Abstract
Tiletamine (12.5 mg), zolazepam (12.5 mg), ketamine (20 mg), and xylazine (5 mg) (TKX; 0.25 ml, IM) combination was evaluated as an anesthetic in 22 male and 67 female adult feral cats undergoing sterilization at high-volume sterilization clinics. Cats were not intubated and breathed room air. Oxygen saturation (SpO2), mean blood pressure (MBP), heart rate (HR), respiration rate (RR), and core body temperature were recorded. Yohimbine (0.25 ml, 0.5 mg, IV) was administered at the completion of surgery. TKX produced rapid onset of lateral recumbency (4±1 min) and surgical anesthesia of sufficient duration to complete surgical procedures in 92% of cats. SpO2 measured via a lingual pulse oximeter probe averaged 92±3% in male cats and 90±4% in females. SpO2 fell below 90% at least once in most cats. MBP measured by oscillometry averaged 136±30 mm Hg in males and 113±29 mm Hg in females. MBP increased at the onset of surgical stimulation suggesting incomplete anti-nociceptive properties. HR averaged 156±19 bpm, and RR averaged 18±8 bpm. Neither parameter varied between males and females or over time. Body temperature decreased significantly over time, declining to 38.0±0.8 °C at the time of reversal in males and 36.6±0.8 °C at the time of reversal in females. Time from anesthetic reversal to sternal recumbency was prolonged (72±42 min). Seven cats (8%) required an additional dose of TKX to maintain an adequate plane of anesthesia at the onset of surgery, and this was associated with significantly longer recovery times (108±24 min).
Introduction
Although the number of unowned, free-roaming domestic cats in the USA is difficult to determine, several sources suggest that it may rival that of the owned pet cat population, estimated at 73 million in 2001 (Alley Cat Allies, 2002; Kirkwood, 1998; National Pet Owner Survey, 2001–2002; Patronek and Rowan, 1995). Whereas sterilization rates of pet cats reach as high as 85% in some areas (Johnson and Llewellyn, 1994, 2002; Levy et al., 2003), a vast majority of unowned, free-roaming cats are not sterilized and are believed to contribute substantially to the millions of cats that enter animal shelters each year. This population includes both socialized stray cats, which are friendly towards people, and unsocialized feral cats, which are fearful and often dangerous to handle. For the purposes of this study, the term “feral” is used to denote unowned free-roaming cats, regardless of socialization status. Veterinarians often are invited to participate in programs that seek to reduce the population of feral cats by a process known as trap-neuter-return (TNR) (Centonze and Levy, 2002; Remfry, 1996; Williams et al., 2002; Zasloff and Hart, 1998). In this situation, cats are trapped for sterilization and then returned to their environment.
Anesthesia of feral cats presents unique challenges. Feral cats are usually of unknown age, history, body weight, and health status. Depending on the situation, they may be malnourished, ill, or heavily parasitized. The wild temperament of feral cats prevents examination prior to chemical immobilization and increases the stress associated with trapping and transportation. Many programs sterilize large numbers of feral cats at a time and incorporate veterinary lay personnel in the processing of the cats. Sterilization clinics may operate in remote locations without access to inhalant anesthetics. Anesthetic protocols for feral cats must accommodate safety considerations for both the animals and for the clinic personnel. Injectable anesthetics allow handlers to administer immobilizing drugs while the cats are confined to the traps, thus eliminating escapes or contact with potentially dangerous animals. The ideal anesthetic agent for feral cats would be delivered in a small volume, induce rapid immobilization, provide a surgical plane of anesthesia, have a predictable and sufficient duration of effect, have a rapid recovery, provide adequate post-operative analgesia, and have a wide margin of safety.
The combination of tiletamine, zolazepam, ketamine, and xylazine (TKX) has been used in several species for surgical anesthesia (Ko et al., 1993a,b; Lin et al., 1994; Williams et al., 2002). The pharmacological profiles of the drugs that comprise TKX have been reviewed elsewhere (Williams et al., 2002). Briefly, tiletamine and ketamine are dissociative agents that produce analgesia, immobilization, and general anesthesia with increasing dose. Zolazepam is a benzodiazepine with anxiolytic and muscle relaxant properties. Xylazine is a mixed α 1- and α 2-adrenergic receptor agonist. Xylazine contributes short duration analgesia, sedation, and muscle relaxation. Each individual drug has been approved by the US Food and Drug Administration for use in cats, however, their combination as TKX has not. Yohimbine is not approved for use in cats.
Specifically, use of TKX in feral cats has been shown to be economical and easily administered, has predictable effects, and is associated with low mortality (Williams et al., 2002). Despite the widespread use of TKX, no information has been published to our knowledge on the effects of this drug combination in feral cat populations. The aim of this study was to evaluate the anesthetic and physiological effects of TKX in feral cats undergoing sterilization at clinics that typically handled up to 160 cats in a single day.
Materials and methods
Animals
Feral cats presented to monthly high-volume sterilization clinics (Operation Catnip, Gainesville, FL, USA) were studied. Feral cats were captured from their colonies in humane cage traps and transported on the morning of each clinic by colony caregivers. Between 15 and 180 cats were sterilized at each clinic (133±30 cats/clinic) in approximately 3 h of surgery time (Scott et al., 2003). Cats were admitted to the study only if they were deemed to be adults based on the presence of permanent canine teeth and physical appearance and only if they required routine sterilization procedures (e.g., no advanced pregnancies or cryptorchidism). Cats were selected based on the time of TKX injection relative to the availability of the monitors. Over the 2-year study period, a total of 22 male and 67 female cats were included in this study. This represents 4% of the 2261 cats sterilized during this period. The study protocol required approximately 2 h of monitoring for each cat, which restricted the number of cats included in the study. Other than the use of noninvasive monitoring equipment, no changes to the standard clinic procedures were made. Sterilization of feral cats under these conditions was approved by the University of Florida Institutional Animal Care and Use Committee.
Anesthetic and other perioperative drugs
Vials of lyophilized tiletamine-zolazepam (250 mg tiletamine HCl and 250 mg zolazepam HCl) (Telazol, Fort Dodge Animal Health, Fort Dodge, IO, USA) were reconstituted with 4 ml ketamine HCl 100 mg/ml (Ketaset, Fort Dodge Animal Health, Fort Dodge, IO, USA) and 1 ml xylazine HCl 100 mg/ml (Xyla-ject, Phoenix Pharmaceuticals Inc, St Joseph, MO, USA). Hence, each ml of reconstituted TKX contained 50 mg tiletamine, 50 mg zolazepam, 80 mg ketamine, and 20 mg xylazine. Each cat received 0.25 ml TKX (12.5 mg tiletamine, 12.5 mg zolazepam, 20 mg ketamine, and 5 mg xylazine) while confined in the traps (Table 1). Although the intention was to deliver the drugs intramuscularly in the lumbar or thigh muscles, this route could not be confirmed. At the completion of surgery, cats received 0.5 mg yohimbine, 2 mg/ml, 0.25 ml/cat, IV (Yobine, Lloyd Laboratories, Shenandoah, IO, USA) to reverse the anesthetic effects.
Table 1.
Body weights and doses of tiletamine, zolazepam, ketamine, and xylazine administered to male and female cats as part of the TKX combination
| Weight (kg) | Tiletamine (mg/kg) | Zolazepam (mg/kg) | Ketamine (mg/kg) | Xylazine (mg/kg) | ||
|---|---|---|---|---|---|---|
| Males | Minimum | 2.2 | 5.6 | 5.6 | 9.0 | 2.2 |
| Maximum | 4.6 | 2.8 | 2.8 | 4.4 | 1.1 | |
| Mean±SD | 3.4±0.6 | 3.8±0.7 | 3.8±0.7 | 6.1±1.2 | 1.5±0.3 | |
| Females | Minimum | 1.9 | 6.6 | 6.6 | 10.5 | 2.6 |
| Maximum | 3.9 | 3.2 | 3.2 | 5.2 | 1.3 | |
| Mean±SD | 2.9±0.5 | 4.4±0.8 | 4.4±0.8 | 7.0±1.2 | 1.8±0.3 |
A petroleum-based eye lubricant was applied to both eyes, and long-acting penicillin 300,000 units/cat (Extended Action Penicillin G Benzathine and Penicillin G Procaine, G.C. Hanford Manufacturing Company, Syracuse, NY, USA) was administered subcutaneously. At the end of the surgical procedure, cats received panleukopenia, rhinotracheitis, calicivirus, leukemia virus (Fel-O-Vax LvK-III, Fort Dodge Animal Health, Fort Dodge, IO, USA), and rabies virus (Rabvac3, Fort Dodge Animal Health, Fort Dodge, IO, USA) vaccines and ivermectin 1 mg/cat SC (Ivomec 1% Injection for Cattle and Swine, Merial Limited, Iselin, NJ, USA).
Animal preparation and instrumentation
Small amounts of food were included in the traps as bait so fasting times ranged from 2 to 18 h prior to anesthetic injection. Cats were restrained at one end of the cage using a metal comb passed through the wire mesh of the cage (Fig. 1). Following TKX administration and the onset of lateral recumbency, cats were removed from the traps and weighed. Cats breathed room air and were not intubated.
Figure 1.
TKX was administered intramuscularly to feral cats restrained by means of a metal comb inserted through the side of the wire trap.
Oxygen saturation (SpO2), mean arterial blood pressure (MBP), heart rate, core body temperature, and respiration rate were recorded. Cats were instrumented using the Vet/BP Plus 6500 Noninvasive Blood Pressure Monitor (Heska Corporation, Waukesha, WI, USA) for measurement of SpO2 and heart rate. The pulse wave was identified using the plethysmographic technique, and SpO2 was measured using spectrophotometric oximetry and a lingual probe. SpO2 values were only accepted if the pulse rate derived from the pulse oximeter matched the heart rate counted from the esophageal stethoscope. The same monitor was used to measure MBP. A blood pressure cuff was positioned around the left antebrachium. The size of the cuff for each cat was approximately 40% of the antebrachial circumference. The Vet/Ox 4403 Patient Monitor (Heska Corporation, Waukesha, WI, USA) was used for core body temperature and heart rate measurement. An esophageal stethoscope with a temperature transducer was positioned with the tip of the probe in the mid-thoracic esophagus. The monitor displayed temperature measurements and amplified cardiac sounds through a speaker. Respiratory rate was counted by observing or palpating thoracic tidal excursions.
Following instrumentation, 1 cm of the distal tip of the left ear was removed by placing a hemostat proximal to this position and cutting off the tip with surgical scissors. This permanent mark identifies sterilized cats and prevents them from being represented for surgery. In female cats, the urinary bladder was manually expressed of urine. The surgical site was prepared using standard aseptic technique. Castrations were performed using a closed procedure, and female cats were spayed via a midline or left lateral flank approach. After all procedures were complete, cats received vaccines, ivermectin, and yohimbine as described above. Cats were placed into their traps and observed until able to sustain sternal recumbency. Cats were returned to their colony caregivers later the same day to be released back to their colony the following morning.
Data collection and statistical analysis
Individual anesthetic records were kept for each cat. Intervals recorded were time from TKX injection until lateral recumbency, time from TKX injection until the start of surgery, surgical duration, time from yohimbine administration to sternal recumbency, and total time recumbent. SpO2, MBP, heart rate, body temperature, and respiratory rate were recorded every 5 min from 10 min after TKX injection until yohimbine administration.
Nonparametric means were compared using Mann–Whitney U-tests. Parametric means were compared using a two factor ANOVA (for the effects of gender and time), with Bonferroni's t-tests for post-hoc multiple comparisons. The α-priori significance level was P<0.05.
Results
Animals
Ninety-one cats (22 males, 67 females) were selected from all cats presented to the clinic. Female cats were further divided into those with (n=15) or without (n=52) early pregnancy. The duration of surgery was significantly longer inpregnant cats (16±7 min) compared to nonpregnant cats (10±8 min) (P<0.05); however, no other variables differed between these groups. Therefore, all female cats were evaluated as a single group.
Doses of all drugs are shown in Table 1. Male cats were heavier than females (P<0.05); therefore they received a smaller dose of TKX per kilogram body weight than females (Table 1) (P<0.05). The surgical procedure was completed on most cats (92%) following a single dose of TKX. Four female and 3 male cats required an additional dose of TKX (0.15 ml, IM) at the start of surgery to achieve a surgical plane of anesthesia.
Time intervals
Lateral recumbency was achieved 4±1 min (mean±SD) after injection with TKX and was not significantly different between male and female cats (P=0.41). No cats vomited during anesthetic induction, and the transition to lateral recumbency was free from signs of CNS excitement.
The time from TKX injection until the start of surgery was significantly longer in female cats (28±8 min) than male cats (16±5 min) (P<0.01) due to the longer surgical preparation time required for abdominal surgery. In addition, the surgical procedure required more time to complete in female cats (11±8 min) compared to males (4±4 min) (P<0.01).
The time from injection of the reversal agent yohimbine until the onset of sternal recumbency was 72±42 min and was not significantly different between male and female cats (P=0.16). This duration was significantly longer if cats received a second dose of TKX at the start of surgery (n=7, 108±24 min) (P<0.05). The total time recumbent (including preparation, surgery, and recovery) did not differ between male and female cats (P=0.34), despite the shorter preparation and surgical time in males. Total time recumbent was significantly longer in cats that had received a second dose of TKX (145±17 min) compared to those that received a single dose (112±42 min) (P<0.05).
Physiological variables
Physiological variables were monitored from 10 min after the administration of TKX until yohimbine was given. The feral nature of the cats precluded safe monitoring beyond this point.
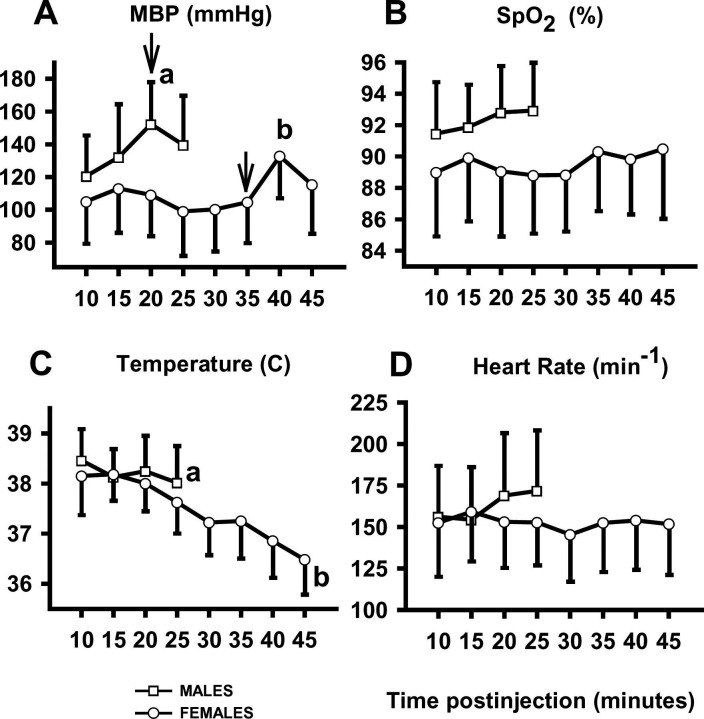
Average MBP over the entire study period was 136±30 mm Hg in males (range 78 to 196 mm Hg) and 113±29 mm Hg in females (range 38 to 186 mm Hg). Blood pressure did not change during surgical preparation (Fig. 2 A), however, MBP increased in both sexes after the onset of surgery compared to the previous measurements (P<0.01) (Fig. 2 A).
Figure 2.
Mean arterial blood pressure (A), hemoglobin oxygen saturation (B), body temperature (C), and heart rate (D) in male (squares) and female (circles) cats during TKX anesthesia. Mean arterial blood pressure increased after the onset of surgery (arrows). a=P<0.05 relative to the initial 10-minute measurement; b=P<0.05 relative to all other time points. Values are mean±SD.
The pulse rate derived from the pulse oximeter did not match the heart rate counted from the esophageal stethoscope in 38% of measurements, so these SpO2 measurements were excluded from analysis as unreliable. The average SpO2 in female cats was 90±4% (range: 67 to 99%). In males, SpO2 averaged 92±3% (range: 86 to 99) (Fig. 2 B). Eighty-eight per cent of female cats had SpO2 values below 90% at least once during the monitored period. In contrast, SpO2 values fell below 90% in only 18% of males. SpO2 did not change over time in either group (P=0.39) (Fig. 2 B).
Body temperature decreased significantly over time, declining to 38.0±0.8 °C at the time of reversal in males and to 36.6±0.8 °C at the time of reversal in females. Body temperature was lower 25 min after administration of TKX compared to the initial measurement in both males and females (P<0.01) (Fig. 2 C). Anesthesia continued in females beyond 25 min during which body temperature further decreased (P<0.01) (Fig. 2 C).
Heart rate averaged 156±19 bpm and did not vary significantly during the study period (P=0.58) (Fig. 2 D). Respiratory rate was measured by counting tidal excursions of the chest until the chest was draped for surgery. The average respiratory rate prior to surgery did not change with time (18±8 resp/min; range: 4 to 48) (P=0.76).
Anesthesia recovery was smooth in all cats. There were no injuries to personnel or to cats, and all cats survived to the time of return to the colony.
Discussion
The mass sterilization of feral cats presents many logistical challenges not present in the routine management of pet cats. Anesthetic protocols for feral cats must be safe for personnel, be easy to administer to trapped cats, provide rapid immobilization and onset of surgical anesthesia, have predictable and sufficient duration of effect, have a wide margin of safety, provide smooth and rapid anesthetic recovery, and impart intraoperative and postoperative analgesia. Although TKX fulfills many of these criteria, there are also limitations.
Importantly, perioperative mortality is extremely low in feral cats anesthetized with TKX (0.3%) (Williams et al., 2002). None of the cats in this study died prior to return to their colony. Furthermore, the combination is inexpensive and practical to administer. However, the current study indicates that some troublesome physiological responses may be commonly encountered.
Severe hypoxemia, particularly in female cats, was common, and could result in residual organ impairment not apparent at the time of discharge. Hemoglobin saturation with oxygen was less than 90% at least once in some male cats and in most female cats. In general, SpO2 values below 90% reflect PaO2 values below 60 mmHg and are considered evidence of clinical hypoxemia (Haskins, 1996). A low SpO2 value may also reflect problems with the instrument detecting a signal or patient factors. Obtaining a reliable pulse signal with the oximeter probe was not always possible. The α-adrenergic receptor agonists (i.e., xylazine) cause intense vasoconstriction (Haskins et al., 1986) and reduce blood flow to many organs including the tongue (Skrbic and Chiba, 1991). Hypoxemia also decreases SpO2 values and can occur if animals breathe room air (low oxygen tension), during hypoventilation, and if ventilation-perfusion mismatching or shunting are present. A previous study reported normal end-tidal CO2 concentrations in cats anesthetized with TKX (Ko et al., 1993a), indicating adequate ventilation, but the dose of TKX in the current study was higher and may have contributed more respiratory depression. The cats in the current study breathed room air, and it is possible that oxygen supplementation would correct the hypoxemia. However, large-scale feral cat clinics frequently sterilize more than 50 cats per h, dozens of which may be under anesthesia simultaneously. The logistical challenge of identifying cats at risk for hypoxemia or of routinely administering oxygen to all cats should not be underestimated.
In the current study, cats received approximately 0.083 ml/kg TKX. In a previous study, less than half this dose was administered to cats undergoing onychectomy and castration; however, every cat required additional TKX to complete the surgical procedures (Ko et al., 1993a). In contrast, only 7 cats in the current study required additional TKX. Higher heart rates were reported in the previous study, but this may reflect the lower dose of TKX and the concurrent administration of atropine (Ko et al., 1993a).
Both hypertension and hypotension were observed at some time points in some cats, although most cats maintained acceptable blood pressures. Mean arterial blood pressure increased at the onset of surgical manipulation in the current study. This suggests TKX may lack sufficient antinociceptive properties at the time of surgery to prevent reflex changes in blood pressure in response to surgical stimulation. Although this does not imply cats were aware of pain during the surgical period, it does suggest that TKX may be limited as an analgesic after the animal regains consciousness.
The duration of analgesia following anesthetic doses of dissociative and α 2 agents is not entirely understood. A recent report describes significant preemptive analgesic effects of ketamine in cats (Nagasaka et al., 2000). No similar study exists for tiletamine. Postoperative analgesia offered by xylazine may be minimal because its duration of analgesia is shorter than its period of sedation, and yohimbine was given to antagonize the effects of xylazine at the α 2-adrenergic receptor. However, 2 recent reports of α 2 agonists in combination with ketamine in cats suggest that TKX may provide some post-operative analgesia (Robertson et al., 1995; Slingsby et al., 1998). Nevertheless, it can be assumed that female cats, undergoing a more invasive surgery than males, do not receive adequate duration postoperative analgesia from a single dose of TKX. The addition of a narcotic or nonsteroidal anti-inflammatory agent might improve postoperative analgesia.
The use of TKX in this setting was associated with prolonged recovery. Long recovery times delay the return of cats to their colony caregivers and represent a considerable logistical problem. The body temperature of all cats declined steadily after anesthetic injection. Although not monitored, body temperature probably declined further after yohimbine administration until cats were able to move. Even mild hypothermia can substantially prolong postanesthetic recovery times (Lenhardt et al., 1997) and may be related to reduced drug metabolism. Application of external heat sources would reduce the severity of hypothermia but imposes considerable logistic problems when large numbers of cats may be recovering from anesthesia at the same time. Although recovery times also may be prolonged if yohimbine fails to reverse the sedative effects of xylazine, rapid reversal of recumbency was reported when yohimbine (0.1 mg/kg) was administered 45 min after xylazine (2.2–4.4 mg/kg) in cats (Hsu and Lu, 1984). The dose of yohimbine administered in the current study was 0.5 mg/cat (0.11–0.26 mg/kg) to reverse the xylazine dose of 5 mg/cat (1.1–2.6 mg/kg), suggesting that recumbency was not due to excessive xylazine sedation.
In summary, TKX fulfills many of the challenging requirements for feral cat anesthesia and facilitates practical and economical mass sterilization. However, SpO2 can be below accepted normal values, recovery times can be long, and postoperative analgesia may be inadequate. Further refinement of injectable anesthetic protocols for feral cats is needed.
Acknowledgements
The authors acknowledge Vincent Centonze, Brian Grossbard, Marianne Orwan, and Chi Ho for technical assistance. Funding for this study was provided in part by the University Scholars Program, University of Florida.
References
- National pet owner survey. 2001–2002. Greenwich: American Pet Products Manufacturing Association, 2002;64. [Google Scholar]
- Alley Cat Allies. Getting ahead of the game. Alley Cat Allies web site: www.alleycat.org. [April, 2002].
- Centonze L.A., Levy J.K. Characteristics of free-roaming cats and their caretakers, Journal of the American Veterinary Medical Association, 220, 2002, 1627–1633. [DOI] [PubMed] [Google Scholar]
- Haskins S.C. Monitoring the anesthetized patient. Thurmon J.C., Tranquilli W.J., Benson G.T. Lumb & Jones' Veterinary Anesthesia, third ed., 1996, Lippincott, Williams & Wilkins: Philadelphia, 409–424. [Google Scholar]
- Haskins S.C., Patz J.D., Farver T.B. Xylazine and xylazine-ketamine in dogs, American Journal of Veterinary Research, 47, 1986, 636–641. [PubMed] [Google Scholar]
- Hsu W.H., Lu Z.X. Effect of yohimbine on xylazine-ketamine anesthesia in cats, Journal of the American Veterinary Medical Association, 185, 1984, 886–888. [PubMed] [Google Scholar]
- Johnson K., Llewellyn L. National Pet Alliance survey report on Santa Clara County's pet population, Cat Fanciers Almanac, January, 1994, 71–77.
- Johnson K., Llewellyn L., 2002. San Diego County: survey and analysis of the pet population. National Pet Alliance, web site: www.fanciers.com/npa/. [April, 2002]. [Google Scholar]
- Kirkwood S. Free-roaming cats: In search of new approaches, Animal Sheltering, 21, 1998, 5–6. [Google Scholar]
- Ko J.C.H., Thurmon J.C., Benson G.J., Tranquilli W.J. An alternative drug combination for the use in declawing and castrating cats, Veterinary Medicine, 88, 1993a, 1061–1065. [Google Scholar]
- Ko J.C.H., Williams B.L., Smith V.L., McGrath C.J., Jacobson J.D. Comparison of Telazol, Telazol-ketamine, Telazol-xylazine, and Telazol-ketamine-xylazine as chemical restraint and anesthetic induction combination in swine, Laboratory Animal Science, 43, 1993b, 476–480. [PubMed] [Google Scholar]
- Lenhardt R., Marker E., Goll V., Tschernich H., Kurz A., Sessler D.I., Narzt E., Lackner F. Mild intraoperative hypothermia prolongs postanesthetic recovery, Anesthesiology, 87, 1997, 1318–1323. [DOI] [PubMed] [Google Scholar]
- Levy J.K., Woods J.E., Turick S.L., Etheridge D.L. Number of unowned free-roaming cats in a college community in the southern United States and characteristics of community residents who feed them, Journal of the American Veterinary Medical Association, 223, 2003, 202–205. [DOI] [PubMed] [Google Scholar]
- Lin H.C., Wallace S.S., Tyler J.W., Robbins R.L., Thurmon J.C., Wolfe D.F. Comparison of tiletamine-zolazepam-ketamine and tiletamine-zolazepam-ketamine-xylazine anaesthesia in sheep, Australian Veterinary Journal, 71, 1994, 239–242. [DOI] [PubMed] [Google Scholar]
- Nagasaka H., Nakamura S., Mizumoto Y., Sato I. Effects of ketamine on formalin-induced activity in the spinal dorsal horn of spinal cord-transected cats: Differences in response to intravenous ketamine administered before and after formalin, Acta Anaesthesiologica Scandinavica, 44, 2000, 953–958. [DOI] [PubMed] [Google Scholar]
- Patronek G.J., Rowan A.N. Editorial: Determining dog and cat numbers and population dynamics, Anthrozoos, 8, 1995, 199–205. [Google Scholar]
- Remfry J. Feral cats in the United Kingdom, Journal of the American Veterinary Medical Association, 208, 1996, 520–523. [PubMed] [Google Scholar]
- Robertson S.A., Richter M., Martinez S., 1995. Comparison of two injectable regimens for onychectomy in cats. Annual Meeting of the American College of Veterinary Anesthesiologist, Atlanta, Georgia.
- Scott K.C., Levy J.K., Crawford P.C. Characteristics of free-roaming cats presented for sterilization, Journal of the American Veterinary Medical Association, 221, 2003, 1136–1138. [DOI] [PubMed] [Google Scholar]
- Skrbic R., Chiba S. Pharmacological features of vascular responses of isolated dog and monkey lingual arteries to vasoactive substances, Japanese Journal of Pharmacology, 57, 1991, 99–107. [DOI] [PubMed] [Google Scholar]
- Slingsby L.S., Lane E.C., Mears E.R., Shanson M.C., Waterman-Pearson A.E. Postoperative pain after ovariohysterectomy in the cat: A comparison of two anaesthetic regimes, Veterinary Record, 143, 1998, 589–590. [DOI] [PubMed] [Google Scholar]
- Williams L.S., Levy J.K., Robertson S.A., Cistola A.M., Centonze L.A. Use of the anesthetic combination of tiletamine, zolazepam, ketamine, and xylazine for neutering feral cats, Journal of the American Veterinary Medical Association, 220, 2002, 1491–1495. [DOI] [PubMed] [Google Scholar]
- Zasloff R.L., Hart L.A. Attitudes and care practices of cat caretakers in Hawaii, Anthrozoos, 11, 1998, 242–248. [Google Scholar]