Abstract
In the December issue of Journal of Feline Medicine and Surgery, the following case report was published with several errors. The corrected version of this case report follows in full. The publishers apologise for this error.
A 7-year-old spayed domestic shorthaired cat presented with a history of having two seizures in the previous 6 weeks. During the seizures, the cat lost consciousness, paddled its limbs, rolled and urinated. There were no pre-ictal signs but the cat was weak and unable to walk normally in the post-ictal period.
Physical examination was unremarkable although there was evidence of overgrooming on the ventral abdomen. Neurological and ophthalmological examinations were considered normal. Abnormalities in routine haematology included a mild leucocytosis due to a mature neutrophilia. Plasma biochemistry revealed a slight increase in creatine kinase activity, consistent with muscle damage in a seizuring patient. Urine was obtained by cystocentesis and a low number of bacteria was detected with microscopic examination; a light growth of a haemolytic Escherichia coli was cultured from the urine. Feline leukaemia antigen and feline immunodeficiency virus antibody assays were negative.
The cat was anaesthetised and cerebrospinal fluid (CSF) collected from the cerebellomedullary cistern. The fluid was clear and colourless with a protein concentration of 0.34 g/l and 13.33 × 106 cells/l, comprising 30% monocytes and macrophages, 64% lymphocytes and 6% neutrophils. CSF culture was not performed. Recovery from anaesthesia was prompt but after extubation the cat started to paddle and bite its paws and became cyanotic before developing opisthotonos. Propofol was administered intravenously (iv) and the cat was re-intubated. However, it still exhibited extensor rigidity when extubation was attempted. The rigidity was controlled initially with midazolam (0.2 mg/kg iv) and subsequently with phenobarbitone (6 mg/kg iv) but mild limb paddling and excessive licking and leg biting continued. As the CSF pleocytosis suggested inflammatory intracranial disease, serum was submitted for toxoplasma serology. The Toxoplasma gondii specific immunoglobulin G (IgG) titre by immunofluorescent assay was 160 and the immunoglobulin M (IgM) titre was greater than or equal to 160. A tentative diagnosis of cerebral toxoplasmosis was made. Seizure activity resolved over 24 h and the cat was discharged from the hospital on clindamycin treatment (20 mg/kg twice daily orally (PO) for 4 weeks).
Two weeks later, another seizure was observed and the anticonvulsant phenobarbitone was added to the treatment (3 mg/kg once daily PO for 4 days increasing to 5.5 mg/kg daily PO in divided doses after 10 days). Toxoplasma serology four weeks later demonstrated the IgG titre had increased to greater than or equal to 640; the IgM titre was unchanged. One week after cessation of clindamycin treatment, another seizure occurred and a further 28-day course of clindamycin (20 mg/kg twice daily PO) was prescribed and phenobarbitone treatment was continued.
Six months after the initial examination, the cat presented for further evaluation. It had continued to seizure once every month, with each seizure lasting about 30 s. The owners commented that it usually held its head to the left when having a seizure. Two days before presentation, the cat had become anorexic, adipsic and was observed straining to urinate and defaecate. It also started shivering, circling to the left, growling when handled and seemed poorly responsive to sound.
Physical examination revealed a very depressed cat with a dull coat and dry mucous membranes. It circled to the left and was mildly ataxic. Neurological examination revealed an absent menace response in the right eye, despite normal direct and consensual pupillary light reflexes, and subtle deficits in right fore- and hindlimb postural reactions. Spinal reflexes and proprioceptive positioning were considered normal. Prehension difficulties were observed although the cat could chew and swallow food placed in its mouth. The neurological findings were consistent with a lesion in the left prosencephalon and magnetic resonance imaging (MRI) of the brain was recommended.
With the cat anaesthetised, a GE Vectra II 0.5 Tesla scanner with a human wrist surface coil was used to obtain T1-weighted gradient echo sequence (SPGR) images in the sagittal, transverse (axial) and dorsal (coronal) planes (Fig 1a, b) and transverse T2-weighted images (Fig 2). T1-weighted transverse images were repeated after administration of gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA) (0.1 mmol/kg) (Fig 3). A large intra-axial lesion (3 × 2 × 1.8 cm) was observed in the left cerebral hemisphere. The lesion was irregular in outline with a multi-lobed appearance. Increased signal density on the T2-weighted images, interpreted as extensive surrounding oedema, was noted throughout the left cerebral hemisphere and there was considerable associated mass effect with compression of the left lateral ventricle and displacement of the midline structures towards the right. There was descending transtentorial herniation on the left compressing the dorsal aspect of the left cerebellar hemisphere and there was also sub-falcine herniation. After Gd-DTPA, there was considerable gyriform enhancement in the margins of the mass and evidence of central necrosis. No other lesions were detected and there was no abnormality involving the ethmoid air cells or the cribriform plate. In view of the slowly progressive clinical signs, the location of the lesion, the extensive image enhancement after Gd-DTPA and the relatively well circumscribed nature of the single large lesion, meningioma and glioma were considered the most likely diagnoses. Excisional biopsy of the lesion was declined and euthanasia was performed.
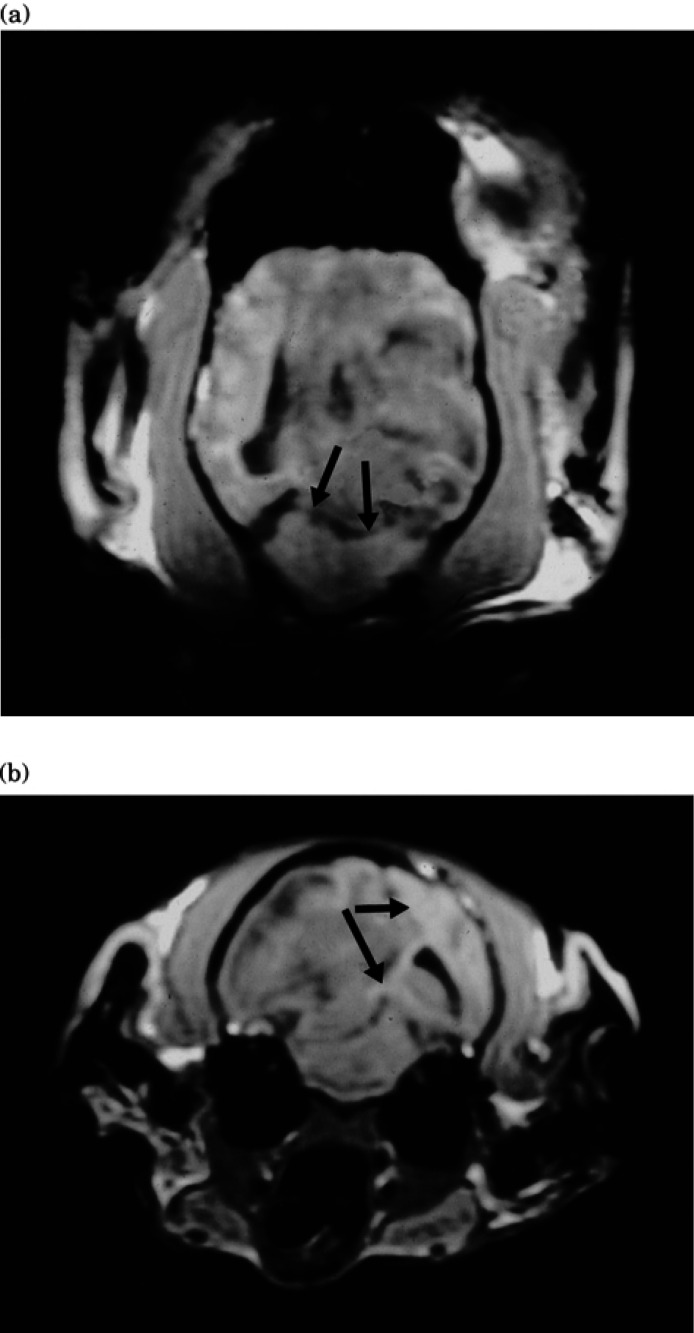
Fig 1.
Transverse (a) and dorsal (b) SPGR images (50/12/45°) showing the hypo-intense lesion with mass effect compressing the lateral ventricle and producing subfalcine (arrows in Fig 1b) and descending transtentorial herniation (arrows in Fig 1a).
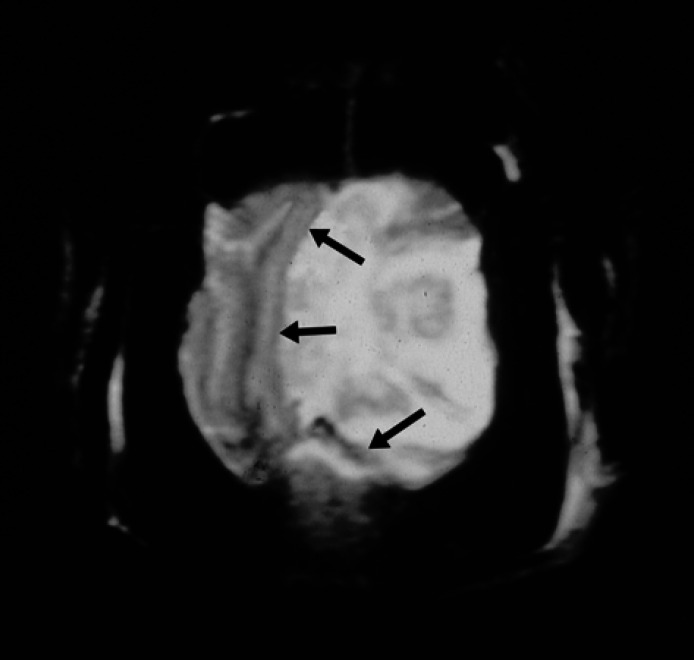
Fig 2.
Transverse (axial) FSE T2-weighted image (4000/110:TR/TC) showing a lobulated mass lesion in the left hemisphere with marked surrounding oedema and central fluid accumulation. There is a severe mass effect with subfalcine herniation (arrows).
Fig 3.
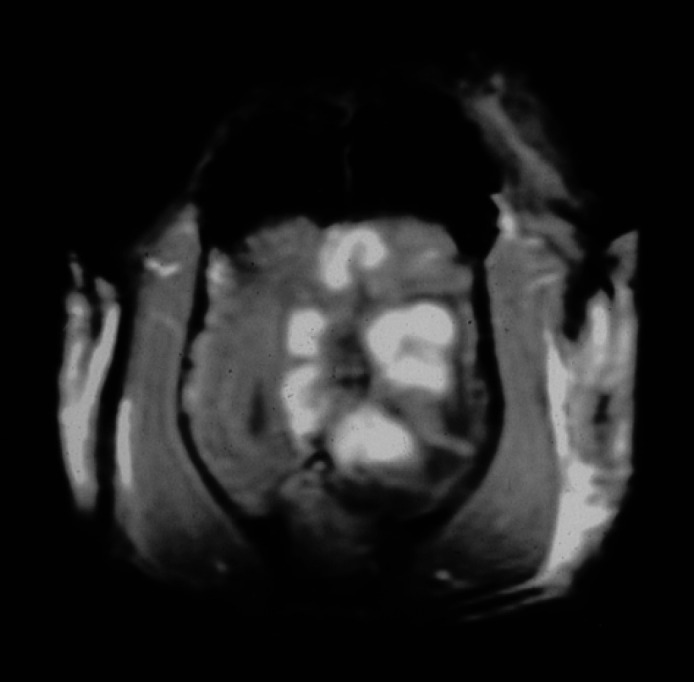
Transverse (axial) SPGR contrast enhanced T1-weighted image (50/12/45°) at the same level as Fig 1. There is enhancement in the subcortical white matter at the margin of the central fluid density cavity producing a striking lobulated appearance to the lesion.
The mass identified by MRI corresponded histologically to several cryptococcal pyogranulomas enveloped by broad zones of oedema and gliosis. The large but variably sized inflammatory foci were present in the left dorsomedial, dorsal and dorsolateral cerebrocortical hemisphere, involving the gyri cinguli, splenialis, suprasplenialis, marginalis, ectomarginalis (supra-sylvianus) and ectosylvius (Fig 4). The foci involved both parietal and temporal lobe cortical grey and white matter and the white matter of the corona radiata, with several foci extending into the overlying leptomeninges to produce adhesions between apposed gyri. There was no extension of infection to involve the lumen of the compressed left lateral ventricle.
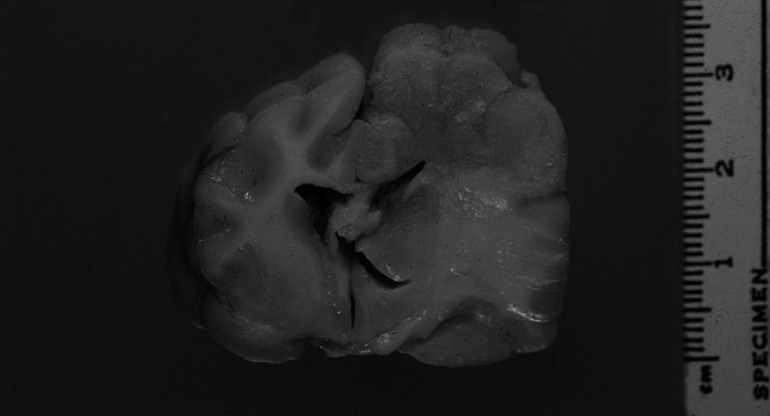
Fig 4.
Transverse section of the brain at the level of the third ventricle and optic tract. Enlargement, distortion and grey discolouration of grey and white matter of the left parietal cortex and left dorsolateral thalamus, with compression of the left lateral ventricle and right displacement of midline structures. Marked expansion of the corona radiata, internal capsule, corpus striatum and lateral thalamus due to reactive oedema and gliosis is evident.
Cryptococcal organisms were present in moderate to regionally high numbers and were of typical appearance, being pale basophilic, round to ovoid structures surrounded by thick clear capsules which stained strongly with periodic acid Schiff. Narrow-based budding was infrequently observed (Fig 5). The organisms were up to 30 μm in diameter, including the capsule. In some areas, there was only a mild neutrophilic response to high cell density sheets of yeasts. In most areas, however, there was intense suppurative to pyogranulomatous exudate enveloping small clusters of yeasts. Dense aggregates of lymphocytes and occasional plasma cells were scattered around the periphery of the inflammatory foci and were also present focally in the overlying leptomeninges and as thick cuffs in the Virchow-Robin spaces of the surrounding neuropil. Broad zones of moderate to severe oedema and reactive gliosis were present in the cortex adjacent to the infectious foci and extending to the margins of the left lateral ventricle and into the left medial corpus callosum. Moderate to severe oedema also radiated into the left dorsolateral and lateral thalamus, corona radiata, internal capsule and corpus striatum. Much of the white matter and, to a lesser extent, grey matter of the left dorsomedial and dorsal cerebral cortex was reduced to glial scar tissue rich in fibrous gemistocytes.
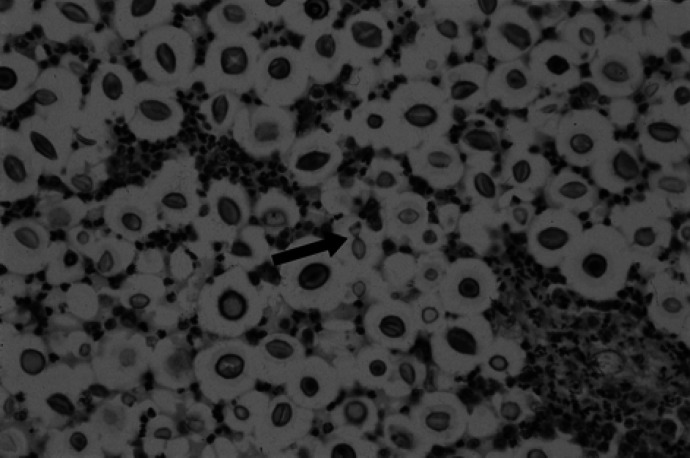
Fig 5.
Left dorsal cerebral cortex. Aggregates of cryptococcal yeasts surrounded by clear halos representing polysac-charide capsules, with infiltrating neutrophils. Central arrow demonstrates narrow-based budding of a yeast. (Haematoxylin and eosin, magnification × 230).
Cryptococcal organisms in the histological sections were typed as Cryptococcus neoformans var. neoformans using immunohistochemistry This new method utilises monoclonal antibodies directed against variety specific capsular antigens (Krockenberger et al., in preparation). A latex cryptococcal antigen agglutination test (LCAT) titre was determined retrospectively on stored serum from the initial presentation and was found to be 32, indicating that cryptococcal infection had most probably been responsible for all the cat's clinical signs.
Discussion
Diseases of the feline central nervous system can be difficult to diagnose as neurological examinations are often difficult to perform and interpret, CSF cytology is typically neither sensitive nor specific and imaging studies are expensive and not easily accessible. This report describes a cat with cerebral disease in which CSF cytology, serology for Toxoplasma gondii and MRI led to incorrect diagnoses.
The seizures for which the cat initially presented were almost certainly referable to a small cerebral cryptococcal granuloma. The lesion presumably acted as an epileptogenic focus but was not large enough to cause signs of prosencephalic dysfunction that could be appreciated on routine neurological examination. The neurological signs which developed as the cat deteriorated were consistent with expansion of a mass lesion in the left prosencephalon. Lesions in this area can result in contralateral menace and postural reaction deficits. The right-sided postural reaction deficits result from interference with ascending conscious proprioceptive pathways or descending upper motor neurons in the left prosencephalon (Glass et al 1996). The overgrooming and agitated licking and biting of the skin associated with seizure activity were consistent with a disturbance to thalamocortical pathways concerned with cutaneous sensation and nociception. Circling towards the side of the lesion is common with prosencephalic lesions (Oliver & Lorenz 1983, Glass et al 1996) and it was interesting that seizure activity invariably resulted in a left head tilt, an ipsilateral vestibular sign. Prosencephalic lesions have also been reported to cause inappropriate urination (Glass et al 1996) but it is not known whether the straining in this case was related to the neurological disorder or a lower urinary tract problem. The cat's clinical signs at the final presentation were almost identical to those described by Glass et al (1996) in a cat with a single, large cryptococcal granuloma documented by computerised tomography (CT) imaging and excisional biopsy.
Central nervous system cryptococcosis is a particularly important neurological problem in humans with acquired immunodeficiency syndrome (AIDS). There are numerous reports of MRI findings in humans with intracranial cryptococcosis but mostly with respect to patients with AIDS (Tien et al 1991, Mathews et al 1992, Andreula et al 1993, Miszkiel et al 1996, Sánchez-Portocarrero & Pérez-Cecilia 1997). Magnetic resonance imaging in humans with intracranial cryptococcosis usually demonstrates meningitis or meningoencephalitis, dilated Virchow-Robin spaces, cyst-like structures (gelatinous pseudocysts) and granulomas (cryptococcomas) (Andreula et al 1993, Ostrow & Hudgins 1994, Miszkiel et al 1996). Lesions are usually hyperintense on T2-weighted scans and have intermediate to low signal intensity on T1-weighted sequences. The lesions are usually in the basal ganglia, thalamus or midbrain and range from 3 mm to several centimetres in diameter (Andreula et al 1993, Ostrow & Hudgins 1994, Miszkiel et al 1996). Enhancement of cryptococcomas or the leptomeninges after Gd-DTPA is not typical (Mathews et al 1992, Andreula et al 1993, Ostrow & Hudgins 1994, Miszkiel et al 1996) although it has been reported (Tiens et al 1991). Doubling the dose of Gd-DTPA has been reported to cause more reliable enhancement (Andreula et al 1993). The reason for this lack of enhancement has been attributed to a poor inflammatory response due to the unique character of the organism and its protective polysaccharide capsule or, more likely, as a result of the profound immunosuppression involved in the majority of human cases of cryptococcosis (Yu et al 1995, Miszkiel et al 1996). Contrast enhancement has been reported in an immunocompetent human patient (Yu et al 1995) and in a dog (Tiches et al 1998).
There are relatively few MRI reports of cryptococcal mass lesions in humans as the frequency of these lesions in AIDS patients is very low (Sánchez-Portocarrero & Pérez-Cecilia 1997). When the literature was reviewed, 9% of HIV patients had focal cerebral mass lesions attributed to cryptococcosis (but which could have been due to other pathology such as toxoplasmosis or lymphosarcoma) compared with 23% of immunocompetent patients (Sánchez-Portocarrero & Pérez-Cecilia 1997). This difference could be related to a difference in cryptococcal varieties involved, as cryptococcosis in immunocompromised humans appears to be almost exclusively due to Cryptococcus neoformans var. neoformans whilst 74% of infections in immunocompetent patients are due to Cryptococcus neoformans var. gattii, (Mitchell et al 1995). Cerebral cryptococcomas have been reported in one study as only occurring with var. gattii infections (Speed 1993, Speed & Dunt 1995) and extrapolating from the human pathology and radiology studies, it was thought that var. gattii was most likely in this cat. Although culture was not performed, the organism was typed as var. neoformans by immunohistochemistry This suggests that cryptococcal mass lesions may reflect a competent cell-mediated response rather than cryptococcal variety.
To our knowledge, this is the first description of MRI of a cerebral cryptococcal granuloma in a cat. Cryptococcosis was not suspected in this patient because of the size and site of the lesion and the intense enhancement on T1-weighted images after Gd-DTPA. This report serves to highlight a number of issues in the diagnosis of CNS disease in cats. Cryptococcus organisms were not seen in the cat's CSF, but organisms are only found in 40–70% of human cases (Dimech 1991) and 60% of veterinary cases (Meric Taylor 1998) of CNS cryptococcosis. The lack of organisms and the relatively subtle pleocytosis may have been because the infection resulted in a cryptococcal granuloma with only late extension to involve the leptomeninges; human patients with solitary cerebral lesions can have normal CSF (Mitchell et al 1995). Culture of the CSF may have been rewarding although negative results of culture have been recorded in human patients with CNS cryptococcosis (Speed & Dunt 1995). As a provisional diagnosis of toxoplasmosis was made on the basis of the CSF analysis and serology cryptococcosis was dismissed as a diagnostic possibility and a LCAT was not performed. It was later revealed that the toxoplasma IgM test procedure used had very low specificity although the rising toxoplasma IgG titre serology was certainly typical of a recent infection with T gondii. The presence of infection does not necessarily correlate with clinical disease (Dubey 1994) and the relatively poor response to prolonged clindamycin treatment should have indicated that toxoplasmosis was unlikely to be responsible for the cat's signs.
A LCAT following pronase treatment (Stockman & Roberts 1983, Medleau et al 1990) should be performed routinely as part of the diagnostic investigation of intra-cranial disease in cats. The LCAT is a sensitive and specific test. It is simple, accessible, inexpensive, and, in this cat's case, would have eliminated the need for CSF collection (a procedure not without risk in cats with intracranial disease) and expensive imaging studies. Interestingly, cryptococcal antigen was not detected in the serum of one cat with a cerebral cryptococcal granuloma (Glass et al 1996) although it was demonstrated retrospectively in our case. If treatment for cryptococcosis had been instituted at the first presentation, the cat's disease may well have resolved. Should imaging be performed in cats with CNS disease, the presence of a single, large, contrast-enhanced cerebral lesion should not preclude a diagnosis of cryptococcal granuloma. Differentiation of cryptococcomas from other brain lesions may be possible in the future with magnetic resonance spectroscopy based on the high levels of α,α trehalose present in the cryptococcal lesions (Dzendrowski et al 1999).
Acknowledgements
Susan Foster was supported by a Lionel Lonsdale Clinical Fellowship. Richard Malik is supported by the Valentine Charlton Bequest of the Post-Graduate Foundation in Veterinary Science of the University of Sydney.
References
- Andreula CF, Burdi N, Carella A. (1993) CNS cryptococcosis in AIDS: spectrum of MR findings. Journal of Computer Assisted Tomography 17, 438–441. [DOI] [PubMed] [Google Scholar]
- Dimech WJ. (1991) Diagnosis, identification and epidemiology of Cryptococcus neoformans infection. Australian Journal of Medical Laboratory Science 12, 13–21. [Google Scholar]
- Dubey JP. (1994) Toxoplasmosis and other coccidial infections. In: The Cat: Diseases and Clinical Management (2nd edn). Sherding R. (ed). New York: Churchill Livingstone, pp 565–584. [Google Scholar]
- Dzendrowski TE, Himmelreich U, Dowd S, Allen C, Mountford C, Sorrell TC. (1999) An animal model for ex vivo discrimination of cerebral cryptococcoma from glioma. In: Programme and Abstracts of the 4th International Conference on Cryptococcus and Cryptococcosis, London, UK. London: The Royal Society, pp 185. [Google Scholar]
- Glass E, deLahunta A, Kent M, Kapatkin A, Joseph R. (1996) A cryptococcal granuloma in the brain of a cat causing focal signs. Progress in Veterinary Neurology 7, 141–144. [Google Scholar]
- Mathews VP, Alo PL, Glass JD, Kumar AJ, McArthur JC. (1992) AIDS-related CNS cryptococcosis: radiologicpathologic correlation. AJNR 13, 1477–1486. [PMC free article] [PubMed] [Google Scholar]
- Medleau L, Marks MA, Brown J, Borges WL. (1990) Clinical evaluation of a cryptococcal antigen latex agglutination test for diagnosis of cryptococcosis in cats. Journal of the American Veterinary Medical Association 196, 1470–1473. [PubMed] [Google Scholar]
- Taylor S Meric. (1998) Diagnostic tests for the neuromuscular system. In: Small Animal Internal Medicine. Nelson RW, Couto CG. (eds). St Louis: Mosby, pp 955–969. [Google Scholar]
- Miszkiel KA, Hall-Craggs MA, Miller RF, Kendall BE, Wilkinson ID, Paley MN, Harrison MJG. (1996) The spectrum of MRI findings in CNS cryptococcosis in AIDS. Clinical Radiology 51, 842–850. [DOI] [PubMed] [Google Scholar]
- Mitchell DH, Sorrell TC, Allworth AM, Heath CH, McGregor AR, Papanaoum K, Richards MJ, Gottlieb T. (1995) Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clinical Infectious Disease 20, 611–616. [DOI] [PubMed] [Google Scholar]
- Oliver JE, Lorenz MD. (1983) Neurologic examination. In: Handbook of Veterinary Neurologic Diagnoses. Philadelphia: WB Saunders, pp 19–57. [Google Scholar]
- Ostrow TD, Hudgins PA. (1994) Magnetic resonance imaging of intracranial fungal infections. Topics in MRI 6, 22–31. [PubMed] [Google Scholar]
- Sánchez-Portocarrero J, Pérez-Cecilia E. (1997) Intracerebral mass lesions in patients with human immunodeficiency virus infection and cryptococcal meningitis. Diagnoses of Microbiological Infectious Disease 29, 193–198. [DOI] [PubMed] [Google Scholar]
- Speed BR. (1993) Comparison of the clinical manifestations of the two varieties of Cryptococcus neoformans var. gattii and var. neoformans. In: Programme and Abstracts of the 2nd International Conference on Cryptococcus and Cryptococcosis, Milan, Italy. Milan: Organising and Scientific Committee, p72. [Google Scholar]
- Speed B, Dunt D. (1995) Clinical and host differences between infections with two varieties of Cryptococcus neoformans. Clinical Infectious Disease 21, 28–34. [DOI] [PubMed] [Google Scholar]
- Stockman L, Roberts GD. (1983) Specificity of the latex test of cryptococcal antigen: a rapid, simple method for eliminating interference factors. Journal of Clinical Microbiology 17, 945–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiches D, Vite CH, Dayrell-Hart B, Steinberg SA, Gross S, Lexa F. (1998) A case of canine central nervous system cryptococcosis: management with fluconazole. Journal of the American Animal Hospital Association 34, 145–151. [DOI] [PubMed] [Google Scholar]
- Tien RD, Chu PK, Hesselink JR, Duberg A, Wiley C. (1991) Intracranial cryptococcosis in immunocompromised patients: CT and MR findings in 29 cases. AJNR 12, 283–289. [PMC free article] [PubMed] [Google Scholar]
- Yu YQ, Jiang XX, Gao YJ. (1995) MRI of a pituitary cryptococcoma simulating an adenoma. Neuroradiology 37, 449–450. [DOI] [PubMed] [Google Scholar]