12581580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.