Abstract
Schizophrenia is a brain disorder that profoundly perturbs cognitive processing. Despite the success in treating many of its symptoms, the field lacks effective methods to measure and address its impact on reasoning, inference and decision making. Prefrontal cortical abnormalities have been well-documented in schizophrenia, but additional dysfunction in the interactions between prefrontal cortex and thalamus have recently been described. This dysfunction may be interpreted in light of parallel advances in neural circuit research using non-human animals, which show critical thalamic roles in maintaining and switching prefrontal activity patterns in various cognitive tasks. Here, we review this basic literature and connect it to emerging innovations in clinical research. We highlight the value of focusing on associative thalamic structures not only to better understand the very nature of cognitive processing, but also to leverage these circuits for diagnostic and therapeutic development in schizophrenia. We suggest that the time is right for building close bridges between basic thalamic research and its clinical translation, particularly in the domain of cognition and schizophrenia.
Introduction:
Real world behavior involving attention, sensorimotor integration, inference, and planning requires coordination of neural activity across multiple cortical areas (Sakai and Passingham 2003; Cole and others 2013; Cohen and D’Esposito 2016). Consider an everyday task of crossing a busy street; if one of the traffic lights suddenly goes out, one would need to integrate inputs from passing cars and other pedestrians in order to dynamically adjust their plans of when to cross. Such ‘on-the-fly’ adjustment requires that a number of specialized cortical areas dynamically interact, resulting in a shift in which sensory streams are prioritized as well as which motor actions are selected. Key to this sensorimotor reorganization is the engagement of associative cortical areas, such as prefrontal and parietal areas (Zanto and Gazzaley 2013; Murray and others 2017; Hwang and others 2019), that add working memory and salience maps to enhance this process and make it efficient. Thus, a simple action of crossing a street and indeed most mammalian behavior requires flexible integration of information computed and stored in a distributed manner across cortical regions. One of the primary goals of neuroscience is to describe the biological basis of distributed cortical processing and inter-areal interactions.
Over the last few decades, research in humans and other animals have revealed a multitude of conditions requiring interaction between cortical areas. For instance, studies have shown that prefrontal areas can direct visual attention by generating ‘top down’ signals that change how sensory inputs are processed in visual cortex (Baldauf and Desimone 2014; Bichot and others 2019). Similarly, prefrontal activity is required for challenging cases of visual object recognition known to be processed in inferotemporal cortex (Kar and DiCarlo 2021). Because of extensive direct connections between cortical areas, a major assumption in the field is that these direct cortico-cortical pathways implement many cognitive operations requiring distributed processing. However, in addition to direct pathways, mounting evidence suggests that the thalamus provides an indirect route for communication between cortical areas, adding both flexibility and efficiency (Murray Sherman and Guillery 2011; Halassa and Kastner 2017; Pergola and others 2018; Wolff and Vann 2019; Bourgeois and others 2020; Fiebelkorn and Kastner 2020; Shepherd and Yamawaki 2021). We argue throughout this perspective that dissecting the circuit mechanisms of these trans-thalamic cortico-cortical interactions is likely to yield a more complete mechanistic description of cognition and its dysfunction in psychiatric disorders (Halassa and Kastner 2017; Ouhaz and others 2018; Parnaudeau and others 2018; Svoboda and Li 2018; Bourgeois and others 2020; Steullet 2020; Avram and others 2021; Roy and others 2022).
In addition to its basic scientific value, understanding the role of the thalamus in regulating interactions across cortical areas is of particular relevance to human health. Recent evidence suggests that large-scale interactions across networks in the human brain are severely perturbed in schizophrenia (Dong and others 2018). These brain-based metrics are in-line with the symptomatology reported for this disorder involving abnormal reasoning (Woodward and others 2009; Weickert and others 2014; Leptourgos and others 2017) as well as more recent computational models that reveal abnormalities in latent variables underlying inference (Baker and others 2019) and decision making (Nassar and others 2021). Adding studies that show selective vulnerability of associative thalamic structures in schizophrenia (Giraldo-Chica and others 2018; Huang and others 2019) and the known role of these subcortical structures in cortical functional organization, it is reasonable to assume that thalamic dysfunction may underlie some of the cognitive abnormalities in this disorder. Equally importantly, because the thalamus has been utilized as a neurostimulation target in a variety of brain disorders, it may be an untapped pathway for disease correction in schizophrenia which we also discuss in this perspective.
In the next sections, we will first review the anatomy of thalamocortical loops with a focus on associative structures. Then we discuss functional evidence in support of their role in regulating cortical communication based on data collected across a range of animals and tasks. Finally, we look at thalamocortical dysfunction reported in schizophrenia and propose that the unique role of the associative thalamus in gating intercortical interaction makes it an attractive target for circuit specific therapeutic interventions.
Anatomical organization of thalamocortical systems – focus on associative thalamus:
The mammalian forebrain is organized into loops connecting the cortex, thalamus and basal ganglia (Foster and others 2021). Broadly, the cortex is largely composed of recurrent excitatory circuits (Peron and others 2020) arranged in layers (Radnikow and Feldmeyer 2018) that can maintain activity patterns across multiple time scales relevant to driving adaptive behavior, while local cortical interneurons gate and sculpt these activity patterns (Hu and others 2014). In relation to the cortex, the thalamus is a centrally located subcortical region divided into nuclei which are primarily composed of excitatory neurons (Jones 2007; Butler 2008; Hunnicutt and others 2014). The thalamus receives inhibitory input from a number of sources, including the thalamic reticular nucleus, basal ganglia and brainstem (Lam and Sherman 2011; Halassa and others 2014; Halassa and Acsády 2016; Crabtree 2018). In primates and carnivores, excitatory thalamic nuclei contain local inhibitory interneurons, but little is known about their function beyond a few well-studied cases (Wang and others 2011; Leist and others 2016). For example, in the lateral geniculate nucleus, interneurons are thought to engage in a push-pull type of operation that ultimately improves signal-to-noise ratio of thalamocortical visual input transmission (Wang and others 2011). Because much basic physiology is carried out in rodents, and the rodent thalamus is devoid of local interneurons outside of the lateral geniculate, we know little about the general properties of these circuits. Instead, considerable focus has been placed on the thalamic reticular nucleus, which forms a shell of inhibitory neurons around the thalamus and provides a major source of inhibition across all thalamic nuclei (Pinault 2004; Crabtree 2018).
The excitatory microcircuits within the thalamus diverges majorly from that of the cortex in one key way – an absence of local excitatory recurrent connectivity (Bickford and others 2008; Hunnicutt and others 2014; Zolnik and Connors 2016). Instead, all excitatory inputs to the thalamus originate from extra thalamic sources. This unique property of the thalamus may be central to its role in inter-cortical communication, as the lack of local excitatory recurrence allows for different task-relevant variables to be effectively separated across neighboring groups of neurons. This may be key for control functions; thalamic ensembles can be conceived as low-dimensional projections of cortical activity patterns that, due to effective separation, maybe well positioned to subsequently exert regulatory effects on cortical dynamics within (Bolkan and others 2017; Guo and others 2017; Schmitt and others 2017; Rikhye and others 2018; Fresno and others 2019) and across areas (Jaramillo and others 2019; Mo and Sherman 2019; Kastner and others 2020; Blot and others 2021). Testing different aspects of this hypothesis will require an integrative approach to thalamic connectivity, physiology, and task-relevant engagement which we will discuss throughout this piece.
Over the last several decades, there have been many attempts at a general classification for thalamocortical circuits (Sherman 2012; Halassa and Sherman 2019). Although there is currently no comprehensive classification that accounts for all features, we think that three broad categories can be conceived in order to assist with functional interpretations. These would sit on a continuum between primary sensory and associative thalamic circuits, with higher-order relays in the middle (Figure 1). Primary sensory thalamic areas, like the lateral geniculate nucleus (LGN) or ventral subdivision of the medial geniculate body (MGBv), mostly form topographic and reciprocal loops with primary sensory cortical areas (Hackett and others 2011; Usrey and Alitto 2015). In addition, they receive their main driving input from subcortical sensory areas while their cortical inputs which originate from layer 6 of their respective sensory cortices are largely modulatory (Homma and others 2017; Usrey and Sherman 2019). For example, in primates, corticothalamic projections from the primary visual cortex to the LGN modulates the timing and precision of thalamic transmission without affecting their receptive field properties (Murphy and others 2021) which are inherited from the retina instead (Hubel and Wiesel 1961; Alonso and others 1996). Due to this organization, primary sensory thalamic areas are best described as high-fidelity relays of information from the sensory organs with minimal intrinsic processing (although, there is quite a bit of literature on temporal visual processing by the LGN (Saalmann and Kastner 2011; Tang and others 2016)).
Figure 1: Schematic representation of different thalamocortical circuits within the brain (bottom) with their differences in input/output connectivity highlighted (top).
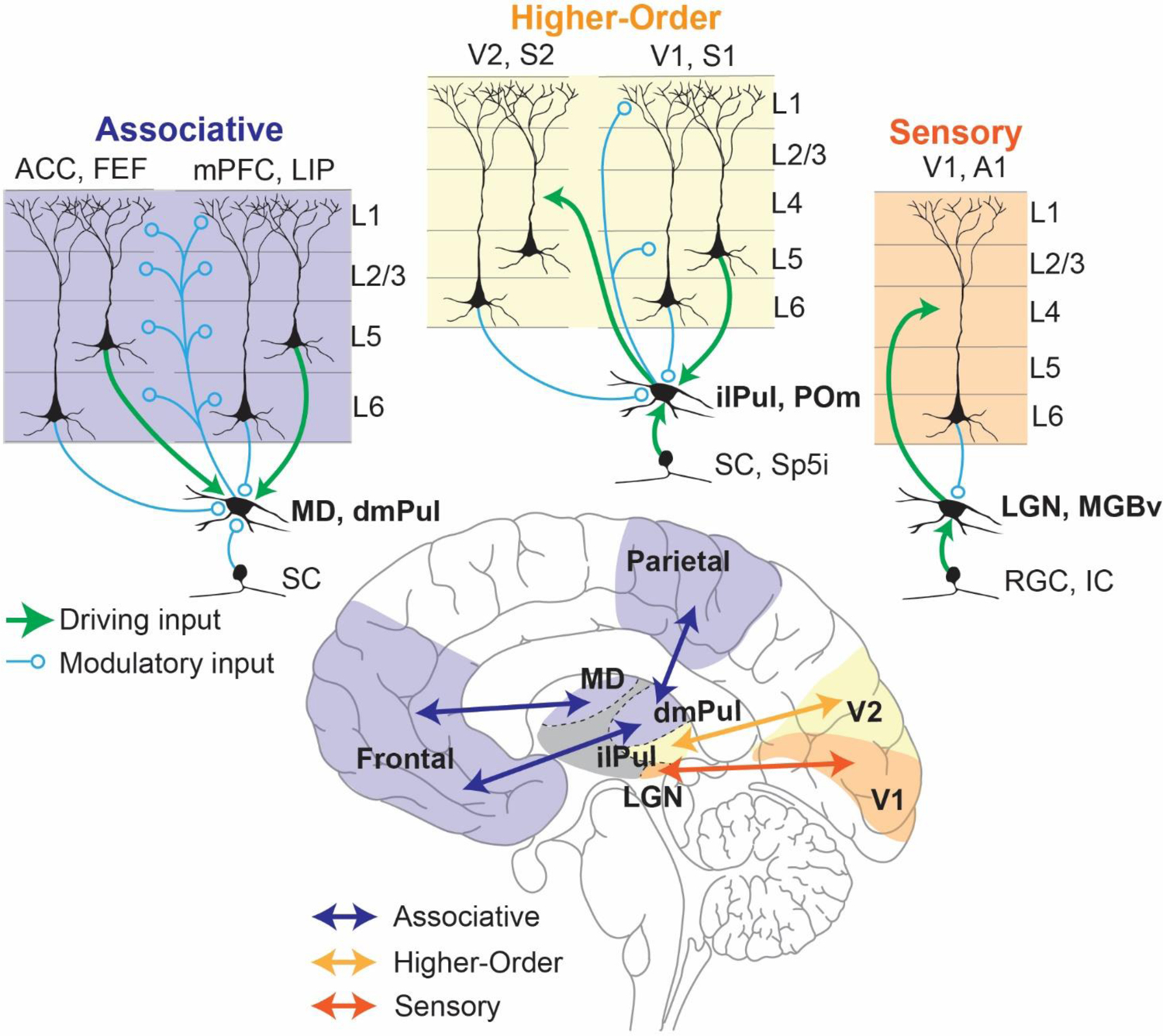
Thalamocortical circuits can be classified in a continuum of circuit motifs based on the origin of their main driving inputs and their cortical outputs. In this scheme (from top-right to top-left) sensory thalamic nuclei (eg: LGN) receive their driving input (green) from sensory systems (eg: retinal ganglion cells (RGC)) and in turn send driving inputs to their cortical targets (eg: V1, orange) that terminate mostly in Layer 4, thus acting as faithful relays of sensory information to the cortex. Such nuclei receive modulatory inputs (blue) from layer 6 of the cortical areas they project to (Alonso and others 2001; Murphy and others 2021). Higher-order thalamocortical nuclei (eg: inferior/lateral pulvinar (ilPul)) can receive their driving inputs from both cortical (eg: V1) layer 5 neurons as well as subcortical areas (eg: superior colliculus (SC)). These nuclei send modulatory cortical outputs to layers 1 and 5 of the cortical area from which their driving input originates and driving inputs to other cortical areas (eg: V2, yellow). Such Higher order thalamic nuclei play a functional role in integrating cortical and subcortical inputs to either modulate cortical dynamics or transfer their integrated output to another cortical region (Groh and others 2014; Mease and others 2017; Blot and others 2021). Finally associative thalamocortical nuclei (eg: MD) receive their main driving inputs entirely from layer 5 neurons of its cortical partners (eg: ACC, mPFC, purple) while the subcortical inputs are mostly modulatory (eg: SC). They in turn modulate cortical dynamics through terminals at all layers of its multiple cortical partners. Associative thalamocortical networks play critical roles in the maintenance and coordination of distributed cortical signals driven by task dependent inputs and outputs (Parnaudeau and others 2013; Bolkan and others 2017; Pergola and others 2018; Fiebelkorn and Kastner 2020; Mukherjee and others 2021). Abbreviations: SC: Superior colliculus; MD: mediodorsal thalamus; dmPulL: dorso-medial Pulvinar; ACC: Anterior cingulate cortex; FEF: Frontal eye fields; mPFC: medial prefrontal cortex; LIP: Lateral intraparietal cortex; SC: superior colliculus; Sp5i: whisker-sensitive cells of the spinal trigeminal subnuclei, interpolar region; ilPul: inferior/lateral Pulvinar; POm: posterior medial thalamus; V1: Primary visual cortex; V2: Secondary visual cortex; S1: Primary somatosensory cortex; V2: Secondary somatosensory cortex; RGC: Retinal ganglion cell; IC: Inferior colliculus; LGN: lateral geniculate nucleus; MGBv: Medial geniculate nucleus, ventral subdivision; A1: Primary auditory cortex.
It may be surprising to note that most of the thalamus, especially that of primates, is composed of associative areas rather than primary sensory ones. In fact, the two largest thalamic nuclei of the human brain, the Pulvinar (dorsomedial division, see below) and mediodorsal thalamus, are largely associative in nature (Halassa and Kastner 2017). Neurons within these areas are largely driven by cortical inputs originating from layer 5 (Usrey and Sherman 2019). Intriguingly, layer 5 cortical neurons that send driving input to associative thalamic nuclei are anatomically distinct from layer 5 neurons that project to other cortical areas, suggesting a distinct function for these pathways from those involved in direct cortico-cortical communication (Petrof and others 2012).
There are at least two types of functional associative thalamocortical loops that we infer: closed and open loops (Figure 2). A closed loop indicates functional interactions that are limited to one cortical area and its thalamic partner, whereas an open loop involves multiple cortical areas indirectly connected through a thalamic node. Closed loop interactions have been described for the rodent prelimbic cortex (primate analogue of dorsolateral prefrontal cortex) and the lateral division of the mediodorsal thalamus (Vertes and others 2015). These interactions involve sustaining cortical representations related to working memory and attentional control signals (Bolkan and others 2017; Guo and others 2017; Schmitt and others 2017), as well as the flexible control/switching of these signals based on changes in input reliability (Mukherjee and others 2021) or temporal context (Rikhye and others 2018). Open loop interactions have been described for the primate Pulvinar (Arcaro and others 2015; Zhou and others 2016; Fiebelkorn and Kastner 2020). Higher-order relays are structures that appear to have hybrid properties of sensory and associative thalamic areas. For example, the Posteriomedial thalamus (POm) of the somatosensory system is driven by both Layer 5 of primary somatosensory cortex as well as the brainstem (Groh and others 2014). It has both driving and modulatory inputs to higher order somatosensory and motor cortex (Casas-Torremocha and others 2017; El-Boustani and others 2020). Of note, the primate Pulvinar complex is composed of subdivisions that can be thought of as associative (the dorsomedial part) and higher-order relay (inferiorlateral part) (Figure 1) (Baldwin and others 2017). In non-primate species, the dorsal equivalent is either under-developed or non-existent (Kaas and Lyon 2007; Zhou and others 2017), rendering the entire Pulvinar as a higher order relay equivalent to the POm. The dorsal Pulvinar may have evolved in primates alongside the evolution of direct fronto-parietal pathways, in line with the ‘replication principle’ which states that any two cortical pathways that are directly connected are also indirectly connected through the thalamus (Shipp 2003). Electrophysiological studies in macaque monkeys have focused on a part of the dorsal Pulvinar that indirectly connects areas V4 and inferotemporal cortex, two regions along the ventral visual hierarchy associated with form vision (Shipp 2003; Saalmann and others 2012; Zhou and others 2016). In a combined feature/spatial attention task (Egly and others 1994), this thalamic region shows elevated activity during the delay period of the task when attentional resources are presumably deployed. This elevation appears to also be associated with rhythmicity in the alpha frequency range (10–14Hz), and accounts for directed functional connectivity between V4 and inferotemporal cortex (Saalmann and others 2012) indicating a role for the Pulvinar in mediating functional cortico-cortical connectivity (Nakajima and Halassa 2017)
Figure 2: Closed vs. open loop associative thalamocortical circuits inferred from function.
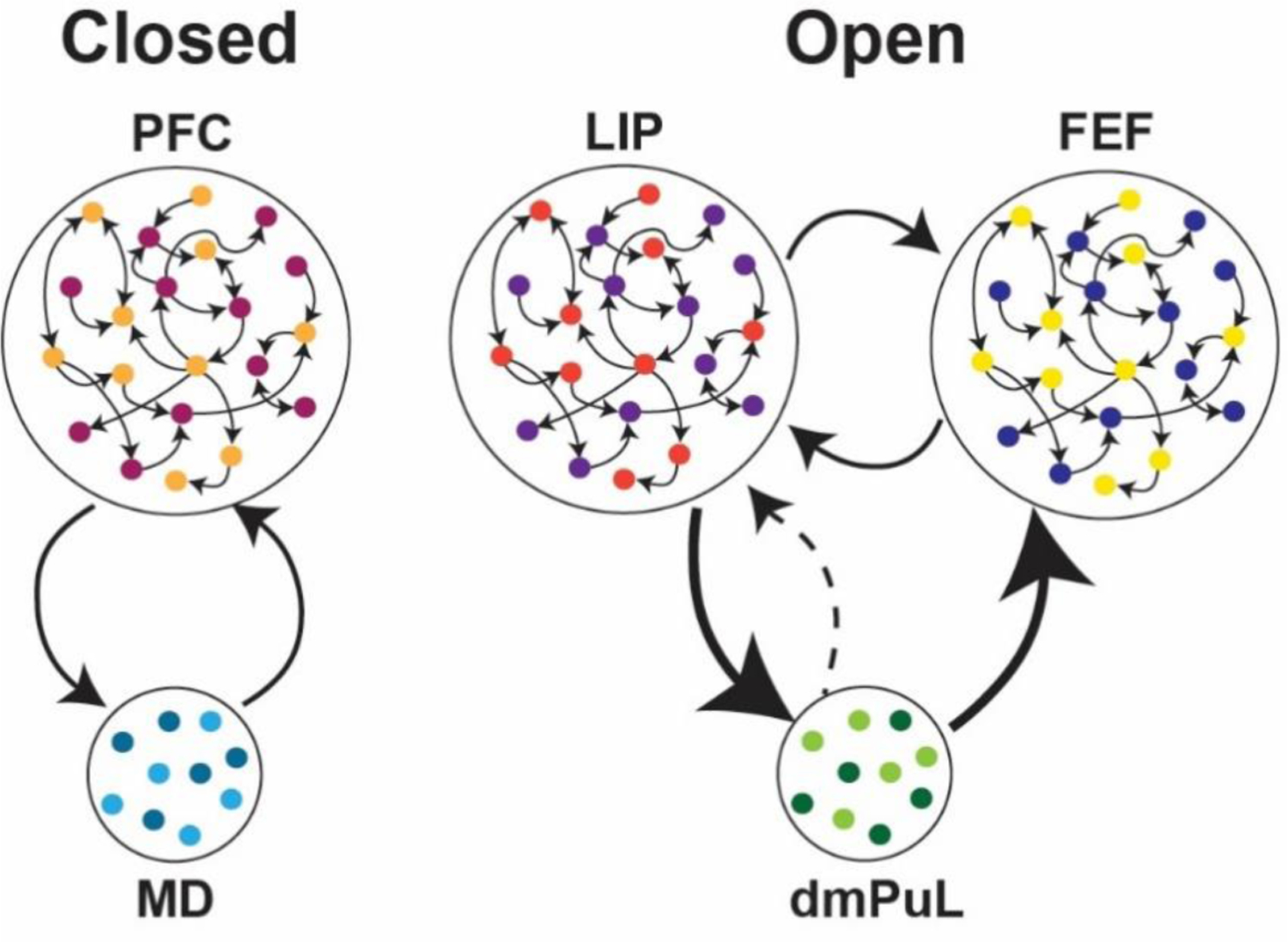
(left) A closed loop circuit can be inferred between the medio dorsal thalamus (MD) and the prefrontal cortex (PFC) where the MD uses inputs from the PFC to generate cognitive signals that sustain prefrontal representations as well as mediate flexible control of these signals based on input reliability or temporal context changes (Bolkan and others 2017; Schmitt and others 2017, Mukherjee and others 2021). (right) A proposed open loop between the lateral interparietal cortex (LIP) and frontal eye fields (FEF) through the dorsomedial Pulvinar (dmPuL) for the control of visual attention. Here dmPuL drives increased functional connectivity with both FEF and LIP during engaged periods of selective visual attention to emphasize sensory processing while LIP drives functional connectivity to the dmPuL during periods of disengagement to facilitate motor processing. (Fiebelkorn and others 2019; Kastner and others 2020).
The functional roles of the associative thalamus in cognition
What exactly is the purpose of these thalamocortical loops? While definitive answers are yet to come, we hypothesize that these loops must rely on the distinct architectural features of the cortex and thalamus. To reiterate, the cortex is characterized by local excitatory recurrence and associated endogenous activity, whereas the thalamus is devoid of such features. In addition, one feature that associative thalamic circuits appear to exhibit is input convergence. Specifically, and in contrast to their primary sensory counterparts, associative thalamic circuits appear to integrate signals from distinct cortical sources (Hwang and others 2017; Ouhaz and others 2018; Parnaudeau and others 2018; Pergola and others 2018; Fiebelkorn and Kastner 2019; Bourgeois and others 2020). In some circumstances, the result of this convergence is a dimensionality reduction of the task-relevant variables found in distributed cortical afferents. In other words, they can integrate and transform these signals into novel representations, including temporal context (Rikhye and others 2018), confidence (Komura and others 2013), and uncertainty (Mukherjee and others 2021). Such representations, in turn, may be utilized as cognitive control signals that functionally regulate distributed cortical dynamics rather than relay signals in the classical sense.
To give a concrete example of the above, we expand the discussion on the Pulvinar mentioned in the previous section. Specifically, work by Kastner and colleagues has shown that interactions between early and late stations of the dorsal visual pathway in the alpha-band range, are temporally coordinated by an intermediary connecting Pulvinar (Saalmann and others 2012). More recently, the same group showed that this relationship is causal; inactivation of the pulvinar diminishes this cortico-cortical alpha band coherence in a task-specific manner (Eradath and others 2021). Work by Komura et al. (Komura and others 2013), also in macaque monkeys, has shown that pulvinar neurons generate ‘summary statistic’ type responses in a classical visual dot motion task (Figure 3A). Specifically, when animals are required to make a perceptual judgment on these well-studied stimuli, Pulvinar neurons show response patterns that are not reflective of the dominant motion direction, as is the case in cortical area LIP (lateral intraparietal) for example (Roitman and Shadlen 2002; Shadlen and Kiani 2013), but rather their responses reflect the coherence as a scalar quantity regardless of motion direction. Computational modeling of these responses suggests they may emerge due to convergence of these two motion-sensitive cortical ensembles onto single pulvinar neurons (Jaramillo and others 2019) (Figure 3B, C). Therefore, responses of Pulvinar neurons are not a simple reflection of upstream inputs, but under certain conditions can reflect a transformation of a vector input to a scalar signal. Adding Kastner’s work to these findings paints a picture in which the pulvinar may not ‘relay’ such signals, but rather generate an output that coordinates activity patterns across distributed cortical ensembles (Bourgeois and others 2020; Kastner and others 2020).
Figure 3: Associative thalamic nuclei integrate convergent cortical inputs into cognitive control signals such as decision confidence or input uncertainty.
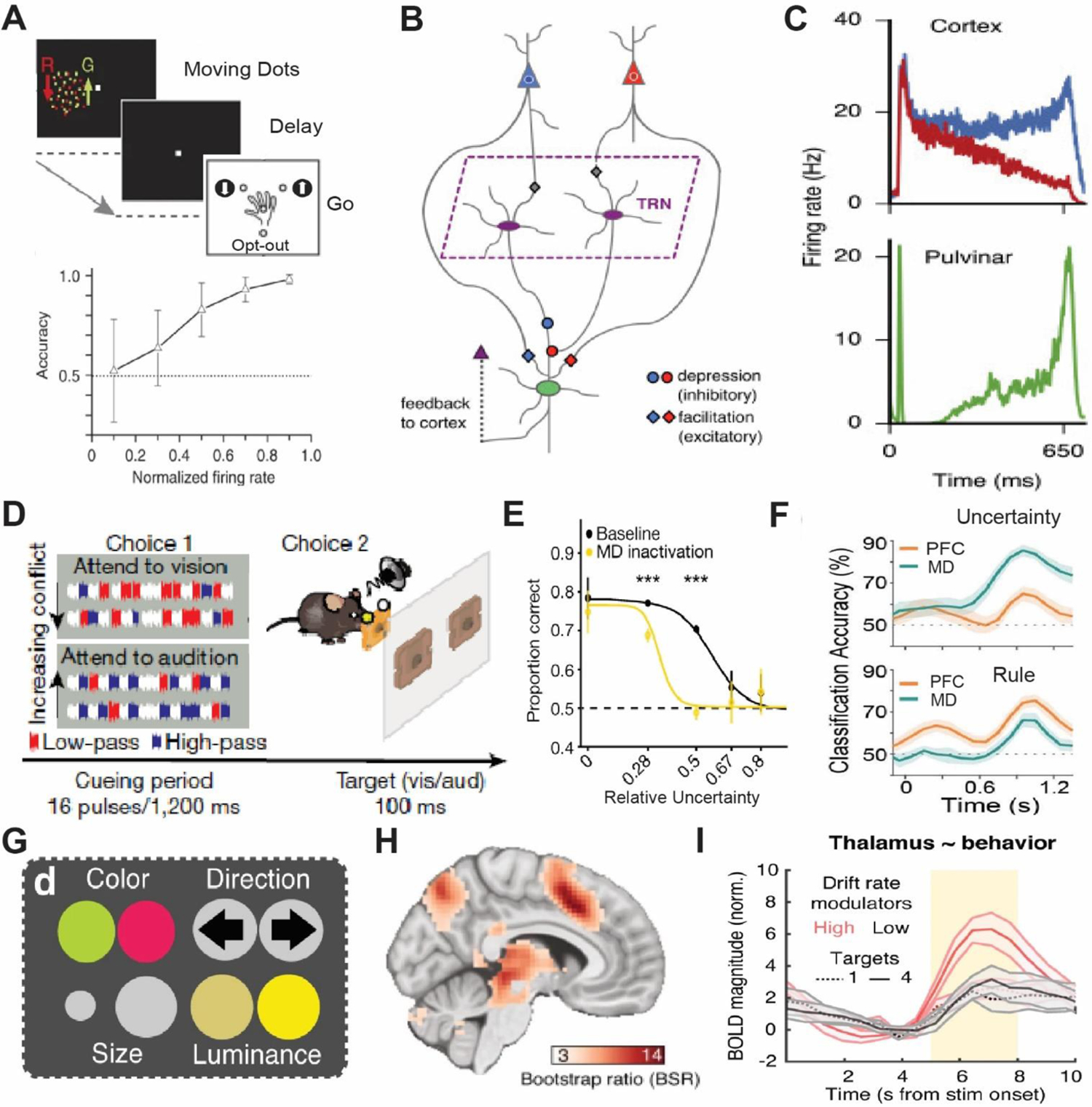
(A) Top: A random dot motion task with three alternative choices where macaque monkeys had to make a categorical choice based on the relative ratio of dots of one color moving in a particular direction (up or down) to get a reward or opt-out for a small but certain reward. Bottom: Activity in the dorsal Pulvinar is predictive of an animal’s confidence in choice accuracy. Adapted from Komura and others 2013. (B) Computational model of the dorsomedial Pulvinar with two cortical ensembles, differentially sensitive for motion, that converge onto single Pulvinar neurons. (C) Simulated Pulvinar neurons from the model (bottom) integrates the cortical activity and approximates the absolute value of the of the difference in rates of the competing cortical neurons (top). Adapted from Jaramillo and others 2019. (D) A rodent attention control task where on each trail an animal has to make a categorical decision on the dominant sound pulse type (high pass; HP vs low pass; LP) in a mixed string of pulses made of HP, LP and non-informative white noise (choice 1). Subsequently the animal selects the target action (attend to vision vs attend to audition) that corresponds to the dominant pulse (choice 2). (E) Optogenetic inactivation the medio dorsal thalamus diminishes performance as a function of tracking uncertainty. (F) Medio dorsal thalamic neurons preferentially track input uncertainty while the PFC encodes rules. Adapted from Mukherjee and others 2021. (G) A modification of the random dot motion task where features of the dots vary across 4 visual dimensions and subjects have to perform a categorical discrimination based on 1 of the pre-cued features. Subjects were pre cued to attend to varying number of features (1 to 4) thus increasing the uncertainty of the feature relevant to the discrimination. (H) Thalamic BOLD magnitude shows correlation to attentional uncertainty and is parametric to it (I). Adapted from Kosciessa and others 2021.
Similar to the Pulvinar, the mediodorsal thalamus establishes widespread connections across multiple cortical areas. However, rather than being primarily connected to posterior cortical areas, the mediodorsal thalamus is connected to multiple frontal cortical areas (Mitchell 2015; Li and others 2022). Recently, multiple studies have examined mediodorsal function in rodents, and showed that neurons within this region have non-relay properties (Parnaudeau and others 2013; Bolkan and others 2017; Schmitt and others 2017; Rikhye and others 2018; Fresno and others 2019; DeNicola and others 2020; Hsiao and others 2020; Ouhaz and others 2022) that might be similar to the Pulvinar studies described above. Specifically, in a cross-modal divided attention task developed in mice, mediodorsal neurons show unique response profiles that are distinct from their connected cortical areas (Figure 3D, E). In the prefrontal cortex, neurons encode the inputs and outputs of the cross-modal task, whereas thalamic neurons encode changes in input patterns over both long and short timescales (Rikhye and others 2018; Mukherjee and others 2021). Encoding of the long-term input changes reflects the statistical regularity of inputs over multi-trial timescale, or the ‘cueing context’. Encoding of short-term input changes reflects their variance or uncertainty (Mukherjee and others 2021). This may reflect an interesting point of divergence between the mediodorsal encoding of uncertainty (Figure 3D–F) versus the Pulvinar encoding the opposite, confidence (Figure 3A–C). Critically, these short-term summary statistic responses have not only been found in non-human animal studies, but also in task-based fMRI in humans (Kosciessa and others 2021) (Figure 3G–I). Therefore, although thalamic microcircuitry may be far simpler than that of the cortex, it is nonetheless likely capable of performing important computational transformations that are critical for coordination of distributed cortical signals and in turn cognitive operations. The rodent studies have also shown that the thalamic outputs do not necessarily drive cortical responses in the classical sense but can drive feedforward inhibition (Ferguson and Gao 2018; Mukherjee and others 2020) to slow cortical dynamics in certain contexts, or alternatively disinhibit cortical ensembles (Anastasiades and others 2021; Mukherjee and others 2021) to implement non-linear gain control of cortical activity patterns in others.
In summary, the fact that associative thalamic structures provide a mechanism for regulating cortical computations in a precise manner, and in turn coordinate activity across large- scale cortical networks lead to our proposal where associative thalamic structures could be leveraged to identify and target cognitive control deficits in psychiatric and neurological disorders. In the next section we focus on schizophrenia as a representative example of this vision.
Mounting evidence for associative thalamocortical dysfunction in schizophrenia
Thalamocortical abnormalities are established in schizophrenia and have been shown across multiple stages of the illness, including early psychosis (Woodward and Heckers 2016; Avram and others 2021; Zhang and others 2021). Specifically, both structural and functional magnetic resonance imaging (fMRI), have demonstrated abnormalities in schizophrenia patients (Figure 4). On the structural end, diffusion tensor imaging (DTI) has shown that the integrity of thalamocortical connections is diminished, particularly between the areas of the thalamus that are connected to the prefrontal cortex (Hamoda and others 2019) (Figure 4A–C). In fact, reduced frontothalamic connectivity is also detectable in subjects at a high risk of developing schizophrenia (Cho and others 2016; Schleifer and others 2019); suggesting that thalamocortical dysconnectivity may be a potential biomarker as it can precede confirmatory diagnosis. On the functional end, the correlated patterns of activity normally observed between the thalamus and large-scale cortical networks are changed (Dong and others 2018). This phenomenon, which is often referred to as resting state functional connectivity, shows reduced correlated activity (hypoconnectivity) between the thalamus and prefrontal areas (Anticevic and others 2014; Woodward and Heckers 2016; Giraldo-Chica and others 2018; Zhang and others 2021) and increased correlated activity (hyperconnectivity) between the thalamus and sensorimotor areas (Woodward and Heckers 2016; Chen and others 2019) (Figure 4D, E). Although the exact mapping between these measures and microcircuit function is currently unknown, it clearly suggests that the nature of thalamocortical interactions is changed in schizophrenia, and that there is a functional ‘dysconnectivity’ between associative thalamic regions and prefrontal cortex. Because the hypoconnectivity phenotype has potential circuit parallels based on non-human animal studies, we will focus on it in this perspective
Figure 4: Structural and functional abnormalities in thalamocortical connectivity in Schizophrenia.
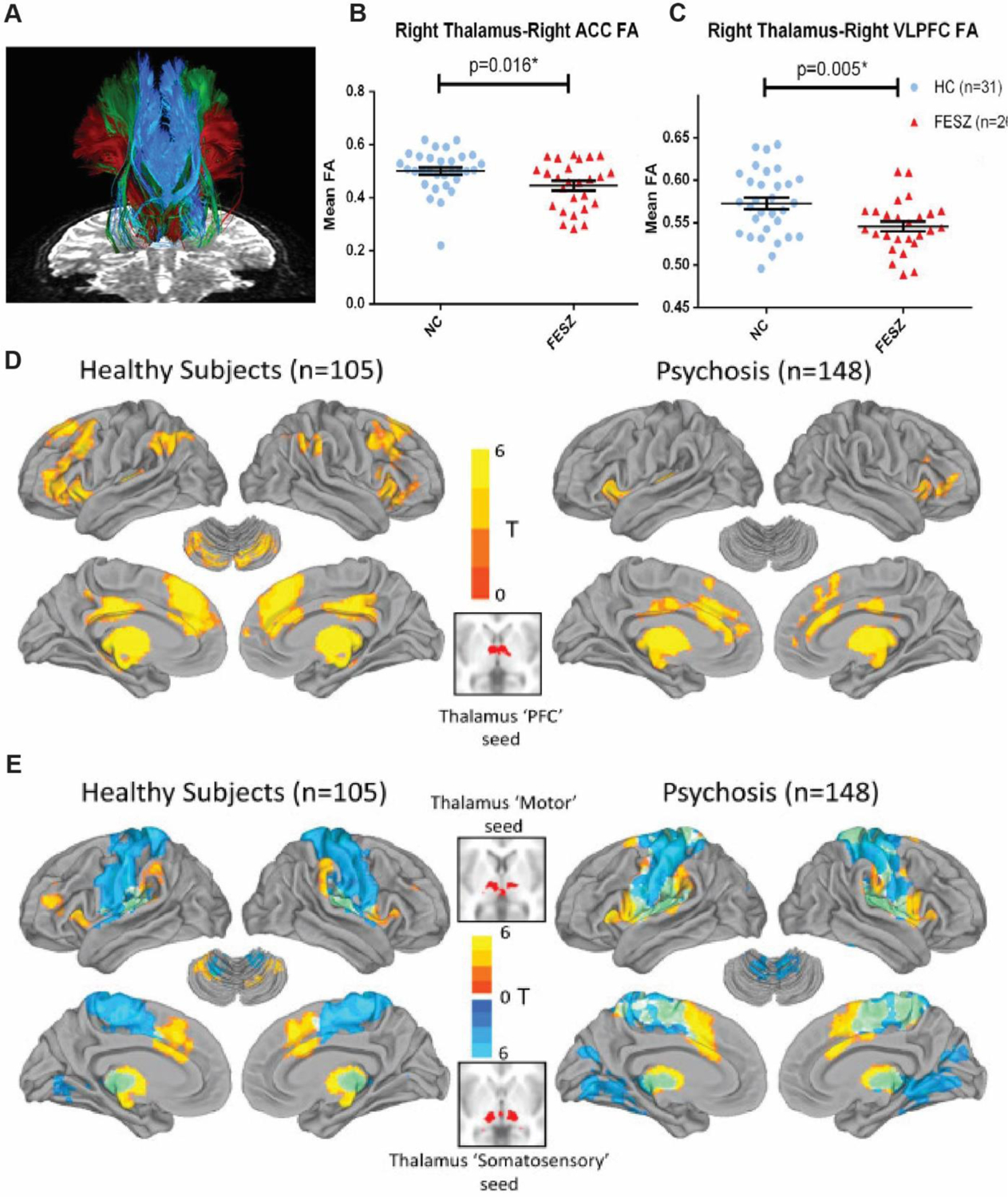
(A) Specific white matter tracts that connects frontal cortical areas, namely, anterior cingulate (ACC), ventrolateral prefrontal cortex (VLPFC) and lateral orbitofrontal cortex (LOFC) to the thalamus as measured with two tensor tractography. Thalamus to ACC (B) and VLPFC (C) show reduced connectivity in schizophrenia patients. Adapted from Hamoda and others 2019. Resting state fMRI comparing functional connectivity between major divisions of the cortex and thalamus of healthy subjects versus schizophrenia patients shows reduced prefrontal-thalamic connectivity (D) and increased motor (warm colors)/somatosensory (cool colors) thalamic connectivity (E). Adapted from Woodward and Heckers 2016.
Frontal thalamocortical hypoconnectivity (or reduced coordinated activity) can be interpreted from a perspective that takes advances in circuit neuroscience highlighted earlier into account. This includes a recent study in mice, which identified two distinct pathways within the mediodorsal thalamus that exert opposing effects on prefrontal circuitry (Mukherjee and others 2021). These two pathways originate from two genetically distinct mediodorsal cell types, one expressing the dopamine D2 receptor and another expressing the kainate receptor Grik4. Leveraging the circuit-accessibility of the mouse and using a number of molecular circuit-tracing and identification techniques, this study discovered that the D2+ mediodorsal neurons preferentially innervate prefrontal disinhibitory neurons, positive for vasointestinal peptide (VIP+) (Guet-McCreight and others 2020). On the other hand, Grik4+ mediodorsal neurons preferentially innervate prefrontal inhibitory neurons positive for parvalbumin (PV+) (Kim and others 2016). This allows these pathways to preferentially activate or suppress prefrontal activity patterns, a functional consequence that plays out differentially in behavior. In fact, these pathways appear to be required for handling uncertainty in decision making (Bach and Dolan 2012) but are required for distinct types of ambiguous inputs. The Grik4+ pathway appears to be recruited when the incoming evidence is ‘noisy’, turning on prefrontal inhibition to reduce its impact on the ongoing deliberation process. In contrast, the D2+ pathway is recruited when the evidence is ‘sparse’, enhancing prefrontal activity to amplify this faint evidence for incorporation into the deliberation process.
It is intriguing that these two pathways straddle two major ideas in schizophrenia, cortical inhibition dependent on parvalbumin interneurons (Lewis and others 2012; Mukherjee and others 2019) and dopamine D2 receptors, the main targets for antipsychotics (Broyd and others 2017). We suggest that these circuit discoveries go one step further, they link these molecular changes to key behavioral and cognitive symptoms in schizophrenia. Specifically, one of the best documented cognitive changes in schizophrenia is the ‘jumping to conclusions’ phenotype, which is associated with patients making high confidence judgments on ambiguous inputs (Woodward and others 2009; Ochoa and others 2014; Nassar and others 2021)(Pytlik and others 2020). This has been recently linked to applying less scrutiny on internally generated hypotheses, and the proneness to delusional thinking (Fouladirad and others 2022). Interestingly, appropriate engagement of prefrontal cortex in schizophrenia is preferentially attenuated in conditions of ambiguity (Krug and others 2014) consistent with the possibility that thalamic mechanisms for appropriately balancing its excitation and inhibition are impaired. Along these same lines, a number of recent studies have shown that prefrontal associated networks show abnormally increased responses during initial evidence acquisition but decreased activity during subsequent evidence accumulation, particularly for disconfirmatory evidence (Figure 5). These brain-behavior changes show correlation to delusional thoughts in clinical ratings (Lavigne and others 2020). Taken together we suggest that the classical notion of a ‘noisy prefrontal cortex’ in schizophrenia (Winterer and others 2004) may thus rely on thalamic mechanisms and in turn be reversed by their targeting. In the final section we discuss how existing techniques for neuromodulation may be implemented to target dysfunctional thalamocortical circuits.
Figure 5: Reduced recruitment of functional brain networks during evidence integration in schizophrenia patients.
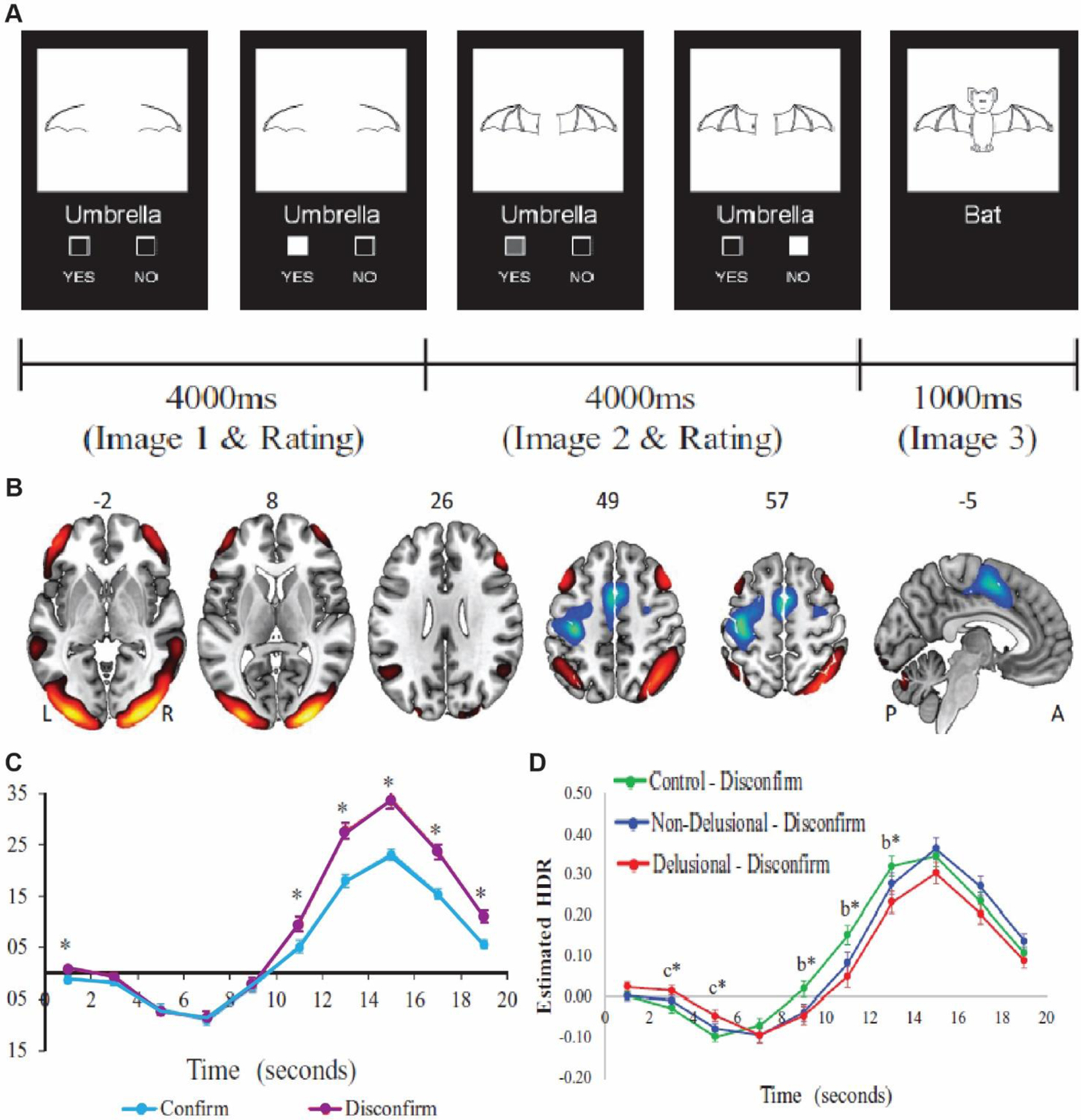
(A) In this task participants are presented with two consecutive partial line drawings of an object and a word describing it, with the second picture being more complete. Subjects were asked if the displayed word described the full picture upon presentation of the first picture and then to confirm/unconfirm, their decision when presented with the second picture. (B) Task induced activation of the cognitive evaluation network (CEN) comprised of bilateral orbitofrontal, dorsolateral PFC, angular gyrus, midline temporal gyrus and occipital lobe. (C) The CEN recruitment measured through fMRI is higher for disconfirmatory evidence integration (purple) than for confirmatory ones. (D) Schizophrenia patients with delusion (Red) show lower activation of the CEN network compared to controls (green) as well as non-delusional patients (blue). Adapted from Lavigne and others 2020.
A framework for targeted thalamocortical neuromodulation in schizophrenia
Over the last several years, neuromodulation techniques like deep brain stimulation (DBS) have evolved into viable therapeutic interventions for treatment of brain disorders (Schiff 2016; Lozano and others 2019; Janson and others 2021). Pioneering work by Schiff and colleagues has shown that central thalamic DBS in a patient, who had been minimally conscious for six years post traumatic brain injury, resulted in improved responses to commands, self-feeding, and functional communication (Schiff and others 2007). While the exact mode of action of DBS, is still under study, the reasons for target selection was grounded in prior research which showed that the central thalamus with its widespread and dense cortical projections is involved in regulating sleep-wake transitions (Brown and others 2011; Bastos and others 2021), enhancing attentional states (Schiff and others 2013; Blumenfeld 2021; Janson and others 2021) and driving forebrain-wide arousal patterns when stimulated (Shirvalkar and others 2006). Thus, could a similar approach be useful in targeting cognitive deficits in schizophrenia?
As highlighted throughout our perspective the associative mediodorsal thalamus occupies a central location in prefrontal cortex connectivity and function. Several lines of research in animal models as well as human neuroimaging studies have established the role of the mediodorsal thalamus in regulating interactions within and across prefrontal cortical areas. These studies have further shown that frontothalamic perturbations correlate with a host of dysfunctions characteristic of schizophrenia. Thus, mediodorsal thalamic stimulation with DBS might prove to be a viable therapy, especially in antipsychotic treatment resistant patients who account for 30% of the patient population (Chan and others 2021). Indeed, in a recent case study DBS was targeted to the substantia nigra pars reticulata, a key subcortical input to the mediodorsal thalamus, in a treatment resistant patient suffering from severe hallucinations and thought distortion. A DBS regimen for 24 weeks led to a near complete cessation of hallucinations and thought distortion and the patient remains stably improved on a 1 year follow up (Cascella and others 2021). These results are an excellent proof-of-principle for using targeted neuromodulation in schizophrenia. It is tantalizing to speculate that this approach may be optimized to preferentially target one of the two mediodorsal thalamic pathways discovered in the mouse work should they have human analogues. However, DBS is an invasive procedure that requires surgical implantation of chronic stimulation electrodes within the brain – a process that can only be performed in a neurosurgical setting and is associated with the risk of infections and hemorrhages (Lozano and others 2019). Thus, in some cases, it may be more viable to look for effective neuromodulation techniques that are minimally invasive.
Transcranial magnetic stimulation (TMS) presents as one such noninvasive technique where an electromagnetically generated electric current is used to depolarize neurons in the brain (Baeken and others 2019). While effective in treating treatment resistant depression, TMS has provided mixed results in schizophrenia where it has been targeted to the frontoparietal cortex to treat auditory hallucinations (Marzouk and others 2020) suggesting that further technical refinement is needed before TMS can be used in schizophrenia. Another major drawback of TMS arises from its lack of target specificity and penetrance - restricting its usage to broad cortical regions. However, more recent developments have resulted in improved target localization (Grossman and others 2017). This method shows enhanced spatial selectivity over traditional TMS, and therefore could evolve as a potential tool for targeted neuromodulation in schizophrenic patients.
A final emerging technology that mitigates the disadvantages of both DBS (invasiveness) and TMS (low penetrance beyond the cortex) is focused ultrasonic stimulation (fUS) (Wang and others 2020; Zhang and others 2022). Here, low intensity ultrasonic sound waves are non-invasively targeted through the skull into the brain with millimeter and millisecond resolution to mediate neuronal activation as well as suppression. While the exact mechanism through which fUS works is yet to be determined, preliminary evidence suggests that it triggers voltage dependent somatic and presynaptic Ca2+ transients within the target area as well as results in plastic changes as seen from the increased expression of neuronal activity induced transcription factor cFos (Tufail and others 2010). Over the last decade the number of publications on animal and human studies utilizing focused ultrasound has expanded tremendously (Fishman and Frenkel 2017; Yang and others 2020; Jeong and others 2022). In line with these Monti and colleagues recently demonstrated that low intensity fUS directed to the thalamus over a 10-minute period resulted in functional recovery of a patient in a minimally conscious state post traumatic brain injury (Monti and others 2016). While it remains to be seen whether fUS based neuromodulation would also work in patients who have been in a minimally conscious state long term, fUS presents us with a potent tool for therapeutic neuromodulation.
We envision a strategy to work similarly for cognitive deficits in schizophrenia, where frontal thalamocortical connectivity is targeted, perhaps even in a closed-loop manner (Figure 6). Specifically, for each patient a thalamocortical connectivity model is generated based on task and resting-state fMRI (Huys and others 2016; Frässle and others 2021). This is followed by fUS of the frontal thalamocortical pathway in the scanner, in which neuromodulation will be titrated using thalamocortical dynamics as neurofeedback signals. A similar approach has been shown to have pre-clinical efficacy in a rodent model of temporal lobe epilepsy (TLE) where the hippocampus was modulated with fUS based on the phase of hippocampal theta rhythms (Yang and others 2020). This stimulation paradigm improved both the power of the theta rhythms as well as latency and duration of epileptic seizures in mice. In schizophrenia, there has been a focus on reduced gamma oscillations (Grent-’t-jong and others 2018) and changes in prefrontal excitatory/inhibitory balance (Wengler and others 2020). Therefore, signatures of these processes and their changes in well-controlled tasks can be used as metrics for closed-loop feedback. Along similar lines, selective deficits in closed vs. open thalamocortical loops may be discovered in the human brain on the basis of tasks known to preferentially engage the former vs. the latter in non-human animals. Beyond modulating circuits directly, focused ultrasounds can also be used for targeted drug delivery to the brain, either by local opening of the blood-brain-barrier or release of drugs from carrier nanoparticles at the site of stimulation (Carpentier and others 2016; Airan 2017; Mainprize and others 2019). Therefore, focal release of D2 antagonists in individual brain regions may better modulate neural circuitry in schizophrenia and allow for increasing dosage with minimal systemic side effects.
Figure 6: Targeted modulation of thalamocortical dysconnectivity in Schizophrenia.
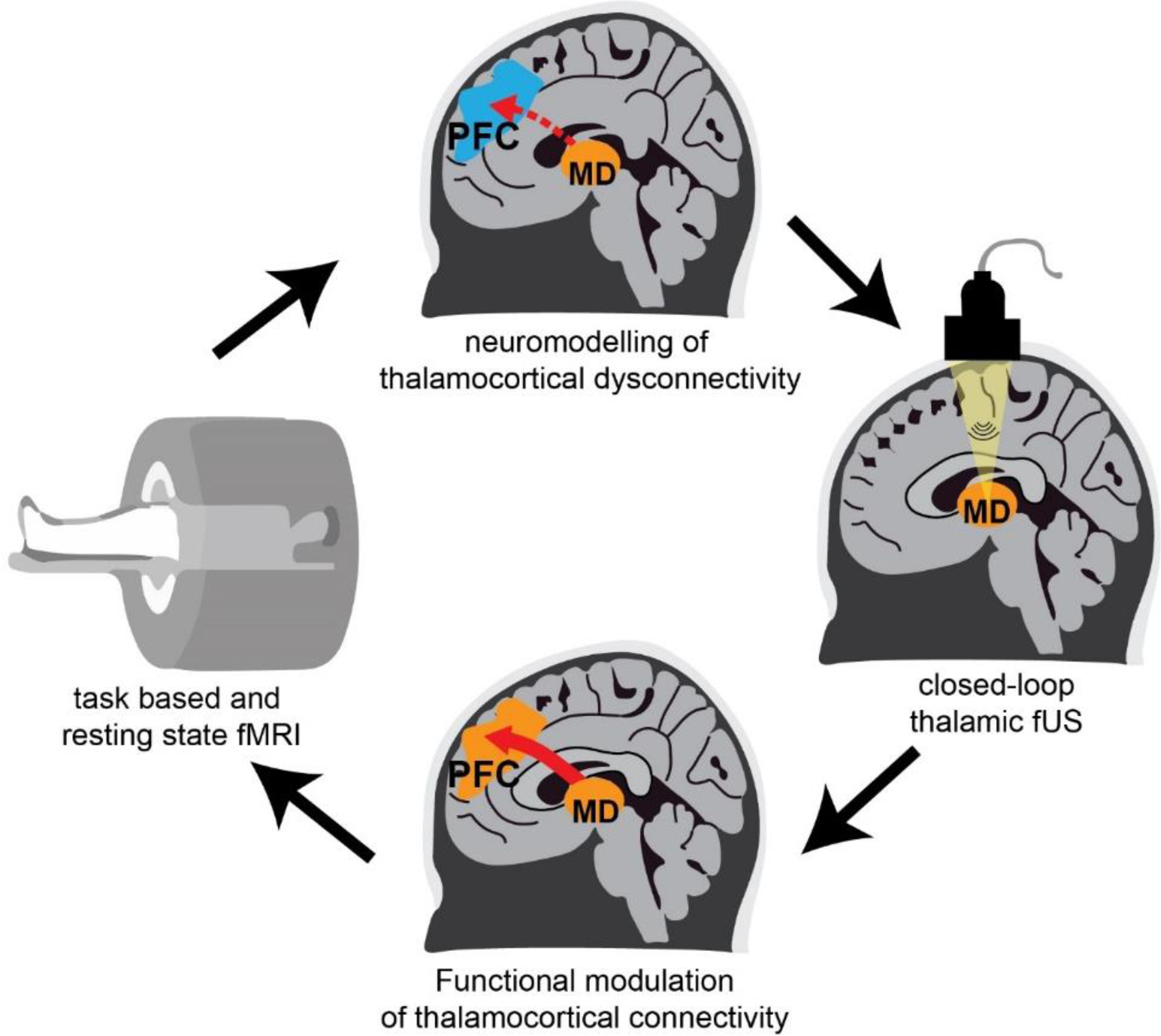
Task based and resting-state fMRI is used to obtain neuroimaging data of patients who are then stratified into different categories of thalamocortical dysconnectivity. Focused ultrasound stimulation of the thalamus is targeted based on this stratification. Functional modulation of dysconnectivity is monitored in real time using fMRI and behavioral testing to titrate stimulation parameters to individual patients.
Conclusions
The associative thalamus has emerged as a critical node for interactions within and across cortical areas subserving diverse cognitive functions. Ongoing exploration, both in humans and animal models has provided mechanistic insights into how the associative thalamus implements cognitive operations in the support of flexible behavior. Additionally, we have argued that perturbation of thalamocortical interactions between the mediodorsal thalamus and the prefrontal cortex may form the basis for delusional thinking in schizophrenia. Given the current advances in targeted neuromodulation through DBS, TMS and focused ultrasound, we are well poised to discover where in the thalamus to target and when in the illness to do so to usher in a new generation of therapy against this disorder.
References:
- Airan R. 2017. Neuromodulation with nanoparticles. Science (1979) 357:465. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Usrey WM, Reid RC. 1996. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature 383:815–819. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Usrey WM, Reid RC. 2001. Rules of connectivity between geniculate cells and simple cells in cat primary visual cortex. Journal of Neuroscience 21:4002–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiades PG, Collins DP, Carter AG. 2021. Mediodorsal and Ventromedial Thalamus Engage Distinct L1 Circuits in the Prefrontal Cortex. Neuron 109:314–330.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, and others. 2014. Characterizing thalamo-cortical disturbances in Schizophrenia and bipolar illness. Cerebral Cortex 24:3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro MJ, Pinsk MA, Kastner S. 2015. The Anatomical and Functional Organization of the Human Visual Pulvinar. Journal of Neuroscience [Internet] 35:9848–9871. Available from: https://www.jneurosci.org/content/35/27/9848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avram M, Rogg H, Korda A, Andreou C, Müller F, Borgwardt S. 2021. Bridging the Gap? Altered Thalamocortical Connectivity in Psychotic and Psychedelic States. Front Psychiatry [Internet] 12:706017. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34721097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Dolan RJ. 2012. Knowing how much you don’t know: A neural organization of uncertainty estimates. Nature Reviews Neuroscience [Internet] 13:572–586. Available from: www.nature.com/reviews/neuro [DOI] [PubMed] [Google Scholar]
- Baeken C, Brem AK, Arns M, Brunoni AR, Filipčić I, Ganho-Ávila A, and others. 2019. Repetitive transcranial magnetic stimulation treatment for depressive disorders: current knowledge and future directions. Current Opinion in Psychiatry [Internet] 32:409. Available from: /pmc/articles/PMC6688778/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SC, Konova AB, Daw ND, Horga G. 2019. A distinct inferential mechanism for delusions in schizophrenia. Brain 142:1797–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldauf D, Desimone R. 2014. Neural mechanisms of object-based attention. Science (1979) 344:424–427. [DOI] [PubMed] [Google Scholar]
- Baldwin MKL, Balaram P, Kaas JH. 2017. The evolution and functions of nuclei of the visual pulvinar in primates. Journal of Comparative Neurology [Internet] 525:3207–3226. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/cne.24272 [DOI] [PubMed] [Google Scholar]
- Bastos AM, Donoghue JA, Brincat SL, Mahnke M, Yanar J, Correa J, and others. 2021. Neural effects of propofol-induced unconsciousness and its reversal using thalamic stimulation. Elife [Internet] 10. Available from: https://pubmed.ncbi.nlm.nih.gov/33904411/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichot NP, Xu R, Ghadooshahy A, Williams ML, Desimone R. 2019. The role of prefrontal cortex in the control of feature attention in area V4. Nature Communications 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford ME, Wei H, Eisenback MA, Chomsung RD, Slusarczyk AS, Dankowsi AB. 2008. Synaptic organization of thalamocortical axon collaterals in the perigeniculate nucleus and dorsal lateral geniculate nucleus. Journal of Comparative Neurology [Internet] 508:264–285. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/cne.21671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot A, Roth MM, Gasler I, Javadzadeh M, Imhof F, Hofer SB. 2021. Visual intracortical and transthalamic pathways carry distinct information to cortical areas. Neuron 109:1996–2008.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H. 2021. Brain Mechanisms of Conscious Awareness: Detect, Pulse, Switch, and Wave. Neuroscientist [Internet]:10738584211049378. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34632846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkan SS, Stujenske JM, Parnaudeau S, Spellman TJ, Rauffenbart C, Abbas AI, and others. 2017. Thalamic projections sustain prefrontal activity during working memory maintenance. Nature Neuroscience 20:987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois A, Guedj C, Carrera E, Vuilleumier P. 2020. Pulvino-cortical interaction: An integrative role in the control of attention. Neuroscience & Biobehavioral Reviews 111:104–113. [DOI] [PubMed] [Google Scholar]
- Brown EN, Purdon PL, van Dort CJ. 2011. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci [Internet] 34:601–628. Available from: https://pubmed.ncbi.nlm.nih.gov/21513454/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd A, Balzan RP, Woodward TS, Allen P. 2017. Dopamine, cognitive biases and assessment of certainty: A neurocognitive model of delusions. Clinical Psychology Review 54:96–106. [DOI] [PubMed] [Google Scholar]
- Butler AB. 2008. Evolution of the thalamus: a morphological and functional review. Thalamus & Related Systems [Internet] 4:35–58. Available from: https://www.cambridge.org/core/journals/thalamus-and-related-systems/article/abs/evolution-of-the-thalamus-a-morphological-and-functional-review/B0D500C7591B6FC801B600D8C43EBD9B [Google Scholar]
- Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, and others. 2016. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med [Internet] 8. Available from: https://pubmed.ncbi.nlm.nih.gov/27306666/ [DOI] [PubMed] [Google Scholar]
- Casas-Torremocha D, Clascá F, Núñez Á. 2017. Posterior Thalamic Nucleus Modulation of Tactile Stimuli Processing in Rat Motor and Primary Somatosensory Cortices. Front Neural Circuits [Internet] 11:69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29021744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella N, Butala AA, Mills K, Kim MJ, Salimpour Y, Wojtasievicz T, and others. 2021. Deep Brain Stimulation of the Substantia Nigra Pars Reticulata for Treatment-Resistant Schizophrenia: A Case Report. Biol Psychiatry [Internet] 90:e57–e59. Available from: https://pubmed.ncbi.nlm.nih.gov/33906736/ [DOI] [PubMed] [Google Scholar]
- Chan SKW, Chan HYV, Honer WG, Bastiampillai T, Suen YN, Yeung WS, and others. 2021. Predictors of Treatment-Resistant and Clozapine-Resistant Schizophrenia: A 12-Year Follow-up Study of First-Episode Schizophrenia-Spectrum Disorders. Schizophrenia Bulletin 47:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Ye E, Jin X, Zhu Y, Wang L. 2019. Association between Thalamocortical Functional Connectivity Abnormalities and Cognitive Deficits in Schizophrenia. Scientific Reports 2019 9:1 [Internet] 9:1–10. Available from: https://www.nature.com/articles/s41598-019-39367-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KIK, Shenton ME, Kubicki M, Jung WH, Lee TY, Yun JY, and others. 2016. Altered Thalamo-Cortical White Matter Connectivity: Probabilistic Tractography Study in Clinical-High Risk for Psychosis and First-Episode Psychosis. Schizophrenia Bulletin [Internet] 42:723. Available from: /pmc/articles/PMC4838094/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, D’Esposito M. 2016. The segregation and integration of distinct brain networks and their relationship to cognition. Journal of Neuroscience 36:12083–12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. 2013. Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience 2013 16:9 [Internet] 16:1348–1355. Available from: https://www.nature.com/articles/nn.3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree JW. 2018. Functional diversity of thalamic reticular subnetworks. Frontiers in Systems Neuroscience 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola AL, Park MY, Crowe DA, MacDonald AW, Chafee M v. 2020. Differential roles of mediodorsal nucleus of the thalamus and prefrontal cortex in decision-making and state representation in a cognitive control task measuring deficits in schizophrenia. Journal of Neuroscience [Internet] 40:1650–1667. Available from: 10.1523/JNEUROSCI.1703-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D, Wang Y, Chang X, Luo C, Yao D. 2018. Dysfunction of Large-Scale Brain Networks in Schizophrenia: A Meta-analysis of Resting-State Functional Connectivity. Schizophrenia Bulletin [Internet] 44:168–181. Available from: https://academic.oup.com/schizophreniabulletin/article/44/1/168/3065825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egly R, Driver J, Rafal RD. 1994. Shifting Visual Attention Between Objects and Locations: Evidence From Normal and Parietal Lesion Subjects. Journal of Experimental Psychology: General [Internet] 123:161–177. Available from: /record/1994-34191-001 [DOI] [PubMed] [Google Scholar]
- El-Boustani S, Sermet BS, Foustoukos G, Oram TB, Yizhar O, Petersen CCH. 2020. Anatomically and functionally distinct thalamocortical inputs to primary and secondary mouse whisker somatosensory cortices. Nature Communications 2020 11:1 [Internet] 11:1–12. Available from: https://www.nature.com/articles/s41467-020-17087-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eradath MK, Pinsk MA, Kastner S. 2021. A causal role for the pulvinar in coordinating task-independent cortico–cortical interactions. Journal of Comparative Neurology 529:3772–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BR, Gao WJ. 2018. Thalamic Control of Cognition and Social Behavior Via Regulation of Gamma-Aminobutyric Acidergic Signaling and Excitation/Inhibition Balance in the Medial Prefrontal Cortex. Biological Psychiatry 83:657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn IC, Kastner S. 2019. The Puzzling Pulvinar. Neuron [Internet] 101:201–203. Available from: https://pubmed.ncbi.nlm.nih.gov/30653933/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn IC, Kastner S. 2020. Functional Specialization in the Attention Network. Annu Rev Psychol [Internet] 71:221. Available from: /pmc/articles/PMC7026883/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn IC, Pinsk MA, Kastner S. 2019. The mediodorsal pulvinar coordinates the macaque fronto-parietal network during rhythmic spatial attention. Nature Communications 2019 10:1 [Internet] 10:1–15. Available from: https://www.nature.com/articles/s41467-018-08151-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman PS, Frenkel V. 2017. Focused Ultrasound: An Emerging Therapeutic Modality for Neurologic Disease. Neurotherapeutics [Internet] 14:393. Available from: /pmc/articles/PMC5398988/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster NN, Barry J, Korobkova L, Garcia L, Gao L, Becerra M, and others. 2021. The mouse cortico–basal ganglia–thalamic network. Nature 2021 598:7879 [Internet] 598:188–194. Available from: https://www.nature.com/articles/s41586-021-03993-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouladirad S, Chen LV, Roes M, Chinchani A, Percival C, Khangura J, and others. 2022. Functional brain networks underlying probabilistic reasoning and delusions in schizophrenia. Psychiatry Research: Neuroimaging [Internet] 323:111472. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0925492722000336 [DOI] [PubMed] [Google Scholar]
- Frässle S, Aponte EA, Bollmann S, Brodersen KH, Do CT, Harrison OK, and others. 2021. TAPAS: An Open-Source Software Package for Translational Neuromodeling and Computational Psychiatry. Frontiers in Psychiatry [Internet] 12:680811. Available from: /pmc/articles/PMC8206497/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresno V, Parkes SL, Faugère A, Coutureau E, Wolff M. 2019. A thalamocortical circuit for updating action-outcome associations. Elife [Internet] 8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31012845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo-Chica M, Rogers BP, Damon SM, Landman BA, Woodward ND. 2018. Prefrontal-Thalamic Anatomical Connectivity and Executive Cognitive Function in Schizophrenia. Biological Psychiatry 83:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grent-’t-jong T, Gross J, Goense J, Wibral M, Gajwani R, Gumley AI, and others. 2018. Resting-state gamma-band power alterations in schizophrenia reveal E/I-balance abnormalities across illness-stages. Elife [Internet] 7. Available from: https://pubmed.ncbi.nlm.nih.gov/30260771/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh A, Bokor H, Mease RA, Plattner VM, Hangya B, Stroh A, and others. 2014. Convergence of cortical and sensory driver inputs on single thalamocortical cells. Cereb Cortex [Internet] 24:3167–3179. Available from: https://pubmed.ncbi.nlm.nih.gov/23825316/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk HJ, and others. 2017. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 169:1029–1041.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guet-McCreight A, Skinner FK, Topolnik L. 2020. Common Principles in Functional Organization of VIP/Calretinin Cell-Driven Disinhibitory Circuits Across Cortical Areas. Frontiers in Neural Circuits 14:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZV, Inagaki HK, Daie K, Druckmann S, Gerfen CR, Svoboda K. 2017. Maintenance of persistent activity in a frontal thalamocortical loop. Nature 545:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Barkat TR, O’Brien BMJ, Hensch TK, Polley DB. 2011. Linking topography to tonotopy in the mouse auditory thalamocortical circuit. Journal of Neuroscience 31:2983–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Acsády L. 2016. Thalamic Inhibition: Diverse Sources, Diverse Scales. Trends Neurosci [Internet] 39:680–693. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27589879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Chen Z, Wimmer RD, Brunetti PM, Zhao S, Zikopoulos B, and others. 2014. State-dependent architecture of thalamic reticular subnetworks. Cell 158:808–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Kastner S. 2017. Thalamic functions in distributed cognitive control. Nat Neurosci [Internet] 20:1669–1679. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29184210 [DOI] [PubMed] [Google Scholar]
- Halassa MM, Sherman SM. 2019. Thalamocortical Circuit Motifs: A General Framework. Neuron 103:762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamoda HM, Makhlouf AT, Fitzsimmons J, Rathi Y, Makris N, Mesholam-Gately RI, and others. 2019. Abnormalities in thalamo-cortical connections in patients with first-episode schizophrenia: a two-tensor tractography study. Brain Imaging Behav [Internet] 13:472–481. Available from: https://pubmed.ncbi.nlm.nih.gov/29667043/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma NY, Happel MFK, Nodal FR, Ohl FW, King AJ, Bajo VM. 2017. A role for auditory corticothalamic feedback in the perception of complex sounds. Journal of Neuroscience 37:6149–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Noble C, Pitman W, Yadav N, Kumar S, Keele GR, and others. 2020. A Thalamic Orphan Receptor Drives Variability in Short-Term Memory. Cell [Internet] 183:522–536.e19. Available from: http://www.cell.com/article/S0092867420311521/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Gan J, Jonas P. 2014. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science (1979) [Internet] 345:1255263–1255263. Available from: https://www.sciencemag.org/lookup/doi/10.1126/science.1255263 [DOI] [PubMed] [Google Scholar]
- Huang AS, Rogers BP, Woodward ND. 2019. Disrupted modulation of thalamus activation and thalamocortical connectivity during dual task performance in schizophrenia. Schizophr Res [Internet] 210:270–277. Available from: https://pubmed.ncbi.nlm.nih.gov/30630706/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1961. Integrative action in the cat’s lateral geniculate body. The Journal of Physiology 155:385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnicutt BJ, Long BR, Kusefoglu D, Gertz KJ, Zhong H, Mao T. 2014. A comprehensive thalamocortical projection map at the mesoscopic level. Nature Neuroscience 17:1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huys QJM, Maia T v., Frank MJ. 2016. Computational psychiatry as a bridge from neuroscience to clinical applications. Nat Neurosci [Internet] 19:404. Available from: /pmc/articles/PMC5443409/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Bertolero MA, Liu WB, D’Esposito M. 2017. The Human Thalamus Is an Integrative Hub for Functional Brain Networks. Journal of Neuroscience [Internet] 37:5594–5607. Available from: https://www.jneurosci.org/content/37/23/5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Shine JM, D’esposito M. 2019. Frontoparietal Activity Interacts With Task-Evoked Changes in Functional Connectivity. Cerebral Cortex [Internet] 29:802–813. Available from: https://academic.oup.com/cercor/article/29/2/802/4836785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson AP, Baker JL, Sani I, Purpura KP, Schiff ND, Butson CR. 2021. Selective activation of central thalamic fiber pathway facilitates behavioral performance in healthy non-human primates. Sci Rep [Internet] 11. Available from: https://pubmed.ncbi.nlm.nih.gov/34845232/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo J, Mejias JF, Wang X-J. 2019. Engagement of Pulvino-cortical Feedforward and Feedback Pathways in Cognitive Computations. Neuron [Internet] 101:321–336.e9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30553546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H, Song I-U, Chung Y-A, Park J-S, Na S-H, Im JJ, and others. 2022. Short-Term Efficacy of Transcranial Focused Ultrasound to the Hippocampus in Alzheimer’s Disease: A Preliminary Study. J Pers Med [Internet] 12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35207738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. 2007. The Thalamus. 2nd ed. Cambridge University Press; Available from: http://books.google.de/books?id=IR0fSgAACAAJ [Google Scholar]
- Kaas JH, Lyon DC. 2007. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Research Reviews [Internet] 55:285–296. Available from: /record/2007-15393-003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar K, DiCarlo JJ. 2021. Fast Recurrent Processing via Ventrolateral Prefrontal Cortex Is Needed by the Primate Ventral Stream for Robust Core Visual Object Recognition. Neuron 109:164–176.e5. [DOI] [PubMed] [Google Scholar]
- Kastner S, Fiebelkorn IC, Eradath MK. 2020. Dynamic pulvino-cortical interactions in the primate attention network. Curr Opin Neurobiol [Internet] 65:10. Available from: /pmc/articles/PMC7770054/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Ährlund-Richter S, Wang X, Deisseroth K, Carlén M. 2016. Prefrontal Parvalbumin Neurons in Control of Attention. Cell [Internet] 164:208–218. Available from: 10.1016/j.cell.2015.11.038 10.1016/j.cell.2015.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura Y, Nikkuni A, Hirashima N, Uetake T, Miyamoto A. 2013. Responses of pulvinar neurons reflect a subject’s confidence in visual categorization. Nature Neuroscience [Internet] 16:749–755. Available from: https://europepmc.org/article/med/23666179 [DOI] [PubMed] [Google Scholar]
- Kosciessa JQ, Lindenberger U, Garrett DD. 2021. Thalamocortical excitability modulation guides human perception under uncertainty. Nature Communications [Internet] 12:1–15. Available from: 10.1038/s41467-021-22511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug A, Cabanis M, Pyka M, Pauly K, Kellermann T, Walter H, and others. 2014. Attenuated prefrontal activation during decision-making under uncertainty in schizophrenia: a multi-center fMRI study. Schizophr Res [Internet] 152:176–183. Available from: https://pubmed.ncbi.nlm.nih.gov/24325976/ [DOI] [PubMed] [Google Scholar]
- Lam YW, Sherman SM. 2011. Functional organization of the thalamic input to the thalamic reticular nucleus. Journal of Neuroscience 31:6791–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne KM, Menon M, Woodward TS. 2020. Functional Brain Networks Underlying Evidence Integration and Delusions in Schizophrenia. Schizophrenia Bulletin [Internet] 46:175–183. Available from: https://academic.oup.com/schizophreniabulletin/article/46/1/175/5485375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M, Datunashvilli M, Kanyshkova T, Zobeiri M, Aissaoui A, Cerina M, and others. 2016. Two types of interneurons in the mouse lateral geniculate nucleus are characterized by different h-current density. Scientific Reports 2016 6:1 [Internet] 6:1–15. Available from: https://www.nature.com/articles/srep24904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptourgos P, Denève S, Jardri R. 2017. Can circular inference relate the neuropathological and behavioral aspects of schizophrenia? Current Opinion in Neurobiology [Internet] 46:154–161. Available from: https://pubmed.ncbi.nlm.nih.gov/28915387/ [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. 2012. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in Neurosciences 35:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Fan L, Cui Y, Wei X, He Y, Yang J, and others. 2022. The human mediodorsal thalamus: Organization, connectivity, and function. Neuroimage 249:118876. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, and others. 2019. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol [Internet] 15:148. Available from: /pmc/articles/PMC6397644/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, and others. 2019. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Scientific Reports 2019 9:1 [Internet] 9:1–7. Available from: https://www.nature.com/articles/s41598-018-36340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzouk T, Winkelbeiner S, Azizi H, Malhotra AK, Homan P. 2020. Transcranial Magnetic Stimulation for Positive Symptoms in Schizophrenia: A Systematic Review. Neuropsychobiology [Internet] 79:384–396. Available from: https://www.karger.com/Article/FullText/502148 [DOI] [PubMed] [Google Scholar]
- Mease RA, Kuner T, Fairhall AL, Groh A. 2017. Multiplexed Spike Coding and Adaptation in the Thalamus. Cell Reports 19:1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS. 2015. The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neurosci Biobehav Rev [Internet] 54:76–88. Available from: https://pubmed.ncbi.nlm.nih.gov/25757689/ [DOI] [PubMed] [Google Scholar]
- Mo C, Sherman SM. 2019. A Sensorimotor Pathway via Higher-Order Thalamus. Journal of Neuroscience [Internet] 39:692–704. Available from: https://www.jneurosci.org/content/39/4/692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Bajwa N, Lam NH, Porrero C, Clasca F, Halassa MM. 2020. Variation of connectivity across exemplar sensory and associative thalamocortical loops in the mouse. Elife 9:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Carvalho F, Eliez S, Caroni P. 2019. Long-Lasting Rescue of Network and Cognitive Dysfunction in a Genetic Schizophrenia Model. Cell 178:1387–1402.e14. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Lam NH, Wimmer RD, Halassa MM. 2021. Thalamic circuits for independent control of prefrontal signal and noise. Nature [Internet]. Available from: https://pubmed.ncbi.nlm.nih.gov/34614503/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AJ, Shaw L, Hasse JM, Goris RLT, Briggs F. 2021. Optogenetic activation of corticogeniculate feedback stabilizes response gain and increases information coding in LGN neurons. J Comput Neurosci [Internet] 49:259–271. Available from: https://pubmed.ncbi.nlm.nih.gov/32632511/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JD, Jaramillo J, Wang XJ. 2017. Working Memory and Decision-Making in a Frontoparietal Circuit Model. The Journal of Neuroscience [Internet] 37:12167. Available from: /pmc/articles/PMC5729190/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray Sherman S, Guillery RW. 2011. Distinct functions for direct and transthalamic corticocortical connections. Journal of Neurophysiology 106:1068–1077. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Halassa MM. 2017. Thalamic control of functional cortical connectivity. Current Opinion in Neurobiology 44:127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar M, Waltz J, Albrecht M, Gold J, Frank M. 2021. All or nothing belief updating in patients with schizophrenia reduces precision and flexibility of beliefs. Brain [Internet]. Available from: https://pubmed.ncbi.nlm.nih.gov/33434284/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa S, Haro JM, Huerta-Ramos E, Cuevas-Esteban J, Stephan-Otto C, Usall J, and others. 2014. Relation between jumping to conclusions and cognitive functioning in people with schizophrenia in contrast with healthy participants. Schizophrenia Research 159:211–217. [DOI] [PubMed] [Google Scholar]
- Ouhaz Z, Fleming H, Mitchell AS. 2018. Cognitive functions and neurodevelopmental disorders involving the prefrontal cortex and mediodorsal thalamus. Frontiers in Neuroscience 12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouhaz Z, Perry BAL, Nakamura K, Mitchell AS. 2022. Mediodorsal Thalamus Is Critical for Updating during Extradimensional Shifts But Not Reversals in the Attentional Set-Shifting Task. eNeuro [Internet] 9. Available from: https://www.eneuro.org/content/9/2/ENEURO.0162-21.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaudeau S, Bolkan SS, Kellendonk C. 2018. The Mediodorsal Thalamus: An Essential Partner of the Prefrontal Cortex for Cognition. Biological Psychiatry 83:648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaudeau S, O’Neill PK, Bolkan SS, Ward RD, Abbas AI, Roth BL, and others. 2013. Inhibition of Mediodorsal Thalamus Disrupts Thalamofrontal Connectivity and Cognition. Neuron 77:1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola G, Danet L, Pitel AL, Carlesimo GA, Segobin S, Pariente J, and others. 2018. The Regulatory Role of the Human Mediodorsal Thalamus. Trends in Cognitive Sciences [Internet] 22:1011. Available from: /pmc/articles/PMC6198112/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peron S, Pancholi R, Voelcker B, Wittenbach JD, Ólafsdóttir HF, Freeman J, and others. 2020. Recurrent interactions in local cortical circuits. Nature 2020 579:7798 [Internet] 579:256–259. Available from: https://www.nature.com/articles/s41586-020-2062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof I, Viaene AN, Sherman SM. 2012. Two populations of corticothalamic and interareal corticocortical cells in the subgranular layers of the mouse primary sensory cortices. J Comp Neurol [Internet] 520:1678–1686. Available from: https://pubmed.ncbi.nlm.nih.gov/22120996/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D. 2004. The thalamic reticular nucleus: Structure, function and concept. Brain Research Reviews 46:1–31. [DOI] [PubMed] [Google Scholar]
- Pytlik N, Soll D, Hesse K, Moritz S, Bechdolf A, Herrlich J, and others. 2020. Problems in measuring the JTC-bias in patients with psychotic disorders with the fish task: a secondary analysis of a baseline assessment of a randomized controlled trial. BMC Psychiatry [Internet] 20:1–10. Available from: https://bmcpsychiatry.biomedcentral.com/articles/10.1186/s12888-020-02959-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radnikow G, Feldmeyer D. 2018. Layer- and cell type-specific modulation of excitatory neuronal activity in the neocortex. Frontiers in Neuroanatomy 12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikhye RV, Gilra A, Halassa MM. 2018. Thalamic regulation of switching between cortical representations enables cognitive flexibility. Nature Neuroscience 21:1753–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. 2002. Response of Neurons in the Lateral Intraparietal Area during a Combined Visual Discrimination Reaction Time Task. Journal of Neuroscience [Internet] 22:9475–9489. Available from: https://www.jneurosci.org/content/22/21/9475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy DS, Zhang Y, Halassa MM, Feng G. 2022. Thalamic subnetworks as units of function. Nature Neuroscience 2022 25:2 [Internet] 25:140–153. Available from: https://www.nature.com/articles/s41593-021-00996-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S. 2011. Cognitive and Perceptual Functions of the Visual Thalamus. Neuron 71:209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. 2012. The pulvinar regulates information transmission between cortical areas based on attention demands. Science [Internet] 337:753–756. Available from: https://pubmed.ncbi.nlm.nih.gov/22879517/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. 2003. Prefrontal interactions reflect future task operations. Nat Neurosci [Internet] 6:75–81. Available from: https://pubmed.ncbi.nlm.nih.gov/12469132/ [DOI] [PubMed] [Google Scholar]
- Schiff ND. 2016. Mesocircuit mechanisms underlying recovery of consciousness following severe brain injuries: Model and predictions. Brain Function and Responsiveness in Disorders of Consciousness:195–204. [Google Scholar]
- Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, and others. 2007. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature 2007 448:7153 [Internet] 448:600–603. Available from: https://www.nature.com/articles/nature06041 [DOI] [PubMed] [Google Scholar]
- Schiff ND, Shah SA, Hudson AE, Nauvel T, Kalik SF, Purpura KP. 2013. Gating of attentional effort through the central thalamus. J Neurophysiol [Internet] 109:1152–1163. Available from: https://pubmed.ncbi.nlm.nih.gov/23221415/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer C, Lin A, Kushan L, Ji JL, Yang G, Bearden CE, and others. 2019. Dissociable Disruptions in Thalamic and Hippocampal Resting-State Functional Connectivity in Youth with 22q11.2 Deletions. The Journal of Neuroscience [Internet] 39:1301. Available from: /pmc/articles/PMC6381244/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt LI, Wimmer RD, Nakajima M, Happ M, Mofakham S, Halassa MM. 2017. Thalamic amplification of cortical connectivity sustains attentional control. Nature 545:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Kiani R. 2013. Decision making as a window on cognition. Neuron 80:791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GMG, Yamawaki N. 2021. Untangling the cortico-thalamo-cortical loop: cellular pieces of a knotty circuit puzzle. Nature Reviews Neuroscience 2021 22:7 [Internet] 22:389–406. Available from: https://www.nature.com/articles/s41583-021-00459-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. 2012. Thalamocortical interactions. Current Opinion in Neurobiology 22:575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S. 2003. The functional logic of cortico-pulvinar connections. Philos Trans R Soc Lond B Biol Sci [Internet] 358:1605–1624. Available from: https://pubmed.ncbi.nlm.nih.gov/14561322/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvalkar P, Seth M, Schiff ND, Herrera DG. 2006. Cognitive enhancement with central thalamic electrical stimulation. Proc Natl Acad Sci U S A [Internet] 103:17007–17012. Available from: https://pubmed.ncbi.nlm.nih.gov/17065322/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steullet P. 2020. Thalamus-related anomalies as candidate mechanism-based biomarkers for psychosis. Schizophr Res [Internet] 226:147–157. Available from: https://pubmed.ncbi.nlm.nih.gov/31147286/ [DOI] [PubMed] [Google Scholar]
- Svoboda K, Li N. 2018. Neural mechanisms of movement planning: motor cortex and beyond. Curr Opin Neurobiol [Internet] 49:33–41. Available from: https://pubmed.ncbi.nlm.nih.gov/29172091/ [DOI] [PubMed] [Google Scholar]
- Tang J, Jimenez SCA, Chakraborty S, Schultz SR. 2016. Visual Receptive Field Properties of Neurons in the Mouse Lateral Geniculate Nucleus. PLOS ONE [Internet] 11:e0146017. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0146017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, and others. 2010. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron [Internet] 66:681–694. Available from: https://pubmed.ncbi.nlm.nih.gov/20547127/ [DOI] [PubMed] [Google Scholar]
- Usrey WM, Alitto HJ. 2015. Visual Functions of the Thalamus. Annual Review of Vision Science 1:351–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Sherman SM. 2019. Corticofugal Circuits: Communication Lines from the Cortex to the Rest of the Brain. J Comp Neurol [Internet] 527:640. Available from: /pmc/articles/PMC6131091/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. 2015. Limbic circuitry of the midline thalamus. Neuroscience and Biobehavioral Reviews 54:89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JB, di Ianni T, Vyas DB, Huang Z, Park S, Hosseini-Nassab N, and others. 2020. Focused Ultrasound for Noninvasive, Focal Pharmacologic Neurointervention. Frontiers in Neuroscience 14:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sommer FT, Hirsch JA. 2011. Inhibitory circuits for visual processing in thalamus. Current Opinion in Neurobiology 21:726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert TW, Terrazas A, Bigelow LB, Apud JA, Egan MF, Weinberger DR. 2014. Perceptual category judgment deficits are related to prefrontal decision making abnormalities in schizophrenia. Frontiers in Psychiatry 4:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler K, Goldberg AT, Chahine G, Horga G. 2020. Distinct hierarchical alterations of intrinsic neural timescales account for different manifestations of psychosis. Elife 9:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, and others. 2004. Prefrontal Broadband Noise, Working Memory, and Genetic Risk for Schizoprenia. American Journal of Psychiatry [Internet] 161:490–500. Available from: https://ajp.psychiatryonline.org/doi/full/10.1176/appi.ajp.161.3.490 [DOI] [PubMed] [Google Scholar]
- Wolff M, Vann SD. 2019. The cognitive thalamus as a gateway to mental representations. Journal of Neuroscience 39:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Heckers S. 2016. Mapping Thalamocortical Functional Connectivity in Chronic and Early Stages of Psychotic Disorders. Biological Psychiatry 79:1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward TS, Mizrahi R, Menon M, Christensen BK. 2009. Correspondences between theory of mind, jumping to conclusions, neuropsychological measures and the symptoms of schizophrenia. Psychiatry Research 170:119–123. [DOI] [PubMed] [Google Scholar]
- Yang H, Yuan Y, Wang X, Li X. 2020. Closed-Loop Transcranial Ultrasound Stimulation for Real-Time Non-invasive Neuromodulation in vivo. Frontiers in Neuroscience [Internet] 14. Available from: /pmc/articles/PMC7235408/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. 2013. Fronto-parietal network: flexible hub of cognitive control. Trends Cogn Sci [Internet] 17:602–603. Available from: /pmc/articles/PMC3873155/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Palaniyappan L, Deng M, Zhang W, Pan Y, Fan Z, and others. 2021. Abnormal Thalamocortical Circuit in Adolescents With Early-Onset Schizophrenia. Journal of the American Academy of Child & Adolescent Psychiatry 60:479–489. [DOI] [PubMed] [Google Scholar]
- Zhang M, Rodrigues A, Zhou Q, Li G. 2022. Focused ultrasound: growth potential and future directions in neurosurgery. Journal of Neuro-Oncology [Internet] 156:23–32. Available from: https://link.springer.com/article/10.1007/s11060-021-03820-9 [DOI] [PubMed] [Google Scholar]
- Zhou H, Schafer RJ, Desimone R. 2016. Pulvinar-Cortex Interactions in Vision and Attention. Neuron [Internet] 89:209. Available from: /pmc/articles/PMC4723640/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou NA, Maire PS, Masterson SP, Bickford ME. 2017. The mouse pulvinar nucleus: Organization of the tectorecipient zones. Vis Neurosci [Internet] 34:E011. Available from: https://pubmed.ncbi.nlm.nih.gov/28965504/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolnik TA, Connors BW. 2016. Electrical synapses and the development of inhibitory circuits in the thalamus. Journal of Physiology 594:2579–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]


