Abstract
Dysregulation of WNT/β-catenin is a hallmark of many cancer types and a key mediator of metastasis in solid tumors. Overactive β-catenin signaling hampers dendritic cell (DC) recruitment, promotes CD8+ T cell exclusion and increases the population of regulatory T cells (Tregs). The activity of WNT/β-catenin also induces the expression of programmed death-ligand 1 (PD-L1) on tumor cells and promotes programmed death-1 (PD-1) upregulation. Increased activity of WNT/β-catenin signaling after anti-PD-1 therapy is indicative of a possible implication of this signaling in bypassing immune checkpoint inhibitor (ICI) therapy. This review is aimed at giving a comprehensive overview of the WNT/β-catenin regulatory roles on PD-1/PD-L1 axis in tumor immune ecosystem, discussing about key mechanistic events contributed to the WNT/β-catenin-mediated bypass of ICI therapy, and representing inhibitors of this signaling as promising combinatory regimen to go with anti-PD-(L)1 in cancer immunotherapy. Ideas presented in this review imply the synergistic efficacy of such combination therapy in rendering durable anti-tumor immunity.
Keywords: β-catenin, Immune checkpoint inhibitor (ICI), Programmed death-1 (PD-1), Programmed death-ligand 1 (PD-L1), Tumor microenvironment (TME), Resistance
Introduction
Wingless-related integration site (WNT)/β-catenin is an immunosuppressive signaling [1] that its activity in a tumor is indicative of low rates of immune infiltration [2]. WNT/β-catenin signaling is a critical mediator of melanoma metastasis [3], orchestrating a T cell exclusion profile [4]. Disruption of WNT/β‐catenin signaling is reported as a strategy for suppression of invasion and metastasis in non-small cell lung cancer (NSCLC) [5]. β-catenin activation shapes the immune desert landscape of hepatocellular carcinoma (HCC) [6]. The suppressive effect of WNT/β-catenin on CCL4 contributed to the cold immune phenotype of melanoma [7]. Mutations in the WNT/β-catenin occur in about 70% of microsatellite stable colorectal cancer (CRC) patients [8]. The frequency of β-catenin+ tumor cells and programmed death-ligand 1 (PD-L1)+ immune cells can be regarded as an indicator of CRC progression [9]. WNT/β-catenin activity promotes CRC progression through induction of epithelial-mesenchymal transition (EMT) [10]. Expression of Frizzled-10 (Fzd-10) receptor and further β-catenin activation promote cancer stem cell (CSC) expansion and predicts weak prognosis in HCC [11]. Enriched activity of this signaling in tumors with cold immunity (non-T cell-inflamed) provides a rationale for development of inhibitors in order to restore immune infiltration and increasing the efficacy of immunotherapy [12]. There are signs of evidence indicating the combination of impact of WNT/β-catenin blockade with immune checkpoint inhibitors (ICIs) for better promotion of anti-tumor immunity against cancers like NSCLC [13] and melanoma [14]. The aim of this review is to justify the mechanistic backbone of WNT/β-catenin-mediated ICI resistance, as well as rationalizing a possibility of the application of WNT/β-catenin blockade as a combinatory regimen with anti-PD-(L)1 aiming at a durable anti-cancer therapy.
WNT/β-catenin
Signaling elements
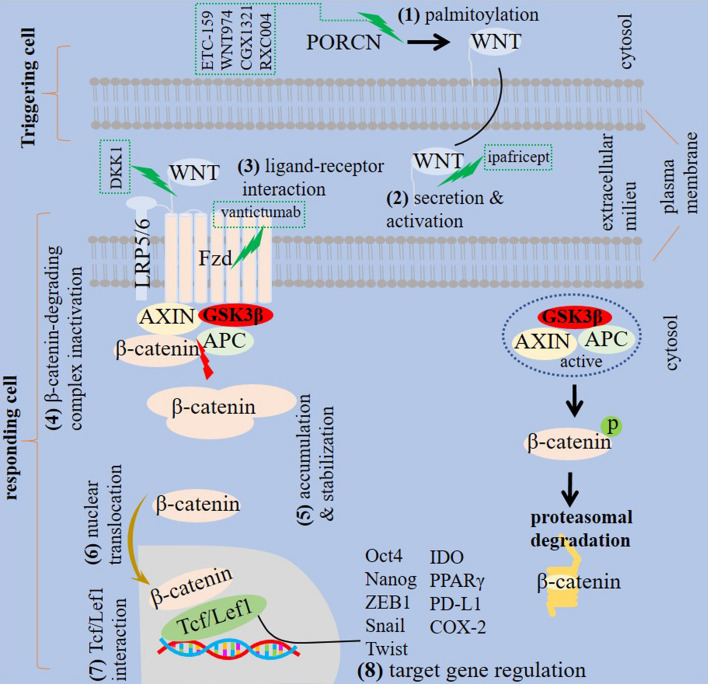
WNT (WNT5a) is a gene assessed to evaluate mesenchymal transition [15], and β-catenin is a critical mediator of WNT signaling [16]. In fact, WNT proteins co-express to act synergistically for activation of β-catenin signaling in several cell types [17]. Adenomatosis polyposis coli, CTNNB1 and AXIN (AXIN1 and AXIN2) are β-catenin signaling elements [12]. Adenomatosis polyposis coli is a gene related to the suppression of WNT/β-catenin [18], which shows mutations in more than 90% of sporadic colon cancer cases [19]. CTNNB1 is a gene encoding β-catenin that is mutated frequently in HCC. CTNNB1 mutation results in the cytoplasmic accumulation of β-catenin, which subsequently causes aberrant activation of WNT [20]. AXIN is a cytoplasmic protein that acts as a negative regulator of WNT pathway and promotes β-catenin degradation [21]. AXIN2 can be assessed as a marker for analyzing the activity of WNT pathway [22]. WNT/β-catenin pathway signals via interaction with Fzd receptor family as well as different co-receptors [23]. Low-density lipoprotein receptor related proteins 5 and 6 (LRP5/6) is a co-receptor located on cell surface that is involved in the initiation of WNT/β-catenin pathway [18]. Upon WNT activation, β-catenin degrading complex is inactivated, which results in β-catenin accumulation within the cytosol and its further stabilization. The stabilized β-catenin further translocated into the nucleus where it bonds to the T cell transcription factor (Tcf)/lymphoid enhancer-binding factor 1 (Lef1) [24, 25]. β-catenin is a Tcf1 transcriptional coactivator in which interactions within the β-catenin/Tcf1 axis are vital for transcriptional regulation. The Tcf1 long isoform contains β-catenin binding domain that mediates β-catenin recruitment to the protein complex [26]. WNT is palmitoylated by porcupine (PORCN). PORCN activity is vital for secretion of WNT and its bondage to Fzd in responder cells [27]. PORCN inhibition disrupts secretion of WNT and hampers stem cell activity in tumors [27], so it can be a target in WNT-driven cancers [28]. Fzd receptors are other targets for WNT pathway suppression in human cancers [23]. Dickkopf-related protein 1 (DKK1) is a known antagonist of WNT that acts through suppression of WNT interaction with Fzd receptors [29]. Hindering the secretion of WNT ligands, interfering with interaction between WNT ligand and receptor, increasing the degradation of β-catenin or blocking interaction between β-catenin with its target genes are strategies for hampering WNT/β-catenin signaling. Monoclonal antibodies against Fzd receptors, such as vantictumab (OMP-18R5), Fzd8 fusion proteins and extracellular traps for WNT ligand signaling, such as ipafricept (OMP-54F28), and PORCN inhibitors, such as ETC-159, LGK974 (WNT974), CGX1321 and RXC004 are targeted inhibitors of WNT/β-catenin signaling [30] (Fig. 1).
Fig. 1.
WNT/β-catenin signaling. Different steps are involved in the activity of WNT/β-catenin signaling. First, WNT palmitoylation occurs under the impact of porcupine (PORCN), which causes WNT secretion and activation. The active WNT interacts with Frizzled (Fzd)/lipoprotein receptor related proteins 5 and 6 (LRP5/6) complex in target cell and subsequently causes inactivation of β-catenin degrading complex and the resultant β-catenin cytosolic accumulation and its stabilization. The stabilized β-catenin translocate into the nucleus where it bonds to the T cell transcription factor (Tcf)/lymphoid enhancer-binding factor 1 (Lef1) for regulation of target genes. β-catenin signaling is inactivated when glycogen synthase kinase 3β (GSK3β) and the inhibitory complex is active, which subsequently promotes β-catenin proteasomal degradation. APC, adenomatosis polyposis coli; ZEB, Zinc finger E-box binding homeobox; IDO, indoleamine 2,3-dioxygenase; PPARγ, peroxisome proliferator-activated receptor-γ; and PD-L1, programmed death-ligand 1. Inhibitors of different paths in this signaling are marked as dashed rectangles
WNT/β-catenin signaling in health and disease
WNT/β-catenin is an evolutionally conserved singling [31] that is important in establishing and maintenance of cell-to-cell adhesion [2]. Dysregulation of WNT/β-catenin accounts for diseases like cancer. When WNT ligand is not present in the environment, β-catenin is assembled in related complex and low level of β-catenin is maintained within cytosol. β-catenin further undergoes phosphorylation and degradation. By contrast, bondage between WNT with related receptors prevent β-catenin degradation and allows its accumulation within cytosol and further translocation into nucleus for activating WNT-related transcription program [32]. WNT/β-catenin maintains stemness in several epithelial tissues, which is important for development and regeneration of body organs [27]. WNT/β-catenin signaling promotes self-renewal potential of hematopoietic stem cells [31], and its sustained activity in epidermal region expands stem cell compartment in the underlying dermis [33]. Survival of immature CD4+ CD8+ T cells in thymus is also associated with β-catenin [34]. The impact of WNT (WNT3a) on self-renewal maintenance of CD8+ T cells, as occurring under normal conditions, represents implications of this signaling in vaccination or adoptive T cell therapy [31].
Increased β-catenin activity is a tumor hallmark [35], which is contributed to the initiation, progression, and invasion and metastasis of cancer [36]. Hyperactive WNT/β-catenin signaling promotes aberrant cellular growth during cancer initiation [18]. WNT/β-catenin is active in areas with vascular endothelial growth factor (VEGF)-related cold immunity [37], and the impact of β-catenin on P-glycoprotein is indicative of its involvement in multi-drug resistance [38, 39]. WNT/β-catenin reduces the expression of epithelial-related markers, such as E-cadherin [40], which is for acquisition of cancer stemness features. CSCs are PORCN+ and provide WNT within their niches for tumor progressive purposes [41].
WNT/β-catenin impact on cellular immunity
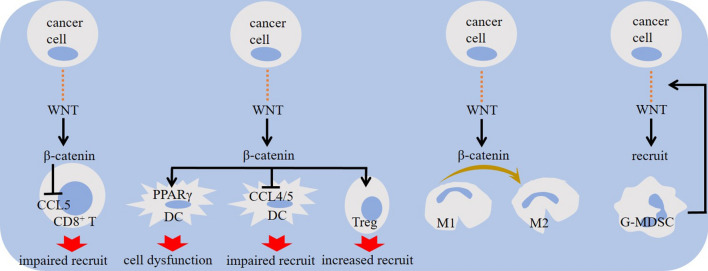
Β-catenin activity mediates cooperation between tumor and stroma to promote cancer growth [42]. Primary tumors show elevated expression of WNT/β-catenin in CD8+ T cells. Increased expression of genes related to the WNT/β-catenin pathway in lymphocytes is contributed to the apoptosis of mature T cells, and increased β-catenin signaling in tumor cells promotes T cell exhaustion [43]. Besides, infiltration of effector CD8+ T cells into the tumor area is diminished under WNT/β-catenin pathway activity [30, 44]. Mutation of adenomatosis polyposis coli is contributed to the elevated β-catenin activity and reduced CD8+ T cell proportion in the TME of CRC [45]. There is a strong correlation between regulatory T (Treg) intra-tumoral recruitment with mutation of adenomatosis polyposis coli in CRC [46]. β-catenin acts on Tcf/Lef, which are transcription factors important for promoting immunosuppressive activity of Tregs [47]. β-catenin also reduces levels of chemokines contributed to the recruitment of dendritic cells (DCs) into tumor area [48, 49]. β-catenin downregulates CCL5 Chemokine (C–C motif) ligand 5 (CCL5) [49], which is involved in T cell [50] and DC [51] recruitment. WNT/β-catenin also promotes DC tolerance [30]. Hampering CD103+ DC recruitment by tumor cell-intrinsic WNT/β-catenin results in defective CD8+ T cell priming [44] (Fig. 2). Increased activity of WNT5a/β-catenin is contributed to the indoleamine 2,3-dioxygenase 1 (IDO1) induction in tumor-associated DCs [30], which is seemingly mediated through peroxisome proliferator-activated receptor-γ (PPARγ) activation [52] and further reprogramming of DC metabolism from glycolysis into oxidative phosphorylation [14] (Fig. 3). Granulocytic-myeloid-derived suppressor cell (G-MDSC) is another cell type highly expressing canonical WNT [53]. WNT signaling promotes G-MDSC recruitment into tumor area [30], and the activity of WNT in G-MDSCs is for the subsequent induction of aberrant WNT/β-catenin activation in malignant cells for promoting breast cancer metastasis [53]. Finally, tumor-associated macrophages (TAMs) are cells upregulating WNT/β-catenin [54]. WNT ligands derived from tumor cells promote macrophage type 2 (M2) polarization through canonical pathway [55]. β-catenin ablation in TAMs by approaches like CD200R1-Ig expressing adenoviral therapy suppresses M2 polarity [56]. β-catenin blockade may even promote a M2-to-M1 shift in macrophages [54] (Fig. 2).
Fig. 2.
The impact of WNT/β-catenin signaling on immune cells within tumor microenvironment (TME). β-catenin activation downregulates chemokine (C–C motif) ligand 5 (CCL5) activity, re-expression of which restores immune surveillance. Defective CD8+ T cell priming, impaired recruitment of dendritic cells (DCs) and CD8+ T cells, and increased recruitment of regulatory T cells (Tregs) and granulocytic-myeloid-derived suppressor cells (G-MDSCs) are outcomes of elevated WNT/β-catenin signaling in cancer. Shifting macrophage reprogramming into pro-tumor type 2 (M2) phenotype is another outcome, which is contributed to the intensification of immunosuppressive tumor profile. PPARγ, peroxisome proliferator-activated receptor-γ
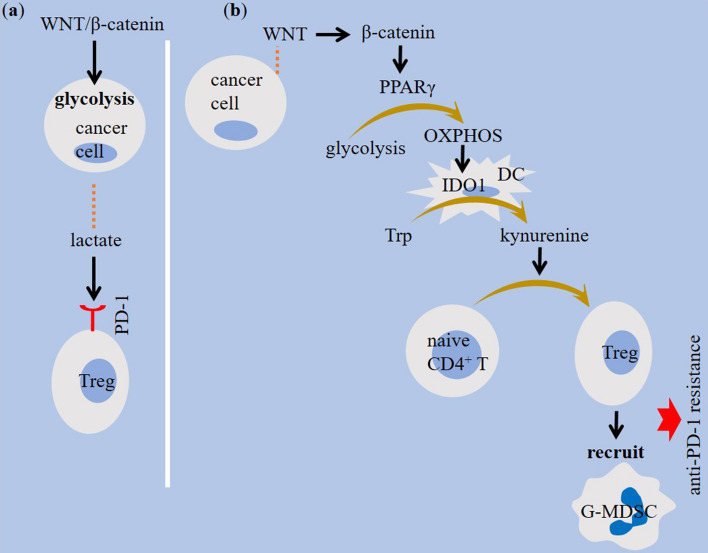
Fig. 3.
WNT/β-catenin signaling in tumor metabolism. A highly glycolytic tumor microenvironment (TME) represents high lactate release, which further acts for expression of programmed death-1 (PD-1) on regulatory T cells (Tregs). WNT5a/β-catenin induces indoleamine 2,3-dioxygenase (IDO)1 in tumor-associated dendritic cells (DCs) through activating peroxisome proliferator-activated receptor-γ (PPARγ). PPARγ reprograms DC metabolism toward oxidative phosphorylation (OXPHOS), which further increases IDO1 activity in DCs. IDO1 catalyzes tryptophan degradation, and the resultant kynurenine accumulation promotes Treg activity
WNT/β-catenin regulatory roles on PD-1/PD-L1 and ICI responses
Increased PD-L1 expression is placed downstream to the β-catenin activity [38]. Bondage of β-catenin/Tcf/Lef complex to the promoter of CD274 gene induces PD-L1 expression on tumor cells [57], and the impact of WNT/β-catenin on PD-L1 activation is indicative of the key role of this signaling in regulation of tumor immune landscape [58]. Increased activity of WNT/β-catenin signaling is the underlying mechanism contributed to the development of non-inflamed TME and low ICI responses in highly mutated cancer type like NSCLC. In such cancer type, high tumor-mutational burden (TMB) is representative of low responses to ICI therapy [13]. This is in contrast with the common belief that a tumor with higher somatic mutations generally shows higher responses to immunotherapy due to being more accessible to be killed by immune system [59]. The high TMB in NSCLC is accompanied by lack of CD8+ T cell in TME and the resultant promotion of ICI resistance. This is due to the increased activity of WNT/β-catenin, which impairs CD8+ T cell infiltration into the tumor area [13]. β-catenin activation is contributed to anti-PD-1 resistance in HCC [49]. B-cell lymphoma 9 (BCL9) is the co-activator of β-catenin. Pharmacological blockade of β-catenin/BCL9 using desired peptides is reported to reduce the proportion of Tregs, increased tumoral infiltration of cytotoxic T lymphocytes and sensitized cancer cells to anti-PD-1 therapy [46]. Lack of T cell genomic signature and T cell infiltrate due to the intrinsic tumor-mediated WNT/β-catenin activity is contributed to the anti-PD-L1 resistance of melanoma [4]. There is a report of increased WNT/β-catenin in CD8+ T cells after anti-PD-1 therapy of primary sarcomas [43]. Constitutive activation of WNT/β-catenin and further decreased expression of the chemokine CCL4 seemingly account for ineffective ICI responses [60]. WNT/β-catenin mediates resistance to ICI therapy in part through blockade of cytokines contributed to the recruitment of immune cells. Targeting CTNNB1 using the nanoparticle drug product DCR-BCAT is attested to augmented T cell infiltration and increased tumor sensitivity to ICI therapy [61].
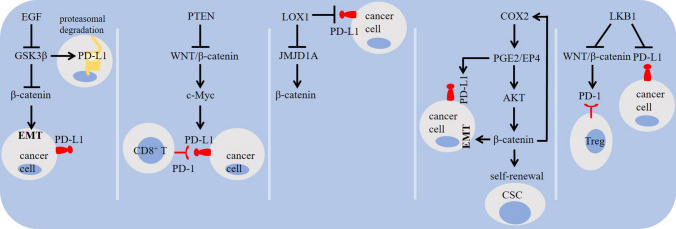
The activity of WNT/β-catenin is hampered by glycogen synthase kinase 3β (GSK3β) [31, 62, 63]. β-catenin is a GSK3β substrate [64]. GSK3β acts for promoting PD-L1 ubiquitination and degradation [65–67] (Fig. 1). GSK3β inhibition, β-catenin induction and PD-L1 glycosylation are mediated under the influence of epidermal growth factor (EGF) [68], and that GSK3β activators can be used for PD-L1 instability and increasing anti-PD-1 efficacy [65]. WNT/β-catenin stimulates glycolysis [69], and the highly glycolytic TME shapes the immune landscape of tumor through inducing the expression of PD-1 on Tregs [70] (Fig. 3). WNT/β-catenin induces PD-L1 transcription and T cell apoptosis through stimulating c-Myc signaling in hepatitis B virus (HBV) mouse model and HBV+ hepatoma cells, which is counteracted by phosphatase and tensin homolog deleted on chromosome 10 (PTEN) [71]. Trujillo and colleagues described mechanistic backbone of resistance to the combined anti-PD-1 and anti-CTLA-4 in two cases of metastatic melanoma and noticed a robust tumoral expression of β-catenin in a one and acquired PTEN loss in another, with both evolving loss of T cell infiltration [72]. β-catenin also cooperate with prostaglandin E2 (PGE2) in cancer [73], and the release of PGE2 from M2 TAMs induces PD-L1 on tumor cells [74]. Study shows a possible correlation between PGE2 generation and increased β-catenin activity for maintaining stemness in glioblastoma tumor cells [75]. Promoter of cyclooxygenase-2 (COX-2) contains Tcf4 binding element to which β-catenin is bonded for further upregulation of COX-2 in colon and liver cancer [76]. β-catenin also interacts with liver kinase B1 (LKB1) to control PD-1 activity [16]. Silencing intracellular LKB1 is also followed by an increase in the level of PD-L1 [77], and the loss of Stk11/Lkb1 is reported to promote resistance to anti-PD-(L)1 in KRAS mutant lung adenocarcinoma [78] (Fig. 4). Finally, β-catenin/Tcf4 induces Zinc finger E-box binding homeobox1 (ZEB1), a known mediator of EMT [79], and that EMT induction is linked positively with PD-L1 expression on tumor cells, as evidenced by the implication of the EMT activator ZEB1 in relieving miR-200-mediated repression of PD-L1 activity on tumor cells [80]. Etoposide is a chemotherapy drug that mediates mesenchymal–epithelial transition (MET) to reduce nuclear β-catenin and the resultant downregulation of PD-L1 on tumor cells [81] (Fig. 5).
Fig. 4.
Signaling pathways related to the WNT/β-catenin activity and checkpoint regulation in cancer. Epidermal growth factor (EGF) inhibits glycogen synthase kinase 3β (GSK3β), induces β-catenin, and stimulates programmed death-ligand 1 (PD-L1) glycosylation. Activation of GSK3β destabilizes PD-L1 through promoting its ubiquitination and proteasomal degradation. β-catenin activity increases c-Myc, the activity of which enforces PD-L1 expression in tumor microenvironment (TME) and the subsequent apoptosis of T cells. The histone demethylase inhibitor 5-carboxy-8-hydroxyquinoline (IOX1) suppresses Jumonji domain-containing 1A (JMJD1A) and its downstream β-catenin, and downregulates PD-L1 on tumor cells. Prostaglandin E2 (PGE2) stimulates the activity of β-catenin for maintaining cancer stemness. PGE2 release from M2 macrophages also induces PD-L1 expression on tumor cells. Promoter of cyclooxygenase-2 (COX-2) contains Tcf4 binding element to which β-catenin is bonded for upregulation of COX-2 expression. PTEN, phosphatase and tensin homolog deleted on chromosome 10; and LKB1, liver kinase B1
Fig. 5.
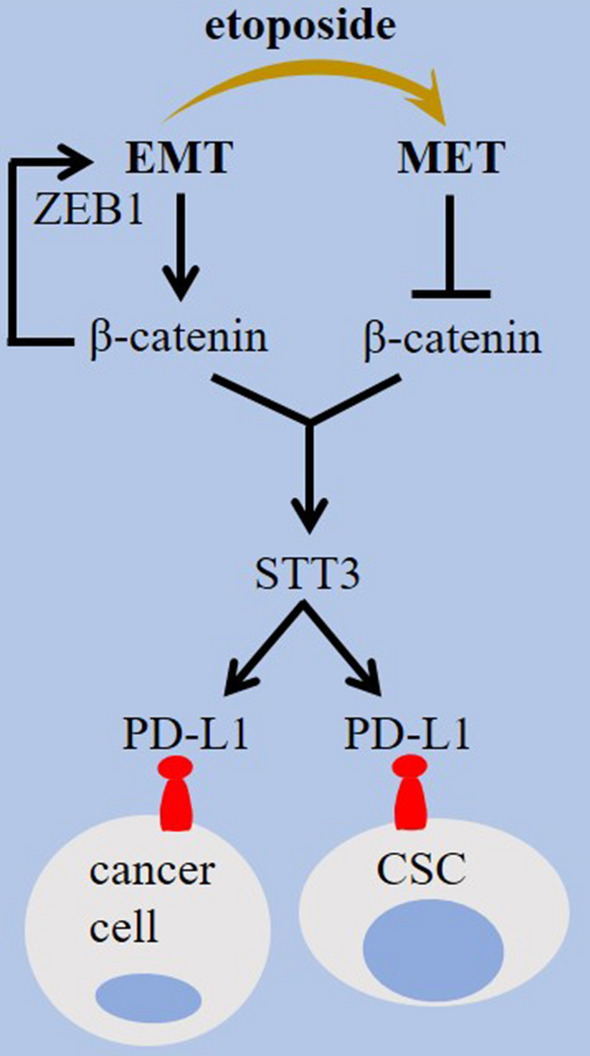
Epithelial mesenchymal plasticity in β-catenin and checkpoint regulation. Zinc finger E-box binding homeobox1 (ZEB1) is an epithelial-mesenchymal transition (EMT)-related transcription factor that its expression is induced by the β-catenin/Tcf4 complex. The N-glycosyltransferase STT3 is stimulated by EMT inducible effect on β-catenin in cancer cells and cancer stem cells (CSCs) to promote programmed death-ligand 1 (PD-L1) upregulation. Conversion into mesenchymal–epithelial transition (MET) phenotype reduces nuclear β-catenin, downregulates PD-L1, and sensitizes tumor cells to immunotherapy
Combination of WNT/β-catenin inhibitors with anti-PD-1/PD-L1
Inhibitors of WNT/β-catenin can be developed to exert synergistic anti-tumor effects with ICIs in cancer immunotherapy [82]. There is a report in HCC mice model indicating potent anti-tumor efficacy of nanoparticles constructed to simultaneously target hyperactive WNT/β-catenin and block endogenous PD-L1 [83]. Takeuchi and colleagues attested a positive impact of WNT/β-catenin on ICI resistance in TMBhigh NSCLC, and the combination therapy with WNT/β-catenin blockade and anti-PD-1 better promoted anti-tumor immunity compared with either agent alone [13]. Microsatellite stable (MSS) CRC shows dismal responses (0%) to ICI therapy. Combination of the PORCN inhibitor ETC-159 with the PD-1 inhibitor nivolumab reduced tumor volume in mice engrafted with MSS CRC. Combination therapy increased the fraction of effector CD4+ and CD8+ T cells and reduced Treg population, and augmented the antigen presentation profile represented by increased tumoral cell expression of major histocompatibility complex class II (MHC II) [84]. Elevated activity of WNT ligand signaling is also responsible for failure of anti-PD-1 in melanoma. Suppression of WNT ligand increases the efficacy of anti-PD-1 in autochthonous animal tumor models through reduction of G-MDSC recruitment and reversion of DC tolerance. The higher suppressive impact of vantictumab or ipafricept over solo anti-PD-1 is reported in animal tumor model of melanoma, which is correlated with higher intra-tumoral infiltration of tumor-specific CD8+ T cells. DeVito and colleagues attested a positive link between anti-PD-1 resistance with increased WNT ligand signaling, which is indicative of the sensitivity of anti-PD-1 refractory melanoma to the WNT ligand blockade, as shown after application of ETC-159 [30]. The efficacy of WNT974 plus the PD-1 inhibitor spartalizumab was evaluated in patients with advanced solid cancers. Treatment-related adverse events (TRAEs) were reported in 78% of patients, with hypothyroidism identified in 19% of cases. 53% of patients who were refractory to prior anti-PD-1 showed stable disease, with uveal melanoma all cases (n = 5) represented stable disease. The outcomes are indicative of a presumable synergistic activity of the combined WNT pathway inhibition with ICI therapy against advanced solid cancers [22] (Table 1).
Table 1.
Targeting WNT-β-catenin in cancer immunotherapy
| Cancer type | Target regimen | Effects | References |
|---|---|---|---|
| NSCLC | WNT/β-catenin blockade plus anti-PD-1 | Combination therapy better promoted anti-tumor immunity | [13] |
| MSS CRC | PORCN inhibitor ETC-159 plus anti-PD-1 (nivolumab) | Combination therapy in in mice engrafted tumor reduced tumor volume, increased the proportion of effector CD4+ and CD8+ T cells and reduced Treg population | [84] |
| Melanoma | ETC-159 plus anti-PD-1 | Anti-PD-1 resistance is linked positively with increased WNT ligand signaling, and anti-PD-1 refractory melanoma is sensitive to the ETC-159 therapy | [30] |
| Advanced solid cancers | WNT974 plus anti-PD-1 (spartalizumab) | Combination therapy resulted in a stable disease in 53% of patients who were refractory to prior anti-PD-1, with uveal melanoma all cases had stable disease | [22] |
| HCC | Nanoparticle-based inhibition of β-catenin and PD-L1 | Nanoparticle delivery increased intra-tumoral proportion and activity of CD8+ T cells, and it showed higher anti-tumor effects compared with anti-PD-L1 in orthotopic homograft animal model | [83] |
| Xenograft model | WNT inhibitors plus anti-PD-L1 | WNT blockade increased anti-PD-L1 efficacy through hampering CAF-related immunotherapy resistance | [85] |
MSS, microsatellite stable; CRC, colorectal cancer; PD-1, programmed death-1; Treg, regulatory T; HCC, hepatocellular carcinoma; PD-L1, programmed death-ligand 1; and CAF, cancer-associated fibroblast
In summary, it is rationale to assert that dysregulation of the WNT/β-catenin occurs in the context of human cancers and is associated with several cellular processes involved in tumor progression. Failure of anti-checkpoint therapy is a multi-mechanistic issue, among which the activity of WNT/β-catenin signaling has recently taken important consideration due to its critical association with cancer stemness. Tight interactions between WNT/β-catenin with different cells within tumor immune ecosystem, close interactions with PD-1/PD-L1 axis, and the promising outcomes from clinical trials targeting the two are all indicative of the application of combination therapies using WNT/β-catenin inhibitors with anti-PD-(L)1 in cancer immunotherapy, particularly in tumors with cold immunity and highly aggressive profile. However, there are points require attention when interpreting outcomes in patients under exposure to the combined WNT/β-catenin inhibitor/anti-PD-(L)1 therapy. First, interactions between WNT with complex receptors can activate signaling either dependent or independent on β-catenin, and a hallmark of a β-catenin-dependent pathway is its stability and nuclear translocation [86]. Second, β-catenin transactivation can also occur independent on WNT [87], and Tcf1/Lef1 can also be activated by other transcription factors, such as ATF2 [88]. Third, genotoxic agents can activate WNT/β-catenin independent on canonical Fzd/LRP receptor complex [89]. Further studies are demanded for surveying other upstream mediators or inhibitors of β-catenin activity. 5-carboxy-8-hydroxyquinoline (IOX1), for instance, is a histone demethylase inhibitor that suppresses Jumonji domain-containing 1A (JMJD1A) and its downstream β-catenin, and downregulates tumoral PD-L1, expressed secondary to the doxorubicin chemotherapy [38] (Fig. 4). The presence of WNT/β-catenin signaling in circulating extracellular vesicles (EVs) [90], and surface representation of PD-L1 by EVs secreted from tumor cells [91] are all indicative of a possibility for application of EVs in cancer immunotherapy targeting both signaling. A key virtue of such strategy is the tendency of EVs for their preferential attraction toward tumor tissue area due to expressing receptors related to that tumor type. Besides WNT/β-catenin, the activity of TGF-β signaling is also contributed to the stemness of tumor cells and cancer resistance to ICI therapy. Bispecific antibodies against TGF-β and PD-L1 are developed, and impressive responses are for PD-L1high platinum refractory NSCLC patients [92].
Acknowledgements
This work received ethical approval from Kurdistan University of Medical Sciences (Ethical Code: IR.MUK.REC.1401.430).
Author contributions
The manuscript has only one author.
Funding
The author has not disclosed any funding.
Declarations
Conflict of interest
None to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Papadas A, Deb G, Cicala A, Officer A, Hope C, Pagenkopf A, Flietner E, Morrow ZT, Emmerich P, Wiesner J. Stromal remodeling regulates dendritic cell abundance and activity in the tumor microenvironment. Cell Rep. 2022;40(7):111201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petralia F, Tignor N, Reva B, Koptyra M, Chowdhury S, Rykunov D, Krek A, Ma W, Zhu Y, Ji J. Integrated proteogenomic characterization across major histological types of pediatric brain cancer. Cell. 2020;183(7):1962–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Rothberg BEG, Taketo MM, Dankort D, Rimm DL, McMahon M. β-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell. 2011;20(6):741–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–5. [DOI] [PubMed] [Google Scholar]
- 5.Li K, Mao S, Li X, Zhao H, Wang J, Wang C, Wu L, Zhang K, Yang H, Jin M. Frizzled-7-targeting antibody (SHH002-hu1) potently suppresses non–small-cell lung cancer via Wnt/β-catenin signaling. Cancer Sci. 2023;114(5):2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berraondo P, Ochoa MC, Olivera I, Melero I. Immune desertic landscapes in hepatocellular carcinoma shaped by β-catenin activation. Cancer Discov. 2019;9(8):1003–5. [DOI] [PubMed] [Google Scholar]
- 7.Dangaj D, Bruand M, Grimm AJ, Ronet C, Barras D, Duttagupta PA, Lanitis E, Duraiswamy J, Tanyi JL, Benencia F. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell. 2019;35(6):885–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44(3):698–711. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi H, Watanabe H, Hashimura M, Matsumoto T, Yokoi A, Nakagawa M, Ishibashi Y, Ito T, Ohhigata K, Saegusa M. A combination of stromal PD-L1 and tumoral nuclear β-catenin expression as an indicator of colorectal carcinoma progression and resistance to chemoradiotherapy in locally advanced rectal carcinoma. J Pathol Clinic Res. 2022;8(5):458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu C, Ye M, Bai J, Liu P, Lu F, Chen J, Yu P, Chen T, Shi X, Tang Q. Methylmalonic acid promotes colorectal cancer progression via activation of Wnt/β-catenin pathway mediated epithelial–mesenchymal transition. Cancer Cell Int. 2023;23(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Yu H, Dong W, Zhang C, Hu M, Ma W, Jiang X, Li H, Yang P, Xiang D. N6-methyladenosine–mediated up-regulation of FZD10 regulates liver cancer stem cells’ properties and lenvatinib resistance through WNT/β-catenin and hippo signaling pathways. Gastroenterology. 2023;164(6):990–1005. [DOI] [PubMed] [Google Scholar]
- 12.Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. WNT/β-catenin pathway activation correlates with immune exclusion across human cancersWNT/β-catenin–associated immune exclusion across cancers. Clin Cancer Res. 2019;25(10):3074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi Y, Tanegashima T, Sato E, Irie T, Sai A, Itahashi K, Kumagai S, Tada Y, Togashi Y, Koyama S. Highly immunogenic cancer cells require activation of the WNT pathway for immunological escape. Sci Immunol. 2021;6(65):eabc6424. [DOI] [PubMed] [Google Scholar]
- 14.Zhao F, Xiao C, Evans KS, Theivanthiran T, DeVito N, Holtzhausen A, Liu J, Liu X, Boczkowski D, Nair S. Paracrine Wnt5a-β-catenin signaling triggers a metabolic program that drives dendritic cell tolerization. Immunity. 2018;48(1):147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang K, Blanco DB, Neale G, Vogel P, Avila J, Clish CB, Wu C, Shrestha S, Rankin S, Long L. Homeostatic control of metabolic and functional fitness of Treg cells by LKB1 signalling. Nature. 2017;548(7669):602–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alok A, Lei Z, Jagannathan NS, Kaur S, Harmston N, Rozen SG, Tucker-Kellogg L, Virshup DM. Wnt proteins synergize to activate β-catenin signaling. J Cell Sci. 2017;130(9):1532–44. [DOI] [PubMed] [Google Scholar]
- 18.Xiao Q, Wu J, Wang W-J, Chen S, Zheng Y, Yu X, Meeth K, Sahraei M, Bothwell AL, Chen L. DKK2 imparts tumor immunity evasion through β-catenin-independent suppression of cytotoxic immune-cell activation. Nat Med. 2018;24(3):262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voloshanenko O, Erdmann G, Dubash TD, Augustin I, Metzig M, Moffa G, Hundsrucker C, Kerr G, Sandmann T, Anchang B. Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells. Nat Commun. 2013;4(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng CK, Dazert E, Boldanova T, Coto-Llerena M, Nuciforo S, Ercan C, Suslov A, Meier M-A, Bock T, Schmidt A. Integrative proteogenomic characterization of hepatocellular carcinoma across etiologies and stages. Nat Commun. 2022;13(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miete C, Solis GP, Koval A, Brückner M, Katanaev VL, Behrens J, Bernkopf DB. Gαi2-induced conductin/axin2 condensates inhibit Wnt/β-catenin signaling and suppress cancer growth. Nat Commun. 2022;13(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janku F, de Vos F, de Miguel M, Forde P, Ribas A, Nagasaka M, Argiles G, Arance AM, Calvo A, Giannakis M. Abstract CT034: phase I study of WNT974+ spartalizumab in patients (pts) with advanced solid tumors. Can Res. 2020;80(16_Supplement):CT034–CT034. [Google Scholar]
- 23.Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci. 2012;109(29):11717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherwood V, Chaurasiya SK, Ekström EJ, Guilmain W, Liu Q, Koeck T, Brown K, Hansson K, Agnarsdóttir M, Bergqvist M. WNT5A-mediated β-catenin-independent signalling is a novel regulator of cancer cell metabolism. Carcinogenesis. 2014;35(4):784–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian M, Wang X, Sun J, Lin W, Chen L, Liu S, Wu X, Shi L, Xu P, Cai X. IRF3 prevents colorectal tumorigenesis via inhibiting the nuclear translocation of β-catenin. Nat Commun. 2020;11(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Debo B, Li M, Shi Z, Sheng W, Shi Y. LSD1 inhibition sustains T cell invigoration with a durable response to PD-1 blockade. Nat Commun. 2021;12(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tammela T, Sanchez-Rivera FJ, Cetinbas NM, Wu K, Joshi NS, Helenius K, Park Y, Azimi R, Kerper NR, Wesselhoeft RA. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature. 2017;545(7654):355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci. 2013;110(50):20224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SR, Won HS, Yang JH, Sun DS, Yim K, Hong M, Hong SA, Yoon J-S, Chun SH, Kim K-H. Prognostic value of Dickkopf-1 and ß-catenin expression according to the antitumor immunity of CD8-positive tumor-infiltrating lymphocytes in biliary tract cancer. Sci Rep. 2022;12(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeVito NC, Sturdivant M, Thievanthiran B, Xiao C, Plebanek MP, Salama AK, Beasley GM, Holtzhausen A, Novotny-Diermayr V, Strickler JH. Pharmacological Wnt ligand inhibition overcomes key tumor-mediated resistance pathways to anti-PD-1 immunotherapy. Cell Rep. 2021;35(5):109071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gattinoni L, Zhong X-S, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng VH, Spencer Z, Neitzel LR, Nayak A, Loberg MA, Shen C, Kassel SN, Kroh HK, An Z, Anthony CC. The USP46 complex deubiquitylates LRP6 to promote Wnt/β-catenin signaling. Nat Commun. 2023;14(1):6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lichtenberger BM, Mastrogiannaki M, Watt FM. Epidermal β-catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Nat Commun. 2016;7(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ioannidis V, Beermann F, Clevers H, Held W. The β-catenin–TCF-1 pathway ensures CD4+ CD8+ thymocyte survival. Nat Immunol. 2001;2(8):691–7. [DOI] [PubMed] [Google Scholar]
- 35.Zingg D, Debbache J, Peña-Hernández R, Antunes AT, Schaefer SM, Cheng PF, Zimmerli D, Haeusel J, Calçada RR, Tuncer E. EZH2-mediated primary cilium deconstruction drives metastatic melanoma formation. Cancer Cell. 2018;34(1):69–84. [DOI] [PubMed] [Google Scholar]
- 36.Wong KY, Seim I, Wang R, He Y, Wu A, Patrick M, Lourie R, Schreiber V, Giri R, Ng CP. MUC13 promotes the development of colitis-associated colorectal tumors via β-catenin activity. Oncogene. 2019;38(48):7294–310. [DOI] [PubMed] [Google Scholar]
- 37.Clark DJ, Dhanasekaran SM, Petralia F, Pan J, Song X, Hu Y, da Veiga Leprevost F, Reva B, Lih T-SM, Chang H-Y. Integrated proteogenomic characterization of clear cell renal cell carcinoma. Cell. 2019;179(4):964–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Zhao Z, Qiu N, Zhou Q, Wang G, Jiang H, Piao Y, Zhou Z, Tang J, Shen Y. Co-delivery of IOX1 and doxorubicin for antibody-independent cancer chemo-immunotherapy. Nat Commun. 2021;12(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen DY, Zhang W, Zeng X, Liu CQ. Inhibition of Wnt/β-catenin signaling downregulates P-glycoprotein and reverses multi-drug resistance of cholangiocarcinoma. Cancer Sci. 2013;104(10):1303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pacheco-Pinedo EC, Durham AC, Stewart KM, Goss AM, Lu MM, DeMayo FJ, Morrisey EE. Wnt/β-catenin signaling accelerates mouse lung tumorigenesis by imposing an embryonic distal progenitor phenotype on lung epithelium. J Clin Investig. 2011;121(5):1935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Y, Ramesh A, Gusev Y, Bhuvaneshwar K, Giaccone G. Molecular predictors of response to pembrolizumab in thymic carcinoma. Cell Rep Med. 2021;2(9):100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasashima H, Duran A, Martinez-Ordoñez A, Nakanishi Y, Kinoshita H, Linares JF, Reina-Campos M, Kudo Y, L’Hermitte A, Yashiro M. Stromal SOX2 upregulation promotes tumorigenesis through the generation of a SFRP1/2-expressing cancer-associated fibroblast population. Dev Cell. 2021;56(1):95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wisdom AJ, Mowery YM, Hong CS, Himes JE, Nabet BY, Qin X, Zhang D, Chen L, Fradin H, Patel R. Single cell analysis reveals distinct immune landscapes in transplant and primary sarcomas that determine response or resistance to immunotherapy. Nat Commun. 2020;11(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spranger S, Dai D, Horton B, Gajewski TF. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell. 2017;31(5):711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen B, Cherie’R S, McKinley ET, Simmons AJ, Ramirez-Solano MA, Zhu X, Markham NO, Heiser CN, Vega PN, Rolong A. Differential pre-malignant programs and microenvironment chart distinct paths to malignancy in human colorectal polyps. Cell. 2021;184(26):6262–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng M, Jin J, Xia L, Xiao T, Mei S, Wang X, Huang X, Chen J, Liu M, Chen C. Pharmacological inhibition of β-catenin/BCL9 interaction overcomes resistance to immune checkpoint blockades by modulating Treg cells. Sci Adv. 2019;5(5):eaau5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xing S, Gai K, Li X, Shao P, Zeng Z, Zhao X, Zhao X, Chen X, Paradee WJ, Meyerholz DK. Tcf1 and Lef1 are required for the immunosuppressive function of regulatory T cells. J Exp Med. 2019;216(4):847–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng W-C, Tsui Y-C, Ragusa S, Koelzer VH, Mina M, Franco F, Läubli H, Tschumi B, Speiser D, Romero P. Uncoupling protein 2 reprograms the tumor microenvironment to support the anti-tumor immune cycle. Nat Immunol. 2019;20(2):206–17. [DOI] [PubMed] [Google Scholar]
- 49.Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, Lindblad KE, Maier B, Sia D, Puigvehi M, Miguela V, Casanova-Acebes M, Dhainaut M. β-catenin activation promotes immune escape and resistance to anti–PD-1 therapy in hepatocellular carcinomaβ-catenin promotes immune resistance in liver cancer. Cancer Discov. 2019;9(8):1124–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ayuso JM, Rehman S, Virumbrales-Munoz M, McMinn PH, Geiger P, Fitzgerald C, Heaster T, Skala MC, Beebe DJ. Microfluidic tumor-on-a-chip model to evaluate the role of tumor environmental stress on NK cell exhaustion. Sci Adv. 2021;7(8):eabc2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, e Sousa CR. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172(5):1022–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin X, Zeng W, Wu B, Wang L, Wang Z, Tian H, Wang L, Jiang Y, Clay R, Wei X. PPARα inhibition overcomes tumor-derived exosomal lipid-induced dendritic cell dysfunction. Cell Rep. 2020;33(3): 108278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen N, Feng Q, Deng J, Xiong Y, Deng Y-J, Wang M-M, Zhou L, Yu Q-W, Hu J-P, Deng H. Hdc-expressing myeloid-derived suppressor cells promote basal-like transition and metastasis of breast cancer. Int J Clin Exp Pathol. 2020;13(6):1431. [PMC free article] [PubMed] [Google Scholar]
- 54.Sarode P, Zheng X, Giotopoulou GA, Weigert A, Kuenne C, Günther S, Friedrich A, Gattenlöhner S, Stiewe T, Brüne B. Reprogramming of tumor-associated macrophages by targeting β-catenin/FOSL2/ARID5A signaling: a potential treatment of lung cancer. Sci Adv. 2020;6(23):eaaz6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y, Ye Y-C, Chen Y, Zhao J-L, Gao C-C, Han H, Liu W-C, Qin H-Y. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018;9(8):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin S-P, Goh A-R, Ju J-M, Kang H-G, Kim S-J, Kim J-K, Park E-J, Bae Y-S, Choi K, Jung Y-S. Local adenoviral delivery of soluble CD200R-Ig enhances antitumor immunity by inhibiting CD200-β-catenin-driven M2 macrophage. Molr Ther-Oncol. 2021;23:138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du L, Lee J-H, Jiang H, Wang C, Wang S, Zheng Z, Shao F, Xu D, Xia Y, Li J. β-Catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion. J Exp Med. 2020;217(11):e20191115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sayaman RW, Saad M, Thorsson V, Hu D, Hendrickx W, Roelands J, Porta-Pardo E, Mokrab Y, Farshidfar F, Kirchhoff T. Germline genetic contribution to the immune landscape of cancer. Immunity. 2021;54(2):367–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mortezaee K, Majidpoor J, Najafi S, Tasa D. Bypassing anti-PD-(L) 1 therapy: mechanisms and management strategies. Biomed Pharmacother. 2023;158:114150. [DOI] [PubMed] [Google Scholar]
- 60.Zhang F, Huang H, Qin Y, Chen C, She L, Wang J, Huang D, Tang Q, Liu Y, Zhu G. MTDH associates with m6A RNA methylation and predicts cancer response for immune checkpoint treatment. Iscience. 2021;24(10):103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ganesh S, Shui X, Craig KP, Park J, Wang W, Brown BD, Abrams MT. RNAi-mediated β-catenin inhibition promotes T cell infiltration and antitumor activity in combination with immune checkpoint blockade. Mol Ther. 2018;26(11):2567–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo C-J, Ma X-K, Xing Y-H, Zheng C-C, Xu Y-F, Shan L, Zhang J, Wang S, Wang Y, Carmichael GG. Distinct processing of lncRNAs contributes to non-conserved functions in stem cells. Cell. 2020;181(3):621–36. [DOI] [PubMed] [Google Scholar]
- 63.Huang D, Wang Y, Thompson JW, Yin T, Alexander PB, Qin D, Mudgal P, Wu H, Liang Y, Tan L. Cancer-cell-derived GABA promotes β-catenin-mediated tumour growth and immunosuppression. Nat Cell Biol. 2022;24(2):230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han H, Jain AD, Truica MI, Izquierdo-Ferrer J, Anker JF, Lysy B, Sagar V, Luan Y, Chalmers ZR, Unno K. Small-molecule MYC inhibitors suppress tumor growth and enhance immunotherapy. Cancer Cell. 2019;36(5):483–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li C-W, Lim S-O, Xia W, Lee H-H, Chan L-C, Kuo C-W, Khoo K-H, Chang S-S, Cha J-H, Kim T. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu M, Xia X, Hu J, Fowlkes NW, Li S. WSX1 act as a tumor suppressor in hepatocellular carcinoma by downregulating neoplastic PD-L1 expression. Nat Commun. 2021;12(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Y, Zhang C, Liu X, He Z, Shan B, Zeng Q, Zhao Q, Zhu H, Liao H, Cen X. ARIH1 signaling promotes anti-tumor immunity by targeting PD-L1 for proteasomal degradation. Nat Commun. 2021;12(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li C-W, Lim S-O, Chung EM, Kim Y-S, Park AH, Yao J, Cha J-H, Xia W, Chan L-C, Kim T. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell. 2018;33(2):187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pate KT, Stringari C, Sprowl-Tanio S, Wang K, TeSlaa T, Hoverter NP, McQuade MM, Garner C, Digman MA, Teitell MA. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014;33(13):1454–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin Y-T, Togashi Y, Kamada T, Irie T, Okumura G, Kono H. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. 2022;40(2):201–18. [DOI] [PubMed] [Google Scholar]
- 71.Sun Y, Yu M, Qu M, Ma Y, Zheng D, Yue Y, Guo S, Tang L, Li G, Zheng W. Hepatitis B virus-triggered PTEN/β-catenin/c-Myc signaling enhances PD-L1 expression to promote immune evasion. Am J Physiol Gastrointest Liver Physiol. 2020;318(1):G162–73. [DOI] [PubMed] [Google Scholar]
- 72.Trujillo JA, Luke JJ, Zha Y, Segal JP, Ritterhouse LL, Spranger S, Matijevich K, Gajewski TF. Secondary resistance to immunotherapy associated with β-catenin pathway activation or PTEN loss in metastatic melanoma. J Immunother Cancer. 2019;7(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-ß-catenin signaling axis. Science. 2005;310(5753):1504–10. [DOI] [PubMed] [Google Scholar]
- 74.Pu Y, Ji Q. Tumor-associated macrophages regulate PD-1/PD-L1 immunosuppression. Front Immunol. 2022;13:874589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yin J, Kim SS, Choi E, Oh YT, Lin W, Kim T-H, Sa JK, Hong JH, Park SH, Kwon HJ. ARS2/MAGL signaling in glioblastoma stem cells promotes self-renewal and M2-like polarization of tumor-associated macrophages. Nat Commun. 2020;11(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Araki Y, Okamura S, Hussain SP, Nagashima M, He P, Shiseki M, Miura K, Harris CC. Regulation of cyclooxygenase-2 expression by the Wnt and ras pathways. Can Res. 2003;63(3):728–34. [PubMed] [Google Scholar]
- 77.Liu Z, Zhou K, Zeng J, Zhou X, Li H, Peng K, Liu X, Li F, Jiang B, Zhao M. Liver kinase B1 in exosomes inhibits immune checkpoint programmed death ligand 1 and metastatic progression of intrahepatic cholangiocarcinoma. Oncol Rep. 2022;48(3):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco SE, Gay L. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinomaSTK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant LUAC. Cancer Discov. 2018;8(7):822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sánchez-Tilló E, de Barrios O, Siles L, Cuatrecasas M, Castells A, Postigo A. β-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci. 2011;108(48):19204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn Y-H, Byers LA, Zhang X, Yi X, Dwyer D, Lin W. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsu J-M, Xia W, Hsu Y-H, Chan L-C, Yu W-H, Cha J-H, Chen C-T, Liao H-W, Kuo C-W, Khoo K-H. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat Commun. 2018;9(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu Y, Ng AH, Chow FE, Everson RG, Helmink BA, Tetzlaff MT, Thakur R, Wargo JA, Cloughesy TF, Prins RM. Resolution of tissue signatures of therapy response in patients with recurrent GBM treated with neoadjuvant anti-PD1. Nat Commun. 2021;12(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou Z, Li X, Yang G, Wang J, Li B, Huang Y, Yan J, Tao K. Targeting β-catenin and PD-L1 simultaneously by a racemic supramolecular peptide for the potent immunotherapy of hepatocellular carcinoma. Theranostics. 2023;13(10):3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bagby SM, Hartman SJ, Navarro NM, Yacob BW, Shulman J, Barkow J, Lieu CH, Davis SL, Leal AD, Messersmith WA. Sensitizing microsatellite stable colorectal cancer to immune checkpoint therapy utilizing Wnt pathway inhibition. Cancer Res. 2020;80(16_Supplement):6647–6647. [Google Scholar]
- 85.Huang T, Li F, Cheng X, Wang J, Zhang W, Zhang B, Tang Y, Li Q, Zhou C, Tu S. Wnt inhibition sensitizes PD-L1 blockade therapy by overcoming bone marrow-derived myofibroblasts-mediated immune resistance in tumors. Front Immunol. 2021;12:619209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murillo-Garzón V, Gorroño-Etxebarria I, Åkerfelt M, Puustinen MC, Sistonen L, Nees M, Carton J, Waxman J, Kypta RM. Frizzled-8 integrates Wnt-11 and transforming growth factor-β signaling in prostate cancer. Nat Commun. 2018;9(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu Z, Hunter T. Wnt-independent?-Catenin transactivation in tumor developement. Cell Cycle. 2004;3(5):569–71. [PubMed] [Google Scholar]
- 88.Grumolato L, Liu G, Haremaki T, Mungamuri SK, Mong P, Akiri G, Lopez-Bergami P, Arita A, Anouar Y, Mlodzik M. β-Catenin-independent activation of TCF1/LEF1 in human hematopoietic tumor cells through interaction with ATF2 transcription factors. PLoS Genet. 2013;9(8):e1003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang W, Li M, Ponnusamy S, Chi Y, Xue J, Fahmy B, Fan M, Miranda-Carboni GA, Narayanan R, Wu J. ABL1-dependent OTULIN phosphorylation promotes genotoxic Wnt/β-catenin activation to enhance drug resistance in breast cancers. Nat Commun. 2020;11(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, Li Y, Yu S, Qian L, Chen K, Lai H, Zhang H, Li Y, Zhang Y, Gu S. Circulating EVs long RNA-based subtyping and deconvolution enable prediction of immunogenic signatures and clinical outcome for PDAC. Mol Ther-Nucleic Acids. 2021;26:488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karami Z, Mortezaee K, Majidpoor J. Dual anti-PD-(L) 1/TGF-β inhibitors in cancer immunotherapy–updated. Int Immunopharmacol. 2023;122:110648. [DOI] [PubMed] [Google Scholar]