Abstract
Lactoferricin includes an 11-amino-acid amphipathic alpha-helical region which is exhibited on the outer surface of the amino-terminal lobe of lactoferrin. Synthetic peptides homologous to this region exhibited potent antibacterial activity against a selected range of both gram-negative and gram-positive bacteria. An analog synthesized with methionine substituted for proline at position 26, which is predicted to disrupt the helical region, abolished antibacterial activity against Escherichia coli and considerably reduced antibacterial activity against Staphylococcus aureus and an Acinetobacter strain. The mode of action of human lactoferrin peptide (HLP) 2 against E. coli serotype O111 (NCTC 8007) was established by using flow cytometry, surface plasmon resonance, and transmission electron microscopy. Flow cytometry was used to monitor membrane potential, membrane integrity, and metabolic processes by using the fluorescent probes bis-1,3-(dibutylbarbituric acid)-trimethine oxonol, propidium iodide, and carbonyl cyanide m-chlorophenylhydrazone, respectively. HLP 2 was found to act at the cell membrane, causing complete loss of membrane potential after 10 min and of membrane integrity within 30 min, with irreversible damage to the cell as shown by rapid loss of viability. The number of particles, measured by light scatter on the flow cytometer, dropped significantly, showing that bacterial lysis resulted. The peptide was shown to bind to E. coli O111 lipopolysaccharide by using surface plasmon resonance. Transmission electron microscopy revealed bacterial distortion, with the outer membrane becoming detached from the inner cytoplasmic membrane. We conclude that HLP 2 causes membrane disruption of the outer membrane, resulting in lysis, and that structural considerations are important for antibacterial activity.
Lactoferrin is an avidly iron-binding glycoprotein of the transferrin family. It is found at mucosal surfaces, within the specific granules of neutrophils (32), and in biological fluids (31), such as milk (6, 7, 22), from which it was first isolated. Lactoferrin has been shown to have antimicrobial activity against a broad range of gram-positive bacteria, gram-negative bacteria, and fungi (3, 5, 24). This activity was attributed to its ability to sequester two atoms of iron (35), an essential bacterial nutrient, and is restricted to the apoprotein, with the diferric form being inactive (3). Lactoferrin has also been shown to act synergistically with other proteins, such as lysozyme (13) and immunoglobulin A (1), suggesting that it may damage the cell wall or outer membrane. Furthermore, lactoferrin binds to lipid A (2) and porins (15) and induces lipopolysaccharide (LPS) release from the bacterial wall (14).
Proteolytic digestion of human lactoferrin in vitro yields a peptide fragment called lactoferricin H, which has enhanced antimicrobial activity (4). The peptide corresponds partially to a surface helix on lactoferrin distinct from the area of iron binding (34) and in the region of LPS binding (12). We have shown that a synthetic 11-amino-acid peptide (previously designated HLT 2 [34] and now renamed human lactoferrin peptide [HLP] 2), which includes this LPS binding site, has potent antibacterial activity against Escherichia coli (34). The RKVR region found within HLP 2 forms the glycosaminoglycan binding site of human lactoferrin (28).
A number of other naturally occurring peptides have been shown to have antibacterial activity. These include magainins (39) from frogs, cecropins (21) from insects, and mellitin (19) from bee venom. Although these peptides have little sequence homology, they have two distinguishing features: a high content of basic amino acids, such as arginine and lysine, and the ability to adopt secondary conformations (namely, α-helices and β-sheet structures) in which there is a hydrophilic face of polar and positively charged residues on an axial plane (20). However, the three-dimensional structures of these peptides are not always apparent, as is the case with melittin, which takes up a different structure in aqueous solution when self-association reactions take place (monomer to tetramer) (37), or with magainin when bound to the phosphatidylserine vesicles (33). Cationic peptides have been shown to form pores in artificial membranes (9, 11, 23), and they can also have specific binding sites on the outer membranes of bacteria (10). Their antimicrobial activity is likely to be determined by the mode of interaction with the bacterial cell membrane (20).
A structural feature of the human lactoferricin loop is the 11-amino-acid α-helical region with an asymmetric cluster of basic amino acids (34). In this study the structure-function relationships of the lactoferricin-related peptides are considered. Synthetic peptides homologous to this region and analogs were synthesized, and their antimicrobial activities against a selected range of gram-negative and gram-positive microorganisms were assessed. By using E. coli, flow cytometry was used to monitor changes in bacterial cell wall potential (29, 30) and cell membrane integrity (17), with specific fluorescent dyes, transmission electron microscopy (TEM) to detect morphological changes of bacterial cells, and surface plasmon resonance to detect specific peptide binding sites. The implications of these findings with respect to the antimicrobial activity of full-length lactoferrin in vivo are discussed.
MATERIALS AND METHODS
Peptide synthesis.
Peptides HLP 1, 6, and 7 were synthesized by using 9-fluorenylmethoxycarbonyl chemistry at Kings College Pharmacy Department, London, United Kingdom. Peptides were assessed to be >95% pure by reverse-phase high-pressure liquid chromatography and mass spectrometry. Peptide HLP 2 was synthesized by Neosystem Laboratoire, Strasbourg, France. Peptide HLP 1 corresponded to the loop region of human lactoferricin (residues 20 to 35; NH2-FQWQRNMRKVRGPPVS-COOH), HLP 2 corresponded to the alpha-helical region of the loop in its native conformation (residues 20 to 30; NH2-FQWQRNMRKVR-COOH), HLP 6 had a proline substituted for methionine in HLP 2 (NH2-FQWQRNPRKVR-COOH), and HLP 7 was the d form of HLP 2.
Bacterial strains.
Staphylococcus aureus NCTC 10571 and E. coli NCTC 8007 and NCTC 10418 were obtained from the National Collection of Type Cultures, Colindale, United Kingdom. Clinical isolates of an Acinetobacter sp., Enterobacter aerogenes, a Klebsiella sp., Providencia stuartii, and Proteus mirabilis were provided by the Department of Microbiology, St. Thomas’s Hospital, London, United Kingdom. Bacterial cultures were stored at −70°C and grown on Columbia blood agar at 37°C.
MIC determination.
MICs of each peptide were determined in 96-well plates. Peptides were dissolved in 1% proteose peptone and serially diluted in microtiter wells to give concentrations of between 1.3 mM and 10 μM in a final volume of 95 μl. Bacteria were incubated at 37°C overnight in 1% proteose peptone to give approximately 108 bacteria/ml, and 5 μl was added to each well. The plates were incubated at 37°C overnight, and growth was determined by absorbance at 620 nm. Antimicrobial activity was expressed as the concentration of the peptide required to give no increase in absorbance at 620 nm following incubation (MIC). The MIC determination for each peptide was repeated in separate plates at least four times.
Effects of HLP 2 on membrane integrity and potential.
The membrane potential and integrity of E. coli NCTC 8007 in the presence of HLP 2 was monitored by using flow cytometry and selected dyes by the method of Mason et al. (30). E. coli incubated overnight in 1% proteose peptone was diluted 1:100 with fresh medium and incubated at 37°C to log-phase growth. Bacteria were washed in fresh medium, and cells were harvested to give a density of 108 cells/ml. Bacteria were incubated in different concentrations of HLP 2 in proteose peptone, and aliquots were withdrawn after 2 h of incubation at 37°C to give a dose-response assay. Time-response assays were performed at the MIC, with aliquots taken for analysis immediately (time zero) and at 15, 30, 60, 90, and 120 min. Bacteria were centrifuged at 13,400 × g for 30 s and resuspended in fresh medium before flow cytometry. Bacterial membrane potential was determined by using the lipophilic anionic membrane potential-sensitive dye bis-1,3-(dibutylbarbituric acid)trimethine oxonol [DiBAC4(3)] (Molecular Probes, Inc.) (30) at a final concentration of 10 μg/ml. Membrane integrity was assessed with the cationic nucleic acid binding fluorescent dye propidium iodide (PI) (Sigma, Poole, United Kingdom) (17) at a final concentration of 10 μg/ml. Fluorescence was measured with an HS Bryte flow cytometer (Bio-Rad, Hemel Hempstead, United Kingdom) and compared to that of control bacteria. Results are the means from four separate experiments.
Metabolic activity.
To investigate the effects of low temperature, cultures of E. coli NCTC 8007 were grown to log phase in 1% proteose peptone at 37°C, the temperature was reduced and equilibrated to 4°C, and the cultures were treated with HLP 2 at the MIC for 2 h. Samples were spun at 13,000 rpm for 30 s at 4°C, and the resulting pellet was resuspended in fresh cold medium. Parallel samples with E. coli NCTC 8007 incubated at 37°C in the presence of HLP 2 and control samples at 4 and 37°C without HLP 2 were also prepared. Aliquots of each sample were taken and subjected to flow cytometry in the presence of either DiBAC4(3) or PI. To determine the effect of the respiratory poison carbonyl cyanide m-chlorophenylhydrazone (CCCP) (Sigma) (26) on the action of HLP 2, bacterial samples were treated with 50 μM CCCP with or without the peptide for 10 min at 37°C, and the cells were washed by centrifugation at 13,400 × g for 30 s and resuspended in fresh medium. Aliquots of each sample were taken, and either DiBAC4(3) or PI was added for flow cytometric analysis.
Effect of HLP 2 on bacterial lysis and cell morphology.
Bacterial lysis was assessed by reduction in particle number. Cell morphology was assessed by comparing forward and side light scattering of bacteria incubated with peptide at the MIC for 2 h with that of control cultures. TEM was employed to confirm morphological changes of the bacterial cells. Log-phase E. coli (approximately 108 bacteria/ml) incubated for various times in 1% proteose peptone with HLP 2 and control cultures were centrifuged at 4,000 × g for 5 min, prefixed in 2.5% glutaraldehyde in 0.2 M phosphate buffer (pH 7.3), postfixed in 0.5% osmium tetroxide in Millonigs constant-osmolarity phosphate buffer (pH 7.4), and dehydrated in graded ethanol solutions. Pellets were embedded in medium Taab Resin and polymerized for 24 h at 60°C. Sections (50 nm) were stained with aqueous uranyl acetate and lead citrate and examined with a Hitatchi H700 transmission electron microscope.
HLP 2 binding to LPS.
The specificity with which peptide HLP 2 bound to LPS was measured by using surface plasmon resonance on a Biacore X (Pharmacia Biosensor AB). This measures the change in refractive index, shown as resonance units, of receptor binding to ligands on a chip surface (27). E. coli serotype O111 LPS (Sigma) was dissolved in phosphate-buffered saline (PBS) (pH 7.4) and equilibrated to saturation over the surface of a hydrophobic HPA sensor chip (Pharmacia Biosensor AB), to which it binds by hydrophobic interactions. Following saturation, peptide at various concentrations was suspended in PBS (pH 7.4) and passed over the coated chip, and peptide binding was measured as a change in resonance units by using BIA software. This was subtracted from binding of the peptide on a blank hydrophobic surface to give the number of resonance units after nonspecific binding had been taken into account. Experiments were carried out three times.
RESULTS
Antibacterial activities of HLPs.
HLP 1, the loop region, was active against E. coli NCTC 8007 and S. aureus NCTC 10571 with the same potency and had greater activity against an Acinetobacter strain but had no activity against P. mirabilis (Table 1). HLP 2, the alpha-helical region, had greater activity than HLP 1 against E. coli NCTC 8007, S. aureus, and the Acinetobacter strain and showed activity against E. aerogenes and a Klebsiella strain but had no activity against P. mirabilis and P. stuartii. HLP 6, in which methionine 26 was replaced by proline, showed no activity, up to 1 mM, against either E. coli NCTC 8007 or P. mirabilis and reduced activity (compared with HLP 2) against S. aureus and the Acinetobacter strain. The d form of HLP 2 (HLP 7) showed increased activity against E. coli NCTC 8007 and the Acinetobacter strain and the same potency against S. aureus as HLP 2 but had no activity against P. mirabilis.
TABLE 1.
MICs of human lactoferrin peptides against a range of bacteriaa
| Species and strain | MIC (μM)b
|
|||
|---|---|---|---|---|
| HLP 1 | HLP 2 | HLP 6 | HLP 7 | |
| E. coli NCTC 8007 | 500 | 290 | >1,000 | 18 |
| E. coli NCTC 10418 | 500 | 579 | 750 | 40 |
| S. aureus NCTC 10571 | 500 | 14 | 250 | 14 |
| Clinical isolates | ||||
| Acinetobacter sp. | 2 | 0.86 | 10 | 0.3 |
| E. aerogenes | >1,000 | 290 | >1,000 | >1,000 |
| Klebsiella sp. strain 3105 | >1,000 | 290 | >1,000 | 80 |
| P. stuartii | >1,000 | >1,000 | >1,000 | 110 |
| P. mirabilis | >1,000 | >1,000 | >1,000 | >1,000 |
Bacteria were grown to log phase and incubated against twofold serial dilutions of HLP 1, HLP 2, HLP 6, or HLP 7 at 37°C overnight in 96-well microtiter plates.
Determined by A620.
Effects of HLP 2 on the membrane potential and integrity.
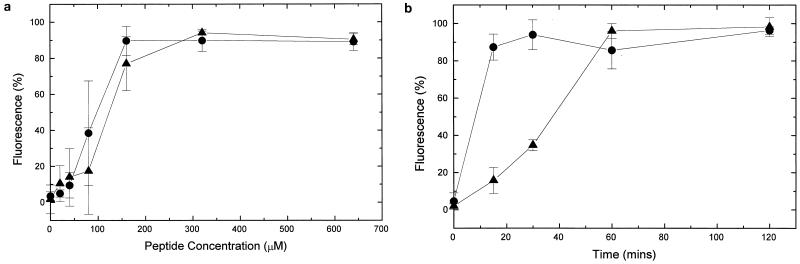
The number of cells in the bacterial population which showed dye-associated fluorescence in the presence of DiBAC4(3) or PI was expressed as a percentage. The dose-response assay (Fig. 1a) demonstrated that the addition of HLP 2 to E. coli NCTC 8007 results in 50% fluorescence at approximately 100 μM (0.15 mg/ml), and in the presence of PI at approximately 140 μM (0.2 mg/ml), showing that the collapse of membrane potential and integrity occurred at approximately the same concentrations. Time-response assays (Fig. 1b) showed that 50% fluorescence is seen after approximately 8 min in the presence of DiBAC4(3) and after approximately 35 min in the presence of PI. Collapse of membrane potential therefore occurred before collapse of membrane integrity.
FIG. 1.
Dose-response (a) and time-response (b) assays, by flow cytometry, of a starting inoculum of 108 E. coli O111 (NCTC 8007) cells per ml versus HLP 2. The percentages of fluorescent bacterial cells in the presence of PI (▴) or DiBAC4(3) (•) are shown. Results are means ± standard errors from at least three experiments.
Metabolic activity.
Inhibition of the metabolic pathways by addition of the respiratory poison CCCP or of enzymatic pathways by reduction of the assay temperature to 4°C had no effect on the activity of HLP 2 (Table 2).
TABLE 2.
E. coli fluorescence in the presence of HLP 2 and CCCP or in the presence of HLP 2 at 4°C determined by using DiBAC4(3) and PI
| Conditiona | Fluorescenceb
|
|||
|---|---|---|---|---|
| Without HLP 2
|
With HLP 2
|
|||
| DiBAC4(3) | PI | DIBAC4(3) | PI | |
| CCCP | ||||
| Absent | 6.7 | 3.3 | 98.3 | 99.4 |
| Present | 46.0 | 54.2 | 90.2 | 95.9 |
| Temp (°C) | ||||
| 37 | 6.4 | 1.2 | 96.3 | 98.2 |
| 4 | 24.7 | 15.5 | 94.1 | 97.2 |
E. coli grown to log phase was used to give a starting inoculum of 108 bacteria/ml and incubated for 2 h in the presence or absence of HLP 2 and either with or without CCCP or at 4 or 37°C.
Fluorescence was monitored by flow cytometry and is expressed as the percentage of fluorescent cells in the whole population. Results are means from at least four experiments.
Rate of cell lysis.
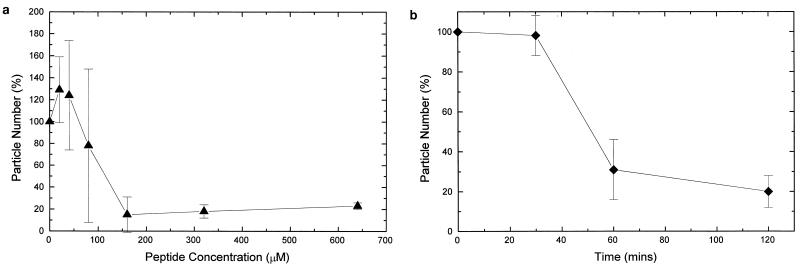
The results were plotted as a percentage of particles compared to that for a control culture which contained no peptide. The dose-response assay (Fig. 2a) showed that 50% of the particles remained at approximately 140 μM (0.2 mg/ml), and the time-response assay (Fig. 2b) showed that 50% of particles remained after 50 min. A comparison of the time-response assays and dose-response assays for particle number with the corresponding DiBAC4(3) and PI assays indicated an inversely proportional relationship between PI fluorescence (indicative of membrane integrity) and particle number.
FIG. 2.
(a) Percentages of particles present after incubation for 2 h of E. coli O111 (NCTC 8007) with various concentrations of HLP 2 compared to that for the control (containing no HLP 2), measured by using flow cytometry. (b) Percentages of particles after incubation of E. coli NCTC 8007 with HLP 2 at the MIC for various time intervals compared to that for control samples containing no HLP 2. Experiments were repeated at least three times, with a starting inoculum of 108 bacteria/ml. Error bars indicate standard errors.
HLP 2 binding to LPS.
After taking into account any nonspecific binding by subtracting binding to a blank chip surface, 450 ± 50 resonance units of HLP 2 bound to 787 ± 100 resonance units of LPS, which is indicative of a specific interaction between HLP 2 and LPS.
Effects of HLP 2 on permeabilization of E. coli.
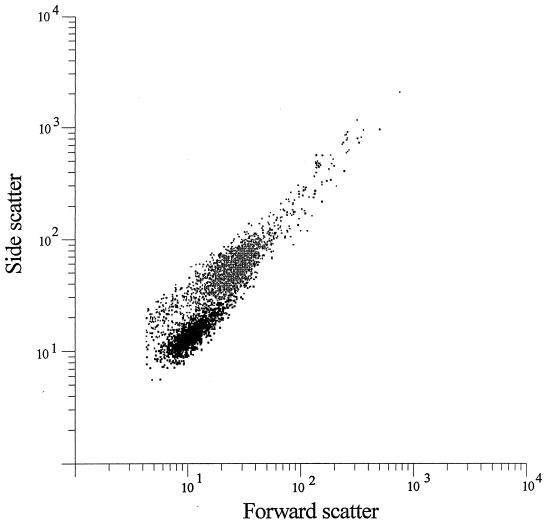
Treatment of E. coli with peptide HLP 2 at the MIC for 2 h resulted in a shift of both forward and side light scatter (Fig. 3), indicating a change in size or morphology (29).
FIG. 3.
Typical side and forward light scatter of control bacteria (black) and bacteria incubated with HLP 2 for 2 h (grey).
Electron microscopy studies of E. coli cells treated with HLP 2.
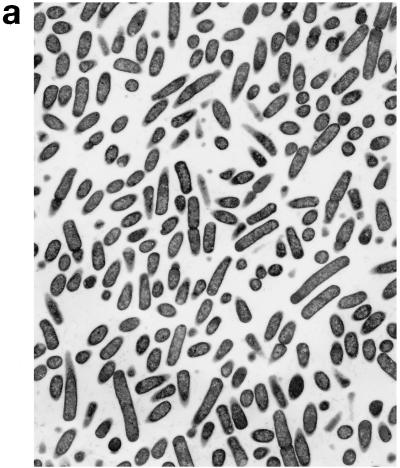
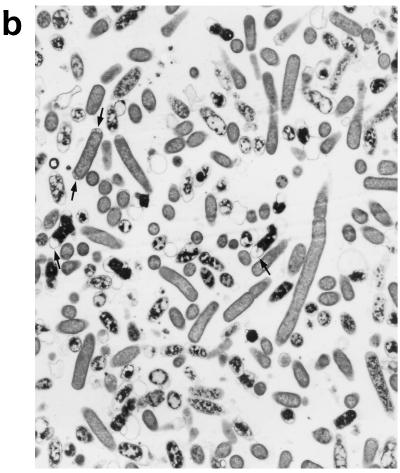
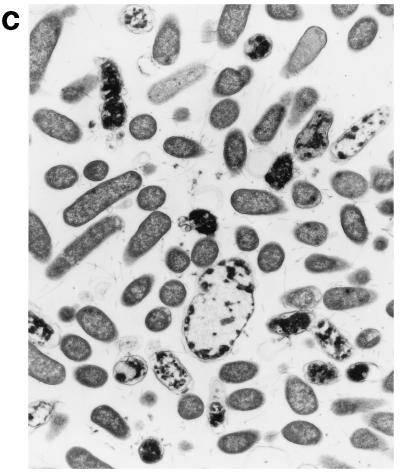
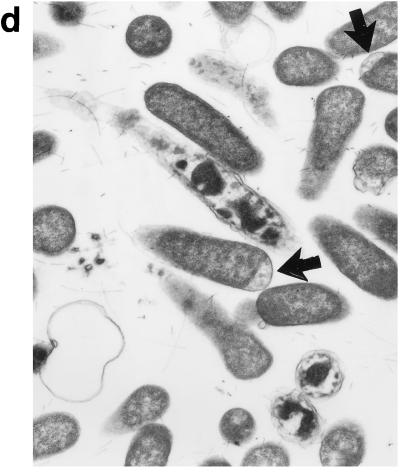
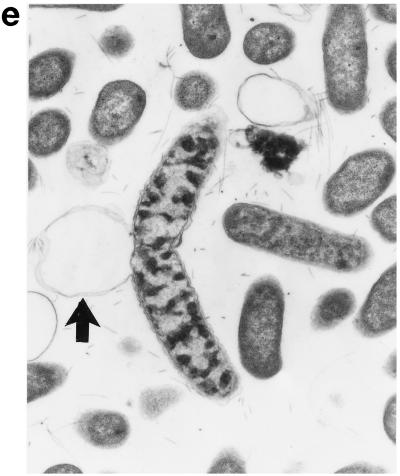
Upon exposure of E. coli to HLP 2 at the MIC and half the MIC for 2 h, TEM revealed clumping of the cytoplasm and the presence of ghost cells (cells which have no cytoplasm but still have a cell wall) (Fig. 4b) as compared to control cells (Fig. 4a). At half the MIC, cytoplasmic clumping and enlargement of the bacteria, caused by blistering of the outer membrane, were observed (Fig. 4c, d, and e). The outer membrane was observed to separate, particularly at the ends of the cells or at the junction of dividing cells. No changes were seen at concentrations lower than half the MIC or after 1 h at the MIC or half the MIC. The change in the morphology of bacteria at the MIC after 120 min is consistent with the forward and side light scatter differences shown in Fig. 3.
FIG. 4.
Electron micrographs of E. coli O111 NCTC 8007. (a) Control (magnification, ×3,600); (b and c) after 2 h of incubation with HLP 2 at the MIC (magnifications, ×3,600 [b] and ×7,200 [c]); (d and e) after 2 h of incubation with HLP 2 at half the MIC (magnification, ×13,500). The original inoculum size was 108 bacteria/ml. Arrows indicate separation of the outer membrane.
DISCUSSION
We have previously shown that the loop region of human lactoferricin contains an amphipathic α-helical region exposed on the outer surface of lactoferrin on helix 1 and that a synthetic 11-amino-acid peptide from this region, HLP 2, has bactericidal activity towards E. coli (34). It has recently been reported that the homologous peptide from bovine lactoferrin adopted an alpha-helical structure in both trifluoroethanol and sodium dodecyl sulfate (25), indicating that hydrophobic conditions are required for maintenance of the alpha-helical structure. We have now demonstrated by using surface plasmon resonance that HLP 2 binds specifically to LPS of E. coli O111, showing the importance of this region to binding and antimicrobial pathogenesis. Previous evidence, obtained by using recombinant human lactoferrin mutated at residues 28 to 34, has shown that this region on the whole molecule is involved in binding to LPS (12). However, other sites may also play a role in binding, such as the arginine cradle proposed by Mann et al. (28), in which arginines 2, 3, and 4 at the amino terminus, in combination with the cationic region on helix 1, form a positively charged cluster on the outer surface of human lactoferrin. The HLP 2 region is likely to play two roles in vivo, first within the whole human lactoferrin molecule and second as the peptide fragment lactoferricin, liberated after enzymatic degradation of lactoferrin. However, the mechanisms of action of lactoferrin and lactoferricin may well be different, as the free peptide fragment has enhanced antibacterial activity compared to lactoferrin (4). Although the bactericidal actions of lactoferrin and lactoferricin may differ, the HLP 2 LPS recognition site probably plays an important role in initial bacterial-protein–peptide interactions.
The orientations of the charged amino acids play an important part in the ability of HLP 2 to exert its antimicrobial activity, as seen by the large reduction in antimicrobial activity when proline is substituted for methionine. This substitution was predicted, by molecular modelling, to disrupt the helix and therefore the orientations of charged amino acids (8). Conformational changes occur within each lobe of human lactoferrin upon binding of iron, when the two domains of each lobe come together, with major structural changes occurring around the hinge region and subtle differences occurring throughout the rest of the molecule (18). The differences in charge orientation at the HLP 2 region on helix 1 between the apolactoferrin and hololactoferrin could account for the inability of the holo form to exert antimicrobial activity (3). It has already been established that the affinity of apo human serum transferrin for the human transferrin receptor is lower than that of the diferric form, and in addition, subtle changes in the conformation of the diferric protein, as a result of a mutation close to one of the metal binding sites, reduce its affinity for the receptor (16).
We have used flow cytometry to investigate how HLP 2 exerts its action on E. coli O111 with the aid of specific markers. Exposure of the bacteria to HLP 2 results in the collapse of membrane potential, leading to pore formation, as shown by PI fluorescence, and causing a collapse in membrane integrity. This is followed by distortion of the cell morphology, as shown by the forward and side light scatter, which finally results in cell lysis. Lysis results in the release of proteases, which could lead to partial proteolysis of the peptide and a reduction in its effective concentration. As the d form of the peptide is likely to be more resistant to proteolysis, this could explain the enhanced potency of HLP 7, the d form of HLP 2, against E. coli O111, a Klebsiella strain, and P. stuartii. From the results of experiments carried out either in the presence of the metabolic inhibitor CCCP or at 4°C, we can conclude that the peptide acts at the membrane surface and not at the metabolic level. The results of our studies using flow cytometry indicate that HLP 2 exerts its antimicrobial effect by a mechanism similar to that of other cationic peptides which act at the bacterial cell membrane. The proposed mechanism by which cationic peptides exert their antimicrobial activity is by interacting with negatively charged divalent cation binding sites on the surface LPS, disrupting these sites and leading to uptake of peptide across the outer membrane. The affected membrane is thought to form channels which allow leakage of cytoplasmic molecules and lead to cell death (20). The differences between HLP 2 and other natural cationic peptides (36) are the comparatively low potency and relatively slow mode of action of HLP 2. We are therefore able to study how the peptide may act and thus gain insight into how HLP 2 and other alpha-helical cationic peptides may exert their activities. The timing of collapse of membrane potential (10 min), collapse of integrity (30 min), and cell lysis (within 2 h) suggests that HLP 2 initially attaches to a site on the LPS, leading to an interaction between the peptide and outer membrane (OM). This is further supported by the TEM micrographs, in which the OM was observed to detach at specific cell sites, either at the point of division or at the peripheral ends of the cells, without loss of structural rigidity. Differences in LPS concentration at different points along the OM could account for the specific detachment. The TEM results obtained in this study are different from these previously shown for lactoferrin (13) or lactoferricin (38), which cause electron-dense blisters, indicating that lactoferrin or lactoferricin simply binds at the cell surface, without penetration, whereas HLP 2 enters the OM.
The increased potency against S. aureus compared with that against E. coli can be explained by differences in the structure of the bacterial cell wall. It is likely that HLP 2 has a different mechanism of action towards S. aureus than towards E. coli because of the absence of LPS. The difference between the activities of HLP 1 and HLP 2 against S. aureus could be explained by differences in peptide size, allowing uptake of HLP 2 rather than HLP 1, but it is more likely to be due to differences in flexibility and conformation if the peptide binds to the peptidoglycan. This would also explain why HLP 6 still has activity against S. aureus. Differences in the composition of the bacterial cell wall and, in the case of gram-negative bacteria, differences in the structure of LPS which prevent bacterial interactions could account for the observations that certain bacteria are resistant to HLP 2 and for the differences in activity between those bacteria which are susceptible to HLP 2. In vivo, this may be important in establishing a bacterial flora.
We have been able to propose for the first time a mechanism of action for how the loop region of helix 1 in human lactoferrin, both in the whole protein and as a liberated peptide, can exert its antimicrobial activity. We have shown that both the helix and the charge are important for antimicrobial activity against E. coli and that subtle differences in charge orientation can cause significant differences in potency. The differences in potency between the free peptide and lactoferrin can be explained by the degree to which the helix can interact with the bacterial membrane as a result of the increased flexibility of the free peptide, to cause bacterial cell wall disruption.
ACKNOWLEDGMENTS
D. S. Chapple was supported by a studentship from the Special Trustees of St. Thomas’ Hospital. Financial support from Bio-Rad Microscience, United Kingdom, is gratefully acknowledged.
We are grateful to A. I. Mallett, St. John’s Institute of Dermatology, UMDS, St. Thomas’s Hospital, for assessing the purity of the peptides by mass spectrometry; to S. Bansal, Kings College, London, for peptide synthesis; and to Ken Brady and his team for their guidance in the use of the electron microscopy facility at UMDS.
REFERENCES
- 1.Akin D T, Lu M Q, Kendall S, Rundegren J, Arnold R R. Bactericidal activity of different forms of lactoferrin. Adv Exp Med Biol. 1994;357:61–70. doi: 10.1007/978-1-4615-2548-6_7. [DOI] [PubMed] [Google Scholar]
- 2.Appelmelk B J, Am Y, Geerts M, Thijs B G, DeBoar H A, MacLaren D M, DeGraff J, Nuijens J H. Lactoferrin is a lipid A-binding protein. Infect Immun. 1994;62:2628–2632. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold R R, Cole R M, McGhee J R. A bactericidal effect for human lactoferrin. Science. 1977;197:263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- 5.Bortner C A, Miller R D, Arnold R R. Bactericidal effect of lactoferrin on Legionella pneumophila. Infect Immun. 1986;51:373–377. doi: 10.1128/iai.51.2.373-377.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brock J H. Lactoferrin in human milk: its role in iron absorption and protection against enteric infection in the new-born infant. Arch Dis Child. 1980;55:417–421. doi: 10.1136/adc.55.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullen J J, Rogers H J, Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972;1:69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapple, D. S., D. J. Mason, C. L. Joannou, J. K. Shergill, E. W. Odell, V. Gant, and R. W. Evans. A helical region on human lactoferrin—its role in antimicrobial pathogenesis. In G. Spik, D. Legrand, J. Mazurier, J.-P. Perraudin, and A. Pierce (ed.), Advances in lactoferrin research, in press. Plenum Publishing Corporation, New York, N.Y.
- 9.Christensen B, Fink J, Merrifield R B, Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci USA. 1988;85:5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David S A, Manthan V I, Balaram P. Interaction of mellitin with endotoxic lipid A. Biochim Biophys Acta. 1992;1123:269–274. doi: 10.1016/0005-2760(92)90006-h. [DOI] [PubMed] [Google Scholar]
- 11.Duclohier H, Molle G, Spach G. Antimicrobial peptide magainin I from Xenopus skin forms anion-permeable channels in planar lipid bilayers. Biophys J. 1989;56:1017–1021. doi: 10.1016/S0006-3495(89)82746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elass-Rochard E, Roseanu A, Legrand D, Trif M, Salmon V, Motas C, Montreuil J, Spik G. Lactoferrin-lipopolysaccharide interaction: involvement of the 28-34 loop region of human lactoferrin in the high affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem J. 1995;312:839–845. doi: 10.1042/bj3120839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison R T, III, Giehl T J. Killing of Gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest. 1991;88:1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellison R T, III, Giehl T J, LaForce F M. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect Immun. 1988;56:2774–2780. doi: 10.1128/iai.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erdei J, Forsgren A, Naidu A S. Lactoferrin binds to porins OmpF and OmpC in Escherichia coli. Infect Immun. 1994;62:1236–1240. doi: 10.1128/iai.62.4.1236-1240.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans R W, Crawley J B, Garratt R C, Grossmann J G, Neu M, Aitken A, Patel K J, Meilak A, Wong C, Singh J, Bomford A, Hasnain S S. Characterisation and structural analysis of a functional human serum transferrin variant and implications for receptor recognition. Biochemistry. 1994;33:12512–12520. doi: 10.1021/bi00207a019. [DOI] [PubMed] [Google Scholar]
- 17.Gant V A, Warnes G, Phillips I, Savage G F. The application of flow cytometry to the study of bacterial responses to antibiotics. J Med Microbiol. 1993;23:83–88. doi: 10.1099/00222615-39-2-147. [DOI] [PubMed] [Google Scholar]
- 18.Grossmann J G, Neu M, Pantos E, Schwab F J, Evans R W, Townes-Andrews E, Appel H, Thies W G, Hasnain S S. X-ray solution scattering reveals conformational changes upon iron uptake in lactoferrin, serum and ovo-transferrins. J Mol Biol. 1992;225:811–819. doi: 10.1016/0022-2836(92)90402-6. [DOI] [PubMed] [Google Scholar]
- 19.Habermann E, Jentsch J. Sequence analysis of melittin from tryptic and peptic degradation products. Hoppe-Seyler’s Z Physiol Chem. 1967;348:37–50. [PubMed] [Google Scholar]
- 20.Hancock R E W. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 21.Hultmark D, Steiner H, Rasmuson T, Boman H G. Insect immunity. Purification and properties of three inducible bactericidal proteins from the hemolymph of immunised pupae of Hyalphora cecropia. Eur J Biochem. 1980;106:7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- 22.Johansson B. Isolation of an iron-containing red protein from human milk. Acta Chem Scand. 1960;14:510–512. [Google Scholar]
- 23.Kagan B L, Selsted M E, Ganz T, Lehrer R I. Antimicrobial defensin peptides form voltage dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci USA. 1990;87:210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalmar J R, Arnold R R. Killing of Actinobacillus actinomycetemcomitans by human lactoferrin. Infect Immun. 1988;56:2552–2557. doi: 10.1128/iai.56.10.2552-2557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang J H, Lee M K, Kim K L, Hahan K S. Structure-biological activity relationship of 11-residue highly basic peptide segment of bovine lactoferrin. Int J Peptide Protein Res. 1996;48:357–363. doi: 10.1111/j.1399-3011.1996.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 26.Kaprelyants A S, Kell D B. Rapid assessment of bacterial viability and vitality by rhodamine 123 and flow-cytometry. J Appl Bacteriol. 1992;72:410–422. [Google Scholar]
- 27.Kuziemko G M, Stroh M, Stevens R C. Cholera toxin binding affinity and specificity for gangliosides determined by surface plasmon resonance. Biochemistry. 1996;35:6375–6384. doi: 10.1021/bi952314i. [DOI] [PubMed] [Google Scholar]
- 28.Mann D M, Romm E, Migliorini M. Delineation of glycosaminoglycan-binding site in the human inflammatory response protein lactoferrin. J Biol Chem. 1994;269:23661–23667. [PubMed] [Google Scholar]
- 29.Mason D J, Power E G M, Talsania H, Phillips I, Gant V. Antibacterial action of ciprofloxacin. J Antimicrob Chemother. 1995;39:2752–2758. doi: 10.1128/aac.39.12.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason D J, Allman R, Stark J M, Lloyd D. Rapid estimation of bacterial antibiotic susceptibility. J Microsc. 1994;176:8–16. doi: 10.1111/j.1365-2818.1994.tb03494.x. [DOI] [PubMed] [Google Scholar]
- 31.Masson P L, Heremans J F, Dive C. An iron-binding protein common to many external secretions. Clin Chim Acta. 1966;14:735–739. [Google Scholar]
- 32.Masson P L, Heremans J F, Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969;130:643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuzaki K, Harada M, Handa T, Funakoshi S, Fujii N, Yajima H, Miyajima K. Magainin 1-induced leakage of entrapped calcein out of negatively-charged lipid vesicles. Biochem Biophys Acta. 1989;981:130–134. doi: 10.1016/0005-2736(89)90090-4. [DOI] [PubMed] [Google Scholar]
- 34.Odell E W, Sarra R, Foxworthy M, Chapple D S, Evans R W. Antibacterial activity of peptides homologous to a loop region in human lactoferrin. FEBS Lett. 1996;382:175–178. doi: 10.1016/0014-5793(96)00168-8. [DOI] [PubMed] [Google Scholar]
- 35.Oram J D, Reiter B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim Biophys Acta. 1968;170:351–365. doi: 10.1016/0304-4165(68)90015-9. [DOI] [PubMed] [Google Scholar]
- 36.Skerlavaj B, Romeo D, Gennaro R. Rapid membrane permeabilization and inhibition of vital functions of gram-negative bacteria by bactenecins. Infect Immun. 1990;58:3724–3730. doi: 10.1128/iai.58.11.3724-3730.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terwilliger T C, Weissman L, Eisenberg D. The structure of melittin in the form I crystals and its implication for melittin’s lytic and surface activities. Biophys J. 1982;37:353–361. doi: 10.1016/S0006-3495(82)84683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamauchi K, Tomita M, Giehl T J, Ellison R T., III Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect Immun. 1993;61:719–728. doi: 10.1128/iai.61.2.719-728.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]