Abstract
Introduction:
Objectively measured physical activity (PA) data were collected in the accelerometry sub-study of the UK Biobank. UK Biobank also contains information about MS diagnosis at the time of and after PA collection. This study aims to: 1) Quantify the difference in PA between prevalent MS cases and matched healthy controls; 2) Evaluate the predictive performance of objective PA measures for incident MS cases.
Methods:
The first analysis compared eight accelerometer-derived PA summaries between MS patients (N=316) and matched controls (30 controls for each MS case). The second analysis focused on predicting time to MS diagnosis among participants who were not diagnosed with MS. A total of 19 predictors including eight measures of objective PA were compared using Cox proportional hazards models (number of events=47; 585,900 person-years of follow-up).
Results:
In the prevalent MS study, the difference between MS cases and matched controls was statistically significant for all PA summaries (p<0.001). In the incident MS study, the most predictive variable of progression to MS in univariate Cox regression models was lower age (C=0.604) and the most predictive PA variable was lower relative amplitude (RA, C=0.594). A two-stage forward selection using Cox regression resulted in a model with concordance C=0.693 and four predictors: age (p=0.015), stroke (p=0.009), Townsend deprivation index (p=0.874), and RA (p=0.004). A model including age, stroke, and RA had a concordance of C=0.691.
Conclusions:
Objective PA summaries were significantly different and consistent with lower activity among study participants who had MS at the time of the accelerometry study. Among individuals who did not have MS, younger age, stroke history, and lower RA were significantly associated with higher risk of a future MS diagnosis.
Keywords: ACCELEROMETER, MULTIPLE SCLEROSIS, UK BIOBANK, WEARABLE DEVICE, COX REGRESSION
INTRODUCTION
Traditionally, physical activity (PA) data have been collected from self-reported questionnaires, which are subject to reporting bias associated with the individual’s ability and will to recall, understand, and communicate their activities of daily life (1,2). In contrast, objective PA measurements collected from wearable accelerometers are essentially unbiased for their target estimand, the amount of vibration at a particular body location (3). The large-scale adoption of wearable devices, such as smart watches, led to an increased interest in objectively measured physical activity for entertainment and research purposes. They have been used in many large studies including BLSA (4,5,6), STURDY (7), NHANES (8,9,10,11,12,13,14), CARDIA (15) and UK Biobank (16,17,18,19). These studies also collect many demographic, behavioral, and exposure variables as well as health outcomes such as disease status, all-cause and cause-specific mortality. Therefore, they provide opportunities for quantifying the association between objective PA and health outcomes while accounting for potential confounders.
This project uses data collected in the UK Biobank (20), the largest long-term biobank study conducted in the United Kingdom with over 500,000 participants. UK Biobank contains a wide variety of potential determinants of health, including objectively measured PA, neuroimaging, and genomics, as well as health outcomes in the UK population. A subset of the UK Biobank participants was asked to wear a wrist-worn device for seven consecutive days, and their objective PA data were collected from these devices; see the Study Population section for a detailed description of the UK Biobank PA data. Here we focus on quantifying the association between eight objective PA summaries and prevalent (current cases) and incident (future cases) Multiple Sclerosis (MS). Given the mutual relationship between the central nervous system (CNS) and mobility (21,22,23), it is biologically plausible that MS, a debilitating disease of CNS, affects the volume and patterns of mobility. Moreover, reduction in physical activity may predict future MS diagnosis if some individuals have undiagnosed MS that is already affecting their PA, if PA is in the causal pathway of another unknown exposure, and/or if reduced PA is a cause for MS.
Multiple Sclerosis (MS) is a neurological disease that in 2020 affected an estimated 2.8 million people in the world (24). MS is an immune-mediated disease of the central nervous system and is characterized by the loss of myelin sheath of the neuronal axons as well as brain and spinal cord lesions (25). MS can lead to motor, visual, balance and cognitive disability, which can decrease an individual’s ability to engage in PA.
Several studies have investigated the differences in PA between individuals with and without MS. For example, Ng & Kent-Braun (26) reported that: (1) there was a statistically significant difference between PA in MS patients and healthy sedentary controls; and (2) accelerometers were more sensitive than the self-reported questionnaires for quantifying the differences between the two groups. This was a small study with 17 MS patients and 15 controls who wore three-dimensional accelerometers to measure PA, and it remains unclear what population is represented by this sample. Other studies reported similar results (27,28,29) using either questionnaires, wearable accelerometers, or both. Exercise has also been shown to be beneficial for managing MS symptoms, promoting mental health, and improving quality of life (30,31,32).
The first goal of this study is to quantify the difference in objective PA summaries between individuals living with and without MS based on data from a very large accelerometry study (UK Biobank). We are using eight summaries of objectively measured PA that include proxies for physical activity volume, fragmentation, and circadian rhythm. The size of the dataset allows us to conduct large case-to-many controls analyses that would not be possible in other data sets or would require lengthy and expensive additional data collection.
The second goal of this study is to quantify the individual and combined prediction performance of PA and traditional risk factors for future MS diagnosis. This is necessary as substantial uncertainty and even disagreement persist with respect to association between PA and future risk of MS diagnosis. For example, Dorans et al. (33) analyzed the Nurses’ Health Study (NHS) and the NHS II questionnaires’ data (34,35) and reported a weak association between higher PA at baseline and reduced risk of MS incidence later in life. Wesnes et al. (36) suggested that vigorous PA is associated with decreased MS risk. In contrast, Warren et al. (37) and Ghadirian et al. (38) reported that individuals who have MS may have participated in more PA before MS diagnosis compared to individuals living without MS. As these studies used self-reported PA data, concerns remain about their bias and measurement error. To bridge this gap, we investigate the predictive performance of objective PA summary measurements on clinically definite MS diagnosis in the UK Biobank and compare the performance of objective PA with other potential predictors of future MS diagnosis.
METHODS
Study Population
UK Biobank is a large prospective study conducted in the UK since 2006, with over 500,000 participants aged between 40 and 69 years at the time of their initial assessment. For this project, we focus on the objectively measured PA data collected from accelerometers and their association with clinically definite MS diagnosis. The research is conducted under UK Biobank Resource Application 33278.
Specifically, among the 502,520 UK Biobank participants, 236,519 participants were invited between February 2013 and December 2015 to wear a wrist-worn device for seven consecutive days, and 106,053 participants accepted the invitation. For each participant who accepted the invitation, an Axivity AX3 wrist-worn triaxial accelerometer was sent to their address (17). Data were measured at 100 Hz and were obtained from 103,712 participants. A subset of 93,370 participants was used for further analyses by including only individuals who had good quality data (according to UK Biobank quality control) for at least 3 days with at least 95% daily wear time. The processed accelerometry data were released at multiple resolutions. Here we focus on data aggregated in five-second non-overlapping intervals using a modified version of the Euclidean norm minus one (ENMO) expressed in milli-gravitational units (milli-g; g = 9.81m/s2). As suggested by Leroux et al. (16), Data were further aggregated at the minute level and transformed to the wide storage format (39). Summary measures were obtained based on the minute-level data for each day and then averaged within individuals across valid days. Here we follow Leroux et al. (16) and use the following eight PA summary measures: total acceleration (TA), total log-transformed acceleration (TLA), total sedentary time (ST), total minutes of light-intensity physical activity (LIPA), total minutes of moderate-to-vigorous physical activity (MVPA), active-to-sedentary transition probability (ASTP), sedentary-to-active transition probability (SATP), and relative amplitude (RA). Here, TA and TLA are measures of total volume of PA, ST, LIPA, and MVPA are measures of time in a given level of activity, ASTP and SATP are measures of PA fragmentation, and RA is a measure for the circadian rhythm. For some of these summaries, the thresholds between sedentary and light and light and moderate PA need to be defined. In this manuscript, we used the 30 milli-g threshold for sedentary and light PA and the 193 milli-g threshold for the light and moderate intensity PA. For more details on the choice of these thresholds see the Supplemental Digital Content (Supplemental Digital Content).
Data
We have constructed two data sets, one for studying differences in PA measures among current (prevalent) MS cases and one for quantifying the prediction performance of PA summaries for future (incident) MS diagnosis. The data, methods, and results sections are organized to reflect the two analyses. Below we describe the construction of the two relevant datasets.
Case-control data for current (prevalent) MS cases
The first data set was constructed to compare PA summaries between individuals with and without MS at the time of accelerometer wear. Data were constructed as a matched case-to-30-controls study because: (1) matching one case to only one control would result in substantial loss of power (throwing away controls); (2) matching one case to as many controls as there are available can result in covariate imbalance; and (3) 30 controls provided a good balance between not losing cases and having the maximum number of controls per MS case. Exclusion criteria were applied before matching was performed (see Supplemental Figure 1, Supplemental Digital Content, Flowchart of data processing for the study of PA among current MS patients). First, study participants with missing diagnostic information, age, sex, or BMI information were excluded. Individuals who had ever (on or before April 1, 2021) been diagnosed with other major diseases, including diabetes, stroke, and coronary heart disease, were also excluded. Next, study participants who were diagnosed with MS after wearing the accelerometers were excluded. Data were then divided into two groups, individuals with and without MS. Individuals without MS were considered as potential candidates for the matched control group.
Each MS patient was matched to 30 controls from the group of individuals without MS on sex, age (± 3 years), and BMI (± 3 BMI units expressed in kg/m2). When there were more than 30 matches for an MS case, 30 controls were selected at random among the matches. A total of 316 MS cases and 9,480 controls were included in further analyses. Table 1 summarizes the characteristics of the two groups after the matching procedure. Among the MS cases, the mean disease duration at the time of the accelerometer wear was 17.58 years (SD = 11.14 years), with the minimum duration being 0.12 years and maximum being 50.56 years.
Table 1.
Characteristics of current (prevalent) MS patients and their matched controls at the time of accelerometer wear
| Control (N = 9,480) |
MS (N = 316) |
|
|---|---|---|
| Age at accelerometer wearing | ||
| Mean (SD) | 60.1(7.35) | 60.0(7.46) |
| Median [Min, Max] | 60.0 [44.0, 78.0] | 60.0 [44.0, 76.0] |
| Sex | ||
| Female | 7, 740 (81.6%) | 258 (81.6%) |
| Male | 1, 740 (18.4%) | 58 (18.4%) |
| BMI | ||
| Mean (SD) | 25.8 (4.23) | 25.8 (4.48) |
| Median [Min, Max] | 25.0 [16.5, 46.1] | 24.8 [17.4, 43.3] |
| Race | ||
| White | 9, 150 (96.5%) | 308 (97.5%) |
| Non-white | 306 (3.2%) | 7 (2.2%) |
| Do not know | 4 (0.0%) | 0 (0%) |
| Prefer not to answer | 16 (0.2%) | 1 (0.3%) |
| Missing | 4 (0.0%) | 0 (0%) |
| Smoking status | ||
| Never | 5, 802 (61.2%) | 160 (50.6%) |
| Previous | 3, 068 (32.4%) | 118 (37.3%) |
| Current | 588 (6.2%) | 38 (12.0%) |
| Prefer not to answer | 18 (0.2%) | 0 (0%) |
| Missing | 4 (0.0%) | 0 (0%) |
| Frequency of drinking alcohol | ||
| Daily or almost daily | 1, 906 (20.1%) | 65 (20.6%) |
| Three or four times a week | 2, 459 (25.9%) | 78 (24.7%) |
| Once or twice a week | 2, 425 (25.6%) | 71 (22.5%) |
| One to three times a month | 1, 125 (11.9%) | 39 (12.3%) |
| Special occasions only | 1, 013 (10.7%) | 42 (13.3%) |
| Never | 547 (5.8%) | 21 (6.6%) |
| Prefer not to answer | 1 (0.0%) | 0 (0%) |
| Missing | 4 (0.0%) | 0 (0%) |
| Cancer (diagnosed before/on April 1, 2021) | ||
| Yes | 1, 393 (14.7%) | 44 (13.9%) |
| Long-standing illness, disability, or infirmity | ||
| Yes | 2, 067 (21.8%) | 253 (80.1%) |
| No | 7, 234 (76.3%) | 54 (17.1%) |
| Do not know | 171 (1.8%) | 9 (2.8%) |
| Prefer not to answer | 4 (0.0%) | 0 (0%) |
| Missing | 4 (0.0%) | 0 (0%) |
| Townsend deprivation index at recruitment | ||
| Mean (SD) | −1.73 (2.78) | −1.76 (2.88) |
| Median [Min, Max] Missing | −2.46 [−6.26, 8.68] | −2.56 [−6.18, 7.86] |
| Missing | 9 (0.1%) | 1 (0.3%) |
Data for studying predictors of future (incident) MS diagnosis
The second data set was constructed to quantify the predictive performance of PA for future (incident) MS diagnosis. Accelerometry data were first processed (see Supplemental Figure 2, Supplemental Digital Content, Flowchart of data processing for studying predictors of future (incident) MS diagnosis). Participants with missing diagnostic information or who had MS before the accelerometry study were excluded. The outcome was the indicator and time to an MS diagnosis among study participants who did not have an MS diagnosis at the time of accelerometer wear. The participants were considered to be censored if they were not diagnosed with MS by April 1, 2021 or if they died before this date without an MS diagnosis. Data contained 50 participants who experienced the event (MS diagnosis) after wearing the device, and 92,944 who did not. The characteristics of these 92,994 participants are summarized in the “Before NA removal” columns of Table 2, grouped by whether they had an MS diagnosis.
Table 2.
Characteristics of the participants. The columns labeled “Before NA removal” include participants with missing or unavailable demographic information. The columns labeled “After NA removal” include data excluding missing or unavailable demographics. The “Censored” columns show summary statistics of participants who had not been diagnosed with MS until the earlier of April 1, 2021, or date of death. The “MS” columns show summary statistics of participants who were diagnosed with MS after wearing the accelerometers.
| Before NA removal | After NA removal | |||
|---|---|---|---|---|
| Censored N = 92,944 |
MS N = 50 |
Censored N = 92,122 |
MS N = 47 |
|
| Age at accelerometer wearing | ||||
| Mean (SD) | 62.4 (7.83) | 60.1 (8.27) | 62.4 (7.83) | 60.0 (8.34) |
| Median [Min, Max] | 63.0 [43.0, 79.0] | 58.5 [48.0, 76.0] | 63.0 [43.0, 79.0] | 58.0 [48.0, 76.0] |
| Sex | ||||
| Female | 52,076 (56.0%) | 32 (64.0%) | 51,645 (56.1%) | 30 (63.8%) |
| Male | 40,868 (44.0%) | 18 (36.0%) | 40,477 (43.9%) | 17 (36.2%) |
| BMI | ||||
| Mean (SD) | 26.7 (4.52) | 26.1 (4.98) | 26.7 (4.53) | 26.2 (4.95) |
| Median [Min, Max] | 26.0 [12.1, 67.3] | 25.2 [18.4, 43.6] | 26.0 [12.1, 67.3] | 25.2 [18.7, 43.6] |
| Missing | 188 (0.2%) | 1 (2.0%) | — | — |
| Race | ||||
| White | 89,792 (96.6%) | 47 (94.0%) | 89,321 (97.0%) | 45 (95.7%) |
| Non-white | 2,836 (3.1%) | 2 (4.0%) | 2,801 (3.0%) | 2 (4.3%) |
| Do not know | 23 (0.0%) | 0 (0%) | — | — |
| Prefer not to answer | 252 (0.3%) | 0 (0%) | — | — |
| Missing | 41 (0.0%) | 1 (2.0%) | — | — |
| Smoking status | ||||
| Never | 52,982 (57.0%) | 27 (54.0%) | 52,656 (57.2%) | 27 (57.4%) |
| Previous | 33,367 (35.9%) | 14 (28.0%) | 33,177 (36.0%) | 13 (27.7%) |
| Current | 6,349 (6.8%) | 7 (14.0%) | 6,289 (6.8%) | 7 (14.9%) |
| Prefer not to answer | 206 (0.2%) | 1 (2.0%) | — | — |
| Missing | 40 (0.0%) | 1 (2.0%) | — | — |
| Frequency of drinking alcohol | ||||
| Daily or almost daily | 21,268 (22.9%) | 13 (26.0%) | 21,112 (22.9%) | 12 (25.5%) |
| Three or four times a week | 24,179 (26.0%) | 9 (18.0%) | 23,996 (26.0%) | 9 (19.1%) |
| Once or twice a week | 23,305 (25.1%) | 14 (28.0%) | 23,133 (25.1%) | 13 (27.7%) |
| One to three times a month | 10,095 (10.9%) | 7 (14.0%) | 10,009 (10.9%) | 7 (14.9%) |
| Special occasions only | 8,791 (9.5%) | 2 (4.0%) | 8,701 (9.4%) | 2 (4.3%) |
| Never | 5,229 (5.6%) | 4 (8.0%) | 5,171 (5.6%) | 4 (8.5%) |
| Prefer not to answer | 36 (0.0%) | 0 (0%) | — | — |
| Missing | 41 (0.0%) | 1 (2.0%) | — | — |
| Long-standing illness, disability or infirmity | ||||
| Yes | 25,826 (27.8%) | 26 (52.0%) | 25,530 (27.7%) | 24 (51.1%) |
| No | 65,326 (70.3%) | 22 (44.0%) | 64,873 (70.4%) | 22 (46.8%) |
| Do not know | 1,676 (1.8%) | 1 (2.0%) | 1,653 (1.8%) | 1 (2.1%) |
| Prefer not to answer | 75 (0.1%) | 0 (0%) | 66 (0.1%) | 0 (0%) |
| Missing | 41 (0.0%) | 1 (2.0%) | 0 (0%) | 0 (0%) |
| Disease history | ||||
| Diabetesa | 4,297 (4.6%) | 2 (4.0%) | 4,236 (4.6%) | 2 (4.3%) |
| Strokea | 2,252 (2.4%) | 4 (8.0%) | 2,221 (2.4%) | 4 (8.5%) |
| Coronary heart diseasea | 3,981 (4.3%) | 2 (4.0%) | 3,932 (4.3%) | 2 (4.3%) |
| Cancer | 13,251 (14.3%) | 7 (14.0%) | 13,134 (14.3%) | 6 (12.8%) |
| Townsend deprivation index at recruitment | ||||
| Mean (SD) Median [Min, Max] |
−1.73 (2.81) | −1.14 (3.06) | −1.74 (2.81) | −1.40 (2.93) |
| Missing | 104 (0.1%) | 0 (0%) | — | — |
subcategories of the diseases can be found in the supplementary material.
Potential predictors were split into two groups. The first group (traditional predictors) contained demographic, behavioral, and disease history variables including: age at accelerometer-wearing, sex, BMI at recruitment, race (binary, white or not), smoking status (binary, previous/current smoker or not), alcohol drinking frequency (binary, drinking at least once a week or not), Townsend deprivation index at recruitment, diabetes (binary, yes or no), stroke (binary, yes or no), coronary heart disease (binary, yes or no), and cancer (binary, yes or no). The second group (PA variables) included TA, TLA, ST, LIPA, MVPA, ASTP, SATP, and RA.
Finally, some of the participants had missing data or responded “do not know” or “prefer not to answer” to some questions. Missing and unavailable data represented a small proportion of the data set and were excluded from further analyses. After implementing this exclusion criterion, there were 47 MS diagnoses and 92,122 censored observations with a total of 585,900 follow-up person years with an MS incidence rate of 8.02 × 10−5 per person-year. The average number of years from accelerometer wear to MS diagnosis was 2.72 years (SD = 2.03 years). For censored observations, the average number of years from accelerometer wear to censoring was 6.36 years (SD = 0.79 years). The characteristics of the 92,169 participants in the analytic data set are summarized in the “After NA removal” columns in Table 2, grouped by MS diagnosis.
Standard Protocol Approvals, Registrations, and Patient Consents
Written informed consent was obtained from all UK Biobank participants in accordance with the Declaration of Helsinki and its later amendments as part of the recruitment process. The study has obtained all necessary ethics approvals from the respective Research Ethics Committees.
Methods for analyzing the case-control data for current (prevalent) MS cases
Exploratory data analysis started with comparing the distributions of the objective PA summaries between the MS case and control groups. To determine if the differences between the two groups were statistically significant, Welch’s two-sample unpaired t-tests were performed on the variables TA, TLA, ST, LIPA, MVPA, ASTP, SATP, and RA. In general, higher TA, TLA, LIPA, MVPA, SATP, or RA is consistent with higher levels of PA, though each measure targets a different dimension of PA. Higher ST or ASTP is consistent with lower levels of PA. In addition, two-proportion z-tests were performed to assess if race, smoking status, or frequency of drinking alcohol differs between cases and controls.
Methods for analyzing predictors of future (incident) MS diagnosis
To investigate which variables could contribute to predicting MS diagnosis, survival analysis methods were employed. Time from accelerometer wear to MS diagnosis was defined as the outcome. We first explored the categorical/binary variables. To visualize the survival functions, Kaplan-Meier curves (40) were constructed for binary variables. The differences between groups were visually compared; see the Supplemental Digital Content for details.
To quantify how a predictor may affect the hazard of MS diagnosis, single predictor Cox proportional hazards models were fitted for each predictor separately. The concordance (41) for each model was compared to quantify the predictor’s performance. A two-stage forward selection procedure was used to assess which combination of predictors had the best combined prediction performance. Specifically, during the first stage, the forward selection procedure was implemented using all the traditional predictors (PA summaries were not included in the first stage). For each selection round, one candidate predictor was added to the selected model from the previous round. The criterion for adding predictors was concordance. The difference between the concordance of the new model and the previous one (ΔC) was then calculated. A stopping rule of 0.01 increase in concordance was used. That means that if ΔC was greater than 0.01, a new attempt was made to add one of the remaining variables. The process was repeated until ΔC was no longer greater than 0.01. During the second stage, the selected traditional predictors from the first stage were forced into the model. To study whether PA predictors add anything to these traditional predictors, a second stage forward selection procedure was used for the PA summaries where each model contained the selected traditional predictors. A final combination of predictors was then determined.
RESULTS
Results for the case-control data for current (prevalent) MS cases
The density plots in Figure 1 indicate that MS cases generally appeared to have lower TA, TLA, RA, LIPA, MVPA, SATP and higher ST and ASTP compared to controls. The MVPA plot indicates a larger proportion of MS cases (5.7%) who had zero minutes of MVPA compared to controls (0.6%). The results of all PA measurements were consistent, indicating that MS cases had lower levels of PA than healthy controls.
Figure 1 -.
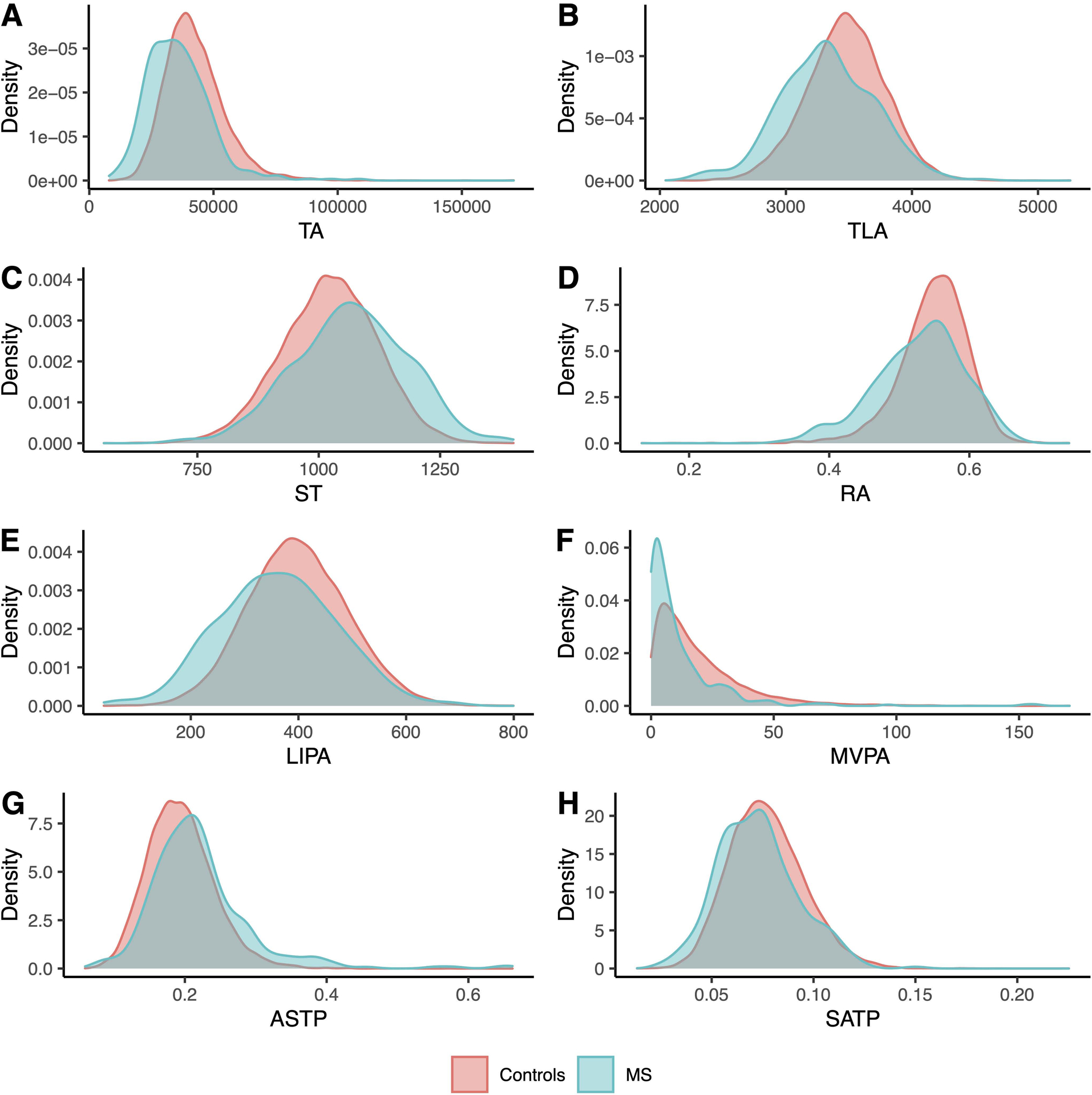
Histogram of eight PA summaries by current (prevalent) MS patients (blue) and their matched controls (red) at the time of accelerometer wear
To quantify these differences, we conducted tests for equality of the group means. The test results are shown in Table 3. The mean TA for the MS group was 15.5% smaller than that of the control group (p < 0.001). The mean TLA for the MS group was 4.3% smaller (p < 0.001). The mean ST for the MS group was 4.4% larger (p < 0.001) (1,067 minutes in the MS group compared to 1,022 minutes in the control group). One reason why the difference expressed in percentages is not that large is that the sedentary time is very long in the control group. The mean time in LIPA for the MS group was 9.5% shorter (p < 0.001) (361 minutes in the MS group compared to 399 minutes in the control group). The mean time in MVPA for the MS group was 39% shorter (p < 0.001) (11.6 minutes in the MS group compared to 19.1 minutes in the control group). MS cases had, on average, significantly higher ASTP compared to controls (p < 0.001), being 15.8% more likely to transition from active to sedentary. Similarly, MS cases had significantly lower SATP (p < 0.001), being 12.5% less likely to transition from sedentary to active. The mean RA for MS cases was about 3.7% lower than that of the control group. In addition, significantly more participants in the MS group were previous or current smokers compared to the control group (p < 0.001), where the proportion was 49.37% for the MS group and 38.66% for the control group. There was also a higher proportion of non-white study participants in the control group (3.24%) compared to the MS group (2.4%), but this was not statistically significant (p = 0.423). This result may be affected by the low proportion of individuals who are not white and had MS (7 individuals) and the low proportion of individuals who are not white in the UK Biobank. A larger proportion of participants in the control group (71.66%) drank at least once a week compared to the MS group (67.72%), but this difference was not statistically significant (p = 0.127).
Table 3.
Results of statistical tests comparing current (prevalent) MS patients and their matched controls at the time of accelerometer wear. For continuous variables, Welch’s two-sample t-tests were performed and p values were reported to determine if the differences were significant between the two groups. For categorical variables, two-proportion z-tests were performed and p values were reported. The proportions were calculated excluding subjects who responded “Prefer not to answer”, “Do not know”, or have missing data. The values in the “Control” and “MS” columns are in the format of “mean (SD)” for continuous variables, and “n (%)” for categorical variables.
| Control | MS | ||
|---|---|---|---|
| Variables | N = 9, 480 | N = 316 | p value |
| TA | 42,477 (12,151) | 35,887 (13,15) | < 0.001*** |
| TLA | 3,476 (311) | 3,327 (368) | < 0.001*** |
| ST | 1,022 (99) | 1,067 (116) | < 0.001*** |
| LIPA | 399 (93) | 361 (109) | < 0.001*** |
| MVPA | 19.1 (17.9) | 11.6 (17.6) | < 0.001*** |
| ASTP | 0.19 (0.05) | 0.22 (0.07) | < 0.001*** |
| SATP | 0.08 (0.02) | 0.07 (0.02) | < 0.001*** |
| RA | 0.55 (0.05) | 0.53 (0.06) | < 0.001*** |
| Race (%non-white) | 306 (3.24%) | 7 (2.4%) | 0.423 |
| Smoking (%previous or current) | 3, 656 (38.66%) | 156 (49.37%) | < 0.001*** |
| Drinking (%at least once a week) | 6, 790 (71.66%) | 214 (67.72%) | 0.127 |
p < 0.05.
p < 0.01.
p < 0.001.
Results for the analysis of predictors of future (incident) MS diagnosis
The results from fitting the single-predictor Cox models are presented in Table 4. The rows with gray background correspond to PA variables. The model with the highest concordance (C = 0.604) was the one with age at the time of accelerometer wear. The rest of the models rounding up the top 5 highest concordance models were the ones using RA (C = 0.594), MVPA (C = 0.568), TA (C = 0.553), and the Townsend deprivation index (C = 0.548), respectively. RA was the best-performing PA variable in terms of concordance calculated based on single-predictor models. The only variables that were significant at the level =0.05 in single-variable models were age (p = 0.048), RA (p = 0.004), and stroke (p = 0.010). For age, the hazard ratio was estimated to be 0.965 (95% CI: [0.931, 1.00]) for each one-year increase in age. The hazard ratio regarding RA was 0.00136 (95% CI: [1.53 × 10−5, 0.121]) for every one-unit increase in RA. The hazard ratio for the stroke indicator variable was 3.82 (95% CI: [1.37, 10.70]) comparing individuals with a history of stroke comparing those without.
Table 4.
Hazard ratios (exponentiated coefficients) and their 95% CIs, p values of the coefficients, and the concordances with SEs of the single-predictor cox proportional hazards models. This table was arranged from the highest concordance to the lowest. The rows with gray background color are rows of PA variables.
| Variable | Hazard ratio (95% CI) | p value | Concordance (SE) |
|---|---|---|---|
| Age | 0.965 (0.931, 1.00) | 0.0478* | 0.604 (0.0440) |
| RA | 0.00136 (1.53 ×10−5, 0.121) | 0.00397** | 0.594 (0.0404) |
| MVPA | 0.980 (0.959, 1.00) | 0.0739 | 0.568 (0.0431) |
| TA | 1.00 (1.00, 1.00) | 0.107 | 0.553 (0.0436) |
| Townsend deprivation index at recruitment | 1.04 (0.945, 1.15) | 0.421 | 0.548 (0.0419) |
| ASTP | 51.3 (0.332, 7933) | 0.126 | 0.539 (0.0439) |
| Sex (male) | 0.726 (0.401, 1.32) | 0.292 | 0.535 (0.0359) |
| Stroke | 3.82 (1.37, 10.7) | 0.0103* | 0.533 (0.0215) |
| BMI | 0.977 (0.914, 1.04) | 0.495 | 0.529 (0.0484) |
| Cancer | 0.896 (0.380, 2.11) | 0.801 | 0.519 (0.0219) |
| ST | 1.00 (0.999, 1.00) | 0.332 | 0.519 (0.0433) |
| Drinking frequency (at least once a week) | 0.916 (0.483, 1.73) | 0.787 | 0.515 (0.0338) |
| Smoking status (previous or current) | 0.993 (0.557, 1.77) | 0.982 | 0.509 (0.0364) |
| LIPA | 0.999 (0.996, 1.00) | 0.483 | 0.509 (0.0438) |
| Race (white) | 0.714 (0.173, 2.95) | 0.642 | 0.507 (0.0156) |
| Coronary heart disease | 1.02 (0.247, 4.19) | 0.981 | 0.501 (0.0156) |
| Diabetes | 0.943 (0.229, 3.89) | 0.935 | 0.500 (0.0156) |
| TLAC | 1.00 (0.999, 1.00) | 0.671 | 0.487 (0.0446) |
| SATP | 0.887 (1.66 ×10−7, 4.73 ×106) | 0.988 | 0.476 (0.0467) |
p < 0.05.
p < 0.01.
p < 0.001.
The best combination of variables based on the two-stage forward selection process is shown in Table 5. The selected traditional predictors in the first stage were age, stroke, and Townsend deprivation index. The model with these three variables had a concordance of C = 0.641. In the second stage of forward selection using PA variables, RA was the only variable selected, contributing a 0.052 change in the cumulative concordance for a combined concordance of C = 0.693. In this model, age, stroke, and RA were significant at the level 𝛼 = 0.05, which is consistent with the results of the single-predictor models. However, the Townsend deprivation index was not significant in this combined model. Specifically, age had an estimated hazard ratio of 0.956 (95% CI: [0.922, 0.991]). Thus, with every one-year greater age at the time of wearing the accelerometer, the hazard of being subsequently diagnosed with MS was 4.39% lower, adjusting for stroke, Townsend deprivation index, and RA. Stroke had an estimated hazard ratio of 4.06 (95% CI: [1.42, 11.57]), indicating that stroke history was independently associated with four times the risk of subsequent MS diagnosis. The hazard ratio of RA was estimated to be 0.0013 (95% CI: [1.44 × 10−5, 0.120]), indicating that independent of other covariates, with every 0.1 greater RA, the risk of MS was 48.55% lower.
Table 5.
Results of the two-stage forward selection process. The variables were divided into the traditional predictors group (first stage) and the PA predictors group (second stage). The selection process columns show the cumulative concordance after adding the additional variable, and the change of concordance compared to the previous step. The selected model columns show the hazard ratios (exponentiated coefficients) and the p values of the variables in the Cox model with all selected variables.
| Selection process | Selected model | |||
|---|---|---|---|---|
| Variable | Cumulative C | ΔC | Hazard ratio (95% CI) | p value |
| Traditional predictors | ||||
| Age | 0.6038 | 0.6038 | 0.9561 (0.9220, 0.9914) | 0.01520* |
| Stroke | 0.6303 | 0.0265 | 4.0571 (1.4231, 11.5664) | 0.00879** |
| Townsend deprivation index at recruitment | 0.6409 | 0.0106 | 1.0079 (0.9143, 1.1110) | 0.87440 |
| PA predictors | ||||
| RA | 0.6925 | 0.0517 | 0.0013 (1.4352 ×10−5, 0.1204) | 0.00400** |
p < 0.05.
p < 0.01.
p < 0.001.
Based on these results, we propose a final model with the three significant predictors: age, stroke, and RA. The concordance of this final model was C = 0.691. The detailed results are reported in Table 6. All three predictors in the model were significant at the level =0.05, independent of one another. The risk of MS diagnosis was 4.43% lower for every one-year greater age (hazard ratio: 0.956, 95% CI: [0.922, 0.991]), 4.07 times higher for those with a history of stroke (hazard ratio: 4.07, 95% CI: [1.43, 11.59]), and 48.96% lower (calculated by (1-0.00120.1) × 100%) for 0.1 greater RA (hazard ratio: 0.0012, 95% CI: [1.43 × 10−5, 0.109]).
Table 6.
Results of the final model (concordance C = 0.691)
| Variable | Hazard ratio (95% CI) | p value |
|---|---|---|
| Age | 0.9557 (0.9220, 0.9907) | 0.01347* |
| Stroke | 4.0677 (1.427, 11.5914) | 0.00864** |
| RA | 0.0012 (1.429 ×10−5, 0.1089) | 0.00337** |
p < 0.05.
p < 0.01.
p < 0.001.
DISCUSSION
We have conducted two main analyses. The first analysis compared the levels of objectively measured physical activity between two groups: individuals with and without MS at the time of accelerometer wear matched on sex, age, and BMI. The second analysis focused on identifying predictors and quantifying their predictive performance of future MS diagnosis among individuals who did not have an MS diagnosis at the time of accelerometer wear.
The results of the first study indicated that MS patients generally engaged in less PA compared to matched controls. All eight objective PA summaries, including TA, TLA, ST, LIPA, MVPA, ASTP, SATP, and RA, showed strong statistically significant associations with prevalent (current) MS case status. MVPA had the largest difference as measured by the proportion of the difference in the means of the MS and control groups relative to the control group mean. On average, study participants who had MS spent almost 40% less time per day in MVPA compared to controls. This is consistent with previous results based on the UK Biobank data, which indicated a 44% lower weekly moderate PA among individuals with MS compared to individuals without MS (42)42. They defined a moderate PA minute as between 100 milli-g and 400 milli-g per minute based on Hildebrand et al. (43). Our cut-off point for moderate to vigorous PA was 193 milli-g per minute, which was obtained from a normalization procedure based on the NHANES data; see the Supplemental Digital Content for details. The 193 milli-g cutoff is likely to be better because it makes the range of daily MVPA minutes among participants who are around 60 years old comparable between UK Biobank and NHANES. However, we have also performed a sensitivity analysis using the 100 milli-g cutoff for MVPA and report the results in the Supplemental Digital Content.
RA is a measure of rest-activity rhythm, which is likely a proxy for the circadian rhythm of a person (44). Since MS patients had lower RA on average, the distinction between their most active 10 hours and least active 5 hours was not as strong as that for individuals without MS. This result complements previous findings by Rietberg et al. (45) regarding daily PA patterns in MS patients.
Smoking status was also significantly different between MS cases and controls. Being a previous or current smoker was previously identified as a risk factor of MS (46). We did not find a statistically significant association between drinking status and MS, which is consistent with a published meta-analysis (47).
We have also investigated the association between both traditional predictors and objectively measured PA predictors and future MS diagnosis. Results indicated that age, stroke history, and RA, both individually and combined, are strong predictors of future MS diagnosis. Greater age and RA were associated with a reduced hazard of a future MS diagnosis, while stroke history was associated with increased hazard of a future MS diagnosis.
The negative association between age and MS may appear counter intuitive. However, the typical age of MS diagnosis is around 20 to 30 years (48) and the average age at the time of accelerometer wear in the analyzed data was around 60. Our results indicate that individuals with a stroke history may be at higher risk of developing MS. This complements previously published results indicating that people with MS have an increased risk of developing stroke (49). However, this association may or may not be causal. For example, individuals who have a history of stroke might be under more intense health surveillance, which may help identify MS cases faster. Another possibility could be that strokes may be misdiagnosed as MS later in life. Alternatively, MS lesions may initially be classified as stroke to only later be correctly classified as MS lesions. Further research is needed to confirm this association. The RA results indicate that individuals with stronger contrasts between their most and least active periods were at lower risk of developing MS. Interestingly, sex was not associated with the risk of developing future MS. This may be surprising, as women are twice as likely as men to have MS (24). One possibility could be that this effect is not as pronounced in new cases among older adults, as suggested by a study in Denmark (50). The small sample size of individuals diagnosed with MS could have also played a role.
Our analysis has several limitations. First, some variables used, including BMI, smoking status, drinking frequency, Townsend deprivation index, were obtained at the time of the initial recruitment, which was earlier than the accelerometry study. This time gap could be substantial (prevalent MS analysis data: mean = 2,078, sd = 397, min = 1,073, max = 3,512; incident MS analysis data: mean = 2,081, sd = 400, min = 1,017, max = 3,537; unit: days). When longitudinal physical activity data are available, the literature suggests that these data should not be modeled independently. In our study, we find insufficient evidence that non-PA variables were significantly different between visits through longitudinal and sensitivity analysis, suggesting that our conclusions are robust after accounting for time gaps. Results are shown in the Supplemental Digital Content. Second, because data were sampled from the UK, some results may not generalize to other populations. For example, the proportion of study participants who identified as white is roughly 95% of the sample. Third, new MS diagnoses are relatively rare in this population, which affects the type and complexity of predictive analyses that could be performed. Overfitting is likely not a major problem in our approach as our final model only included three predictors: age, stroke, and RA. Fourth, reverse causality may be a problem (16). The diagnosis of MS is rather complicated and there could be a long time between when MS started and when it was diagnosed. Therefore, some individuals might initially be asymptomatic or undiagnosed, though their PA may be reduced. So instead of lower PA levels predicting MS, it could be that people with undiagnosed MS may have lower PA levels. Fifth, in this study we use wrist-worn accelerometry data as an objective measure of physical activity intensity for all participants, including MS patients and healthy individuals. However, due to study design limitations, the wrist-worn accelerometers have not been formally validated in people with MS to check if they can reflect the true activity levels of this population.
CONCLUSIONS
This project provides insights into the activity levels of MS patients, which is one of the indicators of their quality of life. The strong associations between a wide variety of objective PA summaries and MS status, as well as future MS diagnosis may help inform the diagnostic and management of MS.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke under Award Number R01 NS060910. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine. Dr. Crainiceanu is consulting with Bayer, Johnson and Johnson, and Cytel on methods development for wearable devices in clinical trials. The details of the contracts are disclosed through the Johns Hopkins University eDisclose system and have no direct or apparent relationship with the current paper.
Conflict of Interest and Funding Source:
This work was supported by the National Institute of Neurological Disorders and Stroke under Award Number R01 NS060910. Dr. Crainiceanu is consulting with Bayer, Johnson and Johnson, and Cytel on methods development for wearable devices in clinical trials. The details of the contracts are disclosed through the Johns Hopkins University eDisclose system and have no direct or apparent relationship with the current paper. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
SUPPLEMENTAL DIGITAL CONTENT
SDC 1: Supplementary Material comments.docx
REFERENCES
- 1.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37(3):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner PS, DeLamater JD. Social desirability bias in self-reports of physical activity: is an exercise identity the culprit? Soc Indic Res. 2014;117(2):489–504. [Google Scholar]
- 3.Bai J, Di C, Xiao L, et al. An activity index for raw accelerometry data and its comparison with other activity metrics. PLoS One. 2016;11(8):e0160644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrack JA, Cooper R, Koster A, et al. Assessing daily physical activity in older adults: unraveling the complexity of monitors, measures, and methods. J Gerontol A Biol Sci Med Sci. 2016;71(8):1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrack JA, Leroux A, Fleg JL, et al. Using heart rate and accelerometry to define quantity and intensity of physical activity in older adults. J Gerontol A Biol Sci Med Sci. 2018;73(5):668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrack JA, Zipunnikov V, Goldsmith J, et al. Assessing the “physical cliff”: detailed quantification of age-related differences in daily patterns of physical activity. J Gerontol A Biol Sci Med Sci. 2014;69(8):973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrack J, Urbanek J, Wanigatunga A, et al. Effects of daily vitamin D supplementation on objectively measured physical activity: results from the STURDY trial. Innov Aging. 2020;4(Suppl 1):760. [Google Scholar]
- 8.Smirnova E, Leroux A, Cao Q, et al. The predictive performance of objective measures of physical activity derived from accelerometry data for 5-year all-cause mortality in older adults: National Health and Nutritional Examination Survey 2003–2006. J Gerontol A Biol Sci Med Sci. 2020;75(9):1779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui E, Crainiceanu CM, Leroux A. Additive functional Cox model. J Computat Graph Stat. 2021;30(3):780–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui E, Thompson EC, Carroll RJ, Ruppert D. A semiparametric risk score for physical activity. Stat Med. 2022;41(7):1191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koster A, Caserotti P, Patel KV, et al. Association of sedentary time with mortality independent of moderate to vigorous physical activity. PLoS One. 2012;7(6):e37696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. [DOI] [PubMed] [Google Scholar]
- 13.Schmid D, Ricci C, Leitzmann MF. Associations of objectively assessed physical activity and sedentary time with all-cause mortality in US adults: the NHANES study. PLoS One. 2015;10(3):e0119591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews CE, Keadle SK, Troiano RP, et al. Accelerometer-measured dose-response for physical activity, sedentary time, and mortality in US adults. Am J clin Nutr. 2016;104(5):1424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitaker KM, Zhang D, Pettee Gabriel K, et al. Longitudinal associations of midlife accelerometer determined sedentary behavior and physical activity with cognitive function: the CARDIA study. JAMA. 2021;10(3):e018350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leroux A, Xu S, Kundu P, et al. Quantifying the predictive performance of objectively measured physical activity on mortality in the UK Biobank. J Gerontol A Biol Sci Med Sci. 2021;76(8):1486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doherty A, Jackson D, Hammerla N, et al. Large scale population assessment of physical activity using wrist worn accelerometers: the UK biobank study. PLoS One. 2017;12(2):e0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowlands AV, Dempsey PC, Gillies C, et al. Association between accelerometer-assessed physical activity and severity of COVID-19 in UK Biobank. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramakrishnan R, Doherty A, Smith-Byrne K, et al. Accelerometer measured physical activity and the incidence of cardiovascular disease: evidence from the UK Biobank cohort study. PLoS Med. 2021;18(1):e1003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark DJ, Rose DK, Ring SA, Porges EC. Utilization of central nervous system resources for preparation and performance of complex walking tasks in older adults. Front Aging Neurosci. 2014;6:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dishman RK, Berthoud HR, Booth FW, et al. Neurobiology of exercise. Obesity (Silver Spring). 2006;14(3):345–56. [DOI] [PubMed] [Google Scholar]
- 23.Varma VR, Hausdorff JM, Studenski SA, et al. Aging, the central nervous system, and mobility in older adults: interventions. J Gerontol A Biol Sci Med Sci. 2016;71(11):1451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS. Mult Scler. 2020. Dec;26(14):1816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobson R, Giovannoni G. Multiple sclerosis–a review. Eur J Neurol. 2019;26(1):27–40. [DOI] [PubMed] [Google Scholar]
- 26.Ng AV, Kent-Braun JA. Quantitation of lower physical activity in persons with multiple sclerosis. Med Sci Sports Exerc. 1997;29(4):517–23. [DOI] [PubMed] [Google Scholar]
- 27.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005;11(4):459–63. [DOI] [PubMed] [Google Scholar]
- 28.Sandroff BM, Dlugonski D, Weikert M, Suh Y, Balantrapu S, Motl RW. Physical activity and multiple sclerosis: new insights regarding inactivity. Acta Neurol Scand. 2012;126(4):256–62. [DOI] [PubMed] [Google Scholar]
- 29.Motl RW, McAuley E, Sandroff BM, Hubbard EA. Descriptive epidemiology of physical activity rates in multiple sclerosis. Acta Neurol Scand. 2015;131(6):422–5. [DOI] [PubMed] [Google Scholar]
- 30.Byrnes KL, Whillier S. Effects of nonpharmaceutical treatments on symptom management in adults with mild or moderate multiple sclerosis: a meta-analysis. J Manipulative Physiol Ther. 2019;42(7):514–31. [DOI] [PubMed] [Google Scholar]
- 31.Suh Y, Motl RW, Mohr DC. Physical activity, disability, and mood in the early stage of multiple sclerosis. Disabil Health J. 2010;3(2):93–8. [DOI] [PubMed] [Google Scholar]
- 32.Motl RW, Gosney JL.Effect of exercise training on quality of life in multiple sclerosis: a meta-analysis. Mult Scler. 2008;14(1):129–35. [DOI] [PubMed] [Google Scholar]
- 33.Dorans KS, Massa J, Chitnis T, Ascherio A, Munger KL. Physical activity and the incidence of multiple sclerosis. Neurology. 2016;87(17):1770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colditz GA, Philpott SE, Hankinson SE. The impact of the Nurses’ Health Study on population health: prevention, translation, and control. Am J Public Health. 2016;106(9):1540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao Y, Bertoia ML, Lenart EB, et al. Origin, methods, and evolution of the three Nurses’ Health Studies. Am J Public Health. 2016;106(9):1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wesnes K, Myhr KM, Riise T, et al. Physical activity is associated with a decreased multiple sclerosis risk: the EnvIMS study. Mult Scler. 2018;24(2):150–7. [DOI] [PubMed] [Google Scholar]
- 37.Warren SA, Warren KG, Greenhill S, Paterson M. How multiple sclerosis is related to animal illness, stress and diabetes. Can Med Assoc J. 1982;126(4):377-82, 385. [PMC free article] [PubMed] [Google Scholar]
- 38.Ghadirian P, Dadgostar B, Azani R, Maisonneuve P. A case-control study of the association between socio-demographic, lifestyle and medical history factors and multiple sclerosis. Can J Public Health. 2001;92(4):281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leroux A, Di J, Smirnova E, et al. Organizing and analyzing the activity data in NHANES. Stat Biosci. 2019;11(2):262–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–81. [Google Scholar]
- 41.Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247(18):2543–6. [PubMed] [Google Scholar]
- 42.Barker J, Smith Byrne K, Doherty A, et al. Physical activity of UK adults with chronic disease: cross-sectional analysis of accelerometer-measured physical activity in 96 706 UK Biobank participants. Int J Epidemiol. 2019;48(4):1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hildebrand MV, Van Hees VT, Hansen BH, Ekelund UL. Age group comparability of raw accelerometer output from wrist-and hip-worn monitors. Med Sci Sports Exerc. 2014;46(9):1816–24. [DOI] [PubMed] [Google Scholar]
- 44.Ferguson A, Lyall LM, Ward J, et al. Genome-wide association study of circadian rhythmicity in 71,500 UK biobank participants and polygenic association with mood instability. EBioMedicine. 2018;35:279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rietberg MB, van Wegen EE, Kollen BJ, Kwakkel G. Do patients with multiple sclerosis show different daily physical activity patterns from healthy individuals? Neurorehabil Neural Repair. 2014;28(6):516–23. [DOI] [PubMed] [Google Scholar]
- 46.Poorolajal J, Bahrami M, Karami M, Hooshmand E. Effect of smoking on multiple sclerosis: a meta-analysis. J Public Health. 2017;39(2):312–20. [DOI] [PubMed] [Google Scholar]
- 47.Zhu T, Ye X, Zhang T et al. Association between alcohol consumption and multiple sclerosis: a meta-analysis of observational studies. Neurol Sci. 2015;36(9):1543–50. [DOI] [PubMed] [Google Scholar]
- 48.McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325(8):765–79. [DOI] [PubMed] [Google Scholar]
- 49.Hong Y, Tang HR, Ma M, Chen N, Xie X, He L. Multiple sclerosis and stroke: a systematic review and meta-analysis. BMC Neurol. 2019;19(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koch-Henriksen N, Thygesen LC, Stenager E, Laursen B, Magyari M. Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology. 2018;90(22):e1954–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


