Abstract
Objective:
We previously reported that participants with atypical anorexia nervosa (atypical AN) had higher historical and admission weights, greater eating disorder psychopathology, but similar rates of amenorrhea and weight suppression at baseline as compared to anorexia nervosa (AN); here we compare one-year outcomes.
Method:
Weight, % median body mass index (%mBMI), Eating Disorder Examination Questionnaire (EDE-Q) scores, resumption of menses, and rehospitalizations were examined at 3, 6, and 12-months post-discharge. Analyses (N=111) compared changes in %mBMI, weight suppression, and EDE-Q scores over time between atypical AN and AN.
Results:
Among participants (48 atypical AN, 63 AN), both groups gained weight but those with atypical AN had lower gains than those with AN in %mBMI (p=.02) and greater weight suppression (p=.002) over time. EDE-Q scores improved over time, independent of weight suppression, with no significant difference between atypical AN and AN. Groups did not differ by rates of resumption of menses (80% atypical AN, 76.9% AN) or rehospitalization (29.2% atypical AN, 37.9% AN). Greater weight suppression predicted longer time to restore menses and more days rehospitalized.
Discussion:
Individuals with atypical AN regained a smaller proportion of body mass and were more weight suppressed over time. Change in eating disorder cognitions, resumption of menses, and rehospitalization rates at one-year follow-up did not differ between groups. There was no significant difference in weight suppression between groups for those who were psychologically improved at 12 months. Findings highlight limitations in our understanding of weight recovery in atypical AN. New metrics for recovery are urgently needed.
Keywords: Anorexia nervosa, atypical anorexia nervosa, course, outcome
Introduction:
Atypical anorexia nervosa (atypical AN), a diagnosis new to the 5th edition of the Diagnostic Manual of Mental Disorders (DSM-5) (American Psychiatric Association, 2013), describes those individuals who meet all criteria for anorexia nervosa (AN), have lost a significant amount of weight, but whose weight is in the normal or above normal range. The number of such patients presenting to specialized eating disorder programs has increased dramatically in recent years (Garber et al., 2019; Kennedy et al., 2017; Lebow et al., 2015; Sawyer et al., 2016; Sim et al., 2013; Whitelaw et al., 2014). Even though individuals with atypical AN weigh in the normal or above normal range, severe or rapid weight loss can lead to medical instability requiring hospitalization for medical stabilization (Garber et al., 2019).
Medical morbidity in atypical AN can be just as severe as in AN with similar rates of bradycardia, hypotension and orthostatic pulse changes on admission to medical stabilization units (Garber et al., 2019; Sawyer et al., 2016). Among participants in the Study of Refeeding to Optimize Inpatient Gains (StRONG), we previously reported that weight suppression and illness severity did not differ between atypical AN and AN (Garber et al., 2019). However, across diagnoses, greater weight suppression (amount, rate and duration of loss) was associated with more severe medical complications upon hospital admission, including more severe bradycardia and lower serum phosphorus. Rates of eating disorder-specific psychopathology and psychological distress in atypical AN can be as high or even higher than in those with AN (Garber et al., 2019; Sawyer et al., 2016). However, because weight is in the normal range, the diagnosis of atypical AN is often missed. As a result, many individuals with atypical AN often present for treatment late in the course of their illness (Lebow et al., 2015). Furthermore, in DSM-5, atypical AN is classified under the category of Other Specified Feeding or Eating Disorders (OSFED), sometimes misinterpreted to imply that it is less severe than AN (Moskowitz & Weiselberg, 2017).
Limited data on course and outcome exist in individuals with atypical AN, and there has been a call for further research on outcome and response to treatment (Crow, 2022; Golden, 2022; Hay, 2023). Two recent systematic reviews comparing atypical AN to AN have confirmed similarities with respect to psychological symptoms, medical complications, illness severity, and degree of impairment (Walsh et al., 2022, Johnson-Munguia et al., 2023). However, only 2 of 24 available studies reported on course of illness (Walsh et al., 2022). Data on outcomes in atypical AN are urgently needed to inform the discussion of whether atypical AN and AN are different clinical conditions or the same condition across the weight spectrum which should be incorporated under a single diagnosis.
In the StRONG, a recent multicenter randomized clinical trial on higher-calorie refeeding in adolescent and young adults hospitalized with medical stability secondary to AN and atypical AN, 43% of enrolled participants met criteria for atypical AN (Garber et al., 2020; Golden et al., 2021). The primary aim of the present study was to compare clinical outcomes (weight gain, psychological symptoms, resumption of menses, and rehospitalization rates) in the StRONG participants, i.e., atypical AN vs. AN, over 12-month follow-up. Secondarily, we sought to explore associations between weight change (gain and suppression) and clinical outcomes (cognitive recovery, resumption of menses, rehospitalization). We hypothesized that there would be no significant differences in absolute amount of weight gain, psychological symptom improvement, rates of resumption of menses, or rehospitalization rates between the two groups at 3, 6, and 12 months post-discharge, but that weight suppression would be associated with clinical outcomes across diagnoses.
Method:
Design:
This is a secondary analysis of data from StRONG, a multicenter randomized-controlled trial comparing higher-calorie refeeding to lower-calorie refeeding in 120 adolescent and young adults hospitalized with medical instability secondary to AN or atypical AN (Garber et al., 2020; Golden et al., 2021). As previously described, enrolled participants were randomly assigned to higher calorie refeeding (2,000 kcals/d increasing by 200 kcals/d) or lower calorie refeeding (1,400 kcals/d, increasing by 200 kcals every other day) until medically stable, at which time they were discharged to outpatient care (Garber et al., 2020; Golden et al., 2021). The study was approved by the institutional review boards of each institution and written informed consent was obtained from each participant with parental consent and assent obtained from minors. Briefly, at each site (Lucile Packard Children’s Hospital, Stanford and Benioff Children’s Hospital, San Francisco), eligible patients with AN or atypical AN who were admitted for medical instability between Feb 8th, 2016 and March 7th, 2019 were approached within 24 hours of admission for enrollment in the study. Methods are detailed elsewhere (Garber et al., 2020; Golden et al., 2021).
Participants:
Inclusion criteria were diagnosis of AN or atypical AN, age 12 to 24 years, and no hospitalization within the prior 6 months. Patients with bulimia nervosa, anorexia nervosa binge-eating/purging type, avoidant restrictive food intake disorder (ARFID), acute suicidality or a comorbid chronic medical illness such as inflammatory bowel disease, diabetes, or celiac disease, and those < 60% median body mass index (%mBMI) were excluded. A weight cutoff > 85% mBMI was used to indicate normal or above normal weight for the diagnosis of atypical AN. Sex was self-reported.
Assessment during follow-up:
This was an open follow-up trial in which participants were able to obtain treatment at their discretion, at the same site as their hospitalization or elsewhere in the community. Participants were followed for study visits at 3, 6, and 12 months after discharge from the hospital. At each visit, height, weight, and vital signs were measured. Height was measured using a wall-mounted stadiometer, and weight was measured with a digital scale, post voiding with the participant wearing only a hospital gown. Weight suppression on admission was defined as [(highest historical %mBMI - admission %mBMI)/highest historical %mBMI] x 100. Percent mBMI was calculated (current BMI/50th percentile BMI for age and sex x 100) using the Centers for Disease Control and Prevention growth charts (Centers for Disease Control (U.S.). 2003). Weight suppression at 12 months was defined as [(highest historical %mBMI – 12-month %mBMI)/highest historical %mBMI] x 100. Girls and women were asked about age of menarche, date of last menstrual period, date of prior menstrual period and current use of hormonal contraception. Resumption of menses was limited to postmenarcheal girls and women who had ≥ 3 months amenorrhea at presentation and who were not on hormonal contraception and was defined as self-reported return of two or more consecutive spontaneous menses (Golden et al., 1997). Participants completed the Eating Disorder Examination Questionnaire (EDE-Q), a measure of eating disorder psychopathology with good validity (Berg et al., 2012; Cooper et al., 1989; Fairburn & Beglin, 1994), where higher global EDE-Q scores indicate more severe eating disorder psychopathology. Participants also completed a health care utilization questionnaire, which asked about hospitalization(s) and outpatient medical and psychiatric care since the last study visit.
Statistical Methods:
The primary outcome variables were changes in %mBMI, weight suppression, and EDE-Q global scores over time. Linear mixed-effect models (LMM) were used to examine whether change in %mBMI, weight suppression, and EDE-Q global score at 3, 6, and 12-month follow-up was different in participants with atypical AN compared to AN. LMM uses less stringent assumption on missing data than complete case analyses and includes all available data in the analysis. Each model included the baseline value of the outcome (%mBMI at discharge, %mBMI at highest historical weight, or EDE-Q on admission), log of time, diagnosis (atypical AN or AN), and interaction log(time)*diagnosis in fixed effects, while accounting for correlation due to two-level nested clustering (sites and patients). The interaction of log(time) and diagnosis indicates the difference in %mBMI, weight suppression, and EDE-Q change over time between atypical AN and AN, and therefore is our main comparison of interest. Groups were compared with independent t-tests for continuous variables and the Wilcoxon rank sum test for data that were not normally distributed. Chi-square analysis or Fisher’s exact test were used as appropriate for categorical variables. Effect size estimates were calculated using Cohen’s d for continuous variables where a Cohen’s d of .2 is considered a “small” effect, .5 is considered a “moderate” effect and ≥ .8 is considered a “large” effect. Cohen’s h was used for proportions (Cohen, 1992).
Secondary analyses examined associations between weight gain/suppression and clinical outcomes (EDE-Q score and resumption of menses). First, groups meeting recovery thresholds were compared by independent t-tests for continuous variables and the Wilcoxon rank sum test for data that were not normally distributed; chi-square analysis or Fisher’s exact test were used as appropriate for categorical variables. Second, separate multivariable linear regression models examined the association of weight suppression (independent variable) with those clinical outcomes (EDE-Q scores, time to resumption of menses, and days spent rehospitalized) as dependent variables), adjusting for %mBMI on admission. Analyses were performed using SPSS for Windows (Version 27), SAS 9.4, and STATA 16.1.
Results:
Of the 111 participants who completed the treatment protocol, 48 (43.2%) had atypical AN and 63 (56.8%) had AN. Demographic data, weight and menstrual history, and EDE-Q scores on admission have been previously published (Garber et al., 2019) and are shown in Table 1. Participants with atypical AN and AN did not differ by any key characteristics other than significantly higher historical and presentation weight and higher EDE-Q global scores in participants with atypical AN. Mean age was 16.3 ± 2.6y for atypical AN and 16.7 ± 2.5y for AN and 10 of the participants were male, 5 in each group. Participants presented for hospitalization after losing approximately 20% body mass and approximately 2/3 of the post-menarcheal girls and women had secondary amenorrhea with no significant differences by diagnosis (Garber et al., 2019). On admission, 44% of those with atypical AN exceeded the 95%mBMI, the weight recovery threshold frequently used for AN (Agras et al., 2014; Le Grange et al., 2016; Lock et al., 2010; Loeb et al., 2007; Madden, Miskovic-Wheatley, Wallis, Kohn, Hay, et al., 2015).
Table 1:
Demographics, weight and menstrual history on admission in atypical anorexia nervosa (atypical AN) vs. anorexia nervosa (AN)
| Atypical AN (N=48) |
AN (N=63) |
p-value | |
|---|---|---|---|
| Demographics | |||
|
| |||
| Age (yrs.), mean (SD) | 16.3 (2.6) | 16.7 (2.5) | .36 |
| Female, n (%) | 43 (89.6%) | 58 (92.0%) | .65 |
| Race, n (%) | |||
| White | 37 (77.1%) | 53 (84.1%) | .37 |
| Black/African American | 0 (0.0%) | 1 (1.6%) | |
| Asian | 6 (12.5%) | 7 (11.1%) | |
| Multiple/Other | 5 (10.4%) | 2 (3.2%) | |
| Ethnicity, n (%) | |||
| Non-Hispanic | 35 (72.9%) | 52 (82.5%) | .26 |
| Hispanic or Latino | 13 (27.0%) | 11 (27.1%) | |
| Weight History, mean (SD) | |||
|
| |||
| Highest historical weight (kg) | 66.8 (15.0) | 54.3 (9.7) | <.001 |
| Highest historical %mBMI* | 129.4 (29.1) | 104.7 (16.0) | <.001 |
| Weight loss (kg) | 14.6 (12.1) | 12.7 (6.8) | .30 |
| % weight loss (%) | 20 (10.5) | 22.3 (8.7) | .21 |
| Rate of weight loss (kg/mo) | 1.65 (1.3) | 1.62 (1.4) | .92 |
| Admission weight (kg) | 52.2 (7.3) | 41.6 (5.3) | <.001 |
| %mBMI on admission | 95.4% (9.0) | 76.9% (5.6) | <.001 |
| Menstrual History | |||
|
| |||
| Amenorrhea ≥ 3m. n (%) | 20 (62.5%) | 33 (68.8%) | .63 |
| Duration of amenorrhea (mo), mean (SD) | 5.18 (7.22) [N=41] |
6.34 (6.7) [N=51] |
.42 |
%mBMI = percent median BMI
Weight change (gain and suppression):
In Table 2, both groups gained weight and there was no difference in absolute weight gained (in kilograms) between individuals with atypical AN and AN (4.7 ± 7.6 vs. 6.1 ± 5.7 kg, mean difference 1.4 kg, 95% CI −1.3 to 4.0, p=.15). However, as shown in Figure 1, changes in %mBMI over time were significantly smaller in participants with atypical AN as compared to those with AN (p=.02). Total change in %mBMI from discharge to 12 months was 5.8% in the atypical AN group (98.3 ± 8.9 to 103.9 ± 10.5 %mBMI) vs. 9.2% (82.3 ± 5.3 to 91.7 ± 10.9 %mBMI) in the AN group. As shown in Figure 2, participants with atypical AN remained significantly more weight suppressed over time as compared to those with AN (p=.002). Weight suppression at 12 months was 16.6 ± 16.9% in those with atypical AN vs. 11.1 ± 12.7% in those with AN, mean difference −5.5, 95% CI −11.7 to .7, p=.08, Cohen’s d= −.38, indicating a small-to-moderate effect.
Table 2:
Weight, %mBMI, EDE-Q global scores, resumption of menses and rehospitalizations by diagnosis at study timepoints
| Atypical AN (N=48) |
AN (N=63) |
Mean difference 95% CI | p-value | Effect size | |
|---|---|---|---|---|---|
| Discharge weight (kg) | 54.3 (7.7) | 44.5 (5.3) | −9.8, −12.4 to −7.2 | <.001 | −1.52 |
| 12 mo. weight (kg) | 59.4 (8.2) | 50.8 (7.1) | −8.6, −11.7 to −5.6 | <.001 | −1.14 |
| Weight gain over follow-up (kg) | 4.7 (7.6) | 6.1 (5.7) | 1.4, −1.3 to 4.0 | .15 | .21 |
| %mBMI gain over follow-up | 5.8 (11.8) | 9.2 (10.1) | 3.4, −1.0 to 7.8 | .13 | .32 |
| Weight suppression 12 mo. (%) | 16.6 (16.9) | 11.1 (12.7) | −5.5, −11.7 to .7 | .08 | −.38 |
| Average %mBMI* over time | |||||
| Discharge | 98.3 (8.9) | 82.3 (5.3) | −16.1, −19.0 to −13.2 | <.001 | −2.26 |
| 3 mo. | 103.0 (9.1) | 89.5 (9.4) | −13.5, −17.3 to −9.8 | <.001 | −1.46 |
| 6 mo. | 104.5 (11.6) | 90.7 (10.0) | −13.8, −18.2 to −9.4 | <.001 | −1.29 |
| 12 mo. | 103.9 (10.5) | 91.7 (10.9) | −12.2, −16.6 to −7.9 | <.001 | −1.14 |
| Change in %mBMI over time | .02 ** | ||||
| EDE-Q global score ***, mean (SD) | |||||
| Admission | 3.80 (1.66) [N=41] |
3.06 (1.65) [N=56] |
−.74, −1.4 to −.1 | .03 | −.45 |
| 3 mo. | 2.0 (1.7) [N=34] |
2.6 (1.7) [N=43] |
.55, −.2 to 1.3 | .16 | .33 |
| 6 mo. | 1.7 (1.5) [N=31] |
2.1 (1.7) [N=36] |
.35, −.4 to 1.1 | .38 | .22 |
| 12 mo. | 1.9 (1.6) [N=33] |
2.7 (1.5) [N=44] |
.76, .1 to 1.5 | .04 | .49 |
| Change in EDE-Q over time | .41 ** | ||||
| Resumption of Menses | |||||
| Proportion with resumption of menses | 16/20 (80%) | 20/26 (77%) | .80 | .07 | |
| Rehospitalizations | |||||
| Proportion rehospitalized over 12 mo. | 14/48 (29.2%) | 22/58 (37.9%) | .41 | .19 | |
| Days spent rehospitalized | 7.7 (16.0) | 3.0 (6.7) | 4.7, .1 to 9.3 | .06 | .37 |
%mBMI = percent median BMI
p-value from linear mixed effect models (LMM)
EDE-Q = Eating Disorder Examination Questionnaire
Estimate of effect size: Cohen’s d =.2 (small effect); d =.5 (moderate effect); d ≥.8 (large effect)
Figure 1. Change in percent median BMI over time. Atypical anorexia nervosa vs. anorexia nervosa.
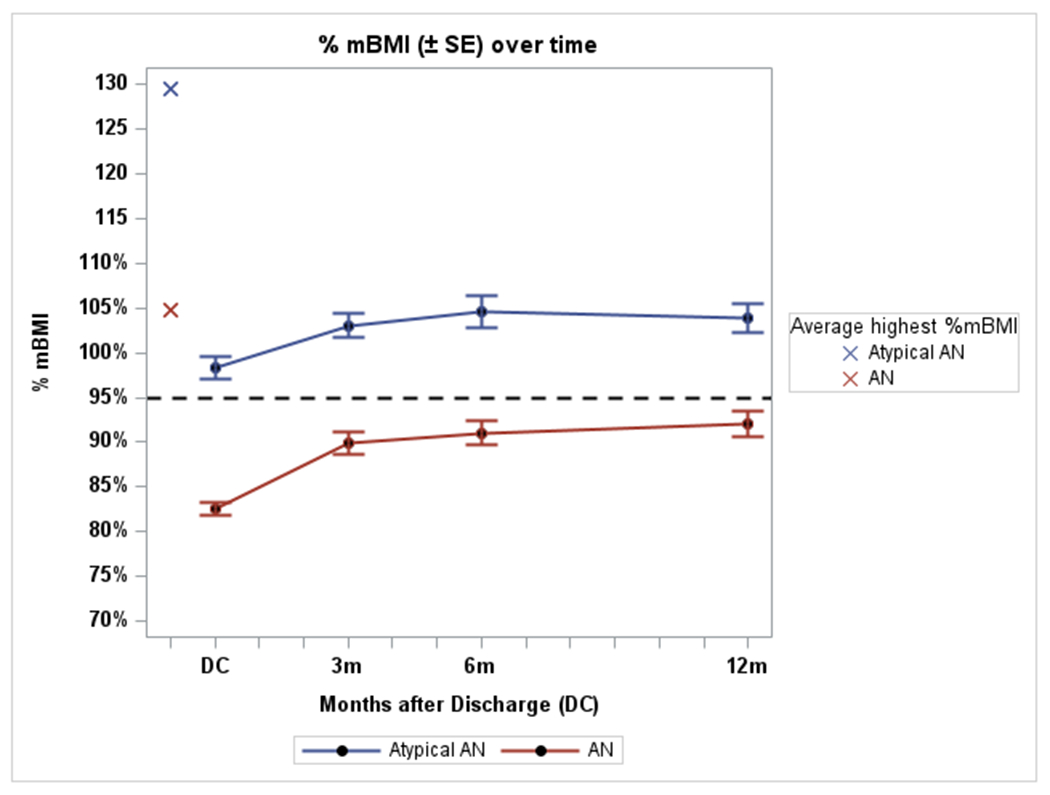
Atypical AN = atypical anorexia nervosa
AN = anorexia nervosa
%mBMI = percent median body mass index
----- Dashed line represents 95% mBMI
Figure 2. Change in weight suppression over time. Atypical anorexia nervosa vs anorexia nervosa.

Atypical AN = atypical anorexia nervosa
AN = anorexia nervosa
Weight suppression defined as [(highest historical %mBMI – 12-month %mBMI)/highest historical %mBMI] x 100.
ED Psychopathology:
Data on cognitive recovery defined as EDE-Q global score within 1 SD of community norms (1.59 ± 1.32) were available for 77 participants at 12 months. EDE-Q global scores at admission, 3, 6, and 12 months are shown in Figure 3 and Table 2. In both participants with atypical AN and AN, EDE-Q global scores improved over time, with no significant differences in the change over time between individuals with atypical AN and those with AN (p=.41). In the atypical AN group, 72.7% (n=24/33) had an EDE-Q global score within 1 SD of community norms at 12 months compared with 52.3% (n=23/44) of those with AN, p=.07. Mean EDE-Q global scores at 12 months were significantly lower in those with atypical AN compared to AN (1.9 ± 1.6 vs. 2.7 ± 1.5, mean difference .76, 95% CI .1 to 1.5, p=.04, Cohen’s d = .49), indicating a moderate effect.
Figure 3. Eating Disorder Examination Questionnaire (EDE-Q) scores (mean ± 1SD) at admission, 3m, 6m, and 12m. Atypical AN, AN and a normative population.
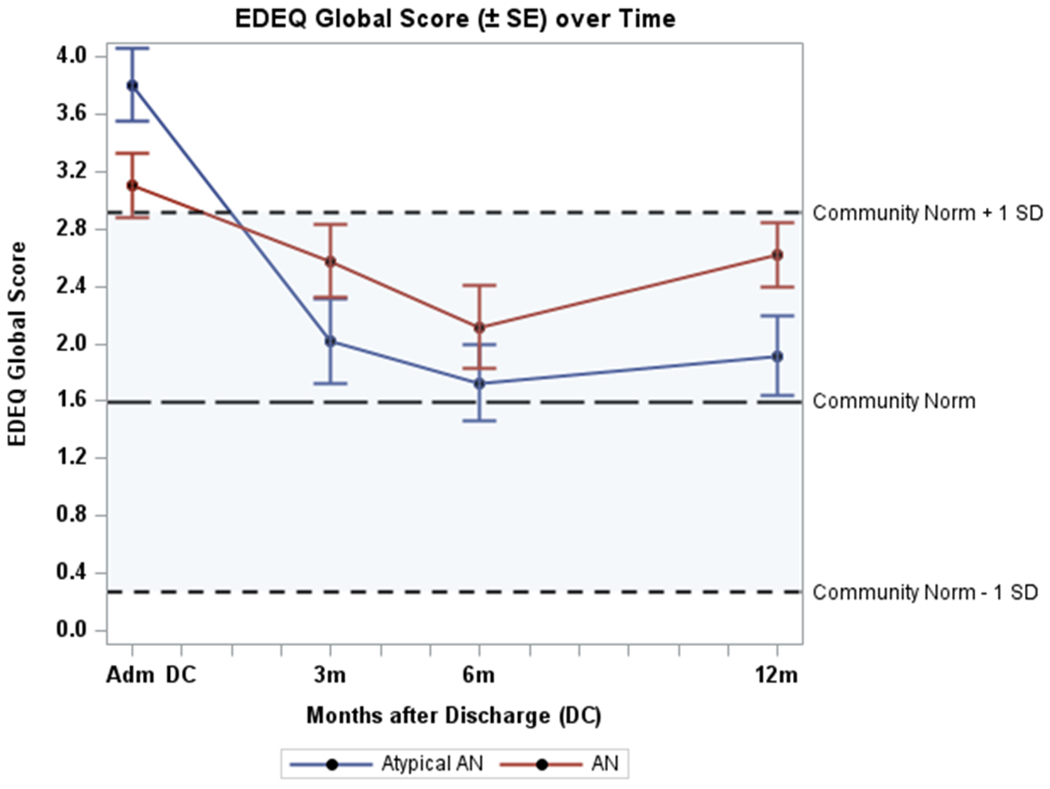
Atypical AN = atypical anorexia nervosa
AN = anorexia nervosa
Comparing participants who met cognitive recovery (n = 47) to those who did not (n = 30), there was no significant group difference in %mBMI gain during follow-up (from discharge to 12 months) (7.3 ± 12.1 vs. 7.7 ± 10.3, mean difference −.3, 95% CI −5.8 to 5.1, p =.91), or weight suppression at discharge (20.7 ± 11.6% vs. 22.1 ± 11.7%, mean difference 1.36, 95% CI −4.1 to 6.8, p=.62) and 12 months (14.1 ± 2.4 vs. 15.1 ± 2.7, mean difference 1.0, 95% CI −6.3 to 8.4, p=.77). Weight suppression at 12 months was not associated with EDE-Q global scores at 12 months (p=.45). For individuals with atypical AN and EDE-Q global scores within community norms, absolute difference between weight at 12 months and highest historical weight was 8.9 ± 13.8 kg, compared with 4.0 ± 7.9 kg for AN, mean difference −4.9 kg, 95% CI −12.0 to 2.1, p=.16. At 12 months, both groups weighed less than their highest historical weight with no significant difference between groups.
Resumption of menses:
Data on resumption of menses were available for 46 post-menarcheal girls or women who were not on hormonal contraception and had been amenorrheic for at least 3 months, 20 with atypical AN and 26 with AN. There was no significant difference in the proportion of participants who resumed menses by diagnostic group: 16/20 participants with atypical AN (80.0%) resumed menses within the year of follow-up compared to 20/26 participants with AN (77.0%), p =.80. Time to resumption of menses after the initial admission did not differ by diagnostic group (atypical AN: 4.3 ± 5.1 months; AN: 4.1 ± 4.0 months, mean difference .4, 95% CI −1.9 to 2.8, p = .88, Cohen’s d =.11).
Among the 39 participants who had resumption of menses during 12-month follow-up, there was no significant difference in gain in %mBMI from discharge to 12 months compared with those who did not resume menses (13.3 ± 7.5 vs. 6.8 ± 9.8, mean difference 6.5, 95% CI −.43 to 13.4, p=.07, Cohen’s d = −.05). Those who resumed menses tended to be less weight suppressed at 12 months than those who did not, however not significantly (11.0 ± 13.7% vs. 18.9 ± 9.7%, mean difference −7.9, 95% CI −19.7 to 3.8, p=.13, Cohen’s d = −.60). In multivariate analysis, greater weight suppression at 12 months was significantly associated with longer time to restore menses regardless of diagnosis: for every 10% greater weight suppression at 12 months, resumption of menses occurred 1.2 months later [B = 0.12 (95% CI .01 to .24), p=.04].
Rehospitalization rates:
There were no differences in the proportion rehospitalized in each group over the 12-month follow-up period (atypical AN: 29.2%, n=14/48; AN: 37.9%, n=22/58; p=.41). Mean duration of hospital readmission in atypical AN was 7.7 ± 16.0 compared to 3.0 ± 6.7 days in AN, mean difference 4.7, 95% CI .1 to 9.3, p=.06, Cohen’s d = .37).
Compared to patients who were not rehospitalized (n = 70), those who were (n = 36) demonstrated greater weight suppression at 12 months (18.4 ± 16.7 vs. 11.1 ± 13.4%, mean difference 7.3, 95% CI 1.1 to 13.5, p=.02, Cohen’s d =.50), despite no differences in %mBMI gain (8.1 ± 11.1 vs. 7.0 ± 10.8, mean difference −1.1, 95% CI −5.8 to 3.6, p=.65, Cohen’s d = −.10). Greater weight suppression at 12 months was also associated with longer rehospitalizations: every 10% greater weight suppression was associated with 2.3 additional days in hospital during the one-year follow-up period [B = 0.23 (95% CI −0.50 to 0.40), p=.01].
Discussion:
We found that participants with atypical AN enrolled in the StRONG trial gained significantly less weight and remained more weight suppressed over the course of one-year follow-up as compared to those with AN. Despite lesser weight gain, there was no difference between groups in the proportion who resumed menses, achieved normal EDE-Q scores, or were rehospitalized at 12 months. However, these findings do not support the assertion that weight gain is not necessary for recovery from atypical AN. On the contrary, we found significant associations between weight suppression, persistent amenorrhea, and rehospitalization regardless of diagnosis. This comports with our prior findings (Garber et al., 2019) in this trial cohort showing that weight suppression predicted illness severity upon hospital admission, including bradycardia and lower serum phosphorus.
An often-used definition of remission in adolescent AN (weight restoration to at least 95% of mBMI plus an EDE score within 1 SD of community norms) (Agras et al., 2014; Le Grange et al., 2016; Le Grange et al., 2021; Lock et al., 2010; Loeb et al., 2007; Madden, Miskovic-Wheatley, Wallis, Kohn, Lock, et al., 2015) does not apply to patients with atypical AN. In our study, nearly half of the participants with atypical AN already met weight criteria for recovery on admission to the hospital. We found that weight restoration was associated with resumption of menses in both diagnoses. The importance of full weight restoration is clear in patients with AN, where greater historical BMI is positively correlated with a higher BMI at resumption of menses (Berner et al., 2017). The need for full weight restoration in individuals with atypical AN is not known. In a prior study, we reported that patients with eating disorders and historical overweight status needed to be at a higher %mBMI than those who were not previously overweight in order to resume menses (Seetharaman et al., 2016). Rastogi et al (Rastogi et al., 2020) examined resumption of menses as a marker of recovery in previously overweight patients with eating disorders and found that resumption of menses occurred at a weight significantly lower than premorbid maximum weight, concluding that restoration to highest premorbid weight was not necessary for resumption of menses. Consistent with those findings, participants with atypical AN in our trial resumed menses while remaining 14% below their highest historical weight. However, it is important to note that the AN group who resumed menses was also significantly weight suppressed (~9% below historical %mBMI). In addition, we showed that for every 10% increase in weight suppression, resumption of menses was delayed by 1.2 months. Thus, while menses can resume below highest historical (pre-illness) weights, these findings suggest that weight suppression may prolong the hypogonadal state.
Global EDE-Q scores were notably different between groups. We previously reported that EDE-Q scores in this study cohort were significantly worse at hospital admission in those with atypical AN than in those with AN (Garber et al., 2019), consistent with an earlier study by Sawyer et al. (Sawyer et al., 2016). Here we report that, despite being more weight suppressed, patients with atypical AN had improved (lower) EDE-Q scores at 12 months, indicating less eating disorder psychopathology than those with AN. We did not find evidence that cognitive recovery was associated with weight suppression. In fact, individuals with atypical AN and EDE-Q scores in the normative range remained nearly 17% below their highest historical weight at 12 months. Again, it must be noted that those with AN who met the EDE-Q recovery threshold were nearly 12% weight suppressed at 12 months. The finding that many individuals with atypical AN met cognitive recovery despite significant weight suppression is consistent with a case series demonstrating improved depressive symptoms and eating disorder psychopathology with almost no weight gain after the hospitalization period (Hughes et al., 2017). However, the EDE-Q was developed for patients with AN and may not capture important facets of ED psychopathology in individuals with atypical AN, such that it cannot necessarily be concluded that cognitive remission can be achieved with little weight gain. Studies are urgently needed in individuals with atypical AN examining broader psychopathology relevant to higher weight individuals (e.g., body acceptance, weight bias internalization).
Our finding that participants with atypical AN tended to be rehospitalized for shorter stays than those with AN did not reach statistical significance, but requires further investigation. Our finding that weight suppression was significantly associated with more time spent readmitted to hospital in both diagnoses underscores the importance of weight gain for recovery.
Individuals with atypical AN comprise a considerable portion of adolescents and young adults with eating disorders. In community samples across the globe, both point prevalence and lifetime prevalence of atypical AN is higher than that of AN (Harrop et al., 2021; Stice et al., 2013). Eating disorders in individuals of higher weight are under recognized and under treated (Ralph et al., 2022). True estimates of population prevalence are probably even higher (Crow, 2022). In one national sample of Canadian youth, atypical AN accounted for half of all eating disorder hospitalizations during the COVID-19 pandemic (Agostino et al., 2021).
New benchmarks of recovery for individuals with atypical AN must be highly individualized and less reliant on weight. We have shown that the variability in historical weights among individuals with atypical AN is double that of AN (Garber et al., 2019), consistent with Sawyer et al. (Sawyer et al., 2016). This suggests that recovery weights in atypical AN will be even more variable than AN, which already vary widely (Golden et al., 2008). Other potential benchmarks could include measures of metabolism such as resting energy expenditure (Schebendach et al., 1997; Sterling et al., 2009), biological markers of recovery e.g., serum leptin (Misra et al., 2004) and triiodothyronine (T3) levels (Aschettino-Manevitz et al., 2012; Kiyohara et al., 1989), hormonal indicators of hypothalamic-pituitary-gonadal (HPG) axis recovery for all genders, such as luteinizing hormone, follicle stimulating hormone, estradiol levels in girls and women (Golden et al., 1997) and testosterone levels in boys and men (Wabitsch et al., 2001), as well as measures of psychological recovery that have been tested and validated in this particular patient population.
Strengths of our study include the prospective, longitudinal, multicenter design, relatively large sample size with a standardized method of data collection, use of mixed effect models, and 12-month follow-up period. A major limitation is that this trial cohort only included 48 participants with atypical AN, which limited us to exploratory analyses of outcomes. Larger studies are urgently needed to power rigorous examination of clinical outcomes in atypical AN. Other limitations include the open follow-up period during which participants engaged in various therapies. Furthermore, the timeline and frequency of follow-up visits was not designed to assess resumption of menses therefore we do not have weight at resumption of menses but rather the date of last menstrual period and the weight at the closest study point. While resumption of menses can be used as a marker of recovery in girls and women, there is no similar marker of recovery for boys and men and as is common in studies of individuals with eating disorders, the proportion of males in the study was small. In addition, while sometimes used as a marker of recovery, the EDE-Q is a measure of eating disorder psychopathology that depends on patient report. Minimization or even denial of symptoms is common, so it is not surprising that some participants reported minimal levels of ED psychopathology (i.e., EDE-Q score within community norms) on admission. Another limitation is that this study focused on a subgroup of individuals who required hospitalization and may not reflect outcome of individuals treated exclusively as outpatients. Given that a relatively smaller proportion of participants with atypical AN may be hospitalized compared to those with AN because of their non-underweight status, it is possible that this study is comparing a more severe subgroup of patients with atypical AN compared to AN. Finally, one-year follow-up may be too short a timeframe to assess outcome.
In summary, individuals with atypical AN regained less weight over time and remained more weight suppressed during recovery as compared to those with AN. However, there was no difference in the proportion who resumed menses, recovered cognitively or were readmitted to hospital. Across both diagnoses, weight suppression was a significant predictor of prolonged amenorrhea and rehospitalization. These findings underscore the limitations of our understanding of weight recovery in atypical AN. There is a pressing need to establish objective markers of physical and cognitive recovery to guide clinical decision making in this diverse patient population.
Public Significance Statement:
Little is known about outcome in atypical anorexia nervosa. We examined recovery metrics in young people with atypical anorexia nervosa and anorexia nervosa one year after medical hospitalization. Individuals with atypical anorexia nervosa showed slower weight gain and remained further from their pre-illness weight. There were no differences in rates of psychological recovery, resumption of menses, or rehospitalization. New metrics are needed to assess recovery in atypical anorexia nervosa.
Funding information:
Funded by the National Institutes of Health, Grant Number: RO1HD082166
Footnotes
Conflict of interest: No
Data Availability:
Study investigators agree to abide by the principles for sharing research resources as described by the NIH in “Principles and Guidelines for Recipients of NIH Research Grants and Contracts on Obtaining and Disseminating Biomedical Research Programs.”
References
- Agostino H, Burstein B, Moubayed D, Taddeo D, Grady R, Vyver E, Dimitropoulos G, Dominic A, & Coelho JS (2021). Trends in the Incidence of New-Onset Anorexia Nervosa and Atypical Anorexia Nervosa Among Youth During the COVID-19 Pandemic in Canada. JAMA Network Open, 4(12), e2137395. 10.1001/jamanetworkopen.2021.37395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agras WS, Lock J, Brandt H, Bryson SW, Dodge E, Halmi KA, Jo B, Johnson C, Kaye W, Wilfley D, & Woodside B (2014). Comparison of 2 family therapies for adolescent anorexia nervosa: a randomized parallel trial. JAMA Psychiatry, 71(11), 1279–1286. 10.1001/jamapsychiatry.2014.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. American Psychiatric Association Publishing. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Aschettino-Manevitz DL, Ornstein RM, Meyer Sterling W, Kohn N, & Fisher M (2012). Triiodothyronine (T3) and metabolic rate in adolescents with eating disorders: Is there a correlation? Eating and Weight Disorders, 17(4), e252–258. 10.3275/8756 [DOI] [PubMed] [Google Scholar]
- Berg KC, Peterson CB, Frazier P, & Crow SJ (2012). Psychometric evaluation of the eating disorder examination and eating disorder examination-questionnaire: a systematic review of the literature. International Journal of Eating Disorders, 45(3), 428–438. 10.1002/eat.20931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner LA, Feig EH, Witt AA, & Lowe MR (2017). Menstrual cycle loss and resumption among patients with anorexia nervosa spectrum eating disorders: Is relative or absolute weight more influential? International Journal of Eating Disorders, 50(4), 442–446. 10.1002/eat.22697 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (U.S.) (2003). CDC, National Center for Health Statistics (NCHS, National Health and Nutrition Examination Survey Analytic Guidelines. Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf.
- Cohen J (1992). A power primer. Psychological Bulletin, 112(1), 155–159. 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Cooper Z, Cooper PJ, & Fairburn CG (1989). The validity of the eating disorder examination and its subscales. British Journal of Psychiatry, 154, 807–812. 10.1192/bjp.154.6.807 [DOI] [PubMed] [Google Scholar]
- Crow SJ (2023). Atypical anorexia nervosa: In need of further study. International Journal ofEating Disorders, 56(4), 824–825. 10.1002/eat.23889 [DOI] [PubMed] [Google Scholar]
- Fairburn CG, & Beglin SJ (1994). Assessment of eating disorders: interview or self-report questionnaire? InternationalJournal of Eating Disorders, 16(4), 363–370. https://www.ncbi.nlm.nih.gov/pubmed/7866415 [PubMed] [Google Scholar]
- Garber AK, Cheng J, Accurso EC, Adams SH, Buckelew SM, Kapphahn CJ, Kreiter A, Le Grange D, Machen VI, Moscicki AB, Saffran K, Sy AF, Wilson L, & Golden NH (2019). Weight Loss and Illness Severity in Adolescents With Atypical Anorexia Nervosa. Pediatrics, 144(6), e20192339. 10.1542/peds.2019-2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber AK, Cheng J, Accurso EC, Adams SH, Buckelew SM, Kapphahn CJ, Kreiter A, Le Grange D, Machen VI, Moscicki AB, Sy A, Wilson L, & Golden NH (2021). Short-term Outcomes of the Study of Refeeding to Optimize Inpatient Gains for Patients With Anorexia Nervosa: A Multicenter Randomized Clinical Trial. JAMA Pediatrics, 175(1), 19–27. 10.1001/jamapediatrics.2020.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden NH (2022). Atypical Anorexia Nervosa is not atypical at all! Commentary on Walsh et al. (2022). International Journal of Eating Disorders, 56(4), 826–827. 10.1002/eat.23871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden NH, Cheng J, Kapphahn CJ, Buckelew SM, Machen VI, Kreiter A, Accurso EC, Adams SH, Le Grange D, Moscicki AB, Sy AF, Wilson L, & Garber AK (2021). Higher-Calorie Refeeding in Anorexia Nervosa: 1-Year Outcomes From a Randomized Controlled Trial. Pediatrics. 147(4), e2020037135. 10.1542/peds.2020-037135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden NH, Jacobson MS, Schebendach J, Solanto MV, Hertz SM, & Shenker IR (1997). Resumption of menses in anorexia nervosa. Archives of Pediatrics & Adolescent Medicine, 151(1), 16–21. https://www.ncbi.nlm.nih.gov/pubmed/9006523 [DOI] [PubMed] [Google Scholar]
- Golden NH, Jacobson MS, Sterling WM, & Hertz S (2008). Treatment goal weight in adolescents with anorexia nervosa: use of BMI percentiles. International Journal of Eating Disorders, 41(4), 301–306. 10.1002/eat.20503 [DOI] [PubMed] [Google Scholar]
- Harrop EN, Mensinger JL, Moore M, & Lindhorst T (2021). Restrictive eating disorders in higher weight persons: A systematic review of atypical anorexia nervosa prevalence and consecutive admission literature. International Journal of Eating Disorders, 54(8), 1328–1357. 10.1002/eat.23519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay P (2023). Disentangling eating disorder diagnostic schemes. Commentary on Walsh et al., “A systematic review comparing atypical anorexia nervosa and anorexia nervosa”. International Journal of Eating Disorders. 10.1002/eat.23895 [DOI] [PubMed] [Google Scholar]
- Hughes EK, Le Grange D, Court A, & Sawyer SM (2017). A case series of family-based treatment for adolescents with atypical anorexia nervosa. International Journal of Eating Disorders, 50(4), 424–432. 10.1002/eat.22662 [DOI] [PubMed] [Google Scholar]
- Johnson-Munguia, Negi S, Chen Y, Thomeczek ML, & Forbush KT (2023). Eating disorder psychopathology, psychiatric impairment, and symptom frequency of atypical anorexia nervosa versus anorexia nervosa: A systematic review and meta-analysis. International Journal of Eating Disorders, 1–19. 10.1002/eat.23989 [DOI] [PubMed] [Google Scholar]
- Kennedy GA, Forman SF, Woods ER, Hergenroeder AC, Mammel KA, Fisher MM, Ornstein RM, Callahan ST, Golden NH, Kapphahn CJ, Garber AK, Rome ES, & Richmond TK (2017). History of Overweight/Obesity as Predictor of Care Received at 1-year Follow-Up in Adolescents With Anorexia Nervosa or Atypical Anorexia Nervosa. Journal of Adolescent Health : official publication of the Society for Adolescent Medicine, 60(6), 674–679. 10.1016/j.jadohealth.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara K, Tamai H, Takaichi Y, Nakagawa T, & Kumagai LF (1989). Decreased thyroidal triiodothyronine secretion in patients with anorexia nervosa: influence of weight recovery. American Journal of Clinical Nutrition, 50(4), 767–772. 10.1093/ajcn/50.4.767 [DOI] [PubMed] [Google Scholar]
- Le Grange D, Hughes EK, Court A, Yeo M, Crosby RD, & Sawyer SM (2016). Randomized Clinical Trial of Parent-Focused Treatment and Family-Based Treatment for Adolescent Anorexia Nervosa. Journal of the American Academy of Child and Adolescent Psychiatry, 55(8), 683–692. 10.1016/j.jaac.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Le Grange D, Pradel M, Pogos D, Yeo M, Hughes EK, Tompson A, Court A, Crosby RD, & Sawyer SM (2021). Family-based treatment for adolescent anorexia nervosa: Outcomes of a stepped-care model. International Journal of Eating Disorders, 54(11), 1989–1997. 10.1002/eat.23629 [DOI] [PubMed] [Google Scholar]
- Lebow J, Sim LA, & Kransdorf LN (2015). Prevalence of a history of overweight and obesity in adolescents with restrictive eating disorders. Journal of Adolescent Health, 56(1), 19–24. 10.1016/j.jadohealth.2014.06.005 [DOI] [PubMed] [Google Scholar]
- Lock J, Le Grange D, Agras WS, Moye A, Bryson SW, & Jo B (2010). Randomized clinical trial comparing family-based treatment with adolescent-focused individual therapy for adolescents with anorexia nervosa. Archives of General Psychiatry, 67(10), 1025–1032. 10.1001/archgenpsychiatry.2010.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb KL, Walsh BT, Lock J, le Grange D, Jones J, Marcus S, Weaver J, & Dobrow I (2007). Open trial of family-based treatment for full and partial anorexia nervosa in adolescence: evidence of successful dissemination. Journal of the American Academy of Child and Adolescent Psychiatry, 46(7), 792–800. 10.1097/chi.0b013e318058a98e [DOI] [PubMed] [Google Scholar]
- Madden S, Miskovic-Wheatley J, Wallis A, Kohn M, Hay P, & Touyz S (2015). Early weight gain in family-based treatment predicts greater weight gain and remission at the end of treatment and remission at 12-month follow-up in adolescent anorexia nervosa. International Journal of Eating Disorders, 48(7), 919–922. 10.1002/eat.22414 [DOI] [PubMed] [Google Scholar]
- Madden S, Miskovic-Wheatley J, Wallis A, Kohn M, Lock J, Le Grange D, Jo B, Clarke S, Rhodes P, Hay P, & Touyz S (2015). A randomized controlled trial of in-patient treatment for anorexia nervosa in medically unstable adolescents. Psychological Medicine, 45(2), 415–427. 10.1017/S0033291714001573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Miller KK, Almazan C, Ramaswamy K, Aggarwal A, Herzog DB, Neubauer G, Breu J, & Klibanski A (2004). Hormonal and body composition predictors of soluble leptin receptor, leptin, and free leptin index in adolescent girls with anorexia nervosa and controls and relation to insulin sensitivity. The Journal of Clinical Endocrinology and Metabolism, 89(7), 3486–3495. 10.1210/jc.2003-032251 [DOI] [PubMed] [Google Scholar]
- Moskowitz L, & Weiselberg E (2017). Anorexia Nervosa/Atypical Anorexia Nervosa. Current Problems in Pediatric and Adolescent Health Care, 47(4), 70–84. 10.1016/j.cppeds.2017.02.003 [DOI] [PubMed] [Google Scholar]
- Ralph AF, Brennan L, Byrne S, Caldwell B, Farmer J, Hart LM, Heruc GA, Maguire S, Piya MK, Quin J, Trobe SK, Wallis A, Williams-Tchen AJ, & Hay P (2022). Management of eating disorders for people with higher weight: clinical practice guideline. Journal of Eating Disorders, 10(1), 121. 10.1186/s40337-022-00622-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi R, Sieke EH, Nahra A, Sabik J, & Rome ES (2020). Return of Menses in Previously Overweight Patients with Eating Disorders. Journal of Pediatric and Adolescent Gynecology, 33(2), 133–138. 10.1016/j.jpag.2019.11.002 [DOI] [PubMed] [Google Scholar]
- Sawyer SM, Whitelaw M, Le Grange D, Yeo M, & Hughes EK (2016). Physical and Psychological Morbidity in Adolescents With Atypical Anorexia Nervosa. Pediatrics, 137(4), e20154080. 10.1542/peds.2015-4080 [DOI] [PubMed] [Google Scholar]
- Schebendach JE, Golden NH, Jacobson MS, Hertz S, & Shenker IR (1997). The metabolic responses to starvation and refeeding in adolescents with anorexia nervosa. Annals of the New York Academy of Sciences, 817, 110–119. [DOI] [PubMed] [Google Scholar]
- Seetharaman S, Golden NH, Halpern-Felsher B, Peebles R, Payne A, & Carlson JL (2017). Effect of a Prior History of Overweight on Return of Menses in Adolescents With Eating Disorders. Journal of Adolescent Health, 60(4), 469–471.. 10.1016/j.jadohealth.2016.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LA, Lebow J, & Billings M (2013). Eating disorders in adolescents with a history of obesity. Pediatrics, 132(4), e1026–1030. 10.1542/peds.2012-3940 [DOI] [PubMed] [Google Scholar]
- Sterling WM, Golden NH, Jacobson MS, Ornstein RM, & Hertz SM (2009). Metabolic assessment of menstruating and nonmenstruating normal weight adolescents. The International Journal of Eating Disorders, 42(7), 658–663. 10.1002/eat.20604 [DOI] [PubMed] [Google Scholar]
- Stice E, Marti CN, & Rohde P (2013). Prevalence, incidence, impairment, and course of the proposed DSM-5 eating disorder diagnoses in an 8-year prospective community study of young women. Journal of Abnormal Psychology, 122(2), 445–457. 10.1037/a0030679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabitsch M, Ballauff A, Holl R, Blum WF, Heinze E, Remschmidt H, & Hebebrand J (2001). Serum leptin, gonadotropin, and testosterone concentrations in male patients with anorexia nervosa during weight gain. Journal of Clinical Endocrinology and Metabolism, 86(7), 2982–2988. 10.1210/jcem.86.7.7685 [DOI] [PubMed] [Google Scholar]
- Walsh BT, Hagan KE, & Lockwood C (2022). A systematic review comparing atypical anorexia nervosa and anorexia nervosa. International Journal of Eating Disorders, 56(4), 798–820. 10.1002/eat.23856 [DOI] [PubMed] [Google Scholar]
- Whitelaw M, Gilbertson H, Lee KJ, & Sawyer SM (2014). Restrictive eating disorders among adolescent inpatients. Pediatrics, 134(3), e758–764. 10.1542/peds.2014-0070 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Study investigators agree to abide by the principles for sharing research resources as described by the NIH in “Principles and Guidelines for Recipients of NIH Research Grants and Contracts on Obtaining and Disseminating Biomedical Research Programs.”


