Abstract
Cytochalasin-induced actin disruption has often been associated with decreased bacterial internalization by cultured epithelial cells, although polarized enterocytes have not been systematically studied. In assays using confluent polarized HT-29 enterocytes, cytochalasin D appeared to increase internalization of wild-type Salmonella typhimurium, Proteus mirabilis, and Escherichia coli. HeLa and HEp-2 epithelial cells, as well as HT-29 and Caco-2 enterocytes, were used to clarify this unexpected observation. Resulting data showed that cytochalasin D was associated with increased internalization of S. typhimurium and P. mirabilis by both HT-29 and Caco-2 enterocytes and with increased internalization of E. coli by HT-29 enterocytes; with either HeLa or HEp-2 cells, cytochalasin was associated with no change or a decrease in internalization of these same bacterial strains. Cytochalasin caused decreased internalization of Listeria monocytogenes by HT-29, Caco-2, HeLa, and HEp-2 cells, indicating that cytochalasin did not consistently augment bacterial internalization by polarized enterocytes. Fluorescein-labeled phalloidin confirmed marked disruption of filamentous actin in cytochalasin-treated HT-29, Caco-2, HeLa, and HEp-2 cells. Cytochalasin had no noticeable effect on epithelial viability but caused distorted apical microvilli, cell rounding, and separation of adjacent enterocytes in confluent cultures (with a corresponding decrease in transepithelial electrical resistance). Scanning electron microscopy showed that cytochalasin-induced enhanced bacterial internalization was associated with preferential bacterial adherence on the exposed enterocyte lateral surface. Colchicine, used to disrupt microtubules, had no noticeable effect on bacterial internalization by HT-29 or Caco-2 enterocytes. These data indicated that for HT-29 and Caco-2 enterocytes, cytochalasin-induced disruption of filamentous actin might augment internalization of some bacterial species by a mechanism that appeared to involve exposure of the enterocyte lateral surface.
Cellular actin exists in several forms within polarized epithelial cells (3, 5, 15). Monomeric actin forms a globular pool (G-actin) distributed diffusely throughout the cell cytoplasm. Oligomeric, polymerized, filamentous actin (F-actin) is found in a number of locations, including (i) in the terminal web and extending into the core of the microvilli and toward regions of intercellular contacts, (ii) in association with cadherins at the zonula adherens forming a peripheral ring surrounding the cell, (iii) in a less organized network of filaments found along the rest of the lateral surface including the tight junctions, and (iv) in larger bundles in the basal cytoplasm in association with integrins at focal adhesion sites (5, 15) defined as regions of the plasma membrane that are so close to the substratum (10 to 15 nm) that they appear as dark areas (focal contacts) by interference reflection microscopy (21). In the last decade, many investigators have attempted to clarify the role of eucaryotic actin in bacterial interactions with cultured epithelial cells.
Cytochalasins are low-molecular-weight fungal metabolites that bind actin and cause a number of effects that depend on the cell type and density, as well as the cytochalasin concentration and type (3, 43). Cytochalasins resemble capping proteins because they bind to the fast-polymerizing barbed ends (as opposed to the pointed ends) of actin filaments, inhibiting the association and dissociation of actin subunits at that end (3). Cytochalasins have been shown to depolymerize F-actin, to cleave filaments, to stabilize oligomers, to stimulate F-actin ATPase activity, and to interact with G-actin to accelerate actin self-assembly (15, 32). A general observation is that cytochalasins change actin organization from an isotropic network to focal accumulations (3).
The binding of cytochalasins to the barbed ends of actin filaments may have an additional function related to cellular focal adhesions. Transmembrane adhesion proteins (integrins) link extracellular matrix components on the substratum to dense cytoplasmic plaques associated with actin bundles, termed stress fibers (21). If actin filaments are held in place by capping proteins at the barbed ends, cytochalasins may release these filaments, permitting them to contract into punctuate aggregates. The observation that cytochalasins can cause contraction of stress fibers supports this theory (3, 5). Cytochalasins influence a variety of eucaryotic cellular activities associated with contractility. Cytochalasins have been reported to prevent cytoplasmic cleavage, to inhibit cell motility, and to cause cells to round up or flatten, to stop membrane ruffling, and to stop translocating molecules (3, 32, 43).
Cytochalasins B and D have been most frequently used to clarify the effect of actin on bacterial internalization by eucaryotic cells, with cytochalasin D used more often than B, likely because D is considered the most specific and potent of the cytochalasins (30). If cytochalasin D interferes with bacterial internalization, it has been inferred that F-actin plays a role and that internalization is directed by the host cell as opposed to the bacterium itself. The effect of cytochalasin D on bacterial internalization has been studied in a variety of bacterial species and a variety of host eucaryotic cells. The most frequent observation has been that cytochalasin D decreases bacterial internalization by eucaryotic cells, implicating the need for functional F-actin in host-directed bacterial endocytosis (1, 2, 4, 6, 7, 10–13, 19, 25, 27, 30, 31, 34, 44). There is ample evidence in the literature to conclude that the uptake of most, if not all, bacteria into eucaryotic cells (including epithelial cells) can be blocked by cytochalasins.
Thus, we were surprised to observe that in polarized HT-29 enterocytes, cytochalasin D increased internalization of several wild-type strains of enteric bacteria. Experiments were then designed to clarify this observation. Resulting data indicated that cytochalasin D was associated with enhanced internalization of some strains of enteric bacteria by polarized HT-29 and Caco-2 enterocytes by a mechanism that appeared to involve cell rounding with exposure of the enterocyte lateral surface.
MATERIALS AND METHODS
Bacteria.
Salmonella typhimurium ATCC 14028, Listeria monocytogenes ATCC 43249 serotype 1/2a, Shigella flexneri ATCC 12022, and Shigella sonnei ATCC 25931 were obtained from the American Type Culture Collection (ATCC), Rockville, Md. Proteus mirabilis M13 and Escherichia coli M21 are rodent isolates. These bacterial species represent a spectrum of virulence, and excluding the shigellae, these strains have been used in several in vivo studies of oral infectivity in rodents (35, 39) as well as in vitro studies of bacterial internalization by cultured enterocytes (37, 38, 40, 41).
Cultured eucaryotic cells.
All epithelial cell lines were obtained from the ATCC and were cultivated in 24-well (2-cm2) plastic dishes. Cells were seeded at 105 per well for use after overnight cultivation, or 2 × 104 per well for use after prolonged (2- to 3-week) cultivation. Unless otherwise stated, all tissue culture reagents were purchased from Sigma Chemical Co., St. Louis, Mo. Epithelial cell viability was assessed with the vital dyes trypan blue (0.36%) and propidium iodide (20 μg/ml).
HT-29 cells are human enterocytes that are relatively undifferentiated when cultivated under standard conditions, but when grown in the absence of glucose, confluent HT-29 cells are highly polarized and contain two phenotypes resembling terminally differentiated absorptive enterocytes and mucus-secreting goblet cells (18, 26, 28). HT-29 cells were used at passages 16 to 18 following removal of glucose; these cells were cultivated as described by Huet et al. (18) in glucose-free Dulbecco’s modified Eagle’s medium supplemented with 15% dialyzed fetal bovine serum, 4 mM l-glutamine, and 5 mM galactose. HT-29 cells were incubated at 37°C in 9.5% CO2 for 22 to 24 days, when these enterocytes are considered highly polarized and differentiated (18, 26, 28). Because there is evidence that HT-29 differentiation is reversible when galactose-adapted enterocytes are seeded back into glucose medium (18, 26), for selected experiments, HT-29 cells were cultivated in the presence of glucose (producing HT-29glu cells), using Dulbecco’s modified Eagle’s medium supplemented with 15% fetal bovine serum and 4 mM l-glutamine. HT-29glu cells have been reported to have a faster doubling time than HT-29 cells (18, 28). HT-29glu cells grew more rapidly than HT-29 cells, achieving confluence in several days, and were cultivated 15 to 17 days at 37°C in 9.5% CO2. HT-29glu were used at passages 2 and 3 from a stock culture obtained directly from the ATCC and not yet cultivated away from glucose and at passage 24 from HT-29 cells that had been cultivated in the absence of glucose for the first 22 passages. Caco-2 cells (human enterocytes) were cultivated in the same medium and under the same conditions as those described for HT-29glu enterocytes. Caco-2 cells were used at passages 5 to 12 and after 15 to 17 days of incubation, when these enterocytes are considered polarized and differentiated (26, 29). Using transmission electron microscopy, we have previously shown that these mature Caco-2 and HT-29 enterocytes have characteristics of polarized cells, i.e., well-developed apical microvilli, distinct apical and basolateral domains, and tight junctions joining adjacent enterocytes (36, 40, 42).
HEp-2 cells (human larynx epithelium) were cultivated in minimal essential medium supplemented with Earle’s salts supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 2.2 g of sodium bicarbonate (Celox Laboratories, Hopkins, Minn.) per liter. HeLa cells (human epithelioid carcinoma) were cultivated in the same medium supplemented with nonessential amino acids (Celox). HEp-2 and HeLa cells were at passages 3 to 7 and after overnight incubation at 37°C in 5% CO2.
Bacterial internalization by cultured epithelial cells.
Epithelial cell internalization of viable bacteria was assayed as described previously (37, 38, 40, 41), with minor modifications. Overnight tryptic soy broth (Difco Laboratories, Detroit, Mich.) cultures of individual bacterial strains were washed twice and diluted in the appropriate tissue culture medium. Maintaining a multiplicity of infection of 100 (bacterium-to-epithelial cell ratio), 1 ml containing 107 or 108 viable bacteria was added to each tissue culture well containing either 105 epithelial cells (overnight nonconfluent cultures) or 106 epithelial cells (2- to 3-week-old confluent cultures), respectively. Bacterial concentrations were determined by densitometry and confirmed by serial dilution, followed by viable plate counts on appropriate agar media. Bacteria were incubated with epithelial cells for 1 h at 37°C. Epithelial cells were then washed five times with Hanks balanced salt solution (HBSS), and tissue culture medium containing gentamicin sulfate (50 μg/ml) was added to kill residual viable extracellular bacteria. After 2.5 h, epithelial cells were washed five times with HBSS and lysed for 3 to 5 min with 1% Triton X-100. Viable intracellular bacteria were quantified following serial dilution and incubation on appropriate agar media. Agar media consistently included both colistin-nalidixic acid agar (Difco) supplemented with 5% sheep blood and MacConkey agar supplemented with 10% lactose (Difco) to verify the purity and concentration of the bacterial inocula, to verify the absence of extracellular bacteria after incubation with gentamicin, and to quantify the numbers of intracellular bacteria while verifying the absence of bacterial contamination after epithelial cell lysis.
Cytochalasin D from Zygosporium mansonii (Sigma) was used to study the effect of actin disruption on bacterial internalization by cultured epithelial cells. In all experiments, epithelial cells were pretreated with cytochalasin for 1 h, and cytochalasin was present throughout the bacterial internalization assay. Results of preliminary experiments using mature HT-29 enterocytes and 0, 0.1, 1.0, and 10 μg of cytochalasin per ml indicated that 1.0 μg/ml was the optimal concentration to modulate the internalization of each of the bacterial strains studied. Working dilutions of cytochalasin were made in the appropriate tissue culture medium, using a stock solution of 1 mg/ml in dimethyl sulfoxide maintained at −20°C. Preliminary experiments showed that pertinent concentrations of dimethyl sulfoxide had no noticeable effect on bacterial internalization and that pertinent concentrations of cytochalasin D had no effect on bacterial viability.
In selected experiments, the effects of cytochalasin were studied in assays using confluent enterocytes that had their junctional complexes previously disrupted by incubation in a calcium-free medium, which does not effect enterocyte viability but reversibly disrupts the junctional complex, exposing the lateral enterocyte membrane (14, 37). It was preferable to use a calcium-free medium to open enterocyte tight junctions, as opposed to calcium chelation with ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, because the latter compound has been reported to artifactually inhibit Caco-2 internalization of L. monocytogenes (14). Enterocytes were incubated 1 h in calcium-free Krebs-Ringer solution (37), washed three times with HBSS, and then incubated 1 h in tissue culture medium supplemented with 1 μg of cytochalasin D per ml. Bacterial internalization was then assayed as described above, with cytochalasin present throughout the assay.
Colchicine was used to study the effects of microtubule disruption (30) on bacterial internalization by enterocytes. Enterocytes were pretreated 1 h with various concentrations of colchicine (Sigma), and colchicine was present throughout the bacterial internalization assay. Working dilutions of colchicine were made in the appropriate tissue culture medium, using a stock solution of 25 mg/ml in absolute ethanol maintained at −20°C.
To quantify bacterial internalization by epithelial cells, each bacterial strain was tested in at least three separate assays, performed on different days, each assay representing the average of triplicate tissue culture wells. Bacterial numbers were converted to log10 prior to statistical analysis. The lower limit of assay detection was 50 bacteria or 1.7 log10; for statistical analysis, values below this limit were assigned a value of 1.7. Differences in bacterial numbers were analyzed by a one-way analysis of variance followed by Scheffe’s test for significant differences. Statistical analyses were performed with StatView 4.5 (Abacus Concepts, Berkeley, Calif.).
Visualization of filamentous actin.
Phalloidin is a phallotoxin that preferentially binds actin filaments as opposed to actin monomers (1, 5). The distribution of F-actin in the epithelial cell cytoskeleton was observed by the method of Howard and Meyer (17), with minor modifications. Epithelial cultures were incubated at 37°C for 30 min with 0.8 μM fluorescein-labeled phalloidin (Sigma) suspended in 5% buffered formalin containing 0.1 mg of lysophosphatidylcholine (Sigma) per ml, then washed three times with HBSS, mounted in phosphate-buffered saline-glycerin (1 part-9 parts) containing 0.1% p-phenylenediamine (Sigma) at pH 8, and viewed by epifluorescence microscopy.
TEER.
Transepithelial electrical resistance (TEER) can be used to monitor changes in epithelial cell culture integrity, presumably caused by loosening of the tight junctions (18). TEER was studied with the Millicell electrical resistance system (Millipore Corp., Bedford, Mass.), using mature enterocyte cultures grown on Falcon 0.45-μm-pore-size Cyclopore membranes with a 0.6-cm2 surface area (Becton Dickinson & Co., Lincoln Park, N.J.). Because TEER values often vary among individual enterocyte cultures, prior to addition of a test medium, each electrical resistance value was recorded after subtracting the average resistance of two membranes in the absence of enterocytes, i.e., membranes equilibrated overnight in tissue culture medium. The decrease in TEER (ΔΩ/cm2) was calculated as ΔΩ/cm2 = 0.6 cm2 (Ω in tissue culture medium − Ω after 1 h of incubation in test medium).
Ultrastructural visualization of cultured enterocytes and enteric bacteria.
High-resolution, low-voltage scanning electron microscopy (SEM) was used to observe the effect of cytochalasin D on nonconfluent HeLa and HEp-2 cells and on confluent Caco-2 and HT-29 cells; bacterial surface interactions with cytochalasin-treated epithelial cells were also observed. For SEM, epithelial cells were grown in wells containing a 12-mm-diameter glass coverslip. Epithelial cells were treated with cytochalasin D and incubated with bacteria as described above for bacterial internalization. Epithelial cells were then washed, fixed, and processed for SEM as described previously (37, 38, 40, 41). Fixed samples were dehydrated in ethanol, critical-point dried with CO2, and sputter-coated with a 1-nm discontinuous layer of platinum. A modified YAG crystal scintillator was used for backscatter electron imaging at 3 to 4 kV in a Hitachi S-900 field emission scanning electron microscope.
RESULTS
Effects of cytochalasin D on bacterial internalization by HeLa, Hep-2, HT-29, Caco-2, and HT-29glu cells.
Data from initial experiments indicated that a 1-h pretreatment of mature, confluent HT-29 enterocytes with cytochalasin resulted in decreased internalization of L. monocytogenes but augmented internalization of S. typhimurium, P. mirabilis, and E. coli (data not shown). These results were surprising because other investigators have typically reported that cytochalasin D was associated with decreased (not increased) bacterial internalization by cultured epithelial cells (10, 30). We therefore compared the effects of cytochalasin on bacterial internalization in mature, confluent HT-29 enterocytes, HeLa and HEp-2 cells (two nonconfluent cell lines often used by others), as well as nonconfluent HT-29 enterocytes. Assay conditions were similar to those most often used by others (1, 2, 4, 6, 7, 10–13, 19, 25, 27, 30, 31, 34, 44), e.g., similar cultivation conditions for the nonconfluent epithelial cells, similar cytochalasin concentrations, and similar bacterium-to-epithelial cell ratios.
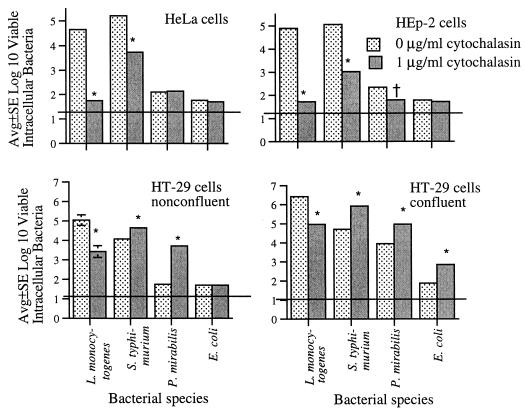
Figure 1 summarizes the effects of cytochalasin on internalization of L. monocytogenes, S. typhimurium, P. mirabilis, and E. coli in mature, confluent HT-29 enterocytes as well as nonconfluent HeLa, HEp-2, and HT-29 cells. Cytochalasin was associated with decreased internalization of L. monocytogenes in each of these four cell lines (Fig. 1). Internalization of S. typhimurium was decreased with HeLa and HEp-2 cells but increased with confluent and nonconfluent HT-29 enterocytes. P. mirabilis internalization was decreased in cytochalasin-treated HEp-2 cells, unchanged in cytochalasin-treated HeLa cells, but increased in both confluent and nonconfluent HT-29 enterocytes (Fig. 1). Cytochalasin had no apparent effect on HeLa or HEp-2 internalization of E. coli, but the numbers of intracellular E. coli were near the lower limit of assay detection and might not be reliable; internalization of E. coli was augmented in confluent but not nonconfluent HT-29 enterocytes (Fig. 1).
FIG. 1.
Effects of cytochalasin D on internalization of L. monocytogenes, S. typhimurium, P. mirabilis, and E. coli by nonconfluent HeLa and HEp-2 epithelial cells and by nonconfluent and confluent HT-29 enterocytes. Horizontal lines indicate lower limit of assay detection. Error bars are not apparent if <0.1. Significant change in the numbers of bacteria internalized in the presence of cytochalasin compared to those internalized in the absence of cytochalasin: ∗, P < 0.01; †, P < 0.05.
Because there is evidence that the effects of cytochalasins are rapidly reversible (22, 32), we attempted to determine if the effects of cytochalasin on bacterial internalization were reversible. Mature HT-29 enterocytes were pretreated for 1 h with cytochalasin D, and cytochalasin was then washed off. Internalization of L. monocytogenes, S. typhimurium, P. mirabilis, and E. coli was assayed immediately (0 h) and 24 and 48 h later. Significant effects of cytochalasin on bacterial internalization were noted only at 0 h; there were no significant differences between the numbers of bacteria internalized by untreated enterocytes and by cytochalasin-treated enterocytes assayed 24 and 48 h after removal of cytochalasin (data not shown).
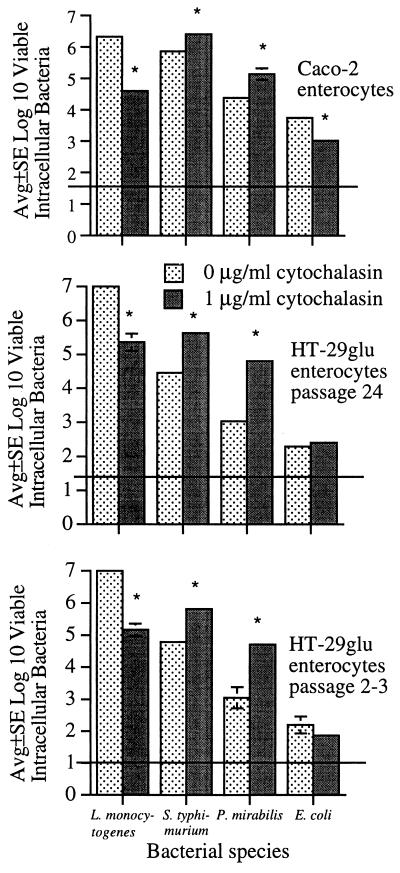
To eliminate the possibility that cytochalasin-induced alterations in bacterial internalization were unique to the HT-29 enterocyte cell line, we tested another polarized enterocyte cell line, Caco-2. Early- and late-passage (described in Materials and Methods) HT-29glu enterocytes were also tested. In confluent HT-29 as well as confluent Caco-2 and HT-29glu enterocytes, L. monocytogenes internalization was dramatically decreased while internalization of S. typhimurium and P. mirabilis was increased (Fig. 2). Cytochalasin was associated with decreased E. coli internalization by confluent Caco-2 enterocytes but appeared to have little effect on E. coli internalization by HT-29glu enterocytes (Fig. 2).
FIG. 2.
Effects of cytochalasin D on internalization of L. monocytogenes, S. typhimurium, P. mirabilis, and E. coli by confluent Caco-2 and HT-29glu enterocytes. Horizontal lines indicate lower limit of assay detection. Error bars are not apparent if <0.1. Significant change in the numbers of bacteria internalized in the presence of cytochalasin compared to those internalized in the absence of cytochalasin: ∗, P < 0.01.
Effect of cytochalasin D on epithelial cell viability and filamentous actin.
Similar to untreated epithelial cells, all epithelial cells remained ≥95% viable following 1 h of incubation in tissue culture medium supplemented with cytochalasin D (1 μg/ml) and following a subsequent 1-h incubation with L. monocytogenes, S. typhimurium, P. mirabilis, or E. coli.
After a 1-h incubation with cytochalasin, F-actin distribution was dramatically altered in each of the cell lines tested (not shown). Following staining with fluorescein-labeled phalloidin, and depending on the plane of focus, untreated Caco-2 and HT-29 enterocytes had (i) finely diffuse apical punctuate staining typical of actin in microvillar cores, (ii) smooth peripheral rings of actin presumably associated with the zonula adherens, (iii) larger actin bundles more basally situated presumably in focal contacts to the substratum, and (iv) a fine meshwork of stress fibers traversing the cells. After cytochalasin treatment, circumferential actin rings lost prominence in enterocytes but were still evident, and actin focal aggregates were noted at all focal planes. In untreated HeLa and HEp-2 cells, actin was most evident at the periphery and in stress fibers, as well as in punctuate staining characteristic of microvilli and focal contacts; after cytochalasin treatment, focal aggregates were prominent at all focal planes.
Effect of cytochalasin D on enterocyte ultrastructural topography and bacterium-enterocyte surface interactions.
Wright-Giemsa stains of confluent Caco-2 and HT-29 enterocytes, observed by light microscopy, indicated that approximately 20 to 40% of the cytochalasin-treated culture had separation of adjacent enterocytes (not shown). Nonconfluent HT-29 cells (consisting primarily of islets of 3 to 10 enterocytes) had smooth cellular outlines, compared to noticeable cytoplasmic blebs following cytochalasin treatment (not shown).
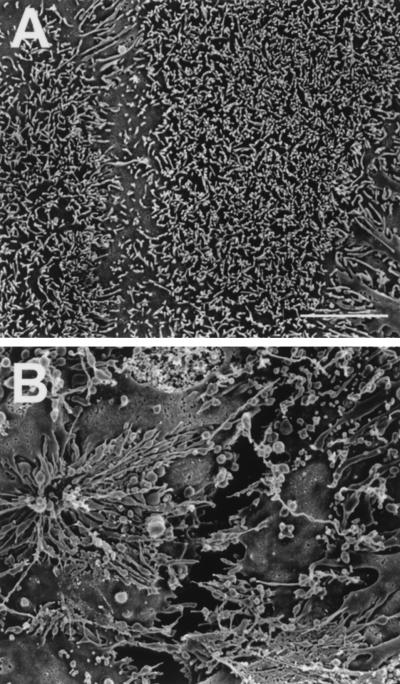
As viewed by SEM, cytochalasin had similar effects on the surface topography of confluent Caco-2 and HT-29 enterocytes. Cytochalasin caused some enterocytes to separate from each other, resulting in noticeable chasms between adjacent enterocytes; there was considerable enterocyte to enterocyte variation in the apical microvilli which appeared relatively sparse, elongated, clumped, or otherwise distorted (Fig. 3). The effects of cytochalasin on nonconfluent HeLa and HEp-2 cells included cell rounding, as well as clumping and distortion of relatively sparse apical microvilli (not shown).
FIG. 3.
High-resolution SEMs of mature, confluent HT-29 enterocytes. (A) Untreated enterocytes with relatively uniform apical microvilli and closely apposed enterocyte borders; (B) enterocytes incubated for 1 h in medium supplemented with 1 μg of cytochalasin D per ml, showing separation of individual enterocytes and grossly distorted apical microvilli. Scale bar, 0.5 μm.
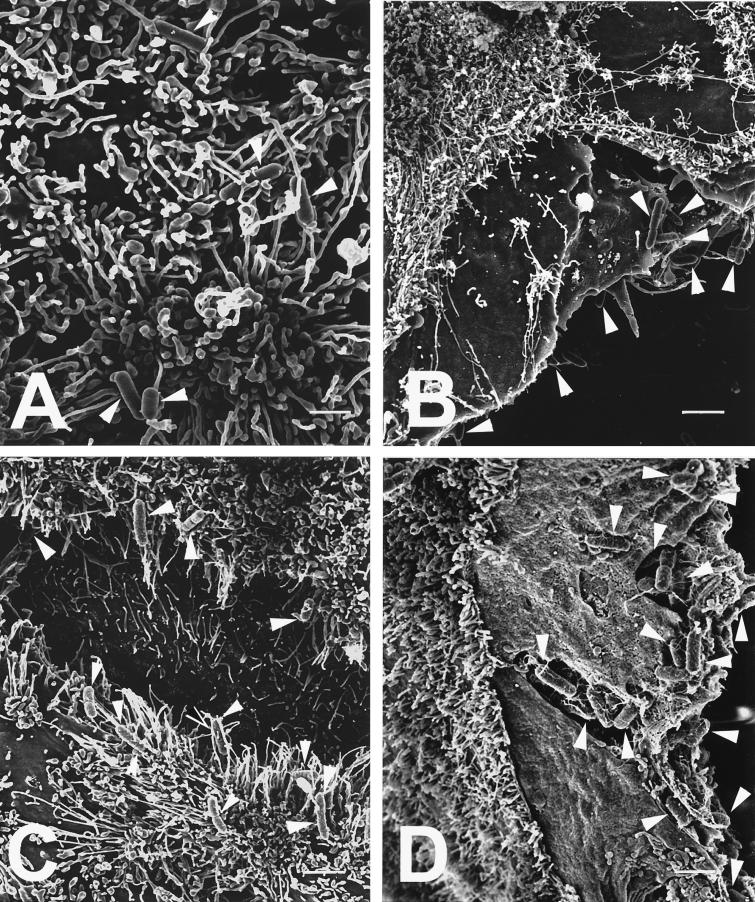
Following 1 h of incubation with cytochalasin-treated enterocytes, surface interactions of L. monocytogenes, S. typhimurium, P. mirabilis, and E. coli were viewed by SEM. (Surface interactions of these bacterial strains with untreated HT-29 and Caco-2 enterocytes have been reported previously [37, 38, 40, 41].) With L. monocytogenes, S. typhimurium, or P. mirabilis, observations were similar for cytochalasin-treated HT-29 and Caco-2 enterocytes: L. monocytogenes appeared diffusely adherent to the apical as well as the lateral surface of separated enterocytes, while S. typhimurium and P. mirabilis appeared preferentially adherent to the enterocyte lateral surface (Fig. 4). Although the numbers of adherent E. coli visualized were low compared to the other three bacterial strains in this study, E. coli appeared diffusely adherent to cytochalasin-treated Caco-2 enterocytes but preferentially adherent to the exposed lateral surface of cytochalasin-treated HT-29 enterocytes.
FIG. 4.
High-resolution SEMs of bacteria (highlighted by white arrowheads) adherent to enterocytes preincubated for 1 h in medium supplemented with 1 μg of cytochalasin D per ml. (A) L. monocytogenes diffusely adherent to the apical surface of a HT-29 enterocyte, showing listerial cells entwined among the apical microvilli; (B) P. mirabilis preferentially adherent to the lateral surface of a Caco-2 enterocyte with its apical surface devoid of bacteria; (C and D) S. typhimurium preferentially adherent to the lateral surfaces of two separated HT-29 enterocytes (C) as well as to the lateral surface of a Caco-2 enterocyte (D), where bacteria appear both tightly adherent to the lateral surface as well as in the process of internalization into the Caco-2 cytoplasm. Scale bars: A, 1 μm; B, 2.5 μm; C, 2.2 μm; and D, 1.7 μm.
Effect of cytochalasin D on bacterial internalization by enterocytes with exposed lateral surfaces.
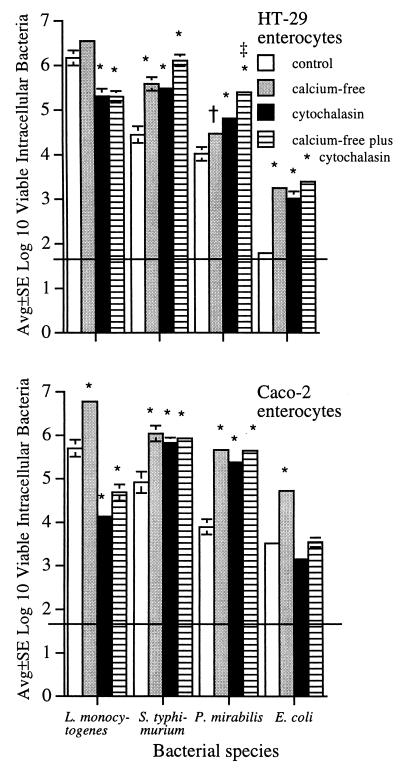
If the effect of cytochalasin D on enhanced bacterial internalization was primarily related to exposure of the enterocyte lateral surface, cytochalasin should have little additional effect on bacterial internalization in enterocytes with lateral membranes previously exposed by another means. Thus, enterocytes were incubated for 1 h in calcium-free medium prior to the 1-h incubation with cytochalasin, and bacterial internalization was then quantified. Such calcium deprivation does not affect enterocyte viability but causes confluent enterocytes to round up, exposing the lateral enterocyte surface (37).
As expected (14, 37), exposure of confluent HT-29 and Caco-2 enterocytes to a calcium-free medium was associated with increased numbers of intracellular L. monocytogenes, S. typhimurium, P. mirabilis, and E. coli cells (Fig. 5), and light microscopy of Wright-Giemsa-stained cultures confirmed that closely apposed enterocytes pulled apart from each other (not shown). Increased bacterial internalization by cytochalasin-treated HT-29 and Caco-2 enterocytes was similar to that described above (Fig. 1 and 2), verifying the reproducibility of these results. For P. mirabilis, exposure to a calcium-free medium and to cytochalasin had an additive effect on bacterial internalization by internalization by HT-29 enterocytes. However, for the remaining seven bacterium-enterocyte combinations, bacteria were internalized in similar numbers by enterocytes treated with cytochalasin alone compared to enterocytes treated with cytochalasin but with lateral membranes previously exposed by another means (i.e., incubation in a calcium-free medium); interestingly, this phenomenon was noted for bacterial strains whose internalization was either increased or decreased by cytochalasin treatment (Fig. 5).
FIG. 5.
Internalization of L. monocytogenes, S. typhimurium, P. mirabilis, and E. coli by confluent Caco-2 and HT-29 enterocytes that had been either untreated or preincubated for 1 h in either calcium-free medium, 1 μg of cytochalasin D per ml, or calcium-free medium followed by an additional 1-h incubation with cytochalasin. Horizontal lines indicate lower limit of assay detection. Error bars are not apparent if <0.1. Significant change in the numbers of bacteria internalized by treated enterocytes compared to the numbers of bacteria internalized by corresponding control, untreated enterocytes: ∗, P < 0.01; †, P < 0.05. For both HT-29 and Caco-2 enterocytes, there are no significant differences in the numbers of bacteria internalized by enterocytes treated with cytochalasin alone compared to the numbers internalized by enterocytes incubated in calcium-free medium prior to cytochalasin treatment, with the exception of P. mirabilis incubated with HT-29 enterocytes, where the numbers of internalized proteus are greater in enterocytes incubated in the calcium-free medium prior to the cytochalasin treatment: ‡, P < 0.05.
Internalization of S. flexneri and S. sonnei by mature Caco-2 enterocytes.
Others have noted that S. flexneri does not generally enter through the apical Caco-2 surface but prefers the basolateral route (24). We therefore tested the effects of cytochalasin and calcium-free medium on internalization of shigellae, using our Caco-2 cultures cultivated under the conditions used in the experiments described above. Because internalization of shigellae was generally undetectable in initial experiments, enterocytes were centrifuged (2,000 × g for 10 min) immediately after addition of the bacterial inoculum to facilitate bacterium-enterocyte contact (24). Compared to numbers of internalized shigellae in untreated enterocytes, centrifugation consistently increased (P < 0.01) internalization of shigellae (Table 1). As expected, numbers of viable intracellular shigellae were consistently greater in Caco-2 cultures exposed to calcium-free medium than in enterocytes incubated in normal tissue culture medium (Table 1). However, cytochalasin had no effect on internalization of shigellae, with the exception of decreased S. sonnei internalization by centrifuged Caco-2 cells (Table 1).
TABLE 1.
Effect of 1 h Caco-2 pretreatment with cytochalasin D or with calcium-free medium on S. sonnei and S. flexneri internalization, where centrifugation (2,000 × g for 10 min) has and has not been used to facilitate initial contact of bacteria with enterocytes
| Shigella species | Treatment | Avg no. of log10 viable bacteria ± SEa
|
|||
|---|---|---|---|---|---|
| Not centrifuged
|
Centrifuged
|
||||
| Control | Cytochalasin | Control | Cytochalasin | ||
| S. sonnei | Cytochalasin | 1.9 ± 0.1 | 2.0 ± 0.1 | 3.9 ± 0.2 | 3.2 ± 0.2b |
| Calcium-free medium | 2.3 ± 0.2 | 3.6 ± 0.1c | 3.8 ± 0.2 | 4.6 ± 0.1c | |
| S. flexneri | Cytochalasin | 1.8 ± 0.1 | 2.0 ± 0.1 | 2.7 ± 0.1 | 2.9 ± 0.1 |
| Calcium-free medium | 1.8 ± 0.1 | 3.6 ± 0.1c | 2.8 ± 0.2 | 4.7 ± 0.1c | |
Average of at least three separate assays, with a lower detection limit of 50 bacteria (1.7 log10). Numbers of internalized shigellae consistently increased in control enterocytes that were centrifuged compared to control enterocytes that were not centrifuged (P < 0.01).
Significantly decreased compared to corresponding control enterocytes, P < 0.05.
Significantly increased compared to corresponding control enterocytes, P < 0.01.
Effect of colchicine on bacterial internalization by mature HT-29 and Caco-2 enterocytes.
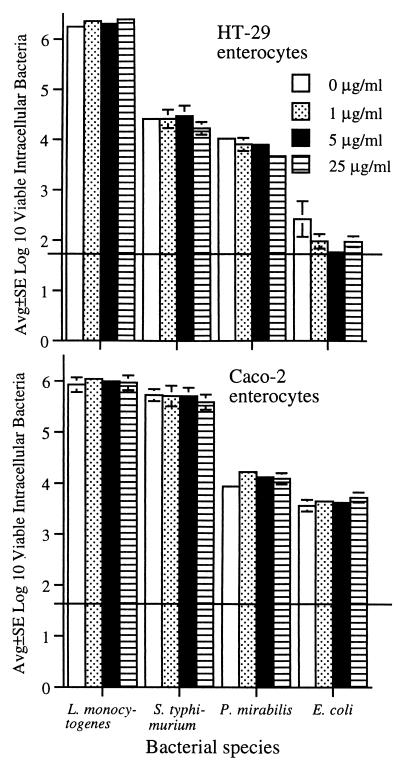
To study the effects of microtubule disruption on bacterial internalization, mature HT-29 and Caco-2 enterocytes were pretreated for 1 h with 0, 1, 5, or 25 μg of colchicine per ml. These concentrations of colchicine were chosen because 8 μg/ml causes marked disruption of Caco-2 microtubules (22). Colchicine had no noticeable effect on internalization of either L. monocytogenes, S. typhimurium, P. mirabilis, or E. coli (Fig. 6). Colchicine had no noticeable effect on enterocyte viability.
FIG. 6.
Effects of various concentrations of colchicine on internalization of L. monocytogenes, S. typhimurium, P. mirabilis, and E. coli by confluent Caco-2 and HT-29 enterocytes. Enterocytes were pretreated for 1 h with either 0, 1, 5, or 25 μg of colchicine per ml, and colchicine was present throughout the subsequent bacterial internalization assay. Horizontal lines indicate lower limit of assay detection.
Effect of cytochalasin D and colchicine on TEER of mature enterocytes.
Exposure of HT-29 cells to calcium-free medium causes rounding of all enterocytes and TEER values decrease to baseline values (37). Calcium-free medium can thus be used as a positive control in studies of the effects of various compounds on enterocyte TEER. We have previously reported decreased TEER in HT-29 cultures incubated for 1 h with cytochalasin D (37). Similar decreases in TEER were noted with cytochalasin-treated Caco-2 cultures, although colchicine had little effect on TEER (Table 2). TEER values were measured 1, 2, and 3 h after cytochalasin or colchicine treatment of Caco-2 cultures, but all decreases appeared maximal after 1 h (data not shown).
TABLE 2.
Decrease in TEER of mature Caco-2 cultures following 1 h of incubation in calcium-free medium or in tissue culture medium supplemented with either cytochalasin or colchicine
| Treatment | % Decrease in TEER (avg ± SE of ≥4 cultures) |
|---|---|
| None | 5 ± 1 |
| Colchicine, 25 μg/ml | 12 ± 2 |
| Cytochalasin | |
| 0.1 μg/ml | 27 ± 2a |
| 1.0 μg/ml | 68 ± 3a |
| 10 μg/ml | 51 ± 3a |
| Calcium-free medium | 94 ± 1a |
Significant decrease in TEER compared to no treatment, analysis of variance followed by Fisher’s test for significant difference, P < 0.01.
DISCUSSION
Cytochalasin-induced actin disruption has been typically associated with decreased bacterial internalization by cultured epithelial cells (1, 2, 4, 6, 7, 10–13, 19, 25, 27, 30, 31, 34, 44). Although Murai et al. (25) reported that cytochalasin B enhanced internalization of Staphylococcus aureus by primary mouse kidney cells, such reports are rare, and we could find no report describing a similar effect of cytochalasin D. Thus, we were surprised that in polarized enterocytes, cytochalasin D was associated with increased internalization of several bacterial species (Fig. 1 and 2).
Because polarized enterocytes have not often been used to study the effects of cytochalasins on bacterium-epithelial cell interactions, results from experiments using confluent HT-29 and Caco-2 enterocytes were compared with results from experiments using other types of epithelial cells. These other epithelial cell lines included two variations of HT-29 enterocytes, namely, nonconfluent HT-29 cells and confluent HT-29glu cells, as well as two cell lines that do not establish polarized monolayers and are used frequently by others, namely, HeLa and HEp-2 cells. Cytochalasin was associated with decreased internalization of S. typhimurium and P. mirabilis in the two nonenterocytic cell lines (HeLa and HEp-2) but with increased internalization in the four types of enterocyte cultures. The effect of cytochalasin on the numbers of internalized E. coli cells showed no pattern, but this E. coli strain was relatively noninvasive and numbers of internalized E. coli cells were often near the lower limit of assay detection and might not be reliable. For each of these epithelial cultures, internalization of L. monocytogenes was consistently decreased in the presence of cytochalasin, indicating that cytochalasin-induced augmentation of bacterial internalization by enterocytes might be species specific.
We cannot explain the similar results obtained with confluent HT-29glu and HT-29 enterocytes and with nonconfluent HT-29 enterocytes. Others have shown that markers of enterocyte differentiation are not fully expressed until enterocytes achieve confluency (18, 26, 28, 29), and several investigators have reported that HT-29glu enterocytes are undifferentiated compared to HT-29 enterocytes (18, 26). Therefore, although we had expected that the effect of cytochalasin on bacterial internalization might differ for confluent HT-29 enterocytes compared to nonconfluent HT-29 and confluent HT-29glu enterocytes, the data suggested that the effect of cytochalasin on bacterial internalization may not depend on the stage of HT-29 differentiation.
As noted by others (3, 32), cytochalasin D had no noticeable effect on epithelial cell viability, and staining with fluorescein-labeled phalloidin confirmed that cytochalasin disrupted F-actin in a characteristic manner (15, 32, 43). As also reported by others (3, 32, 43), cytochalasin D caused cell rounding, and closely apposed epithelial cells pulled apart from each other. It should be noted that nonconfluent HT-29 enterocytes were actually clusters of cells, and there was thus increased exposure of the enterocyte lateral surface in both nonconfluent and confluent HT-29 cultures. The observed cytochalasin-induced disruptions in intercellular junctions of adjacent enterocytes were consistent with decreased TEER following cytochalasin treatment of these confluent cultures. Conversely, colchicine had minimal effect on TEER of confluent enterocyes and had no noticeable effect on bacterial internalization. Ma et al. (22) reported that colchicine-induced disruption of Caco-2 microtubules had no significant effect on actin microfilaments, intercellular junctions, or paracellular permeability.
We have previously reported separation of individual enterocytes in mature confluent HT-29 cultures incubated for 1 h either in a calcium-free medium (37) or in a medium containing purified toxin secreted by enterotoxigenic strains of Bacteroides fragilis (38). These two treatments did not affect enterocyte viability but were associated with increased internalization of the same strains of S. typhimurium, P. mirabilis, and E. coli used in the present study; these increased numbers of intracellular bacteria were also accompanied by electron microscopic visualization of bacterial cells preferentially adherent to the exposed enterocyte lateral surface (37, 38). The fact that calcium-free medium, B. fragilis enterotoxin, and cytochalasin D are disparate treatments with similar outcomes (i.e., exposure of the enterocyte lateral membrane and increased bacterial internalization) argues strongly that the lateral enterocyte surface might be the preferred site of internalization for some strains of enteric bacteria.
The observation that specific strains of enteric bacteria preferentially adhered to the lateral enterocyte surface is not new. Several years ago, Mounier et al. (24) reported that S. flexneri preferentially bound to the exposed lateral membranes of Caco-2 enterocytes when these surfaces were exposed by calcium chelation and that these adherent bacteria more easily invaded enterocytes. Mounier et al. (24) concluded that S. flexneri did not generally enter Caco-2 cells through the apical pole but rather used the basolateral route, which is normally not exposed. We similarly noted that shigellae did not readily enter Caco-2 enterocytes and that internalization was significantly increased in enterocytes that had their lateral surfaces exposed by treatment with low extracellular calcium (Table 1). However, cytochalasin was not associated with increased internalization of shigellae but was associated with decreased internalization of S. sonnei and no change in S. flexneri internalization (Table 1). At first inspection, these effects of cytochalasin on internalization of shigellae appear at odds with the results with other members of the family Enterobacteriaceae (Fig. 1 and 2). However, Finlay and Falkow (8) similarly noted that cytochalasin D inhibited S. flexneri entry into polarized MDCK cells. Strauss and Falkow (33) recently summarized “striking similarities” in the behavior of L. monocytogenes and S. flexneri within host cells; i.e., both are internalized within membrane-bound cytoplasmic vacuoles, and both lyse the vacuole and harness actin, using the force generated by actin polymerization to infect adjacent cells. And, in contrast to shigellae, salmonellae do not lyse the Caco-2 phagosomal membrane, but multiply within the phagosome, and likely move through the enterocyte by transcytosis (9). Thus, in analyzing bacterial interactions with eucaryotic actin, there is evidence that shigellae may have more in common with L. monocytogenes than with other genera of Enterobacteriaceae.
Using the six different types of cultured epithelia, cytochalasin was consistently associated with decreased internalization of L. monocytogenes. Karunasagar et al. (20) noted that listerial uptake appeared favored at the apical enterocyte surface, but basolateral penetration could not be excluded. Gaillard and Finlay subsequently (14) reported that L. monocytogenes bound to and entered both the apical and lateral surfaces of mature Caco-2 cells, although the lateral surface appeared to be the preferred site of entry, based in part on enhanced internalization following disruption of the Caco-2 tight junctions by calcium-free medium. We noted that internalization of L. monocytogenes was augmented in enterocytes with their lateral surfaces exposed by a calcium-free medium (Fig. 5), also indicating that exposure of the lateral enterocyte surface might augment listerial internalization. Thus, there is evidence that internalization of L. monocytogenes occurs at both the apical and lateral surfaces of polarized enterocytes.
If exposure of the lateral enterocyte surface was an important factor in increased bacterial internalization by cytochalasin D-treated enterocytes, then cytochalasin might be expected to have little effect on the numbers of bacteria internalized by enterocytes that had their lateral surfaces previously exposed by another means. In those instances where bacterial internalization was similarly increased in enterocytes pretreated with calcium-free medium, with cytochalasin, or with both, the data indicated that exposure of the enterocyte lateral surface might explain the increased bacterial internalization by cytochalasin-treated enterocytes (Fig. 5). However, these results were not consistent. Perhaps either cytochalasin or exposure to calcium-free medium, or both, triggered a complex cascade of alterations in enterocyte function, and the mechanisms responsible for cytochalasin-induced increases in bacterial internalization cannot be explained solely on the basis of exposure of the enterocyte lateral surface. It is also possible (perhaps likely) that any treatment that opens enterocyte tight junctions (e.g., calcium-free medium or cytochalasin D) results in a redistribution of enterocyte receptors previously localized to the apical or the lateral enterocyte surface, and such receptor redistribution could modulate bacterial internalization by enterocytes. Nonetheless, cytochalasin-induced increases in bacterial internalization seemed to be due, at least in part, to exposure of the enterocyte lateral surface for nearly every bacterium-enterocyte combinations studied because (with exception of L. monocytogenes) bacterial internalization by cytochalasin-treated enterocytes was similar in enterocytes that had their lateral urfaces previously exposed by another means prior to the cytochalasin treatment (Fig. 5).
The combined effects of calcium-free medium and cytochalasin D on enterocyte internalization of L. monocytogenes seemed particularly intriguing and might be explained in view of recent evidence that E-cadherin is the ligand for internalin, a surface protein on L. monocytogenes that is essential for entry into epithelial cells (23). E-cadherin is a calcium-dependent, cell-cell adhesion molecule concentrated in the adherens junctions in association with actin filaments; E-cadherin is expressed basolaterally on enterocytes (16). It was therefore not surprising that exposure of the enterocyte lateral surface (using low concentrations of extracellular calcium) was associated with increased numbers of internalized L. monocytogenes (Fig. 5). The observation that cytochalasin had a dramatic inhibitory effect on listerial internalization, even in enterocytes that had their lateral surfaces previously exposed by incubation in calcium-free medium, indicated that functional actin played a major role in internalization of L. monocytogenes at the enterocyte lateral surface. These data are consistent with recent observations by Mengaud et al. (23), who suggested that the requirement for actin polymerization (blocked by cytochalasin D) and the integrity of the E-cadherin cytoplasmic domain is needed for L. monocytogenes internalization via the internalin–E-cadherin interaction.
If S. typhimurium and P. mirabilis entry is favored at the lateral enterocyte surface, and if the mechanism of entry of these bacteria differs from the L. monocytogenes–E-cadherin interaction, the lack of a cytochalasin-mediated inhibition of S. typhimurium and P. mirabilis internalization could be explained if cytochalasin does not inhibit all forms of endocytosis at the lateral surface. Interestingly, Gottlieb et al. (15) studied the effects of cytochalasin D on polarized MDCK cells and noted a fundamental difference in the process by which endocytotic vesicles are formed at the apical microvillar surface versus the basolateral surface. In a series of elegant experiments, cytochalasin selectively blocked endocytosis of fluid phase and particulate markers at the apical MDCK surface without affecting uptake at the basolateral surface. In addition, cytochalasin did not affect basolateral uptake by MDCK cells that had their tight junctions previously disrupted by depletion of extracellular calcium. Gottlieb et al. (15) concluded that cytochalasin D blocked apical, but not basolateral, endocytosis in polarized MDCK cells and further speculated that the selective inhibitory effect of cytochalasin on apical endocytosis was likely a general effect for all transporting epithelia.
It is important to stress that the results presented herein suggest, but do not prove, that functional F-actin might not be required for internalization of some bacterial species (such as S. typhimurium and P. mirabilis) that preferentially adhere to the enterocyte lateral surface. Although additional studies seem necessary to clarify the role of F-actin in bacterial internalization by enterocytes, the present results do suggest that specific strains of enteric bacteria may preferentially enter enterocytes through the lateral enterocyte surface by a mechanism independent of a pathway mediated by F-actin. Although additional experiments are needed to verify that the lateral enterocyte surface may be the preferred site of entry for some bacterial species, the evidence presented herein combined with evidence in the literature indicates that this is a reasonable working hypothesis to be challenged in future experiments.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI 23484 from the National Institutes of Health.
We thank Muriel Gavin for excellent technical assistance.
REFERENCES
- 1.Benjamin P, Federman M, Wanke C A. Characterization of an invasive phenotype associated with enteroaggregative Escherichia coli. Infect Immun. 1995;63:3417–3421. doi: 10.1128/iai.63.9.3417-3421.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chippendale G R, Warren J W, Trifillis A L, Mobley H L. Internalization of Proteus mirabilis by human renal epithelial cells. Infect Immun. 1994;62:3115–3121. doi: 10.1128/iai.62.8.3115-3121.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper J A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnenberg M S, Newman B, Utsalo S J, Trifillis A L, Hebel J R, Warren J W. Internalization of Escherichia coli into human kidney epithelial cells: comparison of fecal and pyelonephritis-associated strains. J Infect Dis. 1994;169:831–838. doi: 10.1093/infdis/169.4.831. [DOI] [PubMed] [Google Scholar]
- 5.DuBose D A, Haugland R. Comparisons of endothelial cell G- and F-actin distribution in situ and in vitro. Biotech Histochem. 1993;68:8–16. doi: 10.3109/10520299309105570. [DOI] [PubMed] [Google Scholar]
- 6.Elsinghorst E A, Baron L S, Kopecko D J. Penetration of human intestinal epithelial cells by Salmonella: molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:5173–5177. doi: 10.1073/pnas.86.13.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewanowich C A, Melton A R, Weiss A A, Sherburne R K, Peppler M S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989;57:2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlay B B, Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri, and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988;70:1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- 9.Finlay B B, Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990;162:1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- 10.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finlay B B, Ruschkowski S, Dedhar S. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 12.Francis C L, Jerse A E, Kaper J B, Falkow S. Characterization of interactions of enteropathogenic Escherichia coli 0127:H6 with mammalian cells in vitro. J Infect Dis. 1991;164:693–703. doi: 10.1093/infdis/164.4.693. [DOI] [PubMed] [Google Scholar]
- 13.Gaillard J-L, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaillard J-L, Finlay B B. Effect of cell polarization and differentiation on entry of Listeria monocytogenes into the enterocyte-like Caco-2 cell line. Infect Immun. 1996;64:1299–1308. doi: 10.1128/iai.64.4.1299-1308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb T A, Ivanov I E, Adesnik M, Sabatini D D. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993;120:695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermiston M L, Gordon J I. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J Cell Biol. 1995;129:489–506. doi: 10.1083/jcb.129.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard T H, Meyer W H. Chemotactic peptide modulation of actin assembly and locomotion in neutrophils. J Cell Biol. 1984;98:1265–1271. doi: 10.1083/jcb.98.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huet C, Sahuquillo-Merino E, Courdrier E, Louvard D. Absorptive and mucus-screening subclones isolated from a multipotent intestinal cell line (HT-29) provide new models for cell polarity and terminal differentiation. J Cell Biol. 1987;105:345–357. doi: 10.1083/jcb.105.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janda J M, Abbott S L, Oshiro L S. Penetration and replication of Edwardsiella spp. in HEp-2 cells. Infect Immun. 1991;59:154–161. doi: 10.1128/iai.59.1.154-161.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karunasagar I, Senghaas B, Krohne G, Goebel W. Ultrastructural study of Listeria monocytogenes entry into cultured human colonic epithelial cells. Infect Immun. 1994;62:3554–3558. doi: 10.1128/iai.62.8.3554-3558.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luna E J, Hitt A L. Cytoskeleton-plasma membrane interactions. Science. 1992;258:955–963. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- 22.Ma T Y, Hollander D, Tran L T, Nguyen D, Hoa N, Bhalla D. Cytoskeletal regulation of Caco-2 intestinal monolayer paracellular permeability. J Cell Physiol. 1995;164:533–545. doi: 10.1002/jcp.1041640311. [DOI] [PubMed] [Google Scholar]
- 23.Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 24.Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti P J. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992;60:237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murai M, Seki K, Sakurada J, Usui A, Masuda S. Effects of cytochalasins B and D on Staphylococcus aureus adherence to and ingestion by mouse renal cells from primary culture. Microbiol Immunol. 1993;37:69–73. doi: 10.1111/j.1348-0421.1993.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 26.Neutra M, Louvard D. Differentiation of intestinal cells in vitro. In: Satir B H, editor. Functional epithelial cells in culture. New York, N.Y: Alan R. Liss, Inc.; 1989. pp. 363–398. [Google Scholar]
- 27.Oelschlaeger T A, Guerry P, Kopecko D J. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci USA. 1993;90:6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto M, Appay M-D, Simon-Assmann P, Chevalier G, Dracopoli N, Fogh J, Zweibaum A. Enterocyte differentiation of cultured human colon cancer cells by replacement of glucose by galactose in the medium. Biol Cell. 1982;44:193–196. [Google Scholar]
- 29.Pinto M, Robine-Leon S, Apay M, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 30.Rosenshine I, Ruschkowski S, Finlay B B. Inhibitors of cytoskeletal function and signal transduction to study bacterial invasion. Methods Enzymol. 1994;236:467–476. doi: 10.1016/0076-6879(94)36035-9. [DOI] [PubMed] [Google Scholar]
- 31.Russell R G, Blake D C., Jr Cell association and invasion of Caco-2 cells by Campylobacter jejuni. Infect Immun. 1994;62:3773–3779. doi: 10.1128/iai.62.9.3773-3779.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson B R, Begg D A. Concentration-dependent effects of cytochalasin D on tight junctions and actin filaments in MDCK epithelial cells. J Cell Sci. 1994;107:367–375. doi: 10.1242/jcs.107.3.367. [DOI] [PubMed] [Google Scholar]
- 33.Strauss E J, Falkow S. Microbial pathogenesis: genomics and beyond. Science. 1997;276:707–712. doi: 10.1126/science.276.5313.707. [DOI] [PubMed] [Google Scholar]
- 34.Tang P, Rosenshine I, Finlay B B. Listeria monocytogenes, an invasive bacterium stimulates MAP kinase upon attachment to epithelial cells. Am Soc Cell Biol. 1994;5:455–464. doi: 10.1091/mbc.5.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells C L, Feltis B A, Hanson D F, Jechorek R P, Erlandsen S L. Oral infectivity and bacterial interactions with mononuclear phagocytes. J Med Microbiol. 1993;38:345–353. doi: 10.1099/00222615-38-5-345. [DOI] [PubMed] [Google Scholar]
- 36.Wells C L, van de Westerlo E M A, Jechorek R P, Erlandsen S L. Effect of hypoxia on enterocyte internalization of enteric bacteria. Crit Care Med. 1996;24:985–991. doi: 10.1097/00003246-199606000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Wells C L, van de Westerlo E M A, Jechorek R P, Erlandsen S L. Exposure of the lateral enterocyte membrane by dissociation of calcium-dependent junctional complex augments endocytosis of enteric bacteria. Shock. 1995;4:204–210. doi: 10.1097/00024382-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Wells C L, van de Westerlo E M A, Jechorek R P, Feltis B A, Wilkins T D, Erlandsen S L. Bacteroides fragilis enterotoxin modulates epithelial permeability and bacterial internalization by HT-29 enterocytes. Gastroenterology. 1996;110:1429–1437. doi: 10.1053/gast.1996.v110.pm8613048. [DOI] [PubMed] [Google Scholar]
- 39.Wells C L, Maddaus M A, Reynolds C M, Jechorek R P, Simmons R L. Role of the anaerobic flora in the translocation of aerobic and facultatively anaerobic intestinal bacteria. Infect Immun. 1987;55:2689–2694. doi: 10.1128/iai.55.11.2689-2694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells C L, Jechorek R P, Olmsted S B, Erlandsen S L. Bacterial translocation in cultured enterocytes: magnitude, specificity, and electron microscopic observations of endocytosis. Shock. 1994;1:443–451. [PubMed] [Google Scholar]
- 41.Wells C L, Jechorek R P, Erlandsen S L. Inhibitory effect of bile on bacterial invasion of enterocytes: possible mechanism for increased translocation associated with obstructive jaundice. Crit Care Med. 1995;23:301–307. doi: 10.1097/00003246-199502000-00016. [DOI] [PubMed] [Google Scholar]
- 42.Wells C L, Jechorek R P, Olmsted S B, Erlandsen S L. Effect of LPS on epithelial integrity and bacterial uptake in the polarized human enterocyte-like cell line Caco-2. Circ Shock. 1993;40:276–288. [PubMed] [Google Scholar]
- 43.Wodnicka M, Pierzchalska M, Bereiter-Hahn J, Kajstura J. Comparative study on effects of cytochalasins B and D on F-actin content in different cell lines and different culture conditions. Folia Histochem Cytobiol. 1992;30:107–111. [PubMed] [Google Scholar]
- 44.Woods C R, Jr, Mason E O, Jr, Kaplan S L. Interaction of Citrobacter diversus strains with HEp-2 epithelial and human umbilical vein endothelial cells. J Infect Dis. 1992;166:1035–1044. doi: 10.1093/infdis/166.5.1035. [DOI] [PubMed] [Google Scholar]