Abstract
Background:
Catatonia is an under-recognized disorder characterized by psychomotor (increased, decreased, or abnormal) changes, affective symptoms, and disturbance of volition, which may arise in the setting of decompensated psychiatric or non-psychiatric medical disorders. Genetic studies of catatonia are limited, and to the best of our knowledge no prior genome wide association studies of catatonia have been performed to date.
Methods:
First we performed a genome wide association study of catatonia regardless of etiology (psychiatric or non-psychiatric). Secondarily we evaluated whether there was an elevated genetic risk profile for predisposing psychiatric disorders (schizophrenia spectrum disorder, bipolar affective disorder, etc.) in patients with catatonia. We used a matched case control design and applied polygenic risk scores to evaluate for a shared polygenetic contribution to catatonia from common psychiatric phenotypes that show a high prevalence of catatonia in their decompensated states.
Results:
Anxiety, bipolar affective disorder, schizophrenia spectrum disorder and cross disorder polygenic risk scores were significantly associated with catatonia case status in both unadjusted and adjusted logistic regression models for the European Ancestry set even after correcting for multiple comparisons. Depression, Alzheimer’s, Autism Spectrum Disorder and Obsessive Disorder polygenic risk scores were not significantly associated with catatonia status in participants of European Ancestry. In the African Ancestry set, no psychiatric polygenic risk scores were significantly associated with catatonia status in either the unadjusted or adjusted regression models.
Conclusions:
Even after controlling for relevant covariates, anxiety, bipolar affective disorder, schizophrenia spectrum disorder and cross disorders were significantly associated with catatonia status suggesting that there might be a shared genetic risk for those disorders amongst patients with catatonia.
Keywords: catatonia, genetics, schizophrenia, polygenic risk
Introduction
Catatonia is an under-recognized disorder characterized by psychomotor (increased, decreased, or abnormal) behavior, affective symptoms, and disturbance of volition, which may arise in the setting of decompensated psychiatric or primary medical disorders (Walther et al., 2019; Wilson et al., 2017). Risk factors for catatonia may include both predisposing and precipitating factors. Predisposing factors may include demographic factors such as advanced age or clinical factors such as a history of a neurocognitive disorder, psychotic, affective or seizure disorder. Proposed precipitating factors include a variety of substance-related, inflammatory, metabolic, psychiatric, or neurologic conditions that may interplay with predisposing factors to lead to the catatonia phenotype. Understanding predisposition for catatonia, including genetic risk factors, may eventually lead us towards modifying precipitating factors to reduce risk of catatonia.
Genetic studies of patients with catatonia are extremely limited. To the best of our knowledge the only prior genome wide analysis catatonia focused on a rare form of catatonia, referred to as periodic catatonia, which identified significant linkage with regions of chromosome 15q15 (Stober et al., 2000; Stober et al., 2002), chromosome 22q13.33 (Selch et al., 2007) and 22qtel (Stober et al., 2005). The more common presentations of catatonia arise in the setting of severe medical or psychiatric illness and have no prior genome wide association analyses reported in the literature.
The next steps in this line of research involve elucidating genetic risk factors for the development of catatonia in patients with rich phenotypic data. The overall aim of this investigation is to explore the genetic risk factors for the development of catatonia, in a genome wide association study (GWAS) of patients with catatonia regardless of etiology (psychiatric or medical). Secondarily, we aim to determine whether there is an elevated genetic risk profile for predisposing psychiatric disorders in patients who develop catatonia. We employed a matched case control study design and applied polygenic risk scores (schizophrenia, bipolar disorder, major depression, etc.) to test for a shared (latent) polygenetic contribution to catatonia from common psychiatric phenotypes that show a high prevalence of catatonia in their decompensated states.
Methods
Study sample
All analyses were conducted at Vanderbilt University Medical Center (VUMC), a tertiary care center in Nashville, TN. The Vanderbilt electronic health record (EHR) has been in use since 1994 and includes data such as the International Classification of Diseases, 9th and 10th editions (ICD-9 and ICD-10) billing codes, Current Procedural Terminology (CPT) codes, laboratory values, medication administration data and clinical documentation (e.g., admission history and physical, daily progress notes, clinic notes, discharge summaries, as well as others, etc.) by treating providers.
Vanderbilt established a de-identified mirror of their EHR for research purposes termed the Synthetic Derivative (SD), which includes health record data on over 3.5 million individuals and has been scrubbed via deletion or permutation of all identifiers. In 2007, VUMC inaugurated a biobank, termed BioVU, which includes genetic material linked to the SD. Consent for BioVU participation is performed in Vanderbilt’s outpatient clinics, where prospective participants are provided with the BioVU Consent Form and may sign if they wish to donate their excess blood sample(s), or not sign if they do not wish to participate in this repository (Roden et al., 2008). The Vanderbilt Institutional Review Board oversees the SD and BioVU resources and approved these projects. This work was conducted under an approved IRB #220658.
This linkage between the SD and BioVU is important, as this investigation requires high quality phenotyping, which cannot simply be obtained through algorithm refinement, alone. Because catatonia is a diverse condition, there are few ICD or CPT codes to represent the full spectrum of the disorder. Additionally, because catatonia is frequently seen as a complication stemming from a primary psychiatric or medical condition, its presence is sometimes relegated to clinical descriptions in the medical record and oftentimes not coded for in the diagnostic codes, which are relied upon by most SD algorithms. Working in conjunction with the Vanderbilt Phenotyping core, we developed a custom algorithm to identify a target cohort of potential catatonia cases using the SD (Table 1). A prospective set of 1,001 potential cases were returned from the final algorithm iteration.
Table 1.
Catatonia algorithm for BioVU analysis.
| Population: |
| SD |
| Include: |
| [ |
| ICD Code (1 or more of any): |
| ICD-9: |
| • 295.2 Catatonic type schizophrenia |
| • 295.20 Catatonic type schizophrenia, unspecified |
| • 295.21 Catatonic type schizophrenia, subchronic |
| • 295.22 Catatonic type schizophrenia, chronic |
| • 295.23 Catatonic type schizophrenia, subchronic with acute exacerbation |
| • 295.24 Catatonic type schizophrenia, chronic with acute exacerbation |
| • 295.25 Catatonic type schizophrenia, in remission |
| • 293.89 Other specified transient mental disorders due to conditions classified elsewhere, other |
| ICD-10: |
| • F06.1 Catatonic disorder due to known physiological condition |
| • F20.2 Catatonic schizophrenia |
| OR |
| Form (1 or more of either): |
| • Catatonia Rating Scale |
| • Bush Francis Catatonia Rating Scale |
| OR |
| Keyword (2 or more mentions of any keyword, in the same form, See Table 1.2 for list of forms): |
| See Table 1.1 for list of Keywords |
| ] |
| AND |
| [ |
| Medication (1 or more): |
| To be expanded |
| • lorazepam |
| • clonazepam |
| • diazepam |
| OR |
| Keyword (1 or more mention, in any form, See Table 1.2 for list of forms): |
| • ECT |
| • Electroconvulsive |
| OR |
| CPT Code (1 or more): |
| • 90870 Electroconvulsive therapy (includes necessary monitoring) |
| OR |
| ICD Code (1 or more of any): |
| ICD-9: |
| • 94.27 Other electroshock therapy |
| ICD-10: |
| • GZB0ZZZ Electroconvulsive Therapy, Unilateral-Single Seizure |
| • GZB1ZZZ Electroconvulsive Therapy, Unilateral-Multiple Seizure |
| • GZB2ZZZ Electroconvulsive Therapy, Bilateral-Single Seizure |
| • GZB3ZZZ Electroconvulsive Therapy, Bilateral-Multiple Seizure |
| • GZB4ZZZ Other Electroconvulsive Therapy |
Potential cases were reviewed by 2 blinded reviewers and any discrepant assignments were reviewed with an expert reviewer (JEW or SH). Confirmed catatonia cases were identified using pre-determined case criteria including 1) at least 3 catatonia signs / symptoms present using the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) criteria for catatonia, 2) suspected clinical catatonia presence as documented in the EHR by the treating physician and 3) positive response to treatment (e.g., clinical documentation of partial or full remission of catatonia signs after treatment with a benzodiazepine or electroconvulsive therapy, etc.). After final review, there were 147 confirmed catatonia cases, of which 119 (81%) had available genotyped data on the MEGAEX array and covariate information available in SD for use in this analysis. Using a 1:4 ratio, we matched cases to available controls based upon age, sex, and race.
Genotyping and quality control
In BioVU we had access to the genetic information for 94,474 individuals from different ethnic backgrounds who were genotyped on the Illumina MEGAEX array. Using established pipelines, genotypes were filtered for discrepancy between EHR-reported sex and number of X and Y chromosomes, excessive heterozygosity, SNP and individual call rates. Using principal component analyses, implemented in Eigenstrat (Patterson et al., 2006; Price et al., 2006) we selected individuals of European or African ancestry and confirmed the absence of genotyping batch effects using logistic regression with batch as the phenotype. We imputed missing genotypes using the Michigan Imputation Server (Genomes Project et al., 2015) and the Haplotype Reference Consortium (HRC) reference panel. SNPs were filtered for imputation quality (R2 > 0.3) and using the default dosage to hard call parameters. SNPs were limited to autosomes and then filtered for a minor allele frequency (MAF) <0.005 or for differing by >10% for the 1000 Genomes Project phase 3 CEU or ASW set (Genomes Project et al., 2015) and Hardy Weinberg Equilibrium (p > 1×10−10). Using this approach, we were left with a dataset with 6,303,629 SNPs on 72,824 individuals of European genetic ancestries and 12,798,111 SNPs on 15,283 individuals of African genetic ancestries. Matched controls were selected from this sample.
GWAS analyses and Polygenic scoring
We completed a GWAS using SAIGE (Scalable and Accurate Implementation of GEneralized mixed model) as previously described (Zhou et al., 2018) on the 119 catatonia cases and matched controls. We obtained polygenic risk scores (PRS) using PRS-CS (Ge et al., 2019) using SNP weights from the largest meta-analyses for the following psychiatric disorders: depression (Howard et al., 2019), anxiety (Purves et al., 2020), bipolar affective disorder (Mullins et al., 2021), schizophrenia spectrum disorders (Trubetskoy et al., 2022), Alzheimer’s disease (Bellenguez et al., 2022), autism spectrum disorders (Autism Spectrum Disorders Working Group of The Psychiatric Genomics, 2017), obsessive compulsive disorder (International Obsessive Compulsive Disorder Foundation Genetics and Studies, 2018) and cross disorders (Cross-Disorder Group of the Psychiatric Genomics Consortium. Electronic address and Cross-Disorder Group of the Psychiatric Genomics, 2019).
Due to the pleiotropic and highly comorbid nature of psychiatric disorders we elected to include this cross disorder sample, which performed analyses on 232,964 cases and 494,162 controls from genome wide association studies of participants with anorexia nervosa, attention-deficit / hyperactivity disorder, autism spectrum disorder, bipolar disorder, major depressive disorder, obsessive compulsive disorder, schizophrenia spectrum illnesses, and Tourette syndrome (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2019). Meta analysis across these studies detected 109 loci associated with at least two psychiatric disorders, including 23 loci with pleiotropic effects on four or more disorders and 11 loci with antagonistic effects on multiple disorders.
PRS-CS employs a Bayesian polygenic prediction approach that uses an external reference panel from the 1000 Genomes Project phase 3 (Genomes Project et al., 2015) with 503 European ancestry samples to model linkage disequilibrium (LD) and imposes continuous shrinkage priors on SNP effect sizes with these priors representing a mixture of normals, allowing the model to adapt to a variety of genetic architectures. PRS were scaled to have a mean of zero and standard deviation (SD) of one, to allow for clarity of interpretation of effect estimates in analyses to follow.
Once PRS were scaled, using a case control study design, we evaluated the association between polygenic risk scores for depression, anxiety, bipolar affective disorder, schizophrenia spectrum disorders, Alzheimer’s disease, autism spectrum disorders, obsessive compulsive disorder as well as cross disorders with catatonia case status using both an unadjusted traditional logistic regression model, which included covariates for age, race, sex, and top ten principal components estimated from genetic data, but was unadjusted for psychiatric history. Even though cases and controls were matched for age, sex and race, we additionally adjusted for age, sex, race and genetic ancestry principal components to account for additional potential confounding not handled by matching alone as the initial matching was done via a group “best match” approach and was not matched 1-to-1. Principal components generated from the genetic data (PC1–10) were calculated separately for the European and African Ancestry populations using established pipelines and were plotted against each other to ensure quality control.
Adjusted analyses were built upon the unadjusted models and were further adjusted for past psychiatric history of the referent PRS being tested in each model. For example, the logistic model that included a depression PRS term was also adjusted for a history of depression as indicated by ICD 9 and 10 codes for depression. For the model that included the cross disorder PRS, all ICD 9 and 10 covariates such as depression, anxiety, bipolar affective disorder, schizophrenia spectrum disorders, Alzheimer’s disease, autism spectrum disorders, obsessive compulsive disorder as well as an “Other” composite covariate representing all other mental health codes not represented by the ones listed above (e.g., personality disorders, substance use disorders, post-traumatic stress disorder, other cognitive disorders, etc.) were included. ICD 9 and 10 codes for mental disorders in both cases and controls were extracted from the SD and reviewed by psychiatrist JEW. Participants were allowed to have as many ICD 9 or 10 psychiatric diagnoses as were recorded for them in the SD. To correct for multiple comparisons, all p-values were adjusted using the Benjamini-Hochberg Adjusted p-value approach.
To visualize the relationship between the polygenic risk scores of psychiatric disorders and odds of catatonia, decile plots were generated, which illustrate the odds ratio (95% confidence interval) for catatonia by decile of polygenic risk, with the lowest decile as the referent. Decile plots were only generated for the European Ancestry set as the African Ancestry set was underpowered and showed no significant association between psychiatric polygenic risk scores and catatonia status.
European and African Ancestry sets were evaluated in separate logistic regression models. All analyses were conducted in R (R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
Results
Our analysis included 119 BioVU participant catatonia cases matched to 510 controls, for a total of 629 participants. 456 participants were of European Ancestry (86 cases matched to 370 controls) and 173 participants were of African Ancestry (33 cases matched to 140 controls). Participants of European Ancestry had a median (IQR) age of 52 (33 – 75) years whereas participants of African Ancestry had a median age of 38 (24 – 62) years. 345 participants (54.9%) were female, and 284 (45.2%) were male.
The most common psychiatric disorder in participants of both European and African Ancestry was a depressive disorder, with 145 (31.8%) of the European Ancestry and 37 (21.4%) of the African Ancestry participants having an ICD 9 or 10 code for the disorder in the SD. Anxiety, Bipolar Affective Disorder and Schizophrenia Spectrum Disorders were also common in our patient population. Almost a third of the entire cohort had another psychiatric disorder code aside from those diagnoses represented above (e.g., eating disorder, personality disorder, etc.) recorded in their SD chart. No participants of European Ancestry or African Ancestry had a code for Alzheimer’s Disease or obsessive compulsive disorder, respectively. Additional ICD 9 and 10 codes for participants are described in Table 2.
Table 2.
Proportion of ICD 9 and 10 codes amongst participants.
| Psychiatric Disorder Category* |
European Ancestry N = 456, (%) |
African Ancestry N = 173, (%) |
|---|---|---|
| Depression | 145 (31.8%) | 37 (21.4%) |
| Anxiety | 85 (18.6%) | 19 (11%) |
| Bipolar Affective Disorder | 42 (9.2%) | 5 (2.9%) |
| Schizophrenia Spectrum Disorder | 40 (8.8%) | 20 (11.6%) |
| Alzheimer’s Disease | 0 | 1 (0.6%) |
| Autism Spectrum Disorder | 9 (2%) | 1 (0.6%) |
| Obsessive Compulsive Disorder | 17 (3.7%) | 0 |
| Other Psychiatric Disorder | 135 (29.6%) | 54 (31.2%) |
individuals may be diagnosed with >1 disorder and appear under >1 category. As such the % may add up to >100%.
The genomic inflation factor λ (or lambda) for the European Ancestry GWAS was 0.99 and the lambda for the African Ancestry GWAS was 1.04, which was considered adequate. Summary Figures including quantile-quantile (QQ) plots, regression coefficient histograms, and Manhattan plots for both the European Ancestry (Figures 1a–c) and African Ancestry GWAS are provided in Figures 2a–c below. No SNP exceeded the genome-wide threshold for statistical significance.
Figure 1.


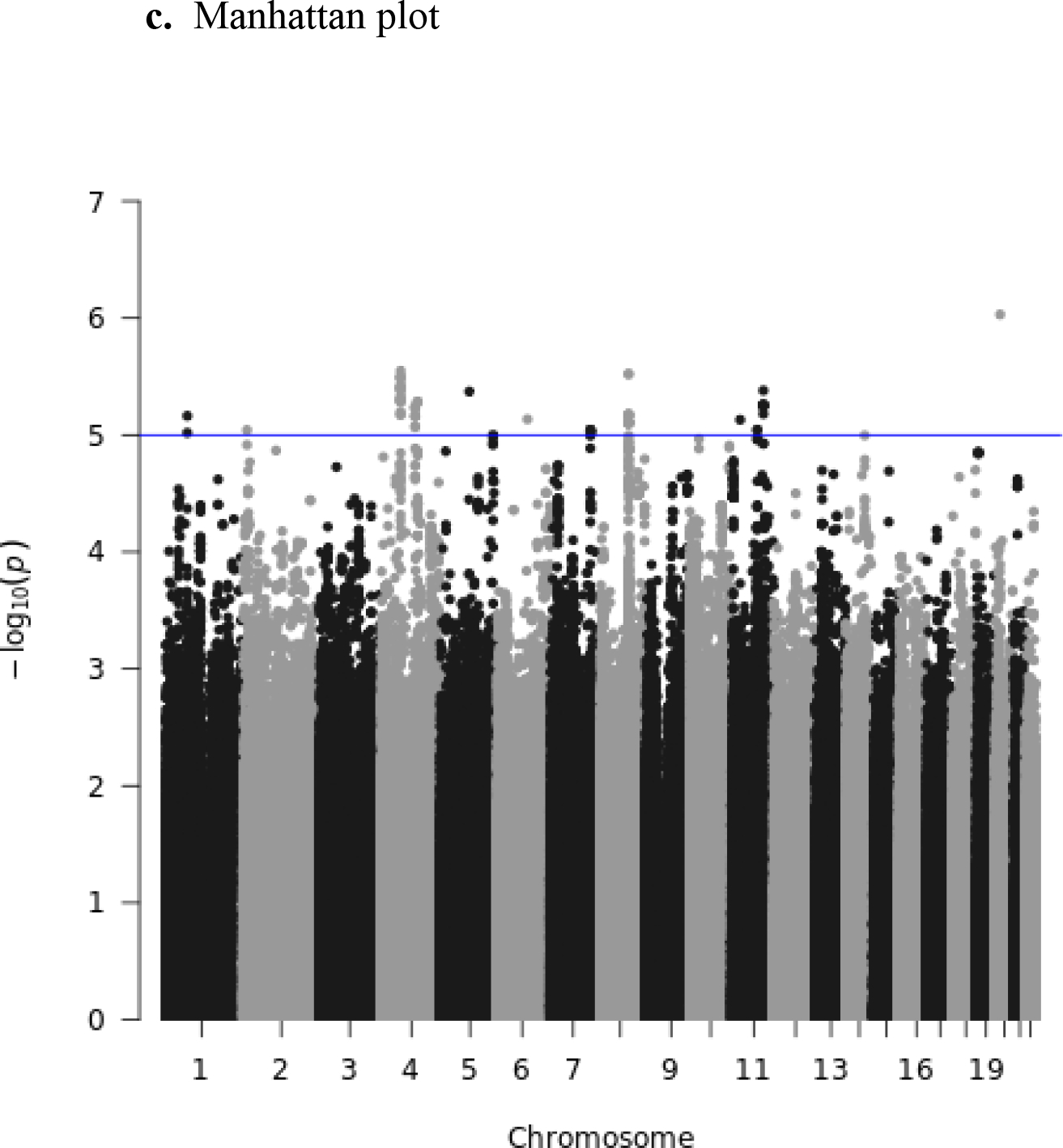
European Ancestry plots.
Figure 2.

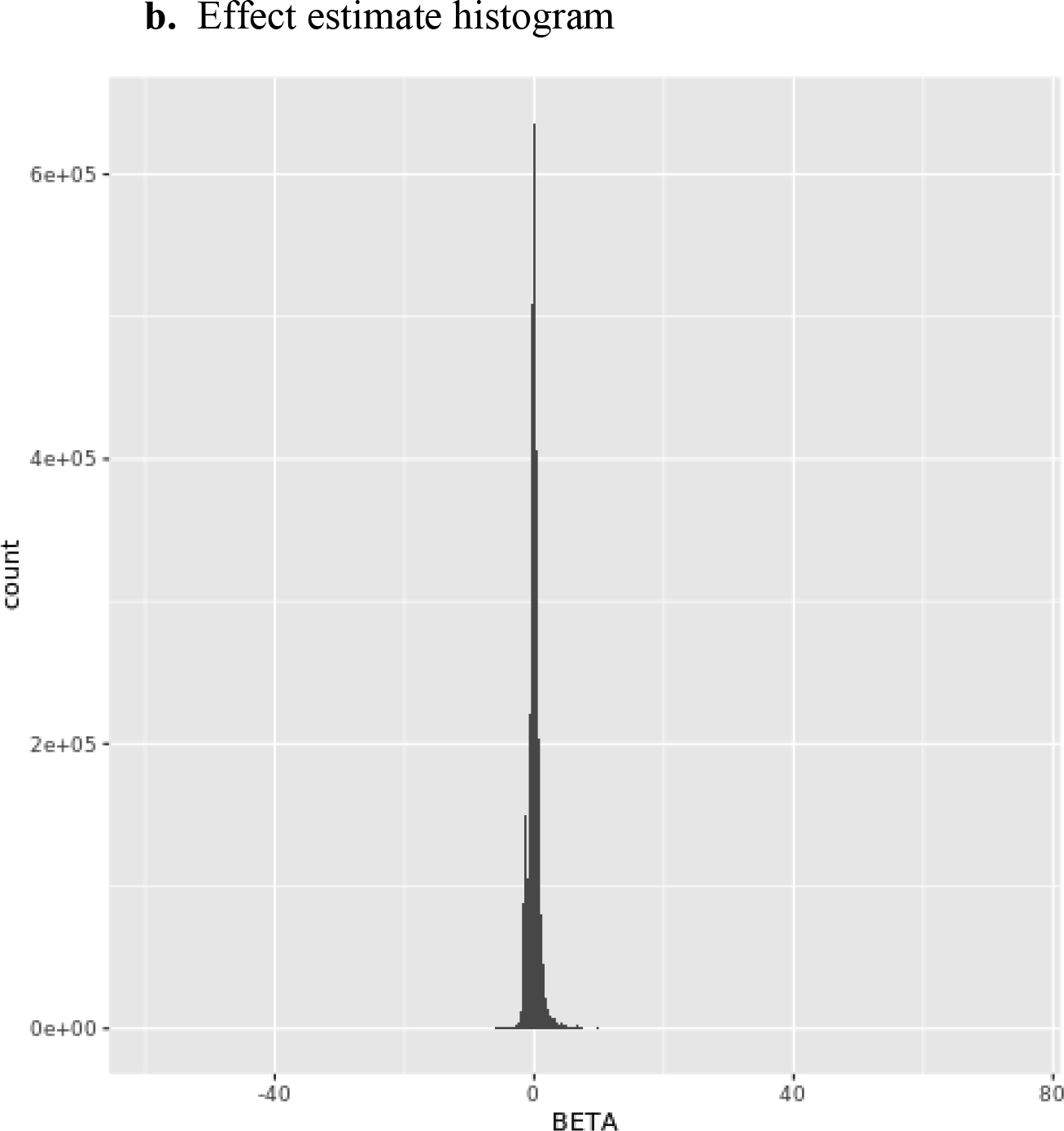

African Ancestry plots.
Anxiety, Bipolar Affective Disorder, Schizophrenia Spectrum Disorder and Cross Disorder polygenic risk scores were significantly associated with catatonia case status in both unadjusted (Table 3) and adjusted (Table 4) logistic regression models for the European Ancestry set even after correcting for multiple comparisons (refer to Figure 3 for decile plots of adjusted logistic regression models for European Ancestry set). Depression, Alzheimer’s, Autism Spectrum Disorder and Obsessive Disorder polygenic risk scores were not significantly associated with catatonia status in participants of European Ancestry. In the African Ancestry set, no psychiatric polygenic risk scores were significantly associated with catatonia status in either the unadjusted (Table 5) or adjusted (Table 6) regression models. Despite the non-significance of the OR for the African ancestry set, the bipolar disorder ORs for both the unadjusted and adjusted analyses were similar in magnitude to the bipolar ORs for the European ancestry analyses.
Table 3.
Unadjusted^ polygenic risk scores for European Ancestry set.
| OR (95% CI) | Standard Error | p-value | Corrected* p-value |
|
|---|---|---|---|---|
| Depression | 1.260 (0.986, 1.610) | 0.1251 | 0.0651 | 0.1042 |
| Anxiety | 1.543 (1.192, 1.997) | 0.1318 | 0.0010 | 0.0020 |
| Bipolar Disorder | 1.771 (1.361, 2.304) | 0.1343 | 0.00002 | 0.00008 |
| Cross Disorders | 1.859 (1.414, 2.444) | 0.1396 | 0.000009 | 0.00007 |
| Schizophrenia SDs | 1.713 (1.297, 2.261) | 0.1418 | 0.0002 | 0.0004 |
| Alzheimer’s Disease | 1.061 (0.815, 1.342) | 0.1278 | 0.6459 | 0.8612 |
| Autism Spectrum Disorders | 1.046 (0.815, 1.342) | 0.1270 | 0.7235 | 0.8269 |
| Obsessive Compulsive Disorder | 1.006 (0.786, 1.287) | 0.1259 | 0.9646 | 0.9646 |
polygenic scores were adjusted for age, sex, race, and ancestral principal components, but were not adjusted for a history of that disorder.
Corrected using Benjamini-Hochberg Adjusted p-value
Table 4.
Adjusted^ polygenic risk scores for European Ancestry set.
| OR (95% CI) | Standard Error | p-value | Corrected* p-value |
|
|---|---|---|---|---|
| Depression | 1.132 (0.866, 1.482) | 0.1374 | 0.3681 | 0.5890 |
| Anxiety | 1.515 (1.162, 1.975) | 0.1354 | 0.0022 | 0.0087 |
| Bipolar Disorder | 1.718 (1.281, 2.304) | 0.1498 | 0.0003 | 0.0025 |
| Cross Disorders | 1.814 (1.185, 2.777) | 0.2172 | 0.0061 | 0.0163 |
| Schizophrenia SDs | 1.586 (1.122, 2.240) | 0.1764 | 0.0090 | 0.0179 |
| Alzheimer’s Disease | 1.061 (0.826, 1.362) | 0.1278 | 0.6459 | 0.8612 |
| Autism Spectrum Disorders | 0.997 (0.774, 1.284) | 0.1289 | 0.9805 | 1 |
| Obsessive Compulsive Disorder | 1.0003 (0.778, 1.287) | 0.1285 | 0.9980 | 0.9980 |
polygenic scores were adjusted for age, sex, race, and ancestral principal components, and were further adjusted for a history of that mental illness, for example: depression scores were adjusted for a history of depression as indicated by ICD 9 and 10 codes for depression, anxiety was adjusted for a history of anxiety according to ICD 9 and 10 codes for anxiety, etc. For cross disorders, all ICD 9 and 10 covariates (depression, anxiety, schizophrenia spectrum disorders, Alzheimer’s disease, autism spectrum disorders, obsessive compulsive disorder and a composite covariate representing all other mental health codes not represented by the ones listed above was included.)
Corrected using Benjamini-Hochberg Adjusted p-value
Figure 3:

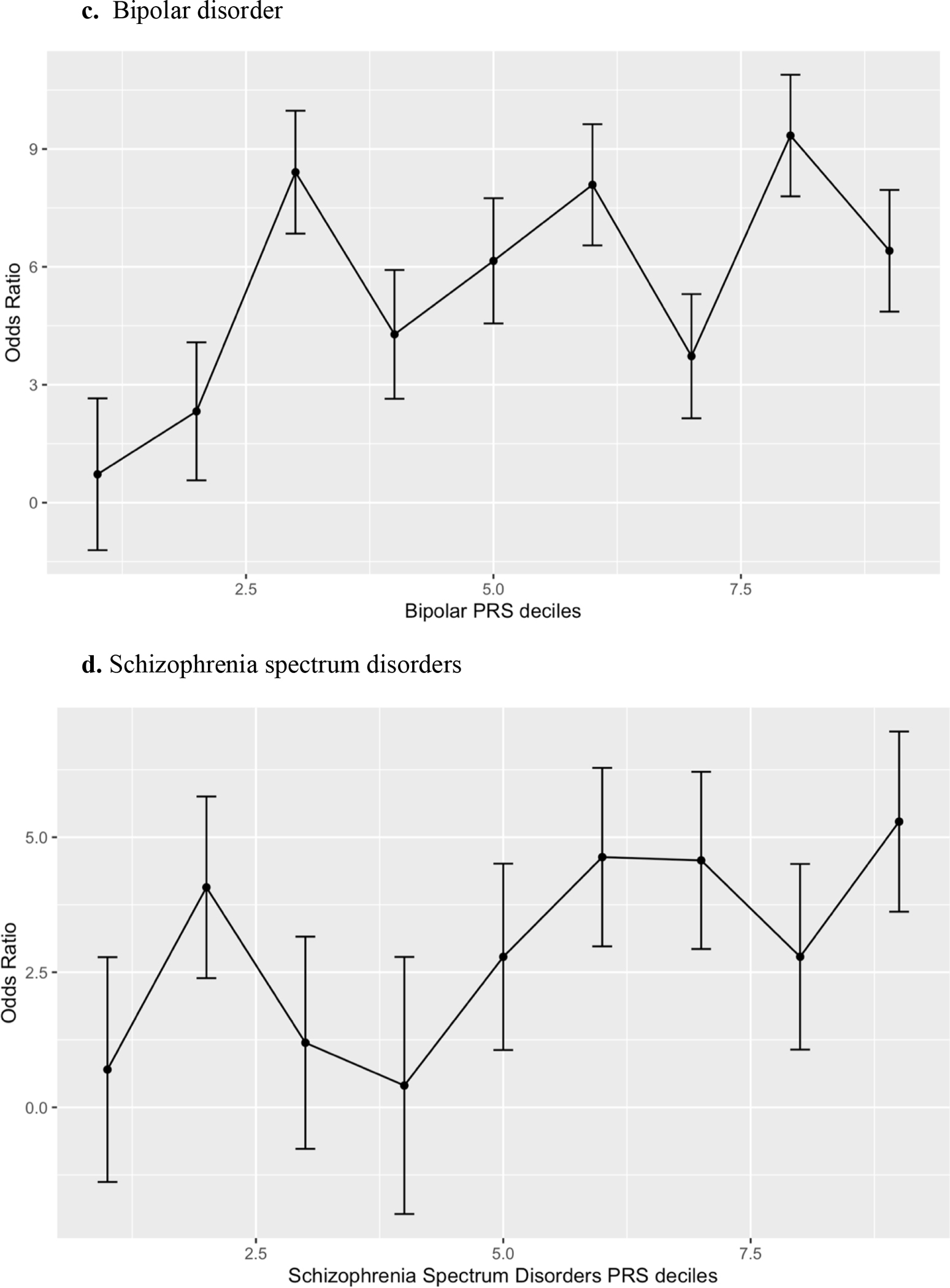

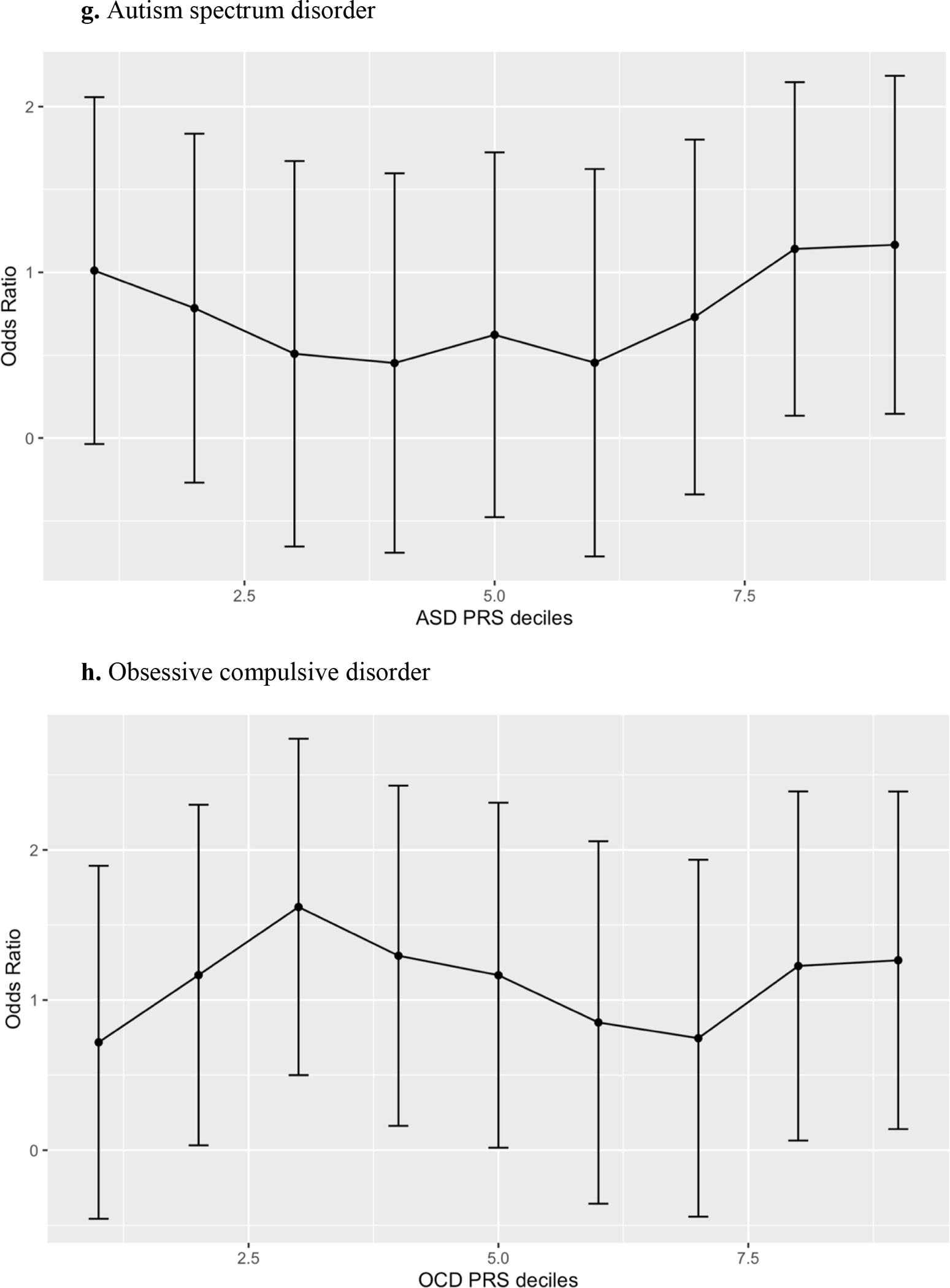
Decile plots* for the European Ancestry PRS models.
*decile of psychiatric disorder polygenic risk score were plotted against the referent (lowest decile).
Table 5.
Unadjusted^ polygenic risk scores for African Ancestry set.
| OR (95% CI) | Standard Error | p-value | Corrected* p-value |
|
|---|---|---|---|---|
| Depression | 0.882 (0.576, 1.353) | 0.2179 | 0.5659 | 0.9054 |
| Anxiety | 0.936 (0.624, 1.406) | 0.2074 | 0.7510 | 0.8583 |
| Bipolar Disorder | 1.741 (1.072, 2.828) | 0.2475 | 0.0251 | 0.2008 |
| Cross Disorders | 1.308 (0.737, 2.324) | 0.2930 | 0.3588 | 0.7176 |
| Schizophrenia SDs | 1.341 (0.863, 2.084) | 0.2249 | 0.1924 | 0.5131 |
| Alzheimer’s Disease | 1.469 (0.936, 2.303) | 0.2297 | 0.0943 | 0.3772 |
| Autism Spectrum Disorders | 1.085 (0.681, 1.729) | 0.2379 | 0.7324 | 0.9765 |
| Obsessive Compulsive Disorder | 0.974 (0.633, 1.499) | 0.2198 | 0.9050 | 0.9050 |
polygenic scores were adjusted for age, sex, race, and ancestral principal components, but were not adjusted for a history of that disorder.
Corrected using Benjamini-Hochberg Adjusted p-value
Table 6.
Adjusted^ polygenic risk scores for African Ancestry set.
| OR (95% CI) | Standard Error | p-value | Corrected* p-value |
|
|---|---|---|---|---|
| Depression | 1.218 (0.748, 1.982) | 0.2486 | 0.428 | 1 |
| Anxiety | 1.032 (0.660, 1.614) | 0.2281 | 0.8907 | 1 |
| Bipolar Disorder | 1.709 (1.025, 2.850) | 0.2609 | 0.0399 | 0.3192 |
| Cross Disorders | 1.390 (0.441, 4.380) | 0.5854 | 0.5735 | 1 |
| Schizophrenia SDs | 1.168 (0.582, 2.344) | 0.3556 | 0.6630 | 1 |
| Alzheimer’s Disease | 1.469 (0.936, 2.303) | 0.2297 | 0.0943 | 0.3772 |
| Autism Spectrum Disorders | 1.054 (0.660, 1.682) | 0.2386 | 0.826 | 1 |
| Obsessive Compulsive Disorder | 0.974 (0.633, 1.499) | 0.2200 | 0.905 | 0.9050 |
polygenic scores were adjusted for a history of that mental illness, for example: depression scores were adjusted for a history of depression as indicated by ICD 9 and 10 codes for depression, anxiety was adjusted for a history of anxiety according to ICD 9 and 10 codes for anxiety, etc. For cross disorders, all ICD 9 and 10 covariates (depression, anxiety, schizophrenia spectrum disorders, Alzheimer’s disease, autism spectrum disorders, obsessive compulsive disorder and a composite covariate representing all other mental health codes not represented by the ones listed above was included.)
Corrected using Benjamini-Hochberg Adjusted p-value
Discussion
In this study, anxiety disorder, bipolar affective disorder, schizophrenia spectrum disorder and cross disorder polygenic risk scores were significantly associated with an increased odds of catatonia, even after controlling for demographic and ethnicity differences as well as past psychiatric history in both cases and controls. Historically, affective disorders, schizophrenia spectrum disorders as well as a variety of other psychiatric disorders have been linked with catatonia in their decompensated states. Prior to the release of DSM-5 in 2013 (Association, 2013), catatonia was recognized as a subtype of schizophrenia. Now however, catatonia can be coded as a specifier for many psychiatric and medical conditions and unspecified catatonia was added for cases where underlying etiology was unclear, making it easier for the clinician to diagnose catatonia. Some have considered catatonia to be a final common pathway (Northoff, 2002) for many psychiatric and non-psychiatric disorders that affect psychomotor behavior, affect and volition, and in part this may explain the shared genetic risk between catatonia and multiple psychiatric disorders (anxiety, bipolar disorder, schizophrenia spectrum disorders, etc.).
The prevalence of catatonia varies between psychiatric disorders. In a prospective cohort study by Kleinhaus et al., 7.6% of cases of schizophrenia had the catatonic subtype (Kleinhaus et al., 2012). An even higher prevalence of catatonia has been described by Taylor and Abrams in affective disorders, with up to 20% or more of patients with bipolar affective disorder experiencing catatonia (Abrams and Taylor, 1976, 1977; Abrams et al., 1979; Taylor and Abrams, 1973). In a clinical sample of patients with catatonia (N=55), two-thirds of patients had affective disorders, typically mania, whereas only 5 patients with catatonia satisfied criteria for schizophrenia (Abrams and Taylor, 1976).
To our knowledge, this is the first reporting of the association between the anxiety polygenic score and catatonia. No major anxiety disorder has been classically linked with catatonia, however catatonia has been described by some as a heightened fear state or a freezing phenomenon described in animals in response to fear inducing stimuli such as a predator, leading some to suggest that catatonia may be a retained evolutionary fear response (Moskowitz, 2004). Additionally, patients who experience catatonia, particularity catatonic stupor describe an intense anxiety or fear phenomenon (Cuevas-Esteban et al., 2020; Northoff et al., 1998; Northoff et al., 1996; Perkins, 1982; Rosebush and Mazurek, 2010). One potentially explanatory theory of the pathophysiology of catatonia is dysfunction of gamma-amino-butyric-acid (GABA) neural circuit. Studies have demonstrated a reduction in GABA-ergic binding in the right orbitofrontal cortex (OFC) (Northoff et al., 1999), as well as GABAA receptor abnormalities in the medial and lateral OFC, prefrontal and posterior parietal cortices (Hirjak et al., 2020) in patients with catatonia. Catatonia’s linkage to anxiety and to abnormal GABA-ergic circuitry could explain in part, why benzodiazepines (potent anxiolytics) are the treatment of choice for catatonia and in many cases bring about resolution of the disorder (Rosebush et al., 1990), as they have also been shown to regulate OFC signaling during emotional processing in catatonia (Richter et al., 2010).
We found no evidence of depression or autism spectrum disorder PRS association with catatonia in our study. Catatonia has long been linked to mood disorders, with Major Depressive Disorder (MDD), being one of only a few DSM-5 diagnoses (Association, 2013) to have some overlap in diagnostic criteria with catatonia. This overlap includes the presence of psychomotor changes (increased or decreased from baseline), and withdrawal (including changes in oral intake, etc.), occurring in both disorders. Catatonic inhibition (mutism, rigidity, staring, posturing / immobility, etc.) could represent a severe form of psychomotor retardation typically seen in more advanced cases of depression. Not described in diagnostic criteria but often seen in both catatonia and MDD are a blunted range of affect and decreased social interaction, with catatonic mutism and withdrawal representing their most severe presentation. Interestingly, Depression PRS were not significantly associated with catatonia in any analysis. It is possible that high genetic heterogeneity in both depression and catatonia may have decreased power to detect an association between genetic variation that contributes to both phenotypes.
Both Autism Spectrum Disorders and catatonia have shared criteria including stereotypies such as repeated motor movements or stereotyped speech such as echolalia (repetition of another’s speech), restricted range of affect, abnormalities in eye contact (staring representing a severe form in catatonia) as well as other deficits in socio-emotional display and reciprocity (Association, 2013). Above and beyond their overlap, patients with autism have a higher prevalence of catatonia (about 1 in 7) (Kakooza-Mwesige et al., 2008) than the general public. Again, no association was detected between the ASD polygenic score and catatonia, suggesting either alternative genetic risk that manifests in similar phenotypic presentations, or genetic heterogeneity that impinged on our power to detect a true association.
None of the African ancestry PRSs were associated with catatonia which is likely due to the smaller sample size and predominantly European ancestry GWAS used to train the PRS. Poor diversity within GWAS is a known problem in the field and has spurred new initiatives to increase diverse representation in genomic studies. Additional age-related heterogeneity may be present in the African ancestry sample for which the median age at catatonia diagnosis was 14 years earlier than in the European ancestry set. Our previous work (Connell et al., 2021) in a prospectively obtained critically ill sample, showed that catatonia prevalence increases with increasing age. The reason for this increased prevalence of catatonia with age is not fully understood but may be related to a number of neurotransmitter level as well as structural level changes that take place in the aging brain, for example altered connectivity within the motor circuit (Walther et al., 2019), reduced GABA-A receptor density (Iseki et al., 2009; Northoff et al., 1999), reduced dopamine levels with aging (Mukherjee et al., 2002) and accumulating vascular changes including white matter lesions (e.g., microinfarcts, microvascular disease, etc.) (Peters, 2006), and cerebrovascular accidents which are also associated with increased dementia risk (Bartzokis et al., 2003; Elias et al., 2004; Head et al., 2004; Lobo et al., 2000), and whose effect on the development of catatonia cannot be wholly adjusted away by matching and controlling for age in the regression model. Given that age is a known risk factor, and the African ancestry sample experienced onset of catatonia at a much earlier age, this raises the hypothesis that this population may also be experiencing additional non-genetic factors that increase risk.
Our study has several important limitations. First the sample size was very small. Future studies should examine these questions in a larger sample. Additionally, although our genetic data came from a large DNA repository, it was from a single site, so this could affect generalizability of our findings. Secondly, although we took great care to try to minimize misclassification of catatonia status through double blinded chart review with expert adjudication, case status was determined retrospectively, thus it is possible that participants who had or did not have catatonia were assigned incorrectly to the wrong case status. Additionally, past psychiatric history was obtained from ICD 9 and 10 codes that were assigned to diagnostic categories. Further misclassification bias could have resulted here, however if so, it would likely have been nondifferential, and would have further biased results toward the null. Robust neuroimaging studies have cast light on some mechanistic contributors to the motor and psychomotor features of catatonia. Future genetic studies could employ an integrative multi-modal inference-based approach to exploring mechanistic pathways involved in catatonia development. Future biomarker studies could explore shared mechanistic pathways via joint genetics and neuroimaging approach to catatonia.
This study has several important strengths. First, to the best of our knowledge, this study represents one of the first GWAS of catatonia in the published literature. The polygenic risk score analysis further allowed us to explore the genetic architecture of catatonia with common psychiatric disorders with existing high quality genetic data.
Conclusions
Anxiety Disorder, Bipolar Affective Disorder, Schizophrenia Spectrum Disorder and Cross Disorder polygenic risk scores were significantly associated with an increased odds of catatonia, even after controlling for relevant covariates, suggesting that there might be a shared genetic risk for those disorders amongst patients with catatonia in our cohort.
Table 1.1.
Keywords (Include like words or misspellings)
| excit* | Rigid* |
| Immobil* | Stiff |
| stupor* | increased tone |
| mutism | Negativis* |
| mute | Waxy |
| staring | Impulsivity |
| stare | automatic obedience |
| Postur* | Mitgehen |
| catalep* | anglepoise lamp |
| Grimac* | gegenhalten |
| echopraxia | ambitendency |
| echolalia | Perseverat* |
| echophenomenon | Combative* |
| echo | autonomic abnormality |
| repetition | trial |
| Repeat* | challenge |
| Stereotyp* | catatonia |
| verbigeration | catatonic |
| verbigerate |
Table 1.2 –
Starforms
| <Hospital Discharge> Discharge Summary (Psychiatry) |
| <Hospital Discharge> Discharge Summary/Note - Psychiatry |
| <Hospital Discharge> Discharge Summary/Note - Psychiatry (Inpatient) |
| <Hospital Discharge> Discharge Summary/Note - Psychiatry (Outpatient) |
| <Clinic Note> Initial Psychiatry Evaluation |
| <Clinic Note> Psychiatry Initial Evaluation |
| <Clinic Note> Psychiatry Initial Outpatient Evaluation for Medication Providers |
| <Clinic Note> Psychiatry Initial Outpatient Evaluation for Clinician Providers |
| <History and Physical Reports> Initial Psychiatric Evaluation (Hospital Based) |
| <Clinic Note> History & Physical - Psychiatry |
| <Clinic Note> History & Physical - Psychiatry (Consultation ) |
| <Clinic Note> History & Physical - Psychiatry (Consultation) |
| <Clinic Note> History & Physical - Psychiatry (Crisis Assessment) |
| <Clinic Note> History & Physical - Psychiatry (Inpatient) |
| <Clinic Note> History & Physical - Psychiatry (Outpatient) |
| <History and Physical Reports> Consultation Note - Psychiatry |
| <History and Physical Reports> History & Physical - Psychiatry |
| <History and Physical Reports> History & Physical - Psychiatry () |
| <History and Physical Reports> History & Physical - Psychiatry (Consultation ) |
| <History and Physical Reports> History & Physical - Psychiatry (Consultation) |
| <History and Physical Reports> History & Physical - Psychiatry (Crisis Assessment) |
| <History and Physical Reports> History & Physical - Psychiatry (Emergency Psychiatry) |
| <History and Physical Reports> History & Physical - Psychiatry (Inpatient) |
| <History and Physical Reports> History & Physical - Psychiatry (Outpatient) |
| <History and Physical Reports> History and Physical - Psychiatry |
| History & Physical - Psychiatry |
| History & Physical - Psychiatry (Crisis Assessment) |
| History & Physical - Psychiatry (Inpatient) |
| History & Physical - Psychiatry (Outpatient) |
| History & Physical: Psychiatry (Consultation) |
| Psychiatric Admission Evaluation |
| Admission Note - Psychiatry |
| VPH Psychiatric Admission Evaluation |
| <Clinic Note> Progress Note - Psychiatry |
| <Clinic Note> Progress Note - Psychiatry (Outpatient) |
| <Clinic Note> Progress Note - Psychiatry (psych) |
| <Clinic Note> Psychiatry Progress Note |
| <Notes> Daily Progress Note (Psychiatry) |
| <Notes> Progress Note - Psychiatry |
| <Notes> Progress Note - Psychiatry (Inpatient) |
| <Notes> Progress Note - Psychiatry (Outpatient) |
| <Notes> Psychiatry Progress Note |
| Psychiatry Progress Note |
| <Nursing Documentation> VPH Nursing Discharge Note |
| <Clinic Note> Initial MHC Evaluation |
| <Notes> VPH Inpatient Progress Note |
| Emergency Psychiatry Consultation Note |
| <Clinic Note> Emergency Psychiatry Consultation Progress Note |
| <Clinic Note> Psychiatry Consult Service Progress Note |
| <Clinic Note> Progress Note - Psychiatry (Consultation) |
| <Notes> Progress Note - Psychiatry (Consultation) |
| <Notes> Psychiatry Consult Service Progress Note |
| <Notes> Progress Note - Psychiatry (Emergency Psychiatry) |
| <Clinic Note> Psychiatry IP Consultation |
| Psychiatry IP Consultation |
| Medical Student Psychiatry C/L Service Follow-up |
| Electroconvulsive Therapy Note (Psychiatry) |
| <Consultation> Consultation Note (ECT) |
| <Clinic Note> Outpatient Psychiatry Progress Note with E/M Services |
| <Clinic Note> Outpatient Psychiatry Progress Note with E/M Services PSL |
| <Clinic Note> Outpatient Psychiatry Progress Note without E/M Services |
| <Clinic Note> Outpatient Psychiatry Medication Provider Progress Note |
| <Clinic Note> Psychosomatic Medicine Consultation Follow-up |
| Child Psychiatry Consult Note |
| Child Psychiatry Consult Progress Note |
| Child Psychiatry Consult-Attending Note |
| Pediatric Psychiatry Consultation |
| Child PsychiatryService Evaluation |
| Psychiatry Intake Evaluation |
| Psychiatry Intake Evaluation-MHC |
| <Clinic Note> Intake Assessment - Psychiatry (Initial Contact) |
| Intake Assessment - Psychiatry (Initial Contact) |
| geropsychiatry clinic progress note |
| <Clinic Note> Geropsychiatry Clinic Note |
| <Clinic Note> Geropsychiatry Initial Evaluation |
| Geropsychiatry Evaluation (Dementia) |
| <Clinic Note> Geropsychiatry Evaluation (Dementia) |
| Stallworth C/L Psychiatry Initial Consult |
| VPH Partial Program Psychiatry Progress Note |
| VUH C/L Psychiatry Initial Consult |
| VUH C/L Psychiatry Progress Note |
| Psychiatry Triage Admission Note |
Acknowledgement:
We would like to thank the generous gift of the Jenkins family (Blake A. Jenkins Discovery award) which helped to fund this research.
Role of the Funding Source:
This work was supported by the Blake A. Jenkins Discovery Award. Additionally, Dr. Wilson, Dittus and Ely receive support for their time by the Veterans Administration Geriatric Research, Education and Clinical Center.
Footnotes
Declaration of Interest: The authors disclose that the research was conducted in the absence of any commercial or financial relationships that could be seen as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Abrams R, Taylor MA, 1976. Catatonia. A prospective clinical study. Arch Gen Psychiatry 33(5), 579–581. [DOI] [PubMed] [Google Scholar]
- Abrams R, Taylor MA, 1977. Catatonia: prediction of response to somatic treatments. Am J Psychiatry 134(1), 78–80. [DOI] [PubMed] [Google Scholar]
- Abrams R, Taylor MA, Coleman Stolurow KA, 1979. Catatonia and mania: patterns of cerebral dysfunction. Biol Psychiatry 14(1), 111–117. [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. The Diagnostic and Statistical Manual of Mental Disorders, 5th ed. [Google Scholar]
- Autism Spectrum Disorders Working Group of The Psychiatric Genomics, C., 2017. Meta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol Autism 8, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J, 2003. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol 60(3), 393–398. [DOI] [PubMed] [Google Scholar]
- Bellenguez C, Kucukali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N, Naj AC, Campos-Martin R, Grenier-Boley B, Andrade V, Holmans PA, Boland A, Damotte V, van der Lee SJ, Costa MR, Kuulasmaa T, Yang Q, de Rojas I, Bis JC, Yaqub A, Prokic I, Chapuis J, Ahmad S, Giedraitis V, Aarsland D, Garcia-Gonzalez P, Abdelnour C, Alarcon-Martin E, Alcolea D, Alegret M, Alvarez I, Alvarez V, Armstrong NJ, Tsolaki A, Antunez C, Appollonio I, Arcaro M, Archetti S, Pastor AA, Arosio B, Athanasiu L, Bailly H, Banaj N, Baquero M, Barral S, Beiser A, Pastor AB, Below JE, Benchek P, Benussi L, Berr C, Besse C, Bessi V, Binetti G, Bizarro A, Blesa R, Boada M, Boerwinkle E, Borroni B, Boschi S, Bossu P, Brathen G, Bressler J, Bresner C, Brodaty H, Brookes KJ, Brusco LI, Buiza-Rueda D, Burger K, Burholt V, Bush WS, Calero M, Cantwell LB, Chene G, Chung J, Cuccaro ML, Carracedo A, Cecchetti R, Cervera-Carles L, Charbonnier C, Chen HH, Chillotti C, Ciccone S, Claassen J, Clark C, Conti E, Corma-Gomez A, Costantini E, Custodero C, Daian D, Dalmasso MC, Daniele A, Dardiotis E, Dartigues JF, de Deyn PP, de Paiva Lopes K, de Witte LD, Debette S, Deckert J, Del Ser T, Denning N, DeStefano A, Dichgans M, Diehl-Schmid J, Diez-Fairen M, Rossi PD, Djurovic S, Duron E, Duzel E, Dufouil C, Eiriksdottir G, Engelborghs S, Escott-Price V, Espinosa A, Ewers M, Faber KM, Fabrizio T, Nielsen SF, Fardo DW, Farotti L, Fenoglio C, Fernandez-Fuertes M, Ferrari R, Ferreira CB, Ferri E, Fin B, Fischer P, Fladby T, Fliessbach K, Fongang B, Fornage M, Fortea J, Foroud TM, Fostinelli S, Fox NC, Franco-Macias E, Bullido MJ, Frank-Garcia A, Froelich L, Fulton-Howard B, Galimberti D, Garcia-Alberca JM, Garcia-Gonzalez P, Garcia-Madrona S, Garcia-Ribas G, Ghidoni R, Giegling I, Giorgio G, Goate AM, Goldhardt O, Gomez-Fonseca D, Gonzalez-Perez A, Graff C, Grande G, Green E, Grimmer T, Grunblatt E, Grunin M, Gudnason V, Guetta-Baranes T, Haapasalo A, Hadjigeorgiou G, Haines JL, Hamilton-Nelson KL, Hampel H, Hanon O, Hardy J, Hartmann AM, Hausner L, Harwood J, Heilmann-Heimbach S, Helisalmi S, Heneka MT, Hernandez I, Herrmann MJ, Hoffmann P, Holmes C, Holstege H, Vilas RH, Hulsman M, Humphrey J, Biessels GJ, Jian X, Johansson C, Jun GR, Kastumata Y, Kauwe J, Kehoe PG, Kilander L, Stahlbom AK, Kivipelto M, Koivisto A, Kornhuber J, Kosmidis MH, Kukull WA, Kuksa PP, Kunkle BW, Kuzma AB, Lage C, Laukka EJ, Launer L, Lauria A, Lee CY, Lehtisalo J, Lerch O, Lleo A, Longstreth W Jr., Lopez O, de Munain AL, Love S, Lowemark M, Luckcuck L, Lunetta KL, Ma Y, Macias J, MacLeod CA, Maier W, Mangialasche F, Spallazzi M, Marquie M, Marshall R, Martin ER, Montes AM, Rodriguez CM, Masullo C, Mayeux R, Mead S, Mecocci P, Medina M, Meggy A, Mehrabian S, Mendoza S, Menendez-Gonzalez M, Mir P, Moebus S, Mol M, Molina-Porcel L, Montrreal L, Morelli L, Moreno F, Morgan K, Mosley T, Nothen MM, Muchnik C, Mukherjee S, Nacmias B, Ngandu T, Nicolas G, Nordestgaard BG, Olaso R, Orellana A, Orsini M, Ortega G, Padovani A, Paolo C, Papenberg G, Parnetti L, Pasquier F, Pastor P, Peloso G, Perez-Cordon A, Perez-Tur J, Pericard P, Peters O, Pijnenburg YAL, Pineda JA, Pinol-Ripoll G, Pisanu C, Polak T, Popp J, Posthuma D, Priller J, Puerta R, Quenez O, Quintela I, Thomassen JQ, Rabano A, Rainero I, Rajabli F, Ramakers I, Real LM, Reinders MJT, Reitz C, Reyes-Dumeyer D, Ridge P, Riedel-Heller S, Riederer P, Roberto N, Rodriguez-Rodriguez E, Rongve A, Allende IR, Rosende-Roca M, Royo JL, Rubino E, Rujescu D, Saez ME, Sakka P, Saltvedt I, Sanabria A, Sanchez-Arjona MB, Sanchez-Garcia F, Juan PS, Sanchez-Valle R, Sando SB, Sarnowski C, Satizabal CL, Scamosci M, Scarmeas N, Scarpini E, Scheltens P, Scherbaum N, Scherer M, Schmid M, Schneider A, Schott JM, Selbaek G, Seripa D, Serrano M, Sha J, Shadrin AA, Skrobot O, Slifer S, Snijders GJL, Soininen H, Solfrizzi V, Solomon A, Song Y, Sorbi S, Sotolongo-Grau O, Spalletta G, Spottke A, Squassina A, Stordal E, Tartan JP, Tarraga L, Tesi N, Thalamuthu A, Thomas T, Tosto G, Traykov L, Tremolizzo L, Tybjaerg-Hansen A, Uitterlinden A, Ullgren A, Ulstein I, Valero S, Valladares O, Broeckhoven CV, Vance J, Vardarajan BN, van der Lugt A, Dongen JV, van Rooij J, van Swieten J, Vandenberghe R, Verhey F, Vidal JS, Vogelgsang J, Vyhnalek M, Wagner M, Wallon D, Wang LS, Wang R, Weinhold L, Wiltfang J, Windle G, Woods B, Yannakoulia M, Zare H, Zhao Y, Zhang X, Zhu C, Zulaica M, Eadb, Gr@Ace, Degesco, Eadi, Gerad, Demgene, FinnGen, Adgc, Charge, Farrer LA, Psaty BM, Ghanbari M, Raj T, Sachdev P, Mather K, Jessen F, Ikram MA, de Mendonca A, Hort J, Tsolaki M, Pericak-Vance MA, Amouyel P, Williams J, Frikke-Schmidt R, Clarimon J, Deleuze JF, Rossi G, Seshadri S, Andreassen OA, Ingelsson M, Hiltunen M, Sleegers K, Schellenberg GD, van Duijn CM, Sims R, van der Flier WM, Ruiz A, Ramirez A, Lambert JC, 2022. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet 54(4), 412–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell J, Kim A, Brummel NE, Patel MB, Vandekar SN, Pandharipande P, Dittus RS, Heckers S, Ely EW, Wilson JE, 2021. Advanced Age Is Associated With Catatonia in Critical Illness: Results From the Delirium and Catatonia Prospective Cohort Investigation. Front Psychiatry 12, 673166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Electronic address, p.m.h.e., Cross-Disorder Group of the Psychiatric Genomics, C., 2019. Genomic Relationships, Novel Loci, and Pleiotropic Mechanisms across Eight Psychiatric Disorders. Cell 179(7), 1469–1482 e1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Esteban J, Iglesias-Gonzalez M, Serra-Mestres J, Butjosa A, Canal-Rivero M, Serrano-Blanco A, Baladon L, 2020. Catatonia in elderly psychiatric inpatients is not always associated with intense anxiety: Factor analysis and correlation with psychopathology. Int J Geriatr Psychiatry 35(11), 1409–1417. [DOI] [PubMed] [Google Scholar]
- Elias MF, Sullivan LM, D’Agostino RB, Elias PK, Beiser A, Au R, Seshadri S, DeCarli C, Wolf PA, 2004. Framingham stroke risk profile and lowered cognitive performance. Stroke 35(2), 404–409. [DOI] [PubMed] [Google Scholar]
- Ge T, Chen CY, Ni Y, Feng YA, Smoller JW, 2019. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun 10(1), 1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR, 2015. A global reference for human genetic variation. Nature 526(7571), 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ, 2004. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex 14(4), 410–423. [DOI] [PubMed] [Google Scholar]
- Hirjak D, Kubera KM, Wolf RC, Northoff G, 2020. Going Back to Kahlbaum’s Psychomotor (and GABAergic) Origins: Is Catatonia More Than Just a Motor and Dopaminergic Syndrome? Schizophr Bull 46(2), 272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, Coleman JRI, Hagenaars SP, Ward J, Wigmore EM, Alloza C, Shen X, Barbu MC, Xu EY, Whalley HC, Marioni RE, Porteous DJ, Davies G, Deary IJ, Hemani G, Berger K, Teismann H, Rawal R, Arolt V, Baune BT, Dannlowski U, Domschke K, Tian C, Hinds DA, andMe Research T, Major Depressive Disorder Working Group of the Psychiatric Genomics, C., Trzaskowski M, Byrne EM, Ripke S, Smith DJ, Sullivan PF, Wray NR, Breen G, Lewis CM, McIntosh AM, 2019. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 22(3), 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Obsessive Compulsive Disorder Foundation Genetics, C., Studies, O.C.D.C.G.A., 2018. Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry 23(5), 1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki K, Ikeda A, Kihara T, Kawamoto Y, Mezaki T, Hanakawa T, Hashikawa K, Fukuyama H, Shibasaki H, 2009. Impairment of the cortical GABAergic inhibitory system in catatonic stupor: a case report with neuroimaging. Epileptic Disord 11(2), 126–131. [DOI] [PubMed] [Google Scholar]
- Kakooza-Mwesige A, Wachtel LE, Dhossche DM, 2008. Catatonia in autism: implications across the life span. Eur Child Adolesc Psychiatry 17(6), 327–335. [DOI] [PubMed] [Google Scholar]
- Kleinhaus K, Harlap S, Perrin MC, Manor O, Weiser M, Harkavy-Friedman JM, Lichtenberg P, Malaspina D, 2012. Catatonic schizophrenia: a cohort prospective study. Schizophr Bull 38(2), 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, Copeland JR, Dartigues JF, Jagger C, Martinez-Lage J, Soininen H, Hofman A, 2000. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 54(11 Suppl 5), S4–9. [PubMed] [Google Scholar]
- Moskowitz A, 2004. “Scared stiff”: catatonia as an evolutionary-based fear response. Psychol Rev(111), 984–1002. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, Mantil J, 2002. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse 46(3), 170–188. [DOI] [PubMed] [Google Scholar]
- Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, Als TD, Bigdeli TB, Borte S, Bryois J, Charney AW, Drange OK, Gandal MJ, Hagenaars SP, Ikeda M, Kamitaki N, Kim M, Krebs K, Panagiotaropoulou G, Schilder BM, Sloofman LG, Steinberg S, Trubetskoy V, Winsvold BS, Won HH, Abramova L, Adorjan K, Agerbo E, Al Eissa M, Albani D, Alliey-Rodriguez N, Anjorin A, Antilla V, Antoniou A, Awasthi S, Baek JH, Baekvad-Hansen M, Bass N, Bauer M, Beins EC, Bergen SE, Birner A, Bocker Pedersen C, Boen E, Boks MP, Bosch R, Brum M, Brumpton BM, Brunkhorst-Kanaan N, Budde M, Bybjerg-Grauholm J, Byerley W, Cairns M, Casas M, Cervantes P, Clarke TK, Cruceanu C, Cuellar-Barboza A, Cunningham J, Curtis D, Czerski PM, Dale AM, Dalkner N, David FS, Degenhardt F, Djurovic S, Dobbyn AL, Douzenis A, Elvsashagen T, Escott-Price V, Ferrier IN, Fiorentino A, Foroud TM, Forty L, Frank J, Frei O, Freimer NB, Frisen L, Gade K, Garnham J, Gelernter J, Giortz Pedersen M, Gizer IR, Gordon SD, Gordon-Smith K, Greenwood TA, Grove J, Guzman-Parra J, Ha K, Haraldsson M, Hautzinger M, Heilbronner U, Hellgren D, Herms S, Hoffmann P, Holmans PA, Huckins L, Jamain S, Johnson JS, Kalman JL, Kamatani Y, Kennedy JL, Kittel-Schneider S, Knowles JA, Kogevinas M, Koromina M, Kranz TM, Kranzler HR, Kubo M, Kupka R, Kushner SA, Lavebratt C, Lawrence J, Leber M, Lee HJ, Lee PH, Levy SE, Lewis C, Liao C, Lucae S, Lundberg M, MacIntyre DJ, Magnusson SH, Maier W, Maihofer A, Malaspina D, Maratou E, Martinsson L, Mattheisen M, McCarroll SA, McGregor NW, McGuffin P, McKay JD, Medeiros H, Medland SE, Millischer V, Montgomery GW, Moran JL, Morris DW, Muhleisen TW, O’Brien N, O’Donovan C, Olde Loohuis LM, Oruc L, Papiol S, Pardinas AF, Perry A, Pfennig A, Porichi E, Potash JB, Quested D, Raj T, Rapaport MH, DePaulo JR, Regeer EJ, Rice JP, Rivas F, Rivera M, Roth J, Roussos P, Ruderfer DM, Sanchez-Mora C, Schulte EC, Senner F, Sharp S, Shilling PD, Sigurdsson E, Sirignano L, Slaney C, Smeland OB, Smith DJ, Sobell JL, Soholm Hansen C, Soler Artigas M, Spijker AT, Stein DJ, Strauss JS, Swiatkowska B, Terao C, Thorgeirsson TE, Toma C, Tooney P, Tsermpini EE, Vawter MP, Vedder H, Walters JTR, Witt SH, Xi S, Xu W, Yang JMK, Young AH, Young H, Zandi PP, Zhou H, Zillich L, Psychiatry HA-I, Adolfsson R, Agartz I, Alda M, Alfredsson L, Babadjanova G, Backlund L, Baune BT, Bellivier F, Bengesser S, Berrettini WH, Blackwood DHR, Boehnke M, Borglum AD, Breen G, Carr VJ, Catts S, Corvin A, Craddock N, Dannlowski U, Dikeos D, Esko T, Etain B, Ferentinos P, Frye M, Fullerton JM, Gawlik M, Gershon ES, Goes FS, Green MJ, Grigoroiu-Serbanescu M, Hauser J, Henskens F, Hillert J, Hong KS, Hougaard DM, Hultman CM, Hveem K, Iwata N, Jablensky AV, Jones I, Jones LA, Kahn RS, Kelsoe JR, Kirov G, Landen M, Leboyer M, Lewis CM, Li QS, Lissowska J, Lochner C, Loughland C, Martin NG, Mathews CA, Mayoral F, McElroy SL, McIntosh AM, McMahon FJ, Melle I, Michie P, Milani L, Mitchell PB, Morken G, Mors O, Mortensen PB, Mowry B, Muller-Myhsok B, Myers RM, Neale BM, Nievergelt CM, Nordentoft M, Nothen MM, O’Donovan MC, Oedegaard KJ, Olsson T, Owen MJ, Paciga SA, Pantelis C, Pato C, Pato MT, Patrinos GP, Perlis RH, Posthuma D, Ramos-Quiroga JA, Reif A, Reininghaus EZ, Ribases M, Rietschel M, Ripke S, Rouleau GA, Saito T, Schall U, Schalling M, Schofield PR, Schulze TG, Scott LJ, Scott RJ, Serretti A, Shannon Weickert C, Smoller JW, Stefansson H, Stefansson K, Stordal E, Streit F, Sullivan PF, Turecki G, Vaaler AE, Vieta E, Vincent JB, Waldman ID, Weickert TW, Werge T, Wray NR, Zwart JA, Biernacka JM, Nurnberger JI, Cichon S, Edenberg HJ, Stahl EA, McQuillin A, Di Florio A, Ophoff RA, Andreassen OA, 2021. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet 53(6), 817–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, 2002. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci 25(5), 555–577; discussion 578–604. [DOI] [PubMed] [Google Scholar]
- Northoff G, Krill W, Wenke J, Gille B, Russ M, Eckert J, Pester U, Bogerts B, Pflug B, 1998. Major Differences in Subjective Experience of Akinetic States in Catatonic and Parkinsonian Patients. Cognitive Neuropsychiatry 3(3), 161–178. [Google Scholar]
- Northoff G, Krill W, Wenke J, Travers H, Pflug B, 1996. [The subjective experience in catatonia: systematic study of 24 catatonic patients]. Psychiatr Prax 23(2), 69–73. [PubMed] [Google Scholar]
- Northoff G, Steinke R, Czcervenka C, Krause R, Ulrich S, Danos P, Kropf D, Otto H, Bogerts B, 1999. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry 67(4), 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson N, Price AL, Reich D, 2006. Population structure and eigenanalysis. PLoS Genet 2(12), e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins RJ, 1982. Catatonia: the ultimate response to fear? Aust N Z J Psychiatry 16(4), 282–287. [DOI] [PubMed] [Google Scholar]
- Peters R, 2006. Ageing and the brain. Postgrad Med J 82(964), 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D, 2006. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38(8), 904–909. [DOI] [PubMed] [Google Scholar]
- Purves KL, Coleman JRI, Meier SM, Rayner C, Davis KAS, Cheesman R, Baekvad-Hansen M, Borglum AD, Wan Cho S, Jurgen Deckert J, Gaspar HA, Bybjerg-Grauholm J, Hettema JM, Hotopf M, Hougaard D, Hubel C, Kan C, McIntosh AM, Mors O, Bo Mortensen P, Nordentoft M, Werge T, Nicodemus KK, Mattheisen M, Breen G, Eley TC, 2020. A major role for common genetic variation in anxiety disorders. Mol Psychiatry 25(12), 3292–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A, Grimm S, Northoff G, 2010. Lorazepam modulates orbitofrontal signal changes during emotional processing in catatonia. Hum Psychopharmacol 25(1), 55–62. [DOI] [PubMed] [Google Scholar]
- Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, Masys DR, 2008. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther 84(3), 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF, 1990. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 51(9), 357–362. [PubMed] [Google Scholar]
- Rosebush PI, Mazurek MF, 2010. Catatonia and its treatment. Schizophr Bull 36(2), 239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selch S, Strobel A, Haderlein J, Meyer J, Jacob CP, Schmitt A, Lesch KP, Reif A, 2007. MLC1 polymorphisms are specifically associated with periodic catatonia, a subgroup of chronic schizophrenia. Biol Psychiatry 61(10), 1211–1214. [DOI] [PubMed] [Google Scholar]
- Stober G, Kohlmann B, Iekiera M, Rubie C, Gawlik M, Moller-Ehrlich K, Meitinger T, Bettecken T, 2005. Systematic mutation analysis of KIAA0767 and KIAA1646 in chromosome 22q-linked periodic catatonia. BMC Psychiatry 5, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stober G, Saar K, Ruschendorf F, Meyer J, Nurnberg G, Jatzke S, Franzek E, Reis A, Lesch KP, Wienker TF, Beckmann H, 2000. Splitting schizophrenia: periodic catatonia-susceptibility locus on chromosome 15q15. Am J Hum Genet 67(5), 1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stober G, Seelow D, Ruschendorf F, Ekici A, Beckmann H, Reis A, 2002. Periodic catatonia: confirmation of linkage to chromosome 15 and further evidence for genetic heterogeneity. Hum Genet 111(4–5), 323–330. [DOI] [PubMed] [Google Scholar]
- Taylor MA, Abrams R, 1973. The phenomenology of mania. A new look at some old patients. Arch Gen Psychiatry 29(4), 520–522. [DOI] [PubMed] [Google Scholar]
- Trubetskoy V, Pardinas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, Bryois J, Chen CY, Dennison CA, Hall LS, Lam M, Watanabe K, Frei O, Ge T, Harwood JC, Koopmans F, Magnusson S, Richards AL, Sidorenko J, Wu Y, Zeng J, Grove J, Kim M, Li Z, Voloudakis G, Zhang W, Adams M, Agartz I, Atkinson EG, Agerbo E, Al Eissa M, Albus M, Alexander M, Alizadeh BZ, Alptekin K, Als TD, Amin F, Arolt V, Arrojo M, Athanasiu L, Azevedo MH, Bacanu SA, Bass NJ, Begemann M, Belliveau RA, Bene J, Benyamin B, Bergen SE, Blasi G, Bobes J, Bonassi S, Braun A, Bressan RA, Bromet EJ, Bruggeman R, Buckley PF, Buckner RL, Bybjerg-Grauholm J, Cahn W, Cairns MJ, Calkins ME, Carr VJ, Castle D, Catts SV, Chambert KD, Chan RCK, Chaumette B, Cheng W, Cheung EFC, Chong SA, Cohen D, Consoli A, Cordeiro Q, Costas J, Curtis C, Davidson M, Davis KL, de Haan L, Degenhardt F, DeLisi LE, Demontis D, Dickerson F, Dikeos D, Dinan T, Djurovic S, Duan J, Ducci G, Dudbridge F, Eriksson JG, Fananas L, Faraone SV, Fiorentino A, Forstner A, Frank J, Freimer NB, Fromer M, Frustaci A, Gadelha A, Genovese G, Gershon ES, Giannitelli M, Giegling I, Giusti-Rodriguez P, Godard S, Goldstein JI, Gonzalez Penas J, Gonzalez-Pinto A, Gopal S, Gratten J, Green MF, Greenwood TA, Guillin O, Guloksuz S, Gur RE, Gur RC, Gutierrez B, Hahn E, Hakonarson H, Haroutunian V, Hartmann AM, Harvey C, Hayward C, Henskens FA, Herms S, Hoffmann P, Howrigan DP, Ikeda M, Iyegbe C, Joa I, Julia A, Kahler AK, Kam-Thong T, Kamatani Y, Karachanak-Yankova S, Kebir O, Keller MC, Kelly BJ, Khrunin A, Kim SW, Klovins J, Kondratiev N, Konte B, Kraft J, Kubo M, Kucinskas V, Kucinskiene ZA, Kusumawardhani A, Kuzelova-Ptackova H, Landi S, Lazzeroni LC, Lee PH, Legge SE, Lehrer DS, Lencer R, Lerer B, Li M, Lieberman J, Light GA, Limborska S, Liu CM, Lonnqvist J, Loughland CM, Lubinski J, Luykx JJ, Lynham A, Macek M Jr., Mackinnon A, Magnusson PKE, Maher BS, Maier W, Malaspina D, Mallet J, Marder SR, Marsal S, Martin AR, Martorell L, Mattheisen M, McCarley RW, McDonald C, McGrath JJ, Medeiros H, Meier S, Melegh B, Melle I, Mesholam-Gately RI, Metspalu A, Michie PT, Milani L, Milanova V, Mitjans M, Molden E, Molina E, Molto MD, Mondelli V, Moreno C, Morley CP, Muntane G, Murphy KC, Myin-Germeys I, Nenadic I, Nestadt G, Nikitina-Zake L, Noto C, Nuechterlein KH, O’Brien NL, O’Neill FA, Oh SY, Olincy A, Ota VK, Pantelis C, Papadimitriou GN, Parellada M, Paunio T, Pellegrino R, Periyasamy S, Perkins DO, Pfuhlmann B, Pietilainen O, Pimm J, Porteous D, Powell J, Quattrone D, Quested D, Radant AD, Rampino A, Rapaport MH, Rautanen A, Reichenberg A, Roe C, Roffman JL, Roth J, Rothermundt M, Rutten BPF, Saker-Delye S, Salomaa V, Sanjuan J, Santoro ML, Savitz A, Schall U, Scott RJ, Seidman LJ, Sharp SI, Shi J, Siever LJ, Sigurdsson E, Sim K, Skarabis N, Slominsky P, So HC, Sobell JL, Soderman E, Stain HJ, Steen NE, Steixner-Kumar AA, Stogmann E, Stone WS, Straub RE, Streit F, Strengman E, Stroup TS, Subramaniam M, Sugar CA, Suvisaari J, Svrakic DM, Swerdlow NR, Szatkiewicz JP, Ta TMT, Takahashi A, Terao C, Thibaut F, Toncheva D, Tooney PA, Torretta S, Tosato S, Tura GB, Turetsky BI, Ucok A, Vaaler A, van Amelsvoort T, van Winkel R, Veijola J, Waddington J, Walter H, Waterreus A, Webb BT, Weiser M, Williams NM, Witt SH, Wormley BK, Wu JQ, Xu Z, Yolken R, Zai CC, Zhou W, Zhu F, Zimprich F, Atbasoglu EC, Ayub M, Benner C, Bertolino A, Black DW, Bray NJ, Breen G, Buccola NG, Byerley WF, Chen WJ, Cloninger CR, Crespo-Facorro B, Donohoe G, Freedman R, Galletly C, Gandal MJ, Gennarelli M, Hougaard DM, Hwu HG, Jablensky AV, McCarroll SA, Moran JL, Mors O, Mortensen PB, Muller-Myhsok B, Neil AL, Nordentoft M, Pato MT, Petryshen TL, Pirinen M, Pulver AE, Schulze TG, Silverman JM, Smoller JW, Stahl EA, Tsuang DW, Vilella E, Wang SH, Xu S, Indonesia Schizophrenia C, PsychEncode, Psychosis Endophenotypes International, C., Syn GOC, Adolfsson R, Arango C, Baune BT, Belangero SI, Borglum AD, Braff D, Bramon E, Buxbaum JD, Campion D, Cervilla JA, Cichon S, Collier DA, Corvin A, Curtis D, Forti MD, Domenici E, Ehrenreich H, Escott-Price V, Esko T, Fanous AH, Gareeva A, Gawlik M, Gejman PV, Gill M, Glatt SJ, Golimbet V, Hong KS, Hultman CM, Hyman SE, Iwata N, Jonsson EG, Kahn RS, Kennedy JL, Khusnutdinova E, Kirov G, Knowles JA, Krebs MO, Laurent-Levinson C, Lee J, Lencz T, Levinson DF, Li QS, Liu J, Malhotra AK, Malhotra D, McIntosh A, McQuillin A, Menezes PR, Morgan VA, Morris DW, Mowry BJ, Murray RM, Nimgaonkar V, Nothen MM, Ophoff RA, Paciga SA, Palotie A, Pato CN, Qin S, Rietschel M, Riley BP, Rivera M, Rujescu D, Saka MC, Sanders AR, Schwab SG, Serretti A, Sham PC, Shi Y, St Clair D, Stefansson H, Stefansson K, Tsuang MT, van Os J, Vawter MP, Weinberger DR, Werge T, Wildenauer DB, Yu X, Yue W, Holmans PA, Pocklington AJ, Roussos P, Vassos E, Verhage M, Visscher PM, Yang J, Posthuma D, Andreassen OA, Kendler KS, Owen MJ, Wray NR, Daly MJ, Huang H, Neale BM, Sullivan PF, Ripke S, Walters JTR, O’Donovan MC, Schizophrenia Working Group of the Psychiatric Genomics, C., 2022. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604(7906), 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Stegmayer K, Wilson JE, Heckers S, 2019. Structure and neural mechanisms of catatonia. Lancet Psychiatry 6(7), 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JE, Carlson R, Duggan MC, Pandharipande P, Girard TD, Wang L, Thompson JL, Chandrasekhar R, Francis A, Nicolson SE, Dittus RS, Heckers S, Ely EW, Delirium, Catatonia Prospective Cohort, I., 2017. Delirium and Catatonia in Critically Ill Patients: The Delirium and Catatonia Prospective Cohort Investigation. Crit Care Med 45(11), 1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Nielsen JB, Fritsche LG, Dey R, Gabrielsen ME, Wolford BN, LeFaive J, VandeHaar P, Gagliano SA, Gifford A, Bastarache LA, Wei WQ, Denny JC, Lin M, Hveem K, Kang HM, Abecasis GR, Willer CJ, Lee S, 2018. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat Genet 50(9), 1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]


