Abstract
Purpose
Streptococcus pyogenes (mostly termed group A Streptococcus - GAS) is the most important bacterial causative of pharyngitis. However, epidemiology of GAS pharyngitis is not widely established. This study describes GAS pharyngitis cases and emm-type distribution in a prospective study covering over 2 years in two Hospital Districts in Finland.
Methods
A prospective, systematic collection of GAS pharyngitis isolates was conducted between March 2018 and December 2020 in two large Hospital Districts in Finland. Patient characteristics (age, gender) were included if available. All GAS isolates collected were emm typed.
Results
Altogether 1320 GAS pharyngitis strains were collected, 904 in the Hospital District 1 (HD1) and 416 in Hospital District 2 (HD2). In HD1, age and gender data were available. Females were overrepresented (58% of all cases). In addition, the age and gender distributions were noted to be significantly different (p < 0.0001) with females having a more uniform distribution until age of 40. emm28 was common among the age group of 20–29-year-olds and emm89 in children under 10 years of age, respectively. In HD1, most of the isolates were collected during winter and autumn months. Significant differences by season in the frequency of emm12, emm89, emm75 and group of “others” were observed.
Conclusion
Age distribution among GAS pharyngitis cases was significantly different between genders (p < 0.0001). In addition, age group specific and seasonal variations in emm GAS types causing the disease were observed. These findings warrant further investigation, especially for understanding population-based spread of GAS even in more detail.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10096-023-04714-6.
Keywords: Group A streptococcus, S. pyogenes, GAS, Pharyngitis, emm type, Seasonality, Epidemiology
Introduction
Group A Streptococcus (Streptococcus pyogenes, GAS) is an important human pathogen causing infections from mild pharyngitis to life-threatening invasive infections (iGAS) [1–3]. Pharyngitis is the most common disease manifestation of GAS. In 5–15% of adults and 15–35% of children with pharyngitis, GAS is found as the causative agent [1–4]. Asymptomatic throat carriage is also recognized especially in young children [5]. Since late 2022, several European countries have reported increased numbers of GAS infections, especially scarlet fever and iGAS infections in children [6–8].
M-protein is the most important virulence factor of GAS. Emm typing is based on sequencing of the hypervariable region of emm gene that codes the M-protein. There are currently over 260 different emm types recognized [9]. Same emm types may cause both invasive and mild infections, and associations between specific emm types and certain infection foci have been reported [3, 10]. Currently, there is no vaccine available against GAS. However, there are several vaccine candidates in clinical trials [11]. Reports on epidemiology and distribution of emm types and among iGAS are available [12], but those on GAS pharyngitis are scarcer [3].
Here we describe the results from a prospective over 2-year study on epidemiology of GAS pharyngitis in two large hospital districts in Finland. Our results show a significant difference in the prevalence of GAS pharyngitis between different age groups and gender. In addition, a clear pattern of seasonal variation in emm-type distribution was observed.
Methods
Study settings
GAS throat cultures were collected from two clinical microbiology laboratories in Finland: Turku University Hospital, Clinical microbiology laboratory in Hospital district of Southwest Finland (hereafter HD1), serving a population of 470,000 and Fimlab laboratories in Pirkanmaa Hospital district (hereafter HD2), serving a population of 520,000. At the time when this study was conducted, the Finnish Current Care Guidelines on diagnostics of acute pharyngitis recommended a throat culture to be performed especially if GAS infection and subsequent antimicrobial treatment was considered. This practise was commonly followed.
In HD1, the clinical laboratory randomly selected 10 MALDI-TOF confirmed Streptococcus pyogenes cultures from their routine pharyngitis diagnostics to be included in this study. Isolates were collected weekly for a 32-month period (March 2018–December 2020). Collection halted for 6 weeks (16.3.–26.4.2020) due to the COVID-19 pandemic. The culture plates were transferred to the University of Turku, for analysis and storage. If more than 10 cultures were delivered, all were included to have a good presentation of the circulating isolates. The isolation date and age and gender of the patients were recorded. The data was anonymized, and only arbitrary study codes were used.
For comparison, simultaneous collection of S. pyogenes pharyngitis isolates from HD2 was conducted. Similarly, to HD1, MALDI-TOF confirmed S. pyogenes isolates were sent in agar transport tubes to University of Turku in larger batches. The year of isolation was provided. The strains were processed with an arbitrary study code and analysed similarly to isolates from HD1.
Microbiological analysis and emm typing
Beta-haemolytic bacterial colonies were selected from the original throat culture plates and from isolates provided on transport tubes after reculturing on blood agar (TSA with sheep blood, BD). GAS isolates were confirmed with Lancefield antigen agglutination test (RemelTM StreptexTM Latex Group A, ThermoFisher). All isolates were emm typed using the CDC protocol [9].
For analysis, emm subtypes of the main emm types (emm1, emm4, emm12, emm28 and emm89) were grouped under the corresponding emm type (for example emm12.0 and emm12.37 were grouped into emm12). Due to the high prevalence of emm1.25 subtype, it was analysed separately and considered as an emm type in this study (Online Resource 1). The seven most common emm types (emm1, emm1.25, emm4, emm12, emm28, emm75 and emm89) were studied individually and the rest jointly under the group “others”.
In addition to emm types, isolates were analysed based on the emm cluster classification [13].
Seasonality analysis
Seasonality analysis was performed only for isolates from HD1. The study period was divided into quarters representing the seasons: spring (March to May), summer (June to August), autumn (September to November) and winter (December to February). For the seasonality analysis, months after the onset of the COVID-19 pandemic (3/20–12/20) were excluded. The quarters of the remaining 2 years (3/18–2/20) were combined by season (6 months each). Seven most common emm types (emm1, emm1.25, emm4, emm12, emm28, emm75 and emm89) were studied individually and the rest jointly under the group “others”. In addition, seasonality was analysed on emm cluster level.
Vaccine coverage analysis
The coverage of emm types of pharyngitis isolates collected in this study was evaluated in relation to composition of the 30-valent M-protein-based GAS vaccine candidates under development [14].
Invasive GAS isolates
Clinical microbiological laboratories notify iGAS cases (isolations from blood and cerebrospinal fluid) and send the isolates to the National Infectious Disease Register (NIDR) maintained by the Finnish Institute of Health and Welfare (THL). THL performs emm typing for the isolates [9]. In this study, emm-type distribution data and year of isolation on all registered iGAS isolates in HD1 and HD2 covering January 2018–December 2020 were retrieved from NIDR.
Statistical methods
Age and gender distribution analysis and seasonality analysis were only performed with the data from HD1. Due to asymmetrical distribution, median age was reported with range. For further analysis, age was categorized in 10-year age groups. Categorical data (emm type, gender, age group, season, months after onset of the COVID-19 pandemic) was summarized with counts (n) and percentages. Associations between categorical data were analyzed by the chi-square test. The prevalence of emm types in relation to patient age and gender was analyzed using binary logistic regression. First gender, age group and the interaction term between gender and age group were added to the multivariate models and from these models, non-significant factors were gradually omitted. Odds ratios (OR) with 95% Wald confidence intervals (95%CI) were reported. Because of the limited amount of data from iGAS isolates, only descriptive statistics were reported and it was not possible to carry out the association between seasonality and age and gender. All tests were performed as two-sided with a significance level set at 0.05. The analyses were carried out using SAS System, version 9.4 for Windows (SAS Institute Inc., Cary, NC, US).
Results
Overall, 1320 GAS isolates were collected during the 32-month study period, 904 from HD1 and 416 from HD2, respectively. This represents 14% of all GAS-positive pharyngitis cultures performed in these two laboratories during this period: 22% (of 4023 isolates) in HD1 and 7.4% (of all 5597 isolates) in HD2, respectively (Fig. 1 A).
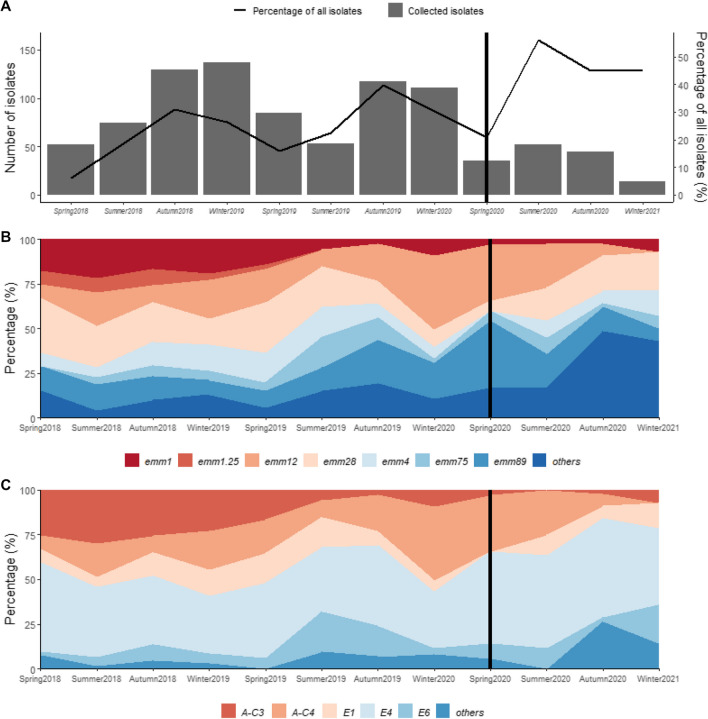
Fig. 1.
A The number of pharyngitis GAS isolates collected to our study by season from Hospital District 1 between March 2018 and December 2020 (bar chart) in relation to all GAS-positive pharyngitis findings from the same period collected in the same hospital district (line chart). Sample collection was halted for 6 weeks in Spring 2020 (16.3–26.4.2020). The vertical black bar marks the start of the COVID-19 pandemic (Spring 2020). Data is shown for each 3-month period; however, Winter 2021 includes only the month of December. The aim was to collect 10 isolates per week. The fulfilment was on average 7 isolates per week (range 0–19). B and C Seasonal fluctuations of main emm types (B) and emm clusters (C) among GAS pharyngitis isolates collected in Hospital District 1. Isolates collected after March 2020 (black bar) were not included in the seasonality analysis. *Significant difference (p < 0.05) observed for emm12, emm75, emm89 and the group ”others”
Hospital district 1
The median number of weekly collected strains was seven (range 0–19). Before the COVID-19 pandemic, the proportion of collected isolates in relation to all GAS isolates varied between 6.0 % (spring 2018, 52/865) and 40% (autumn 2019, 117/293). After the start of COVID-19 pandemic, the amount of isolates decreased, but the proportion remained high (37%, 146/392, Fig. 1 A).
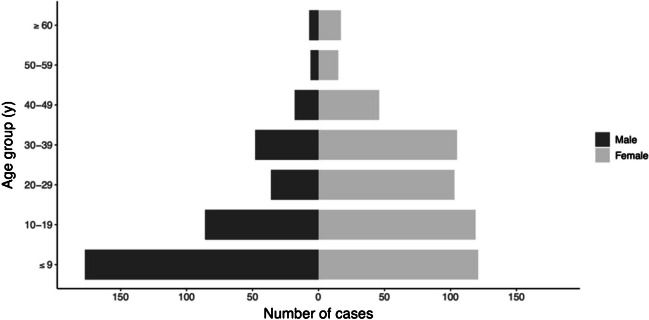
The median age of patients was 17 years (range < 1–81 years). The most common age group was under 10-year-olds (298/904, 33% of all) and 71% of these were 5–9-year-olds (212/904, 23% of all). 58 % of the study subjects were female (526/904, 58%). The age distribution was significantly different between genders (p < 0.0001). Median age of males was 11 years (range 1–72 years), whereas within females it was 21 years (range < 1–81 years). Within males, most cases occurred in the age group under 10 years (177/378, 47%). Within females, the distribution was more uniform until age of 40 years (20–23% of the cases in each of the 10-year age group), whereas within males the distribution was skewed to right (Fig. 2).
Fig. 2.
The age distribution in years (y) by gender, male (n = 378) and female (n = 526), of GAS pharyngitis cases in Hospital District 1 between March 2018 and December 2020. The age distribution was significantly different between genders (p < 0.0001)
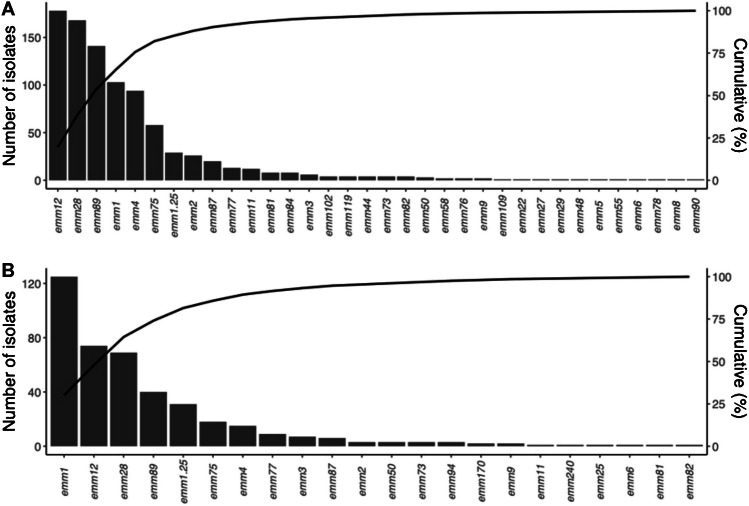
Altogether, 34 different emm types were identified (Fig. 3 A, Online Resource 1). The four most common types were emm12 (20%), emm28 (19%), emm89 (16%) and emm1 (15%), and they covered 70% of the isolates. Two major emm subtypes dominated within emm1; emm1.0 (n = 96, 11% of all) and emm1.25 (n = 29, 3.2%), respectively, and within emm12; emm12.0 (n = 83, 9.2%) and emm12.37 (n = 63, 7.0%), respectively. Five of the most common emm cluster patterns were E4 (367/904 isolates, 41%), A-C4 (n = 178, 20%), A-C3 (n = 132, 15%), E1 (n = 95, 11%) and E6 (n = 79, 8.7%) (Online resorce 1).
Fig. 3.
Emm types and cumulative percentage of GAS pharyngitis isolates collected A in Hospital District 1 (n = 904) and B in Hospital District 2 (n = 416) during the study period
97% of the isolates shared emm types putatively covered by the 30-valent GAS M-protein-based vaccine [14].
Age group was associated with prevalence of emm28 (p = 0.005) and emm89 (p = 0.028). emm28 was most common in the age group of 20–29 years. Compared to the rarest group among > 60 years old, the OR for the age group of 20–29 years is 7.0 (95%CI [2.5–19.6]). Also, all other age groups between 10 and 39 years old differed from the rarest age group (> 60 years old). emm89 was most common at the age group < 10 years; statistically significant difference was to all age groups between 20–49 years, ORs varying from 0.4 to 0.5 (Online Resource 2). No other statistical difference was observed between any of the identified emm type and age group or gender.
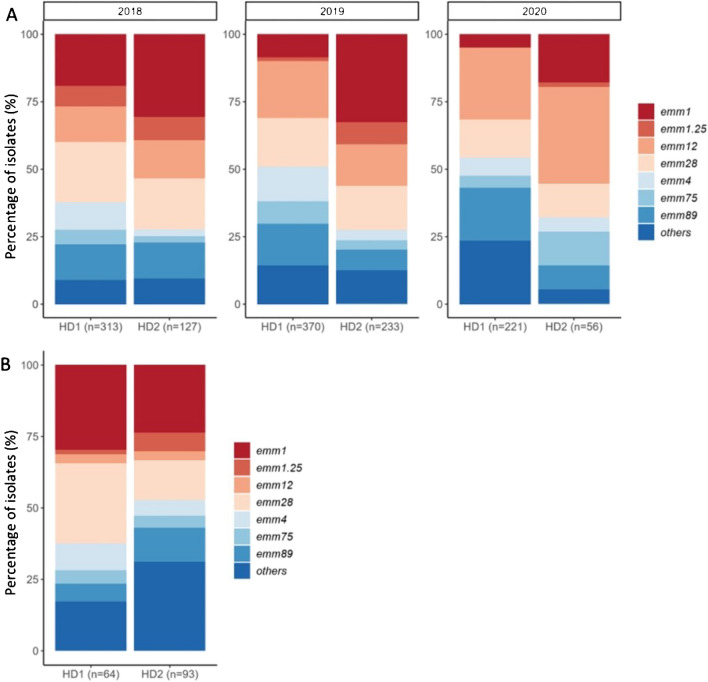
The emm-type distributions varied over time (Fig. 4). Frequency of emm1 decreased during the study (n = 60 (58%) in 2018, n = 11 (11%) in 2020), whereas emm12 increased (n = 41 (23%) vs n = 59 (33%)), respectively.
Fig. 4.
A Distribution of emm types among GAS pharyngitis isolates collected in Hospital District (HD) 1 (n = 904) and HD2 (n = 416) by year. The annual number of isolates is shown under each column. B Distribution of emm types of invasive GAS isolates reported from HD1 (n = 64) and HD2 (n = 93) during 2018–2020. The total number of isolates is shown under the column. In group “others” in iGAS isolates, the proportion of emm77 isolates was high in HD2 (13% of the isolates) and emm84 in HD1 (7.8%). Seven most common emm types (emm1, emm1.25, emm4, emm12, emm28, emm75 and emm89) were studied individually and the rest jointly under the group “others”
The number of isolates varied significantly in relation to season (p < 0.001, Fig. 1 A). Most isolates were collected during autumn (n = 246, 27% of all) and winter (n = 248, 27%). As to emm types, seasonality was noted to be significant within emm12 (p < 0.0001), emm75 (p = 0.003), emm89 (p = 0.003) and the group “others” (p = 0.0007) (Fig. 1 B). Most of the emm12 isolates were observed in winter (n = 76, 43%), emm75 in autumn (n = 23, 40%), emm89 in winter (n = 45, 32%) and “others” in autumn (n = 36, 38%). For emm1, emm1.25, emm4 and emm28, there was no statistically significant changes between seasons. Of note is the rise of the group “others” during the COVID-19 pandemic months.
Hospital district 2
In total, 21 emm types were identified among the 416 isolates collected (Fig. 3 B, Online Resource 1). The four most common emm types were emm1 (38%), emm12 (18%), emm28 (17%) and emm89 (9.6%) covering 75% of the isolates. Two major emm1 subtypes dominated: emm1.0 (n = 119, 29%) and emm1.25 (n = 31, 7.5%). The annual distribution of emm types differed (Fig. 4A). emm1 decreased (n = 39 (31%) in 2018 vs n = 10 (18%) in 2020) and emm12 increased (n = 18 (14%) vs n = 20 (36%)), respectively. Five of the most common emm cluster patterns were A-C3 (n = 156, 38% of all), E4 (n = 124, 30%), A-C4 (n = 74, 18%), E6 (n = 23, 5.5%) and E1 (n = 15, 3.6%). One emm type (emm240.3) did not belong to any known emm cluster (Online resorce 1).
Ninety-seven percent of the isolates shared emm types putatively covered by the 30-valent GAS M-protein-based vaccine (14).
Emm type distribution among iGAS cases
Altogether, 157 iGAS cases were reported (64 in HD1 and 93 in HD2) between 2018–2020. The four most common emm types in HD1 were emm1 (30%), emm28 (28%), emm4 (9.3%) and emm84 (7.8%) and in HD2 emm1 (24%), emm28 (14%), emm77 (13%) and emm89 (12%) (Fig. 4 B). Compared by source of specimen, emm12 was found to be more common among pharyngitis than iGAS isolates in both hospital districts (Figs. 3 and 4 B). While emm77 and emm84 were common in iGAS, they were rare among pharyngitis isolates (grouped to “others”).
Discussion
This study describes the epidemiology of group A streptococcal pharyngitis in two hospital districts in Southern Finland covering approximately one million inhabitants. Systematic, prospective collection allowed to study variation in the emm-type distribution and the seasonality.
From the HD1, a clear difference was observed in the age distributions and prevalence between the genders. In females, the cases occurred more uniformly until the age of 40, whereas in males most cases were in early childhood. Similar observations have recently been reported from a retrospective, register-based study from Canada [15]. The reasons behind these differences remain unknown and can only be speculated. Social and occupational factors may also affect the findings such as contacts with children in general. Our observation that young boys were overrepresented is worth further investigation; the distribution of GAS pharyngitis between genders in relation to age is not often studied. Asymptomatic carriage of GAS has been reported to be over 10% for children over 5 years of age [16, 17], which may reflect the higher disease burden as well. In our study, most of the isolates were collected from 5 to 9-year-olds.
In Western countries, same emm types such as emm1, emm89 and emm28, have been observed to associate with both iGAS and pharyngitis [8, 12, 17–22]. The same was noticed also in our study.
Interestingly, emm28 was found to be common within 20–29-year-olds. Noteworthy, emm28 has previously been associated to iGAS infections in fertile aged women and puerperal sepsis [18, 23]. No statistical differences between gender and age groups were observed in this study, which might be due to the small number of cases per group.
The finding that emm89 was more common in children under 10 years of age, is new, but not unexpected. A new acapsular clone of emm89 emerged in the mid-2010s in many countries, including Finland [24]. This clone has an advantage in persistence and transmission also among pharyngitis cases [25, 26].
Most of the isolates were cultured during autumn and winter, which is supported by previous studies [15, 27]. Interestingly, the prevalence of emm1, emm1.25, emm4 and emm28 were not affected by season. The dominance of emm1 in our collection might reflect to the contemporary emm1 iGAS epidemic in Finland [28]. Likewise, the disappearance of emm1.25 from both hospital districts by 2020 may reflect the same. Similarly, emm4 and emm28 have been associated with epidemic behaviour [21].
Two hospital districts were included to broaden the epidemiological and geographical coverage of the study. The major difference observed was that emm1 dominated only in HD2. An emm1 iGAS epidemic occurred in HD2 in 2019, whereas in HD1 just before our study [29]. Interestingly, the emm type distributions in these two districts varied also between the pharyngitis and iGAS isolates. This underlies the fact that regional epidemiology may vary also within relatively short distances (c. 160 kilometres in between).
Due to overall high morbidity of GAS infections, a vaccine against GAS would be important. In our study, the putative coverage of the M-protein-based GAS vaccines would be over 97%, which is in line with other studies [3, 10, 21]. Due to the cross protection between emm types, the coverage might even be wider [11]. However, regional differences in emm distribution occur, which complicate putative vaccination strategies [10].
The study period included the start of the COVID-19 pandemic, which changed the epidemiology of GAS pharyngitis. The number of GAS-positive pharyngitis cultures decreased, and the emm type distribution diversified with previously less common emm types arising. As however the proportion of GAS pharyngitis isolates included into our study during these months remained high, we find this observation of interest. A similar switch in emm types has been reported within the iGAS cases in Finland and elsewhere [8, 28, 30]. Recently, a surge of GAS infections has been noted in many countries, probably linked to a higher proportion of individuals susceptible to these infections due to less exposure to GAS as result of pandemic lockdown measures [6, 7].
Our study has some limitations. The study protocol aimed to collect a scientifically representative set of GAS pharyngitis isolates within a certain time frame. We acknowledge that the study material covers only part of all culture positive GAS findings in the respective clinical laboratories. Cultures were collected from GAS-positive pharyngitis patients, but GAS carriers suffering from viral pharyngitis may have though been included. Our collection may include multiple isolates from one individual, as these could not be excluded during the collection process. The collection procedure differed slightly between the hospital districts, which limited the analysis of seasonality and patient characteristics to include only HD1. Lastly, we acknowledge that the clinical microbiological laboratories which collected the GAS isolates serve mainly the public health care system leaving private sector and occupational health care neglected.
Conclusions
The prevalence of GAS pharyngitis was different between genders, particularly in different age groups. Females were overrepresented, whereas in young children, males were clearly dominating. Seasonal fluctuation was observed, but some emm types behaved more epidemically. emm28 was more common in the age group of 20–29-year-olds, and emm89 in under 10-year-olds. COVID-19 pandemic changed the epidemiology of GAS pharyngitis resulting in a wider spectrum of emm types among the fewer strains cultured.
Supplementary information
Online Resource 1. Emm types and emm clusters of pharyngitis GAS isolates collected in Hospital District (HD) 1 (n=904) and HD2 (n=416) during the study period. (DOCX 31 kb)
Online Resource 2. The prevalence of emm28 and emm89 in different age groups, corresponding odds ratios (OR) and 95% confidence intervals (CI). (DOCX 13 kb)
Acknowledgements
We thank Tuula Rantasalo, Desiree Corander, Mari Virta and Natalie Tomnikov for excellent technical assistance with sample processing and bacteriological assays. The material is original and has not been published elsewhere. Parts of this study were presented as a poster at the Annual meeting of the Nordic Society of Clinical Microbiology and Infectious Diseases (NSCMID) on 3–6 September 2021 in Turku, Finland.
Ville Kailankangasa,b, Jaana Syrjänena,b, Johanna Vilhonenc, Jarmo Oksic, Risto Vuentod
aDepartment of Internal Medicine, Tampere University Hospital, Finland
bFaculty of Medicine and Health Technology, Tampere University, Finland
cDepartment of Infectious Diseases, Turku University Hospital, Finland
dFimlab laboratories, Tampere, Finland
Code availability
Not applicable.
Author contribution
M.V. analysed the data, conducted bacteriological assays and drafted the manuscript; K.G.Y.H. and J.V. were involved in the conception and design of the article, contributed to the analysis and interpretation of the data and edited the manuscript; T.R. and T.K. performed statistical analysis; K.R.J. and T.S. organized the collection of clinical bacterial isolates and edited the manuscript; E.L. aided in bacterial assays; H.L.H. provided national data on invasive GAS isolates and DICAR study group contributed to the design of the work. All authors were involved in revising and approved the final version of the manuscript.
Funding
Open Access funding provided by University of Turku (including Turku University Central Hospital). This project was funded by Academy of Finland (grant no 308482) and Competitive State Research Financing of the Expert Responsibility area of Turku University Hospital (grant no 8TO5/13285), both to JV.
Data availability
The datasets generated during the current study are not publicly available as they contain health related data but limited datasets (without any identifiable, person-related data) are available from the corresponding author on reasonable request.
Declarations
Ethics approval
The ethical and research permission were provided by Pirkanmaa Hospital District (R18062) and Turku University Hospital (latest T12/001/2022). Data was analysed anonymised. The study was conducted in accordance with the Declaration of Helsinki and national and institutional standards.
Consent to participate
According to the Finnish Medical Research Act (488/1999), Act of the Medical Use of Human Organs, Tissues and Cells (101/2001) and Biobank Act (688/2012) as amended, and as confirmed by the Hospital District of Southwest Finland Research Ethics Committee, no ethical committee approvals or informed consent was needed for this study.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kirsi Gröndahl-Yli-Hannuksela, Email: kagron@utu.fi.
DICAR study group:
Ville Kailankangas, Jaana Syrjänen, Johanna Vilhonen, Jarmo Oksi, and Risto Vuento
References
- 1.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 2.Lamagni TL, Darenberg J, Luca-Harari B, Siljander T, Efstratiou A, Henriques-Normark B, et al. Epidemiology of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2008;46(7):2359–2367. doi: 10.1128/JCM.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouwer S, Rivera-Hernandez T, Curren BF, Harbison-Price N, De Oliveira DMP, Jespersen MG, et al. Pathogenesis, epidemiology and control of group A streptococcus infection. Nat Rev Microbiol. 2023;9:1–17. doi: 10.1038/s41579-023-00865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mustafa Z, Ghaffari M. Diagnostic methods, clinical guidelines, and antibiotic treatment for group A streptococcal pharyngitis: a narrative review. Front Cell Infect Microbiol. 2020;10:563627. doi: 10.3389/fcimb.2020.563627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver J, Malliya Wadu E, Pierse N, Moreland NJ, Williamson DA, Baker MG. Group A streptococcus pharyngitis and pharyngeal carriage: a meta-analysis. PLoS Negl Trop Dis. 2018;12(3):e0006335. doi: 10.1371/journal.pntd.0006335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagcchi S. Surge of invasive Group A streptococcus disease. Lancet Infect Dis. 2023;23(3):284. doi: 10.1016/S1473-3099(23)00043-9. [DOI] [PubMed] [Google Scholar]
- 7.de Gier B, Marchal N, de Beer-Schuurman I, Te Wierik M, Hooiveld M, et al. Increase in invasive group A streptococcal (Streptococcus pyogenes) infections (iGAS) in young children in the Netherlands, 2022. Euro Surveill. 2023;28(1):2200941. doi: 10.2807/1560-7917.ES.2023.28.1.2200941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alcolea-Medina A, Snell LB, Alder C, Charalampous T, Williams TGS, Synnovis Microbiology Laboratory Group et al. The ongoing Streptococcus pyogenes (group A streptococcus) outbreak in London, United Kingdom, in December 2022: a molecular epidemiology study. Clin Microbiol Infect. 2023;29(7):887–890. doi: 10.1016/j.cmi.2023.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Web page: “Streptococcus Laboratory, M protein Gene (emm) Typing”, accessed 6.4.2023 [Internet]. Available from https://www.cdc.gov/streplab/groupa-strep/emm-background.html
- 10.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009;9(10):611–616. doi: 10.1016/S1473-3099(09)70178-1. [DOI] [PubMed] [Google Scholar]
- 11.Dale JB, Walker MJ. Update on group A streptococcal vaccine development. Curr Opin Infect Dis. 2020;33(3):244–250. doi: 10.1097/QCO.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gherardi G, Vitali LA, Creti R. Prevalent emm types among invasive GAS in Europe and North America since Year 2000. Front Public Health. 2018;9(6):59. doi: 10.3389/fpubh.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanderson-Smith M, De Oliveira DM, Guglielmini J, McMillan DJ, Vu T, Holien JK, Henningham A, Steer AC, Bessen DE, Dale JB, Curtis N, Beall BW, Walker MJ, Parker MW, Carapetis JR, Van Melderen L, Sriprakash KS, Smeesters PR, M Protein Study Group A systematic and functional classification of Streptococcus pyogenes that serves as a new tool for molecular typing and vaccine development. J Infect Dis. 2014;210(8):1325–1338. doi: 10.1093/infdis/jiu260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dale JB, Penfound TA, Chiang EY, Walton WJ. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine. 2011;29(46):8175–8178. doi: 10.1016/j.vaccine.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mponponsuo K, Church DL, Lu SJ, Viczko J, Naugler C, McDonald T, et al. Age and sex-specific incidence rates of group A streptococcal pharyngitis between 2010 and 2018: a population-based study. Future Microbiol. 2021;16:1053–1062. doi: 10.2217/fmb-2021-0077. [DOI] [PubMed] [Google Scholar]
- 16.Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics. 2010;126(3):e557–e564. doi: 10.1542/peds.2009-2648. [DOI] [PubMed] [Google Scholar]
- 17.Shea PR, Ewbank AL, Gonzalez-Lugo JH, Martagon-Rosado AJ, Martinez-Gutierrez JC, Rehman HA, et al. Group A streptococcus emm gene types in pharyngeal isolates, Ontario, Canada, 2002-2010. Emerg Infect Dis. 2011;17(11):2010–2017. doi: 10.3201/eid1711.110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luca-Harari B, Darenberg J, Neal S, Siljander T, Strakova L, Tanna A, et al. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2009;47(4):1155–1165. doi: 10.1128/JCM.02155-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shulman ST, Tanz RR, Dale JB, Beall B, Kabat W, Kabat K, et al. Seven-year surveillance of North American pediatric group a streptococcal pharyngitis isolates. Clin Infect Dis. 2009;49(1):78–84. doi: 10.1086/599344. [DOI] [PubMed] [Google Scholar]
- 20.Vähäkuopus S, Vuento R, Siljander T, Syrjänen J, Vuopio J. Distribution of emm types in invasive and non-invasive group A and G streptococci. Eur J Clin Microbiol Infect Dis. 2012;31(6):1251–1256. doi: 10.1007/s10096-011-1436-2. [DOI] [PubMed] [Google Scholar]
- 21.Tamayo E, Montes M, García-Arenzana JM, Pérez-Trallero E. Streptococcus pyogenes emm-types in northern Spain; population dynamics over a 7-year period. J Infect. 2014;68(1):50–57. doi: 10.1016/j.jinf.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Koutouzi F, Tsakris A, Chatzichristou P, Koutouzis E, Daikos GL, Kirikou E, et al. Streptococcus pyogenes emm types and clusters during a 7-year period (2007 to 2013) in pharyngeal and nonpharyngeal pediatric isolates. J Clin Microbiol. 2015;53(7):2015–2021. doi: 10.1128/JCM.00301-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gröndahl-Yli-Hannuksela K, Beres SB, Hyyryläinen HL, Kallonen T, Musser JM, Vuopio J. Genetic evolution of invasive emm28 Streptococcus pyogenes strains and significant association with puerperal infections in young women in Finland. Clin Microbiol Infect. 2021;27(3):420–427. doi: 10.1016/j.cmi.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latronico F, Nasser W, Puhakainen K, Ollgren J, Hyyryläinen HL, Beres SB, et al. Genomic characteristics behind the spread of bacteremic group A streptococcus type emm89 in Finland, 2004-2014. J Infect Dis. 2016;214(12):1987–1995. doi: 10.1093/infdis/jiw468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner CE, Abbott J, Lamagni T, Holden MT, David S, Jones MD, et al. Emergence of a new highly successful acapsular group A streptococcus clade of genotype emm89 in the United Kingdom. mBio. 2015;6(4):e00622. doi: 10.1128/mBio.00622-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu L, Olsen RJ, Nasser W, Beres SB, Vuopio J, Kristinsson KG, et al. A molecular trigger for intercontinental epidemics of group A Streptococcus. J Clin Invest. 2015;125(9):3545–3559. doi: 10.1172/JCI82478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennis M, Tagawa A, Kung VM, Montalbano G, Narvaez I, Franco-Paredes C, et al. Seasonal variations and risk factors of Streptococcus pyogenes infection: a multicenter research network study. Ther Adv Infect Dis. 2022;19(9):20499361221132101. doi: 10.1177/20499361221132101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finnish Institute for Health and Welfare. Web page: “Prevalence of group A streptococcus”, accessed 6.3.2023 [Internet]. Available from: https://thl.fi/fi/web/infektiotaudit-ja-rokotukset/taudit-ja-torjunta/taudit-ja-taudinaiheuttajat-a-o/a-ryhman-streptokokki/a-ryhman-streptokokin-esiintyvyys-suomessa. Available only in Finnish
- 29.Vilhonen J, Vuopio J, Vahlberg T, Gröndahl-Yli-Hannuksela K, Rantakokko-Jalava K, Oksi J. Group A streptococcal bacteremias in Southwest Finland 2007-2018: epidemiology and role of infectious diseases consultation in antibiotic treatment selection. Eur J Clin Microbiol Infect Dis. 2020;39(7):1339–1348. doi: 10.1007/s10096-020-03851-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brueggemann AB, Jansen van Rensburg MJ, Shaw D, McCarthy ND, Jolley KA, Maiden MCJ, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health. 2021;3(6):e360–e370. doi: 10.1016/S2589-7500(21)00077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1. Emm types and emm clusters of pharyngitis GAS isolates collected in Hospital District (HD) 1 (n=904) and HD2 (n=416) during the study period. (DOCX 31 kb)
Online Resource 2. The prevalence of emm28 and emm89 in different age groups, corresponding odds ratios (OR) and 95% confidence intervals (CI). (DOCX 13 kb)
Data Availability Statement
The datasets generated during the current study are not publicly available as they contain health related data but limited datasets (without any identifiable, person-related data) are available from the corresponding author on reasonable request.