Abstract
Purpose
Cefepime is recommended for treating infections caused by AmpC beta-lactamase-producing Enterobacterales (AmpC-PE), though supporting evidence is limited. Therefore, this study compared outcomes associated with cefepime versus carbapenem therapy for bloodstream infections (BSIs) caused by AmpC-PE after phenotypic exclusion of ESBL-co-producing isolates.
Methods
This retrospective cohort study compared definite cefepime versus carbapenem treatment for AmpC-PE BSI in hospitalized patients of the University Hospital Basel, Switzerland, between 01/2015 and 07/2020. Primary outcomes included in-hospital death, renal impairment and neurologic adverse events; secondary outcomes included length of hospital stay and recurrent infection.
Results
Two hundred and seventy episodes of AmpC-PE BSI were included, 162, 77 and 31 were treated with a carbapenem, cefepime and other antibiotics, respectively. Patients treated with carbapenems were more likely to be transferred to the ICU on admission and more frequently had central venous catheter as a source of infection. In uni- and multivariable analyses, primary and secondary outcomes did not differ between the two treatment groups, except for more frequent occurrence of neurological adverse events among patients treated with carbapenems and shorter length of hospital stay among survivors treated with cefepime.
Conclusion
After excluding isolates with phenotypic ESBL-co-production, cefepime was not associated with adverse outcomes compared to carbapenems when used to treat BSIs caused by AmpC-PE. Our study provides evidence to support the use of cefepime as a safe treatment strategy for AmpC-PE BSI, particularly in clinically stable patients without initial renal impairment or increased susceptibility to neurological adverse events.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10096-023-04715-5.
Keywords: AmpC beta-lactamase, Enterobacterales, Cefepime, Carbapenem-sparing regimen, Bloodstream infection
Introduction
Increasing resistance of Enterobacterales to beta-lactam antibiotics, especially to carbapenems, is a global concern [1, 2]. Thus, carbapenem-sparing treatment strategies should be prioritized when there are alternative agents with evidence to support their safety and efficacy.
The Infectious Diseases Society of America (IDSA) recommends the use of cefepime for the treatment of infections caused by Enterobacterales at moderate to high risk of significant AmpC production, such as Enterobacter cloacae, Klebsiella aerogenes, and Citrobacter freundii – particularly for those isolates with minimal inhibitory concentrations (MICs) of 2 µg/ml or less for cefepime to reduce the likelihood of extended spectrum beta-lactamase (ESBL) co-production [3]. However, the guidance document acknowledges the lack of evidence supporting their recommendation, which is based on observational data with limited sample sizes [4–6]. The guideline for treatment of multidrug-resistant gram-negative bacilli published by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) acknowledges a very low certainty of evidence regarding the non-inferiority of cefepime as compared to carbapenems for treatment of AmpC-producers [7].
Cefepime, a fourth generation cephalosporin with enhanced stability against AmpC-producing Enterobacterales, has favorable pharmacokinetic and pharmacodynamic properties when its dosing is optimized [8, 9]. Despite its activity against AmpC, cefepime is not stable to extended-beta-lactamases (ESBL) which can be co-expressed by Enterobacterales [10, 11], leading to clinical failures [10, 11]. Cefepime has also been associated with neurotoxicity, particularly in patients with impaired renal function; carbapenems are often selected over cefepime to avoid these neurologic effects [12, 13]. As of yet, there are no randomized clinical trials evaluating cefepime for the treatment of infections caused by AmpC-producing Enterobacterales (AmpC-PE); therefore, we aim to contribute to the available literature by comparing clinical outcomes associated with cefepime versus carbapenem therapy for bloodstream infections (BSI) caused by AmpC-PE.
Materials and methods
Study design and setting
This was a retrospective observational cohort study conducted at the University Hospital Basel (USB), a Swiss tertiary care center with over 35,000 hospital admissions per year, to compare definitive cefepime versus carbapenem therapy for BSI caused by AmpC-PE. Enterobacterales were classified as AmpC-producers based on species identification and no further confirmatory testing for presence of AmpC was performed. Study participants were identified by systematically extracting all consecutive patients with blood cultures positive for AmpC-PE from the electronic database of the Microbiology Laboratory between January 1st, 2015 and July 31st, 2020. Hospitalized patients ≥ 18 years of age with AmpC-PE BSI and receiving definite therapy with either cefepime or a carbapenem for AmpC-PE BSI were included Patients were excluded if they were transferred to another hospital within 48 h of receipt of the positive blood culture result, if antibiotics were withheld per recommendation of the palliative care team, or if they died prior to receipt of the study drug. All positive blood cultures are reviewed by the infectious disease consultation service on a daily basis (including weekends) at our institution, and local treatment guidelines discourage cefepime use if renal function is impaired (creatinine clearance < 30 mL/min). Our guidelines point to cefepime’s potential to cause adverse events affecting the central nervous system, including lowering the seizure threshold [14]. The standard dosing of cefepime was 2 g twice daily, dosing was reduced if renal function was impaired (i.e. 1 g three times daily for a creatinine clearance between 40-70 ml/min, 1 g twice daily for a creatinine clearance between 10-40 ml/min, and 1 g once daily for a creatinine clearance < 10 ml/min). The standard dosing of meropenem was 1 g three times daily, dosing was reduced if renal function was impaired (i.e. 1 g twice daily for a creatinine clearance between 26-50 ml/min, 500 mg twice daily for a creatinine clearance between 10-25 ml/min and 500 mg once daily for a creatinine clearance between < 10 ml/min). The standard dosing of ertapenem was 1 g once daily, dosing was reduced to 500 mg once daily if creatinine clearance was below 30 ml/min. The standard dosing of Imipenem/Cilastatin was 500 mg four times daily, dosing was reduced if renal function was impaired (i.e. 500 mg three times daily for a creatinine clearance between 30-50 ml/min, 500 mg twice daily for a creatinine clearance between 10-30 ml/min and 250 mg twice daily for a creatinine clearance between < 10 ml/min).
This study is registered at ClinicalTrials.gov with no deviation from the overall protocol (NCT04577989) and approval by the local ethics committee was obtained (EKNZ-2020–02251). We adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [15].
Data collection and outcomes
Pertinent clinical data was collected from electronical medical records and encoded in a secure web-based REDCap database [16]. The primary outcomes included in-hospital all-cause mortality, new renal impairment, and new neurologic adverse events. We considered all three events as clinically important and therefore choose to define them all as primary outcomes. Secondary outcomes included length of hospital stay – overall and among survivors only—as well as recurrent infections with AmpC-PE during the following six months of the initial episode.
The following data were collected: demographics: age, gender, hospital admission and discharge, length of stay, ICU-stay, hospitalization prior to current hospital stay, discharge destination, outcome and cause of death; comorbidities based on the Charlson Comorbidity Index and Elixhauser Comorbidity Score; renal function during hospitalization; antibiotic therapy within the previous 3 months (as determined by reviewing patient’s in-and outpatient records, if available); immunosuppressing medications (as defined below) or immunocompromising condition within the prior 12 months; evidence and type of recurrent infection with AmpC-producing Enterobacterales in the following six months; ICU transfer within 24 h of AmpC-PE BSI onset and need of vasopressors or mechanical ventilation; maximum body temperature within 12 h of blood culture collection; source of infection and presence of source control (source control was defined as drainage/debridement of abscesses and/or wounds, removal of hardware combined with clinical signs of improvement, such as cessation of fever, declining inflammatory markers and no signs of residual infection in imaging if available); results of antimicrobial susceptibility test and emergence of pathogen resistance to study drug during antibiotic therapy, Clostridioides difficile infections in the following six months, dates of initiation, discontinuation, and changes in antibiotic therapy, adverse events attributed to antibiotic therapy reported during hospital stay.
The collection date of the positive blood culture was the reference time point for subsequent time specifications, including infection onset. ESBL-production was suspected based on the detection of resistance to cefpodoxime, ceftriaxone, ceftazidime, or aztreonam. Phenotypic confirmation of the ESBL-PE was performed by Etest® strips (bioMérieuex, Marcy-l’Etoile, France) using cefotaxime, ceftazidime, or cefepime, each tested with and without clavulanic acid or with ROSCO disks (Rosco, Taastrup, Denmark).
Definitions
Recurrent infections included any infection with AmpC-PE within a follow-up period of six months after diagnosis of an initial AmpC-PE episode. Immunosuppressive treatment or condition within the past 12 months was defined as chemotherapy in the last 6 months, untreated or insufficiently treated (CD4 cell count < 500/μL) HIV infection as well as primary immunodeficiency or immunomodulatory therapy (glucocorticoids, calcineurin inhibitors, mTOR inhibitors, cytostatics, monoclonal antibodies, mycophenolate) in the last 30 days or prednisone > 10 mg (or equivalent) for > 14 days within the last 30 days [4].
Empiric antibiotic treatment was defined as the antibiotic regimen administered prior to the diagnosis of AmpC-PE infection while identification and susceptibility testing results were pending [17]. The susceptibility of pathogens was classified according to the EUCAST criteria valid during the study period. The revised EUCAST definitions of susceptibility testing categories was introduced after the study period in autumn 2020, therefore, we considered the intermediate category as resistant within the clinical context [18].
An empiric antibiotic treatment to which the identified pathogen was found non-susceptible was defined as inadequate. Definitive antibiotic treatment applied to the first administered antibiotic regimen after confirmation of AmpC-PE and receipt of susceptibility test results from blood cultures. All AmpC-PE were confirmed susceptible to the definitive treatment regimens.
Renal function was expressed as glomerular filtration rate (GFR) and categorized using the KDIGO classification [19]. The degree of acute kidney injury (AKI) during antibiotic therapy was defined according to the KDIGO Acute Kidney Injury Work Group as meeting criteria for stage 1, stage 2 and stage 3 [20]. New onset renal impairment was considered a KDIGO-defined AKI in patients with no baseline renal dysfunction or an increase of the KDIGO class by at least one category in those already meeting KDIGO criteria at baseline.
Neurological adverse events included all reported events of an epileptic seizure, an encephalopathic state or delirium during the treatment period for AmpC-PE BSI as diagnosed by a physician and recorded as a diagnosis in the medical record.
Further details on microbiological characteristics of AmpC-PE BSI stratified by definite antimicrobial therapy and AmpC-PE are included in the supplementary material.
Statistical analysis
Categorical and numerical variables were compared using the Fisher’s exact and Wilcoxon rank-sum test, respectively. Crude and adjusted associations between definite antimicrobial therapy and binary outcomes (i.e. in-hospital death, recurrent infection, new renal impairment, and neurological adverse events) were estimated using logistic regression models. For the outcome length of stay, we fitted generalized linear models with a log link and gamma distribution. Based on baseline differences between exposures (the groups were already quite balanced at baseline) and data sparsity/multicollinearity, exposure-outcome associations were adjusted for ICU admission within 24 h and the following source of infection: skin/soft tissue, abdomen, and central venous catheters. All models were based on complete cases. As 13 patients contributed twice and two patients contributed to three episodes during the study period resulting in 224 (patient) clusters overall, a sensitivity analysis was performed for the different exposure-outcome associations using cluster robust standard errors. All analyses were performed on a multicore system with Stata/MP version 16 (Stata Corp., College Station, Texas, United States). Reported P-values are two-sided.
Results
Cohort and microbiological data
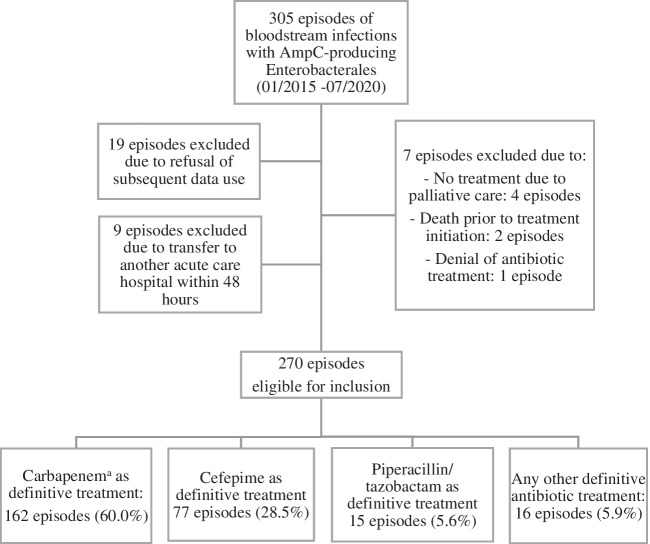
Within the study period, 305 episodes of BSI caused by AmpC-PE were identified among 288 patients, of which 270 episodes met eligibility criteria. Reasons for exclusion are summarized in Fig. 1. Carbapenems were administered as definitive antimicrobial treatment in 162 episodes, cefepime in 77 episodes, piperacillin/tazobactam in 15 episodes and other antibiotic agents in 16 episodes (Fig. 1). Therefore, 239 episodes were included in the comparative analysis between carbapenems and cefepime. Twelve BSI episodes were polymicrobial, 11 with two different AmpC-PE and one with three different AmpC-PE, resulting in 283 AmpC-PE isolates identified.
Fig. 1.
Flow chart of case selection; ameropenem, imipenem, or ertapenem combined
Among the 283 AmpC-PE isolates, 47.3% were identified as Enterobacter cloacae group, 27.4% as Serratia marcescens, 14.4% as Klebsiella aerogenes, 10.4% as Citrobacter freundii group and 7.0% as Morganella morganii. ESBL-production was confirmed in six out of the 283 total AmpC-PE isolates (2.1%) and none produced a carbapenemase (Supplementary table 1). Forty-eight AmpC-PE (16.9%) were intermediate or resistant to piperacillin/tazobactam, 35 (12.5%) were intermediate or resistant to any carbapenem [17 (6.1%) against imipenem, 15 (5.3%) against ertapenem and three (1.1%) against meropenem only] and 20 (7.0%) were intermediate or resistant to cefepime (Supplementary table 1).
Baseline characteristics
Comparisons of baseline characteristics and risk factors between patients treated with carbapenems and cefepime are presented in Table 1. Patients treated with carbapenems were more likely to be transferred to the ICU within 24 h (35.0% vs 11.8%; p < 0.001) and more frequently required vasopressors (23.8% vs 7.9%; p = 0.004) or mechanical ventilation (21.9% vs 7.9%; p = 0.009). Central venous catheter-associated infections were more frequent in the carbapenem group (18.1% vs 3.9%, p = 0.002). Both groups were well balanced in terms of comorbidity scores (median 3.0 vs 2.0, p = 0.734) and receipt of adequate empirical antibiotic therapy (77.2% vs 79.2%, p = 0.868).
Table 1.
Baseline characteristics stratified by definite antimicrobial therapy
| Definite carbapenem treatment (n = 162) | Definite cefepime treatment (n = 77) | p-value | Missing data (n = 239) | |||
|---|---|---|---|---|---|---|
| n/ median | %/ IQR | n/ median | %/IQR | n / % | ||
| Demographics | ||||||
| Age | 70.5 | 59.0–79.0 | 70.0 | 60.5–78.0 | 0.923 | 0 / 0.0 |
| Female gender | 59 | 36.4 | 25 | 32.5 | 0.566 | 0 / 0.0 |
| Admission from | 0.297 | 0 / 0.0 | ||||
| Home | 103 | 63.6 | 58 | 75.3 | ||
| Acute care hospital (Switzerland) | 45 | 27.8 | 12 | 15.6 | ||
| Long-term care facility | 6 | 3.7 | 4 | 5.2 | ||
| Nursing home | 3 | 1.9 | 2 | 2.6 | ||
| Residency abroad | 4 | 2.5 | 1 | 1.3 | ||
| Acute care hospital abroad | 1 | 0.6 | 0 | 0.0 | ||
| Ward group | 0.276 | 0 / 0.0 | ||||
| Surgery | 61 | 37.7 | 21 | 27.3 | ||
| Medicine | 74 | 45.7 | 42 | 54.5 | ||
| Other | 27 | 16.7 | 14 | 18.2 | ||
| Comorbidities | ||||||
| Charlson Comorbidity Index Score | 3.0 | 1.0–4.0 | 2.0 | 2.0–4.5 | 0.734 | 0 / 0.0 |
| Elixhauser Comorbidity Score | 10.0 | 4.0–15.0 | 9.0 | 4.5–13.0 | 0.782 | 0 / 0.0 |
| Solid organ transplantation | 5 | 3.1 | 3 | 3.9 | 0.715 | 0 /0.0 |
| Stem cell transplantation | 5 | 3.1 | 3 | 3.9 | 0.716 | 1 / 0.4 |
| Exposures | ||||||
| Previous antibiotic treatment a | 102 | 69.4 | 42 | 60.0 | 0.219 | 22 / 9.2 |
| Previous PPI therapy a | 101 | 62.7 | 47 | 61.8 | 0.887 | 2 / 0.8 |
| History of immunosuppression b | 41 | 25.3 | 25 | 32.5 | 0.279 | 0 / 0.0 |
| Microbiological history | ||||||
| Colonization or infection with any resistant pathogen c | 12 | 7.4 | 4 | 5.2 | 0.593 | 30 / 12.5 |
| ESBL-producing bacteria | 9 | 5.6 | 2 | 2.6 | ||
| Carbapenemase-producing bacteria | 0 | 0.0 | 0 | 0.0 | ||
| Vancomycin-resistant Enterococcus | 0 | 0.0 | 0 | 0.0 | ||
| Methicillin-resistant Staphylococcus aureus | 1 | 0.6 | 0 | 0.0 | ||
| Others | 3 | 1.9 | 2 | 2.6 | ||
| Colonization or infection with Clostridioides difficile c | 9 | 5.6 | 3 | 3.9 | ||
| Clinical characteristics | ||||||
| Focus of infection | 0.014 | 3 / 1.3 | ||||
| Urogenital | 30 | 18.8 | 17 | 22.4 | ||
| Skin/ Soft tissue | 11 | 6.9 | 10 | 13.2 | ||
| Bone | 1 | 0.6 | 1 | 1.3 | ||
| Abdominal | 18 | 11.3 | 11 | 14.5 | ||
| Pulmonal | 16 | 10.0 | 4 | 5.3 | ||
| Central venous catheter | 29 | 18.1 | 3 | 3.9 | ||
| Others (incl. unclear) | 55 | 34.4 | 30 | 39.5 | ||
| ICU transfer d | 56 | 35.0 | 9 | 11.8 | < 0.001 | 3 / 1.3 |
| Vasopressor d | 38 | 23.8 | 6 | 7.9 | 0.004 | 3 / 1.3 |
| Mechanical ventilation d | 35 | 21.9 | 6 | 7.9 | 0.009 | 3 / 1.3 |
| Systolic blood pressure ≤ 100mmHgd | 82 | 51.6 | 31 | 40.8 | 0.128 | 4 / 1.7 |
| Highest body temperature e | 38.0 | 37.2–38.7 | 38.3 | 37.1–38.9 | 0.436 | 6 / 2.5 |
| Treatment characteristics | ||||||
| Adequate empirical therapy | 125 | 77.2 | 61 | 79.2 | 0.868 | 0 / 0.0 |
| Source control achieved f | 63 | 39.4 | 22 | 28.9 | 0.147 | 3 / 1.3 |
Abbreviations: ICU intensive care unit, IQR interquartile range, ESBL extended-spectrum beta-lactamases, n number, PPI proton pump inhibitor
ain the prior 3 months, bin the prior 12 months, c in the prior 6 months, dwithin 24 h of bloodstream infection onset, ewithin 12 h before or after blood sample, fwithin 5 days
Clinical outcomes
In-hospital mortality (15.4% vs 7.8%), new onset renal impairment (13.6% vs 7.8%), and recurrent infection with AmpC-PE (11.7% vs 6.5%) did not differ between the two treatment groups. Fewer neurological adverse events were reported in patients receiving cefepime versus those receiving carbapenems (5.2% vs 21.0%). The overall length of hospital stay was shorter for survivors in the cefepime group (median 17.0 vs 23.0) (Table 2). Uni- and multivariable analyses revealed similar results and were further substantiated by the sensitivity analysis confidence estimates based on robust standard errors (Table 3).
Table 2.
Outcome characteristics stratified by definite antimicrobial therapy
| Definite carbapenem treatment (n = 162) | Definite cefepime treatment (n = 77) | p-value | |||
|---|---|---|---|---|---|
| n/ median | %/ IQR | n/ median | %/IQR | ||
| Primary outcomes | |||||
| In-hospital death | 25 | 15.4 | 6 | 7.8 | 0.148 |
| New renal impairment | 22 | 13.6 | 6 | 7.8 | 0.281 |
| Neurological adverse events | 34 | 21.0 | 4 | 5.2 | 0.001 |
| Secondary outcomes | |||||
| Length of stay (survivors) | 23.0 | 12.0–37.0 | 17.0 | 9.0–30.0 | 0.031 |
| First recurrent infection with AmpC-PE a | 19 | 11.7 | 5 | 6.5 | 0.255 |
| Second recurrent infection with AmpC-PE a | 3 | 15.8b | 2 | 40.0b | 0.270 |
Abbreviations: AmpC-PE AmpC-producing Enterobacterales, IQR interquartile range, n number
ain the following 6 months, bout of total first recurrent infection with AmpC-PE
Table 3.
Associations between definite antimicrobial therapy and the different outcomes (reference definite therapy with carbapenems)
| Univariable | Multivariablea | Univariable robust | Multivariable robusta | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Primary outcomes | ||||||||||||
| In-hospital death | .46 | .18; 1.18 | 0.107 | .77 | .28; 2.18 | 0.628 | .46 | .19 1.16 | 0.101 | .77 | .27; 2.19 | 0.630 |
| New renal impairment | .54 | .21; 1.39 | 0.199 | .54 | .20; 1.47 | 0.230 | .54 | .21; 1.41 | 0.208 | .54 | .20; 1.48 | 0.233 |
| Neurological adverse events | .21 | .07; .61 | 0.004 | .26 | .09; .79 | 0.018 | .21 | .07; .61 | 0.004 | .26 | .09; .80 | 0.018 |
| Secondary outcomes | ||||||||||||
| Length of stay (survivors) b | -27.50 | -44.87; -4.61 | 0.022 | -27.48 | -45.97; -2.66 | 0.032 | -27.50 | -45.59; -3.35 | 0.028 | -27.48 | -46.63; -1.46 | 0.040 |
| First recurrent infection | 0.51 | 0.18; 1.42 | 0.195 | 0.69 | 0.23; 2.05 | 0.501 | 0.51 | 0.18; 1.45 | 0.206 | 0.69 | 0.21; 2.21 | 0.528 |
| Second recurrent infection c | 3.56 | 0.40; 31.23 | 0.253 | 6.64 | 0.37; 118.18 | 0.198 | 3.56 | 0.38; 33.57 | 0.268 | 6.64 | 0.54; 82.32 | 0.141 |
Abbreviations: OR Odds Ratio, CI Confidence interval
acomplete case analyses (missing co-variables for three episodes)
bestimates shown as % change, i.e. (exponentiated coefficients—1)*100
camong episodes with a first recurrence
Microbiological outcomes
Within the following six months, a first recurrent infection with an AmpC-PE occurred after 19 episodes (11.7%) in the carbapenem group compared to five episodes (6.5%) in the cefepime group (p = 0.255). A second recurrent AmpC-PE infection occurred after three episodes (1.8%) of the carbapenem group and after two episodes (2.5%) of the cefepime group (p = 0.270). The corresponding type of infections are presented in Supplementary table 2. Further microbiological characteristics on the AmpC-PE causing the recurrent infections are included in Supplementary table 3.
During definite antimicrobial treatment of the initial episode of AmpC-PE BSI, seven (4.2%) pathogens in the carbapenem group became intermediate or resistant against any carbapenem compared to two (2.6%) in the cefepime group. Three (1.8%) pathogens in the carbapenem group and one (1.3%) in the cefepime group became intermediate or resistant to cefepime. In the carbapenem group, four (20.0%) pathogens causing a first recurrent infection showed increasing resistance and two (10.0%) decreasing resistance compared to the susceptibility test result of their initial BSI. In the cefepime group as well as for all second recurrent infections], there was no change in the resistance profil between BSI and first recurrent infection (Supplementary table 4).
Discussion
After excluding ESBL-producing isolates, patients with renal impairment, and patients at risk for neurotoxicity, this cohort study found the use of cefepime versus carbapenems for treating BSI caused by AmpC-PE was not associated with in hospital mortality, recurrent infection, or adverse effects.
These results are consistent with the findings of previous studies reporting cefepime as effective alternative treatment for AmpC-PE infections, and add to the existing evidence supporting cefepime as a safe treatment strategy [4, 6, 21].
Cefepime was not associated with a higher rate of treatment related renal impairment and had significantly less neurological adverse events compared to carbapenems. The latter might be explained by our institutional guidelines cautioning against the use of cefepime in patients at increased risk for side effects of the central nervous system and increased vulnerability to neurological adverse events in case of circulatory instability or need of intensive care. However, our findings are consistent with previous literature indicating that relevant neurological adverse events of cefepime are rare, even in severely ill patients [12]. Furthermore, our study showed that the length of stay in the cefepime group, particularly among survivors, was around 30% shorter compared to the carbapenem group but the respective confidence intervals were wide – ranging from -46% to -3%. Our findings indicate that cefepime may be a valuable alternative to carbapenems, not only as a carbapenem-sparing regimen, but also for potentially improving patient outcomes. These results need to be substantiated in a randomized trial.
Interestingly, our analysis showed that the adequacy of empirical antibiotic treatment did not have a significant effect on outcomes. This finding is consistent with recent literature on the relationship between inadequate empirical therapy and mortality where the authors found no association and referred to residual confounding as a possible explanation [22]. In contrast, inappropriate initial antibiotic treatment is known to be associated with increased hospital mortality in Gram-negative bacteremia complicated by severe sepsis or septic shock [23]. As only 27% of BSI episodes within our cohort required ICU-referral within 12 h of blood sample collection, our finding may be attributable to the majority of patients not presenting with severe sepsis at BSI-onset.
Recurrent infections with an AmpC-PE occurred more frequently among patients treated with carbapenems as compared to patients treated with cefepime; however, this did not reach statistical significance likely due to the low rate of recurrences in this study population. Notably, emergence of resistance of AmpC-PE was more commonly noted after treatment of the initial infection episode with carbapenems. Recently, imipenem has been shown to promote AmpC expression and may induce an adaptive response to carbapenems by regulating key genes involved in the control of efflux pumps and porins, which could lead to a multidrug-resistant profile in clinical isolates, contributing to possible treatment failure [24, 25]. Further studies are needed to compare the potential of different beta-lactams in inducing resistance by induction of AmpC or other resistance mechanisms, such as the up-regulation of efflux pumps or down-regulation of the expression of porins.
The inclusion of a relatively large number of patients with BSI caused by AmpC-PE and the systematic exclusion of ESBL-production among all causative isolates are strengths of this study. In addition, patients treated with cefepime and carbapenems were well balanced regarding baseline characteristics and risk factors.
Our study has some relevant limitations, most importantly its retrospective single center design relying on data collection from electronical medical records. Despite best efforts to minimize potential confounding, residual confounding may have occurred. Furthermore, our findings may only be generalizable to similar healthcare settings and patient populations. Recurrent infections may have been missed in patients subsequently not presenting to our institution. Changes in susceptibility of AmpC-PE recovered in the context of recurrent infections were assessed based on Eucast classifications – changes in MICs were not evaluated as at our institution susceptibility testing is routinely performed using the VITEK® system, which lakes the accuracy to determine precise changes in MIC levels. Duration of inadequate treatment prior to establishment of definitive treatment was not assessed and differences in duration of inadequate treatment may act as an important confounder when assessing differences in outcomes between the two treatment groups. As, however, all positive blood culture results are assessed by the infectious disease consultation service on a daily basis and definite treatment with either cefepime or carbapenems was assigned based on pre-defined criteria as indicated in our institutional guidelines, we consider it unlikely that duration of inadequate treatment would differ between the two groups. We further acknowledge that no sample size calculations were performed a priori and that based on our sample size the study may be underpowered to detect differences between the two treatment groups regarding the outcomes investigated (in line post-hoc power analyses reveals a power of 35.9% of our study to detect a significant difference for mortality between the two treatment groups).
In summary, our study provides further evidence that cefepime is a useful carbapenem-sparing agent for the treatment of AmpC-PE BSI if ESBL-production can be excluded. Importantly, our results indicate that cefepime treatment may not be associated with a negative impact on relevant clinical outcomes, including mortality, recurrent infections, and length of hospital stay. These findings present further evidence to support treating AmpC-PE BSI with cefepime as a safe strategy, particularly in clinically stable patients without initial renal impairment or increased susceptibility to neurological adverse events. Robust randomized controlled trials should be conducted to validate these findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all patients for giving their permission for subsequent use of data and the University Hospital Basel, Switzerland as well as the University of Basel, Switzerland for the financial support.
Authors’ contribution
Julia Herrmann constructed the database, recorded the data and started analysing the dataset. Sarah Tschudin-Sutter initiated the study, developed the methodology and assisted with the analysis of the data. Jan Roth analysed the dataset and provided valuable feedback on the methodology. Julia Herrmann wrote the manuscript and Sarah Tschudin-Sutter critically revised and edited the manuscript. Anne-Valérie Burgener-Gasser, Daniel Goldberger, Jan Roth, Pranita D. Tamma, Maja Weisser revised the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by University of Basel. This work was funded in part by the University Hospital Basel, Switzerland and the University of Basel, Switzerland.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
This study is registered at ClinicalTrials.gov with no deviation from the overall protocol (NCT04577989) and approval by the local ethics committee was obtained (EKNZ-2020–02251).
Patient consent
Patients informed consent was obtain using the current version (at time period of hospitalization) of the USB internal “Forschungskonsent”. In case of missing “Forschungskonsent”, we applied for an exception according to HRA Art. 34 a-c, especially regarding critical ill patients at ICU and patient who died prior to signing the USB internal “Forschungskonsent”. Patients, that refused the subsequent use of their data, were excluded from this study.
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.CJ Murray, Shunji Ikuta K, Sharara F et al (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399:629–655. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed]
- 2.Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America Guidance on the treatment of AmpC β-Lactamase-Producing Enterobacterales, Carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis. 2022;74:2089–2114. doi: 10.1093/cid/ciab1013. [DOI] [PubMed] [Google Scholar]
- 4.Tamma PD, Girdwood SCT, Gopaul R, Tekle T, Roberts AA, Harris AD, et al. The use of cefepime for treating AmpC β-lactamase-producing enterobacteriaceae. Clin Infect Dis. 2013;57:781–788. doi: 10.1093/cid/cit395. [DOI] [PubMed] [Google Scholar]
- 5.Blanchette LM, Kuti JL, Nicolau DP, Nailor MD. Clinical comparison of ertapenem and cefepime for treatment of infections caused by AmpC beta-lactamase-producing Enterobacteriaceae. Scand J Infect Dis. 2014;46:803–808. doi: 10.3109/00365548.2014.954262. [DOI] [PubMed] [Google Scholar]
- 6.Siedner MJ, Galar A, Guzmán-Suarez BB, Kubiak DW, Baghdady N, Ferraro MJ, et al. Cefepime vs other antibacterial agents for the treatment of Enterobacter species bacteremia. Clin Infect Dis. 2014;58:1554–1563. doi: 10.1093/cid/ciu182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul M, Carrara E, Retamar P, Tängdén T, Bitterman R, Bonomo RA, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine) Clin Microbiol Infect. 2022;28:521–547. doi: 10.1016/j.cmi.2021.11.025. [DOI] [PubMed] [Google Scholar]
- 8.D’Angelo RG, Johnson JK, Bork JT, Heil EL. Treatment options for extended-spectrum beta-lactamase (ESBL) and AmpC-producing bacteria. Expert Opin Pharmacother. 2016;17:953–967. doi: 10.1517/14656566.2016.1154538. [DOI] [PubMed] [Google Scholar]
- 9.Sanders WE, Tenney JH, Kessler RE. Efficacy of cefepime in the treatment of infections due to multiply resistant Enterobacter species. Clin Infect Dis. 1996;23:454–461. doi: 10.1093/clinids/23.3.454. [DOI] [PubMed] [Google Scholar]
- 10.Choi SH, Lee JE, Park SJ, Kim MN, Choo EJ, Kwak YG et al (2007) Prevalence, microbiology, and clinical characteristics of extended-spectrum β-lactamase-producing Enterobacter spp., Serratia marcescens, Citrobacter freundii, and Morganella morganii in Korea, Eur. J. Clin. Microbiol. Infect. Dis. 26:557–561. 10.1007/s10096-007-0308-2 [DOI] [PubMed]
- 11.Gottlieb J, Wolfson C. Comparison of the MICs of cefepime for extended-spectrum β-lactamase-producing and non-extended-spectrum β-lactamase-producing strains of Enterobacter cloacae. J Antimicrob Chemother. 2000;46:330–332. doi: 10.1093/jac/46.2.330. [DOI] [PubMed] [Google Scholar]
- 12.Khan A, DeMott JM, Varughese C, Hammond DA. Effect of cefepime on neurotoxicity development in critically Ill adults with renal dysfunction. Chest. 2020;158:157–163. doi: 10.1016/j.chest.2020.01.051. [DOI] [PubMed] [Google Scholar]
- 13.Lau C, Marriott D, Gould M, Andresen D, Reuter SE, Penm J. A retrospective study to determine the cefepime-induced neurotoxicity threshold in hospitalized patients. J Antimicrob Chemother. 2020;75:718–725. doi: 10.1093/jac/dkz476. [DOI] [PubMed] [Google Scholar]
- 14.Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs : a systematic review. Neurology. 2015;85:1332–1341. doi: 10.1212/WNL.0000000000002023. [DOI] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tschudin-Sutter S, Fosse N, Frei R, Widmer AF (2018) Combination therapy for treatment of Pseudomonas aeruginosa bloodstream infections. PLoS One 13. 10.1371/journal.pone.0203295 [DOI] [PMC free article] [PubMed]
- 18.The European Committee on Antimicrobial Susceptibility Testing., Breakpoint tables for interpretation of MICs and zone diameters., Version 5.0 - 8.1. (n.d.) 2015–2018. http://www.eucast.org. Accessed 08 Aug 2023
- 19.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group (2013) KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 3:1–150. 10.1038/kisup.2012.74It
- 20.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138. 10.1038/kisup.2012.6
- 21.Harris PNA, Wei JY, Shen AW, Abdile AA, Paynter S, Huxley RR, et al. Carbapenems versus alternative antibiotics for the treatment of bloodstream infections caused by Enterobacter, Citrobacter or Serratia species: a systematic review with meta-analysis. J Antimicrob Chemother. 2016;71:296–306. doi: 10.1093/jac/dkv346. [DOI] [PubMed] [Google Scholar]
- 22.Schuttevaer R, Alsma J, Brink A, van Dijk W, de Steenwinkel JEM, Lingsma HF, et al. Appropriate empirical antibiotic therapy and mortality: Conflicting data explained by residual confounding. PLoS ONE. 2019;14:1–12. doi: 10.1371/journal.pone.0225478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson MT, Reichley R, Hoppe-Bauer J, Dunne WM, Micek S, Kollef M. Impact of previous antibiotic therapy on outcome of Gram-negative severe sepsis. Crit Care Med. 2011;39:1859–1865. doi: 10.1097/CCM.0b013e31821b85f4. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Wang Z, Liu M, Yu X, Zhong Y, Wang F, et al. Cefazolin and imipenem enhance AmpC expression and resistance in NagZ-dependent manner in Enterobacter cloacae complex. BMC Microbiol. 2022;22:1–11. doi: 10.1186/s12866-022-02707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavez M, Vieira C, de Araujo MR, Cerda A, de Almeida LM, Lincopan N, et al. Molecular mechanisms of membrane impermeability in clinical isolates of Enterobacteriaceae exposed to imipenem selective pressure. Int J Antimicrob Agents. 2016;48:78–85. doi: 10.1016/j.ijantimicag.2016.04.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.