Abstract
Opioids have an unjustified reputation for causing mania in cats, but with refinements in dosing they are now used successfully in this species. The mu-opioid agonists are generally considered the best analgesics. Morphine (0.1–0.3 mg/kg) is effective in a clinical setting. Methadone (up to 0.5 mg/kg) has a similar profile to morphine. Pethidine (Demerol, meperidine; 2–5 mg/kg) is a useful analgesic with a faster onset but shorter duration of action than morphine. Oxymorphone and hydromorphone (0.05–0.1 mg/kg) are widely used in the USA. These opioids are more potent (up to 10 times), and longer acting than morphine in cats. Butorphanol (0.1–0.4 mg/kg) is a mu-opioid antagonist that produces its analgesic actions through kappa agonist activity. It rapidly reaches a ceiling effect, is short acting and is a weaker analgesic than pure mu opioids. Buprenorphine (0.01–0.02 mg/kg), a partial mu-agonist, is the most popular opioid used in small animal practice in the UK, other parts of Europe, Australia and South Africa. In clinical studies it has produced better analgesia than several other opioids and appears to be highly suitable for perioperative pain management in cats. NSAIDs are also used in cats for pain management, although cats metabolise these differently from other species. With appropriate dosing, carprofen (1–4 mg/kg) and meloxicam (0.3 mg/kg) have proved highly effective with few side effects. The use of ketoprofen (2 mg/kg), tolfenamic acid (4 mg/kg) and vedaprofen (0.5 mg/kg) has been reported in cats. Other less traditional analgesics such as ketamine, medetomidine and local anaesthetics are also used for clinical pain management. The transmucosal, transdermal and epidural routes offer novel methods for administration of analgesic drugs and have considerable potential for improving techniques in feline pain management.
Introduction
Feline pain has been under treated largely as a result of fear of side effects of traditional analgesics and lack of pharmaceutical products with market authorisation for this species. The cat's reputation for opioid-induced mania lives on, in spite of the fact that it stems largely from gross overdosage described in historical reports. The cat's deficient metabolic pathways leading to the real possibility of non steroidal anti inflammatory drug (NSAID) toxicity has resulted in reticence to use this major group of analgesics in feline patients. However, pain management in cats has progressed considerably in the last few years, and this paper reviews recent studies of traditional analgesics in cats and examines the potential role of alternative agents and routes of administration.
Opioids
In spite of reluctance to use this group of drugs (Lascelles et al., 1999), there is now sufficient experience with several opioids to recommend their use in cats. In contrast to many other species, in cats, opioids cause marked mydriasis (Fig. 1). Resultant effects on their vision may cause them to bump into other objects and they may not see a handler approaching. Hence they should be approached slowly, while being spoken to, to avoid startling. They should also be kept away from bright light while their pupils are dilated. Opioid-induced mydriasis does not correlate with the duration of analgesia. Under laboratory conditions (Dixon et al., 2002), we have noted that pupil dilation occurred within minutes of opioid administration, before an increase in thermal threshold, and persisted after thresholds had returned to baseline. Cats rarely become excited, particularly when opioids are used for treatment of severe pain. It is more common to see euphoria, with purring, rolling, and kneading with the front paws. When used alone for premedication in pain-free cats some opioids may cause nausea, vomiting and salivation; this is common after morphine and hydromorphone but not after buprenorphine. We have also noted that incidence of nausea and vomiting depends on route of administration; subcutaneous hydromorphone results in a higher incidence of vomiting than intravenous.
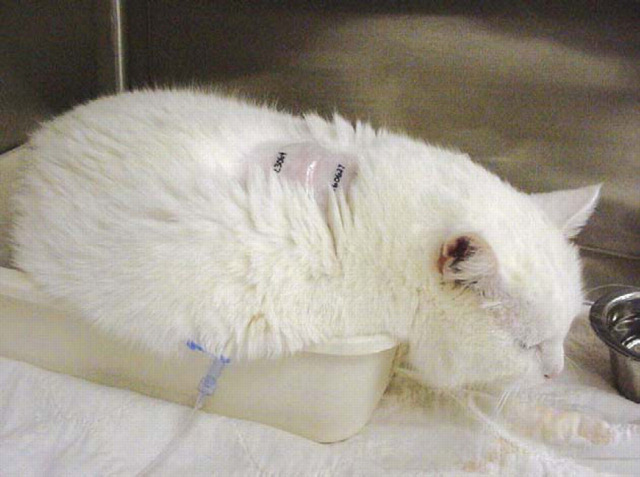
Figure 1.
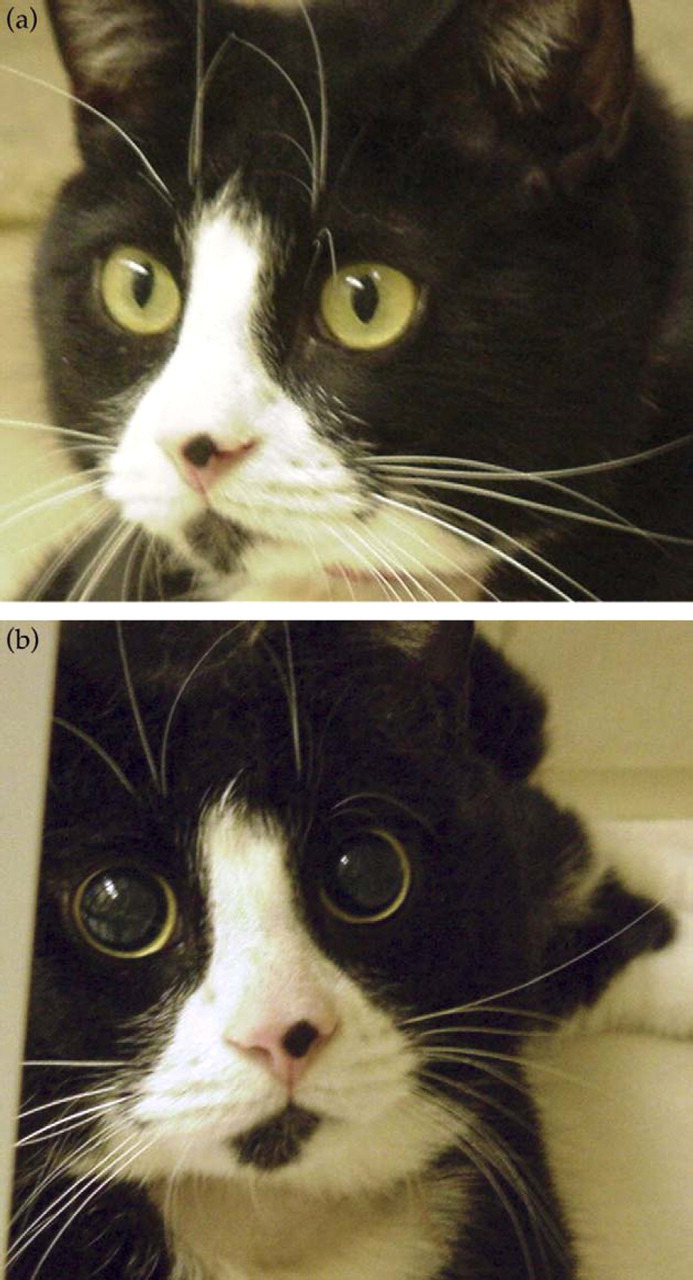
Opioids cause mydriasis. Cat before (a) and after (b) administration of hydromorphone.
Morphine, a mu-receptor agonist opiate, has been widely used in cats and does not produce excitation at doses of 0.1–0.2 mg/kg, that are effective in clinical use (Lascelles and Waterman, 1997). In thermal threshold models, morphine produces significant hypoalgesia (Davis and Donnelly, 1968; Robertson et al., 2003c). Both clinically (Lascelles and Waterman, 1997), and in research models (Robertson et al., 2003c) onset of action is slow. Clinically, morphine appears less effective in cats when compared to dogs and this may be related to their limited production of morphine metabolites (Taylor et al., 2001) compared with other species. Cats produce very little morphine-6-glucuronide, which may contribute significantly to morphine's overall analgesic effect in humans (Murthy et al., 2002).
Pethidine (meperidine, Demerol), also a mu agonist opioid, has been widely used in cats and is the only licensed opioid for use in this species in the UK. It should only be given intramuscularly or subcutaneously, as intravenous injection can produce excitement. Pethidine rarely causes vomiting (Booth and Rankin, 1954). The main drawback of pethidine is its short duration of action. In clinical practice it performs as predicted in experimental studies, producing good analgesia for little more than 1–2 h (Dixon et al., 2002; Lascelles et al., 1995; Slingsby et al., 2001).
Although not widely documented, there are reports of the use of methadone (0.1–0.5 mg/kg, normally IM or SC) for analgesia in cats (Dobromylskyj, 1996). The effects and duration are generally similar to morphine, but there is probably less vomiting.
Oxymorphone has been a popular analgesic for many years in the USA (Dobbins et al., 2002; Palminteri, 1963) and is licensed for use in the cat; it is gradually being replaced by the cheaper alternative hydromorphone (Pettifer and Dyson, 2000). Oxymorphone and hydromorphone are up to 10 times more potent than morphine. Using a visceral pain model, Briggs et al. (1998), reported that a combination of oxymorphone and butorphanol produced a greater degree of analgesia than either drug used alone and that this could be further enhanced by adding acepromazine. Doses of 0.1–0.2 mg/kg of hydromorphone are generally recommended and provide several hours of intense analgesia (Pettifer and Dyson, 2000; Wegner and Robertson, 2003). Hydromorphone combined with acepromazine (0.05–0.2 mg/kg) produces excellent sedation and chemical restraint (Pettifer and Dyson, 2000). In research cats, hydromorphone produced a significant rise in skin temperature (Wegner and Robertson, 2003), and has been implicated in post-anaesthetic hyperthermia (study in progress, University of Florida Veterinary Medical Teaching Hospital). In contrast to the study by Briggs et al. (1998) combination of hydromorphone and butorphanol did not have additive effects on thermal antinociception, but rather produced a longer lasting (up to 9 h) but less intense effect than hydromorphone alone (Lascelles et al., 2003b).
Fentanyl is a potent, short acting pure mu agonist which is most commonly used to supplement general anaesthesia where it can be given as intermittent boluses or by infusion (Lamont, 2002). Alfentanil infusions reduce the minimum alveolar concentration of isoflurane and improve some cardiovascular variables and may be useful in sick cats (Ilkiw et al., 1997; Pascoe et al., 1997).
Transdermal fentanyl (TDF) patches that release fentanyl over several days are intended for treatment of cancer related pain in humans (Muijsers and Wagstaff, 2001), but have been used for acute perioperative pain in dogs (Kyles et al., 1998) and cats (Franks et al., 2000; Gellasch et al., 2002; Glerum et al., 2001; Scherk-Nixon, 1996). They provide a “hands-off” approach to pain management that is especially attractive in cats that are difficult to medicate (Fig. 2). The plasma concentrations associated with analgesia are not known in cats, but are reported to be >1 ng/ml in dogs (Robinson et al., 1999) and humans (Gourlay et al., 1988). Plasma fentanyl concentrations are variable after patch placement in cats (Franks et al., 2000; Gellasch et al., 2002) and in one study (Lee et al., 2000), 2 out of 6 cats had undetectable plasma fentanyl concentrations, emphasising the need for careful evaluation of each patient for pain. The variability may be related to the size of the patch compared to the weight of the cat, skin permeability and body temperature. In general, cats achieve steady state plasma concentration faster than dogs (6–12 h compared to 18–24 h respectively) (Riviere and Papich, 2001) and this persists longer after patch removal in cats (up to 18–20 h, (Lee et al., 2000) compared to the rapid decline seen in dogs (Kyles et al., 1996). TDF patches have proved useful in a clinical setting for routine ovariohysterectomy (Glerum et al., 2001) and were at least as good or better than butorphanol for onychectomy (Franks et al., 2000; Gellasch et al., 2002). TDF treatment in cats for routine procedures cost more than twice that of butorphanol (Franks et al., 2000). The dangers of accidental or deliberate human ingestion must be considered and TDF patches should not be placed on cats that are being discharged to a home with young children.
Figure 2.
A fentanyl transdermal patch provides persistent pain relief for several days without regular injections.
The use of various drugs including fentanyl compounded in transdermal creams has become popular in veterinary medicine but is only based on empirical information (Marks and Taboada, 2003). The American Veterinary Medical Association has stated that “no published scientific data exist to document the proper regimen of a gel product necessary to deliver a safe, yet effective, dose of any drug in any species”. For instance, although widely used for treatment of hyperthyroidism, methimazole in pluronic lecithin organogel (PLO) applied to the inner pinnae of cats produces no measurable plasma drug levels (Hoffman et al., 2002). In our laboratory, fentanyl compounded in PLO cream failed to be absorbed through the skin of the inner pinnae or dorsum of the shaved neck even after a dose of 30 μg/kg; measurable plasma levels were obtained in one cat after it was observed licking the application site.
Pure mu-agonist opioids are subject to stringent controls on purchase, storage and use because they may result in physical dependence in man. Opioids not subject to such controls, for example butorphanol, are popular for veterinary use because of their convenience. Butorphanol is a mu-antagonist, which produces analgesia through its kappa agonist activity. It is the most commonly used opioid in cats in North America, generally given at 0.1–0.4 mg/kg (Dohoo and Dohoo, 1996) although its analgesic properties have been questioned (Wagner, 1999). Agonist-antagonist opioids such as butorphanol exhibit a “ceiling” effect after which increasing doses do not produce any further analgesia. Butorphanol appears to be an effective visceral, but poor somatic analgesic (Sawyer and Rech, 1987). Both clinical studies and experimental investigations indicate that butorphanol is very short acting and requires frequent dosing to be effective (Robertson et al., 2003b,c). In addition to an injectable formulation, butorphanol is also available as a tablet. This produced better analgesia than placebo when used for several days after declaw surgery (Carroll et al., 1998). Butorphanol is a poor analgesic choice for surgery patients where there will be both somatic and visceral pain, but would be a reasonable choice for acute visceral pain such as that associated with interstitial cystitis. Its ceiling effect limits its use to minor procedures, and frequent dosing is inconvenient and expensive.
Buprenorphine is a partial mu-agonist. It acts at the mu receptor but its maximal effect is less than that of the pure mu-agonists. Buprenorphine is the most popular opioid used in small animals practice in the UK (Lascelles et al., 1999) and is also widely used in the rest of Europe, Australia and South Africa (Watson et al., 1996; Joubert, 2001). Transmucosal absorption through oral mucous membranes is more effective in cats than in humans, with almost 100% bioavailability by this route. This is most likely a result of the alkaline (pH 8–9) environment of the cat's mouth (Robertson et al., 2003d). Buccal administration has proved to be both effective and acceptable in cats and can be mastered by owners for at-home treatment. Compared to intramuscular administration, onset of analgesia is faster after buccal administration (Robertson et al., 2003c). Buprenorphine is given at 0.01–0.02 mg/kg and at 0.02 mg/kg the buccal route was as effective as the intravenous route, providing analgesia for more than 6 h (Lascelles et al., 2003a). In clinical studies, buprenorphine has produced better analgesia than morphine (Stanway et al., 2002), oxymorphone (Dobbins et al., 2002) and pethidine (Slingsby and Waterman-Pearson, 1998). Buprenorphine rarely causes vomiting or dysphoria in cats and is highly suitable for perioperative pain management in cats as it is easily administered, highly effective, and long acting (Fig. 3).
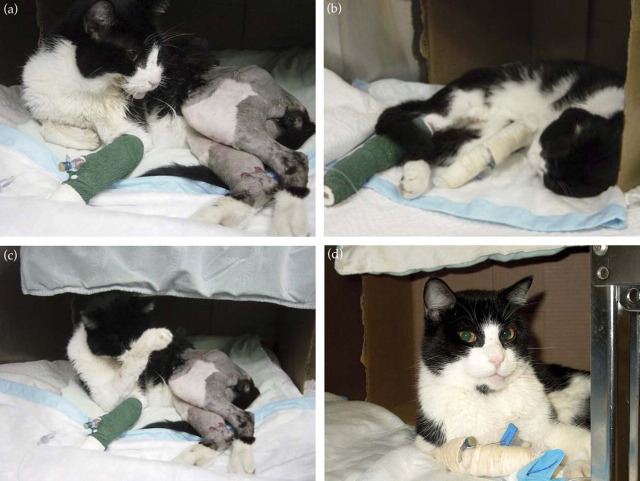
Figure 3.
Oral buprenorphine three times daily provided excellent pain relief for this cat with a fractured jaw and pelvis. He ate, drank, groomed and responded enthusiastically to petting throughout treatment (four small frames, a–d).
A transdermal delivery system for buprenorphine is now available for use in humans in several European countries (Buprenorphine TDS, Transtec®, Gruenenthal GmbH, Germany). Radbruch (2003) reported that 81% of over three thousand patients with chronic pain received good pain relief. This system remains to be evaluated in cats.
Recent studies in laboratory species have investigated the efficacy of long-acting formulations of systemically administered opioids. Liposome-encapsulated oxymorphone was effective for seven days in a rat model of neuropathic pain (Smith et al., 2003). Such preparations would be ideal under clinical conditions in cats, providing sustained levels of analgesia without the discomfort or inconvenience of repeated injections.
Opioids exert their major analgesic effect in the dorsal horn of the spinal cord and intrathecal or epidural administration provides long lasting analgesia with fewer systemic side effects (Fig. 4). Morphine (0.1 mg/kg), fentanyl (4 μ/kg), pethidine and methadone have been used successfully via the epidural route in cats (Duke et al., 1994a,b; Golder et al., 1998; Jones, 2001; Troncy et al., 2002; Tung and Yaksh, 1982). Morphine is probably the most appropriate with regard to duration of action and quality of analgesia combined with the fewest systemic effects. Analgesia may be intensified and prolonged with the addition of the local anaesthetic bupivacaine (Troncy et al., 2002). Liposome-based sustained release preparations of morphine significantly extended the duration of action of a single epidural injection in dogs (Yaksh et al., 1999), but these formulations have not yet been tried in cats. Epidural injection is technically more challenging in cats because of their small size and because the spinal cord ends more caudally, entering the subarachnoid space is more likely. If this occurs, half of the epidural dose may still be administered (Lamont, 2002).
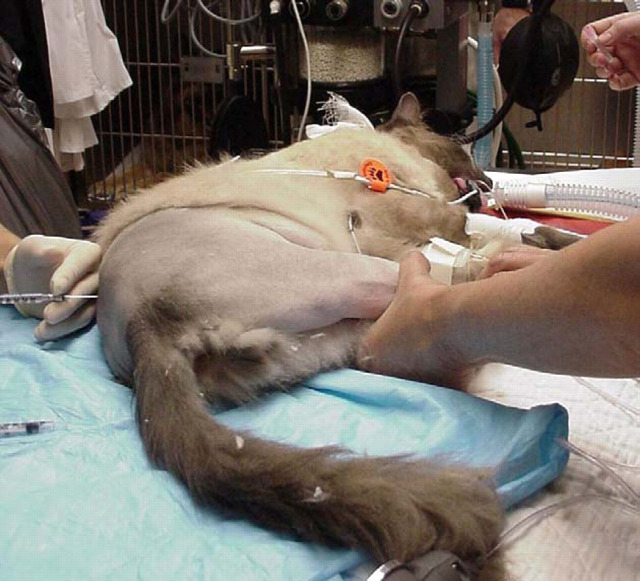
Figure 4.
Epidural administration of morphine (which can be given with local anaesthetic) after induction of anaesthesia before surgery begins. This provides preemptive analgesia in addition to up to 24 h post operative pain relief. Technically more difficult than in the dog, the procedure can be performed with the cat in either lateral or sternal recumbency.
Some opioids are associated with an increase in body temperature in cats. High doses of meperidine (30 mg/kg) produced rectal temperatures of 41.6 °C (Booth and Rankin, 1954). Gellasch et al. (2002) reported significantly elevated temperatures (1.0 °C above baseline) 4–12 h after transdermal fentanyl patch application. Cats had to be actively cooled during alfentanil infusion under isoflurane anaesthesia to prevent hyperthermia (Ilkiw et al., 1997). An elevation in skin temperature has been reported following intravenous hydromorphone (Wegner and Robertson, 2003). In clinical settings temperatures as high as 42 °C have been recorded following surgery when hydromorphone has been administered to cats (study in progress, University of Florida Veterinary Medical Teaching Hospital). In these situations panting and open mouth breathing have been observed. Intervention included injection of acepromazine, active cooling with fans and cold water and opioid reversal with naloxone.
NSAIDs
Until recently NSAIDs have not been widely used in cats. This is largely due to the fear of toxicity. NSAID effects result from inhibition of cyclooxygenase (COX) enzymes that are responsible for prostaglandin synthesis. COX exists in at least two isoforms, COX1 and COX2. In general, COX1 is produced by many organs in small, consistent quantities and is responsible for normal homeostasis. In particular, it is responsible for maintenance of gastric mucosal integrity, normal platelet function and it contributes to renal autoregulation. In contrast, COX2 is generally produced in response to various stimulants of inflammation, resulting in the production of large quantities of prostaglandins which cause inflammation and pain. Hence COX inhibition relieves inflammation and pain but may also lead to gastrointestinal erosion, platelet dysfunction and impaired renal function. In recent years, development of COX2 selective NSAIDs has been heralded as a breakthrough in preventing toxicity from these drugs. However, although gastric erosion in human medication is undoubtedly reduced by the use of COX2 selective NSAIDs, the situation is not as simple as first envisaged (Khan et al., 2002). Some constitutive COX2 is produced, particularly in the kidney, and there is also considerable species variation, so that safety in one species cannot be assumed in another (Khan et al., 2002). This is particularly relevant to the cat, where many of the necessary investigations have not yet been carried out. It appears likely that COX2 selective NSAIDs may be safer in animals but there is little clinical experience yet to substantiate this.
There is considerable potential for NSAID toxicity in cats. Their deficiency of glucuronidation pathways results in slow metabolism of several NSAIDs, particularly the phenolic compounds. This prolongs the duration of effect and may lead to drug accumulation. For example, the half-life of carprofen in cats is approximately 20 h, twice that of the dog (Parton et al., 2000; Taylor et al., 1996). However, with appropriate dose and dosing intervals, NSAIDs can be used safely. In recent years, a number of newer NSAIDs have become available for veterinary use and several of these have been investigated in cats and are now widely used in the perioperative period (Slingsby and Waterman-Pearson, 2000b). NSAIDs have the advantage of being long acting, providing up to 24 h of analgesia, and they are not subject to the purchase and storage restrictions of the controlled mu-agonist opioids. NSAIDs have compared favourably with several opioids in providing postoperative analgesia, particularly after the first few postoperative hours (Balmer et al., 1998; Lascelles et al., 1995; Slingsby and Waterman-Pearson, 1998). The development of toxic effects is dependent on inadequate elimination of the drug, therefore establishing the pharmacokinetic profile of each drug in the cat has been an important component of developing safe dosing protocols for this species. Pharmacokinetic data developed in other species cannot be safely extrapolated to the cat.
Carprofen (Rimadyl; Pfizer) was one of the first “newer generation” NSAIDs to be studied in cats, and pharmacokinetic and pharmacodynamic data are available (Parton et al., 2000; Taylor et al., 1996). Carprofen causes limited COX inhibtion (Taylor et al., 1996), which may explain its good safety record in widespread clinical use in cats and dogs. Clinical studies have demonstrated its efficacy. It provides good postoperative analgesia for at least 24 h which outlasts pethidine by over 20 h (Balmer et al., 1998; Lascelles et al., 1995). It is now licensed for the cat in the UK (injectable formulation; 4 mg/kg, one dose) and widely used for routine perioperative analgesia (Taylor, 1999). Renal autoregulation may be particularly important during anaesthesia, as volatile anaesthetics and blood loss may cause hypotension. NSAIDs that affect renal autoregulation through COX inhibition are thus often avoided during anaesthesia so that renal autoregulation is preserved. However, because of its limited potential for COX-inhibition, carprofen has been licensed for preoperative use. There have been anecdotal reports of toxicity, generally associated with concurrent disease and prolonged administration of the oral formulation (Runk et al., 1999). Problems with repeated dosing may be a result of very variable inter-cat pharmacokinetics; for example in one study the half life of carprofen after intravenous administration to healthy adult cats ranged from 9 to 49 h (Parton et al., 2000).
Meloxicam (Metacam; Boehringer), a COX2 selective NSAID, has recently been licensed in the UK for analgesia in cats (0.3 mg/kg then 0.1 mg/kg for 4 days) and, like carprofen, it is licensed for preoperative use. Its clinical efficiency has been demonstrated for both musculoskeletal pain (Lascelles et al., 2001) and for routine soft tissue surgery (Slingsby and Waterman-Pearson, 2002). Postoperative meloxicam provided analgesia of similar effect and duration to carprofen (Slingsby and Waterman-Pearson, 2002). Five days oral treatment of cats with painful locomotor disorders with meloxicam or ketoprofen provided similar analgesia, but meloxicam drops were more palatable than ketoprofen tablets. Meloxicam is available as an injectable and oral formulation and the honey flavour of the latter is well tolerated by cats, although only the injectable formulation is licensed for this species. The liquid preparation is easy for owners to administer and this formulation allows for accurate dosing. No NSAID is licensed for long term use in cats, but meloxicam has been used successfully in this way (unpublished observations). Doses as low as 0.1 mg/CAT/day appear to be well tolerated, but the cat should be carefully monitored by measuring haematocrit and making clinical chemistry analyses every 8–12 weeks.
Ketoprofen (Ketofen; Merial) has been used as an analgesic in cats for some years (Mathews, 2000; Taylor, 1999). It is a potent COX1 inhibitor and is not licensed for preoperative use. However, its pharmacokinetics and clinical efficacy are well documented (Lees et al., 2003). Ketoprofen has proved to be an effective analgesic for use in cats where, given at the end of anaesthesia, 2 mg/kg provided postoperative analgesia for at least 18 h comparable to carprofen, meloxicam and tolfenamic acid (Slingsby and Waterman-Pearson, 1998, 2000b). Again, it is important that the recommended dose and duration of treatment are not exceeded.
Tolfenamic acid (Tolfedine; Vetoquinol) is another COX1 selective NSAID that is licensed as an anti-inflammatory agent for the cat in the UK and a few other countries. It has, however, also been used successfully as an analgesic in cats (Taylor, 1999), and, given at the end of anaesthesia, 4 mg/kg produced postoperative analgesia comparable to carprofen, meloxicam and ketoprofen for at least 18 h (Slingsby and Waterman-Pearson, 2000b).
Vedaprofen (Quadrisol; Intervet) is not licensed for use in cats but the gel formulation marketed for dogs has been used orally in cats. It produced effective analgesia following ovariohysterectomy when given at 0.5 mg/kg daily for three days (Horspool et al., 2001). It has also been used successfully at the same dose for five days as an anti inflammatory agent for upper respiratory tract disease (Horspool et al., 2000). It appears well tolerated in healthy animals.
There seems to be little difference in the efficacy of the NSAIDs described above in the acute perioperative setting (Slingsby and Waterman-Pearson, 2000b), and choice of agent will depend on personal preference, convenience of dosing, and duration of use.
A number of older NSAIDs have been used in cats, and some investigations have established pharmacokinetic data and clinical effect. Flunixin has been studied under laboratory conditions in cats and found to suppress thromboxane as expected of a COX1 selective NSAID (Lees and Taylor, 1991;Taylor et al., 1991, 1994). Its half life of 1–1.5 h was actually shorter in the cat than in the dog and it may be more suitable for use in cats than dogs. Fonda (1996) reported that intravenous flunixin (1 mg/kg) provided postoperative analgesia as good as pethidine for at least the first 90 min and no toxic effects were seen. Phenylbutazone was used historically for chronic pain management in many cats prior to market authorisation of carprofen, and although there are only anecdotal reports, toxicity was rarely reported. In our laboratory, thromboxane suppression was similar to that produced by flunixin but there are no published reports of analgesic efficacy. Pharmacokinetic and toxicity data for aspririn are published and its long half life and potential for toxicity in cats is well known. Lees et al. (1985) urged care in prescribing aspirin. With the availability of newer NSAIDs, particularly carprofen, meloxicam, ketoprofen and tolfenamic acid, whose efficacy and safety under clinical conditions are established, there appears little indication for using the older NSAIDs as analgesics in cats today, with the possible exception of aspirin and flunixin. Paracetamol, ibuprofen, indomethacin and naproxen are all extremely toxic in cats and should not be used.
Ketamine
NMDA receptors in the spinal cord are involved in the process of “wind up” and ketamine, a non-competitive NMDA antagonist, has been the subject of numerous investigations to establish its ability to prevent or treat pain.
Ketamine is widely used in cats as a dissociative anaesthetic agent, but few studies have been conducted in this species to investigate its analgesic effects. One study demonstrated a weak visceral analgesic effect (Sawyer et al., 1993). Anaesthetic protocols incorporating ketamine provide better postoperative analgesia than those using thiopentone and halothane with or without butorphanol (Robertson et al., 1995; Slingsby et al., 1998). Simply using ketamine as part of the anaesthetic protocol may provide preemptive analgesia. In sheep, induction of anaesthesia with ketamine prevented a decrease in thermal and mechanical thresholds after abdominal surgery, whereas thiopentone resulted in postoperative hyperalgesia (Welsh and Nolan, 1995). In dogs, sub-anaesthetic doses of ketamine (2.5 mg/kg) given preoperatively provided better postoperative analgesia than the same dose given at the end of surgery (Slingsby and Waterman-Pearson, 2000a). Wagner et al. (2002) have demonstrated the opioid sparing effects of low dose ketamine infusion in dogs after major surgery.
Ketamine (2 mg/kg IV) resulted in a brief increase in thermal antinociception followed by a later period of significant hyperalgesia (Robertson et al., 2001, 2003a). It should be noted that in these studies cats did not undergo any painful procedures and the effect of ketamine may be different when used for sedation alone compared to its use in anaesthesia.
Other NMDA antagonists including dextromethorphan, amantadine and memantine have been suggested for treatment of chronic pain syndromes (Fisher and Coderre, 2000). Amantadine, at a dose of 3–5 mg/kg PO has been suggested by Gaynor (2002) for use in cats.
Alpha2-adrenoceptor agonists
Alpha2-agonists, primarily medetomidine (Domitor, Pfizer), more recently dexmedetomidine (Dexdomitor; Orion Pharmos) and originally xylazine (Rompun; Bayer), are commonly used in cats for their sedative and anaesthetic sparing properties. These drugs provide sedation, muscle relaxation and analgesia. The analgesic effect is mediated primarily through alpha2 receptors in the dorsal horn of the spinal cord. The analgesic effect undoubtedly contributes to their role in anaesthesia and sedation but they are not commonly used for their analgesic effect alone, because of the profound sedation and cardiovascular depression that accompanies their use. Because of the latter effect, alpha2-agonists are best reserved for healthy animals. There are however, a number of ways that the analgesic properties of the alpha2-adrenoceptor agonists may be exploited to provide pain relief under conditions where anaesthesia and sedation are not required. Ansah et al. (2002), investigated analgesia in the 2 h following ovariohysterectomy in cats and reported better analgesia when 15 μg/kg medetomidine was given at the end of surgery compared to placebo. Use of an alpha2 agent as part of the anaesthetic protocol also appears to contribute to postoperative analgesia; although where ketamine is also given it is impossible to distinguish the individual contribution of each drug to the overall analgesia. Slingsby et al. (1998) reported better post ovariohysterectomy analgesia after medetomidine–ketamine than acepromazine–thiopentone–halothane anaesthesia. Robertson et al. (1995) also described better analgesia after onychectomy when xylazine–ketamine anaesthesia was used compared with acepromazine–butorphanol–thiopentone.
Other routes of administration have been described for medetomidine. Oral administration most likely results in transmucosal uptake and is a useful technique in fractious cats (Ansah et al., 1998, Grove and Ramsay, 2000). The epidural route has also been studied; Duke et al. (1994b) reported increased pain thresholds in cats for over 4 h after medetomidine (10 μg/kg) was given by this route and found it to be superior to fentanyl (4 μg/kg). Medetomidine produced some short-lived systemic effects but these were not profound. In contrast to other species, including the horse, where epidurally administered alpha2-agonists may be used for long-term pain management (Sysel et al., 1997), there are as yet no published reports of clinical pain management in cats using this technique.
Local anaesthetics
Local anaesthetics can be used to provide both preemptive and postoperative analgesia in cats. They can be given by specific nerve blocks (Fig. 5), local infiltration and regional blocks including epidural administration. Ideally these should be performed after induction of anaesthesia but before surgery, as this will lower the amount of inhalant agent required to maintain anaesthesia. Lidocaine and bupivacaine are most commonly used. Lidocaine dosing should not exceed 2–6 mg/kg total dose and should produce 1–1.5 h of analgesia. Bupivacaine is longer acting, lasting up to 4–5 h. Bupivacaine is more toxic than lidocaine and the total dose should not exceed 2 mg/kg.
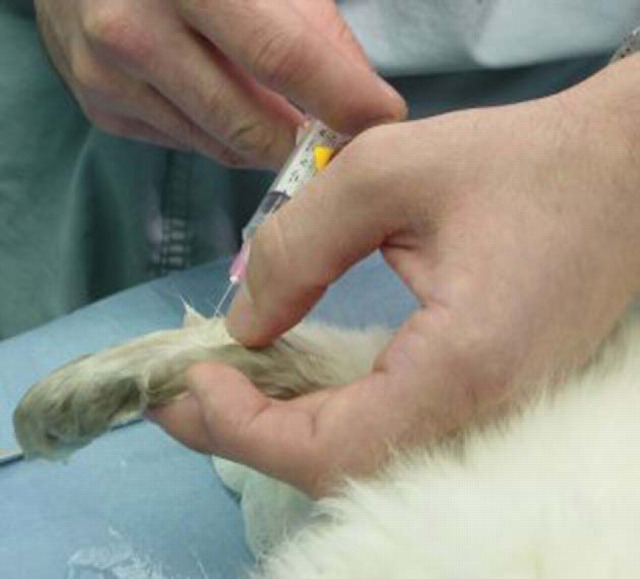
Figure 5.
A regional nerve block provides preemptive analgesia for digital surgery.
Phospholipid-encapsulated bupivacaine has a long residence time at the site of application and has provided effective analgesia following onychectomy in cats (Dodam et al., 2001). A topical liposome-encapsulated formulation is available (ELA-Max, Ferndale Laboratories, Michigan, USA). A eutectic mixture of lidocaine and prilocaine (EMLA cream; Astra pharmaceuticals, Herts, UK) is also available. These preparations may be applied to shaved skin to provide analgesia in advance of venipuncture, catheter placement and skin biopsies. Transdermal absorption did occur after application of 15 mg/kg (ELA-Max), but plasma concentrations remained significantly below toxic values (Fransson et al., 2002).
Administration of lidocaine and bupivacaine directly into the peritoneal cavity provided good analgesia in dogs following ovariohysterectomy (Carpenter et al., 2003), but has not been reported in cats. Continuous intravenous infusion may also provide systemic analgesia. In dogs, lidocaine infusions decreased the minimum alveolar concentration of isoflurane in a dose dependant fashion but the authors could not determine if this was a result of an analgesia or sedative effect (Valverde et al., 2002). This technique may be a useful method of providing analgesia to cats in severe pain where other treatments appear ineffective. Lamont (2002) recommends infusions of 0.025 mg/kg/min of lidocaine. Bupivacaine is cardiotoxic and should not be used intravenously.
Local anaesthetics may be used alone or in combination with opioids (in particular morphine) in the epidural space. Lamont (2002) recommends 4 mg/kg lidocaine or 1.0 mg/kg bupivacaine given in a volume of 1 ml/4.5 kg body weight, with or without morphine (0.1 mg/kg). Most commonly this technique is used after induction of anaesthesia and prior to surgery because of the difficulty in performing an epidural injection in a conscious cat. Lidocaine has a rapid onset and short duration so the effect of motor paralysis will wear off in the recovery period while the morphine provides long lasting postoperative analgesia with no loss of motor function. Morphine will provide analgesia up to the level of the forelimb and can be used for hindlimb, abdominal, thoracic and forelimb surgery. Local anaesthetics can be used for hindlimb and abdominal procedures, but larger volumes that would provide more cranial analgesia cannot be used because of the concomitant respiratory and cardiac depression.
Other analgesic agents
Tricyclic antidepressants such as amitriptyline, clomipramine and imipramine have been used in humans for many years to treat chronic pain, in particular neuropathic pain, and are thought to act by altering the actions of serotonin and noradrenaline both centrally and peripherally (Sawynok, 2003). Amitriptyline (2.5–12.5 mg/kg PO, SID) has been used to treat feline interstitial cystitis with few side-effects (Chew et al., 1998), and there are anecdotal reports of its use for cancer and neuropathic pain management.
In humans, the anticonvulsant gabapentin has a wide margin of safety and is clinically effective in chronic neuropathic pain, although the mechanism of action is not clear (Morello et al., 1999). Based on individual case reports this drug looks promising, (Lamont et al., 2000), and suggested doses have been published (Gaynor, 2002).
Although not classified as an opioid, tramadol has weak binding affinity at mu-receptors and is thought to activate monoaminergic spinal inhibition of pain although this may not apply to non-primate species. It can be administered by multiple routes and is effective for moderately severe acute and chronic pain in humans and seems remarkably devoid of the usual undesirable side-effects of opioids such as respiratory depression and tolerance (Lee et al., 1993). A dose of 1–2 mg/kg IV has been suggested for cats, but there are as yet no published reports of controlled clinical studies (Table 1).
Table 1.
Analgesics in current use in cats
| Drug | Dose | |
|---|---|---|
| Opioids | Morphine | 0.1–0.3 mg/kg |
| Methadone | 0.1–0.5 mg/kg | |
| Pethidine | 2–5 mg/kg | |
| Hydromorphone | 0.1–0.2 mg/kg | |
| Butorphanol | 0.1–0.4 mg/kg | |
| Buprenorphine | 0.01–0.02 mg/kg | |
| NSAIDs | Carprofen | 4 mg/kg |
| Meloxicam | 0.3 mg/kg | |
| Ketoprofen | 2 mg/kg | |
| Vedaprofen | 0.5 mg/kg oral | |
| Tolfenamic acid | 4 mg/kg | |
| Others | Ketamine | • As used for induction of anaesthesia |
| • 1–2 μg/kg pre op IV | ||
| • 2–10 μg/kg/min IV | ||
| Medetomidine | • As used for premed and sedation | |
| • Epidural | ||
| Lidocaine | • Infiltration-maximum 4–6 mg | |
| • IV infusion 0.025 mg/kg/min | ||
| • Epidural 4 mg/kg lidocaine | ||
| Bupivacaine | • Infiltration-maximum 4 mg | |
| • Epidural 1.0 mg/kg bupivacaine | ||
Conclusions
The process of nociception and perception of pain involves numerous steps and pathways and is a robust system. A single analgesic agent is therefore unlikely to alleviate pain completely. The most effective analgesia is provided by “multi-modal analgesia”, where several analgesics of different classes are used simultaneously.
For maximal perioperative pain control, the cat should be premedicated with an opioid and a NSAID, ketamine and medetomidine can be incorporated into the anaesthetic protocol and the surgical site may be blocked with a local anaesthetic. Further doses of one or more of these agents should be given postoperatively according to their expected duration of effect and based on assessment of the cat's pain. It is important to continue providing analgesia because of the continued input from the surgical site that will occur as a result of postoperative inflammation. Preemptive analgesia alone will not be sufficient to cover this post surgical period. In the face of intractable pain a lidocaine or ketamine infusion may be given. Combination of drugs in this way leads to better overall analgesia with smaller doses of individual drugs, thereby also reducing the potential for toxicity.
Management of trauma pain is similar to that of perioperative pain. Lidocaine or ketamine infusions may be particularly valuable, and a fentanyl patch may also be applied. Restraint for diagnosis and stabilisation of fractures may incorporate medetomidine but this must be used with extreme care and at low doses in cats that may be hypovolaemic or otherwise systemically ill. Doses of 2–5 μg/kg of medetomidine with an opioid such as buprenorphine 0.01 mg/kg or butorphanol 0.1 mg/kg will provide good analgesic sedation without serious compromise. If butorphanol is used it will provide good sedation but analgesia will be short lived.
Chronic pain is more difficult to treat in cats. NSAIDs are the mainstay of chronic pain management in most species but none are licensed for this purpose in cats and care must be taken with the dose and dosing interval to avoid toxicity. Some of the less conventional analgesics including the tricyclic anti-depressants, gabapentin and nutraceuticals may prove to play a useful role in chronic pain management, but controlled clinical trials are needed to establish the best doses for maximum efficacy.
References
- Ansah O., Raekallio M., Vainio O. Comparing oral and intramuscular administration of medetomidine in cats, Journal of Veterinary Anaesthesia, 25, 1998, 41–46. [DOI] [PubMed] [Google Scholar]
- Ansah O., Vainio O., Hellsten C., Raekallio M. Postoperative pain control on cats: Clinical trials with medetomidine and butorphanol, Veterinary Surgery, 31, 2002, 99–103. [DOI] [PubMed] [Google Scholar]
- Balmer T.V., Irvine D., Jones R.S., Roberts M.J., Slingsby L., Taylor P.M., Watermana E., Waters C. Comparison of carprofen and pethidine as postoperative analgesics in the cat, Journal of Small Animal Practice, 39, 1998, 158–164. [DOI] [PubMed] [Google Scholar]
- Booth N., Rankin A. Evaluation of meperidine hydrochloride in the cat, Veterinary Medicine, 49, 1954, 249–252. [Google Scholar]
- Briggs S.L.P., Sneed K.L., Sawyer D.C.D. Antinociceptive effects of oxymorphone-butorphanol-acepromazine combination in cats, Veterinary Surgery, 27, 1998, 466–472. [DOI] [PubMed] [Google Scholar]
- Carpenter R., Wilson D., Evans A. Evaluation of intraperitoneal and subcutaneous lidocaine and bupivacaine for analgesia following ovariohysterectomy in the dog, Veterinary Anaesthesia and Analgesia, 30, 2003, 110. [DOI] [PubMed] [Google Scholar]
- Carroll G., Howe L., Slater M., Haughn L., Martinez E., Hartsfield S., Matthews N. Evaluation of analgesia provided by postoperative administration of butorphanol to cats undergoing onychectomy, Journal of the Americal Veterinary Medical Association, 213, 1998, 246–250. [PubMed] [Google Scholar]
- Chew D.J., Buffington C.A., Kendall M.S., Dibartola S.P., Woodworth B.E. Amitriptyline treatment for severe recurrent idiopathic cystitis in cats, Journal of the Americal Veterinary Medical Association, 213, 1998, 1282–1286. [PubMed] [Google Scholar]
- Davis L., Donnelly E. Analgesic drugs in the cat, Journal of the Americal Veterinary Medical Association, 159, 1968, 1161–1167. [PubMed] [Google Scholar]
- Dixon M.J., Robertson S.A., Taylor P.M. A thermal threshold testing device for evaluation of analgesics in cats, Research in Veterinary Science, 72, 2002, 205–210. [DOI] [PubMed] [Google Scholar]
- Dobbins S., Brown N.O., Shofer F.S. Comparison of the effects of buprenorphine, oxymorphone hydrochloride, and ketoprofen for postoperative analgesia after onychectomy or onychectomy and sterilization in cats, Journal of the American Animal Hospital Association, 38, 2002, 507–514. [DOI] [PubMed] [Google Scholar]
- Dobromylskyj P. Cardiovascular changes associated with anaesthesia induced by medetomidine combined with ketamine in cats, Journal of Small Animal Practice, 37, 1996, 169–172. [DOI] [PubMed] [Google Scholar]
- Dodam J., Boedeker B., Gross M., Branson K., Carroll G., 2001. Phosphlipid-encapsulated bupivacaine and analgesia after onychectomy in cats. Annual Meeting of the American College of Veterinary Anesthesiologists New Orleans, LA. [DOI] [PubMed]
- Dohoo S., Dohoo I. Postoperative use of analgesics in dogs and cats by Canadian veterinarians, Canadian Veterinary Journal, 37, 1996, 546–551. [PMC free article] [PubMed] [Google Scholar]
- Duke T., Cox A., Remedios A., Cribb P. The cardiopulmonary effects of placing fentanyl or medetomidine in the lumbosacral space of isoflurane-anesthetized cats, Veterinary Surgery, 23, 1994a, 149–155. [DOI] [PubMed] [Google Scholar]
- Duke T., Cox A., Remedios A., Cribb P. The analgesic effects of administering fentanyl or medetomidine in the lumbosacral epidural space in cats, Veterinary Surgery, 23, 1994b, 143–148. [DOI] [PubMed] [Google Scholar]
- Fisher K., Coderre T. Targeting the N-Methyl-D-Asparate receptor for chronic pain management: Preclinical animal studies, recent clinical experience and future research directions, Journal of Pain and Symptom Management, 20, 2000, 358–373. [DOI] [PubMed] [Google Scholar]
- Fonda D. Post operative analgesic actions of flunixin in the cat, Journal of Veterinary Anaesthesia, 23, 1996, 52–55. [Google Scholar]
- Franks J.N., Boothe H.W., Taylor L., Geller S., Carroll G., Cracas V., Boothe D.M. Evaluation of transdermal fentanyl patches for analgesia in cats undergoing onchyectomy, Journal of the Americal Veterinary Medical Association, 217, 2000, 1013–1019. [DOI] [PubMed] [Google Scholar]
- Fransson B.A., Peck K.E., Smith J.K., Anthony J.A., Mealey K.L. Transdermal absorption of a liposome-encapsulated formulation of lidocaine following topical administration in cats, American Journal of Veterinary Research, 63, 2002, 1309–1312. [DOI] [PubMed] [Google Scholar]
- Gaynor J.S. Other drugs used to treat pain. Gaynor J.S., Muir W.W. Handbook of Veterinary Pain Management, 2002, Mosby: St Louis, 251–260. [Google Scholar]
- Gellasch K.L., Kruse-Elliott K.T., Osmond C.S., Shiha N.C., Bjorling D.E. Comparison of transdermal administration of fentanyl versus intramuscular administration of butorphanol for analgesia after onychectomy in cats, Journal of the Americal Veterinary Medical Association, 220, 2002, 1020–1023. [DOI] [PubMed] [Google Scholar]
- Glerum L.E., Egger C.M., Allen S.W., Haag M. Analgesic effect of the transdermal fentanyl patch during and after feline ovariohysterectomy, Veterinary Surgery, 30, 2001, 351–358. [DOI] [PubMed] [Google Scholar]
- Golder F., Pascoe P., Bailey C., Ilkiw J., Tripp L. The effect of epidural morphine on the minimum alveolar concentration of isoflurane in cats, Journal of Veterinary Anaesthesia, 25, 1998, 52–56. [Google Scholar]
- Gourlay G.K., Kowalski S.R., Plummer J.L., Cousins M.J., Armstrong P.J. Fentanyl blood concentration-analgesic response relationship in the treatment of postoperative pain, Anesthesia and Analgesia, 67, 1988, 329–337. [PubMed] [Google Scholar]
- Grove D., Ramsay E. Sedative and physiologic effects of orally administered alpha 2-adrenoceptor agonists and ketamine in cats, Journal of the Americal Veterinary Medical Association, 216, 2000, 1929–1932. [DOI] [PubMed] [Google Scholar]
- Hoffman S.B., Yoder A.R., Trepanier L.A. Bioavailability of transdermal methimazole in a pluronic lecithin organogel (PLO) in healthy cats, Journal of Pharmacology and Therapeutics, 25, 2002, 189–193. [DOI] [PubMed] [Google Scholar]
- Horspool L.J., Hoeijmakers M., Van Laar P., Rutten A. Efficacy of vedaprofen oral gel in cats with upper respiratory tract infections, Journal of Veterinary Phanrmacology and Therapeutics, 23, 2000, E12. [Google Scholar]
- Horspool L.J., Hoeijmakers M., Van Laar P., Bergman J. The Efficacy and Safety of Vedaprofen Oral Gel in Postoperative Pain Management in Cats, 2001, Voorjaarsdagen Congress: Amsterdam. [Google Scholar]
- Ilkiw J., Pascoe P., Fisher L. Effect of alfentanil on the minimum alveolar concentration of isoflurane in cats, American Journal of Veterinary Research, 58, 1997, 1274–1279. [PubMed] [Google Scholar]
- Jones R.S. Epidural analgesia in the dog and cat, The Veterinary Journal, 161, 2001, 123–131. [DOI] [PubMed] [Google Scholar]
- Joubert K. The use of analgesic drugs by South African veterinarians, Journal of the South African Medical Association, 72, 2001, 57–60. [DOI] [PubMed] [Google Scholar]
- Khan K.N.M., Paulson S.K., Verburg K.M., Lefkowith J.B., Maziasz J. Pharmacology of cyclooxygenase-2 inhibition in the kidney, Kidney International, 61, 2002, 1210–1219. [DOI] [PubMed] [Google Scholar]
- Kyles A.E., Papich M., Hardie E.M. Disposition of transdermally administered fentanyl in dogs, AmericanJournal of Veterinary Research, 57, 1996, 715–719. [PubMed] [Google Scholar]
- Kyles A.E., Hardie E.M., Hansen B.D. Comparison of transdermal fentanyl and intramuscular oxymorphone on post-operative behaviour after ovariohysterectomy in dogs, Research in Veterinary Science, 65, 1998, 245–251. [DOI] [PubMed] [Google Scholar]
- Lamont L.A., Tranquilli W.J., Mathews K.A. Adjunctive analgesic therapy, Veterinary Clinics of North America: Small Animal Practice, 30, 2000, 805–813. [DOI] [PubMed] [Google Scholar]
- Lamont L.A. Feline perioperative pain management, Veterinary Clinics of North America: Small Animal Practice, 32, 2002, 747–763. [DOI] [PubMed] [Google Scholar]
- Lascelles B., Cripps P., Mirchandani S., Waterman A. Carprofen as an analgesic for postoperative pain in cats: Dose titration and assesment of efficacy in comparison to pethidine hydrochloride, Journal of Small Animal Practice, 36, 1995, 535–541. [DOI] [PubMed] [Google Scholar]
- Lascelles D., Waterman A. Analgesia in cats, In Practice, 1997, 203–213.
- Lascelles B.D., Capner C.A., Waterman-Pearsona E. A survey of current British veterinary attitudes to peri-operative analgesia for cats and small mammals, Veterinary Record, 145, 1999, 601–604. [DOI] [PubMed] [Google Scholar]
- Lascelles B., Henderson A., Hackett I. Evaluation of the clinical efficacy of meloxicam in cats with painful locomotor disorders, Journal of Small Animal Practice, 42, 2001, 587–593. [DOI] [PubMed] [Google Scholar]
- Lascelles B., Robertson S., Taylor P., Hauptman J. Comparison of the pharmacokinetics and thermal antinociceptive pharmacodynamics of 20 ug/kg buprenorphine administered sublingually or intravenously in cats, Veterinary Anaesthesia and Analgesia, 30, 2003a, 109. [DOI] [PubMed] [Google Scholar]
- Lascelles B.D., Robertson S.A., Taylor P.M., Hauptman J.G. Thermal antinociceptive pharmacodynamics of 0.1 mg/kg hydromorphone administered intramuscularly in cats, and effect of concurrent butorphanol administration, Veterinary Anaesthesia and Analgesia, 30, 2003b, 108–109. [DOI] [PubMed] [Google Scholar]
- Lee C.R., Mctavish D., Sorkin E.M. Tramadol. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in acute and chronic pain states, Drugs, 46, 1993, 313–340. [DOI] [PubMed] [Google Scholar]
- Lee D., Papich M., Hardie E. Comparison of pharmacokinetics of fentanyl after intravenous and transderaml administration in cats, American Journal of Veterinary Research, 61, 2000, 672–677. [DOI] [PubMed] [Google Scholar]
- Lees P., Higgins A.J., Sedgwick A.D. Aspirin in cats (correspondence), Veterinary Record, 116, 1985, 479. [DOI] [PubMed] [Google Scholar]
- Lees P., Taylor P.M. Pharmacodynamics and pharmacokinetics of flunixin in the cat, British Veterinary Journal, 147, 1991, 298–305. [DOI] [PubMed] [Google Scholar]
- Lees P., Taylor P.M., Landoni F.M., Arifaha K., Waters C. Ketoprofen in the cat: Pharmacodynamics and chiral pharmacokinetics, The Veterinary Journal, 165, 2003, 21–35. [DOI] [PubMed] [Google Scholar]
- Marks S.L., Taboada J. Transdermal therapeutics, Journal of the American Animal Hospital Association, 39, 2003, 19–21. [DOI] [PubMed] [Google Scholar]
- Mathews K.A. Pain assessment and general approach to management, Veterinary Clinics of North America: Small Animal Practice, 30, 2000, 729–755. [DOI] [PubMed] [Google Scholar]
- Morello C.M., Leckband S.G., Stoner C.P., Moorhouse D.F., Sahagian G.A. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain, Archives of Internal Medicine, 159, 1999, 1931–1937. [DOI] [PubMed] [Google Scholar]
- Muijsers R.B., Wagstaff A.J. Transdermal fentanyl: An updated review of its pharmacological properties and therapeutic efficacy in chronic cancer pain control, Drugs, 61, 2001, 2289–2307. [DOI] [PubMed] [Google Scholar]
- Murthy B.R., Pollack G.M., Brouwer K.L. Contribution of morphine-6-glucuronide to antinociception following intravenous administration of morphine to healthy volunteers, Journal of Clinical Pharmacology, 42, 2002, 569–576. [DOI] [PubMed] [Google Scholar]
- Palminteri A. Oxymorphone, an effective analgesic in dogs and cats, Journal of the Americal Veterinary Medical Association, 143, 1963, 160–163. [PubMed] [Google Scholar]
- Parton K., Balmer T.V., Boyle J., Whittem T., Machon R. The pharmacokinetics and effects of intravenously administered carprofen and salicylate on gastrointestinal mucosa and selected biochemical measurements in healthy cats, Journal of Pharmacology and Therapeutic, 23, 2000, 73–79. [DOI] [PubMed] [Google Scholar]
- Pascoe P., Ilkiw J., Fisher L. Cardiovascular effects of equipotent isoflurane and alfentanil/isoflurane minimum alveolar concentration multiple in cats, American Journal of Veterinary Research, 58, 1997, 1267–1273. [PubMed] [Google Scholar]
- Pettifer G., Dyson D. Hydromorphone: A cost-effective alternative to the use of oxymorphone, Canadian Veterinary Journal, 41, 2000, 135–137. [PMC free article] [PubMed] [Google Scholar]
- Radbruch L. Buprenorphine TDS: Use in daily practice, benefits for patients, International Journal of Clinical Practice, 133 (Supplement, 2003, 19–24. [PubMed] [Google Scholar]
- Riviere J., Papich M. Potential and problems of developing transdermal patches for veterinary applications, Advances in Drug Delivery Reviews, 50, 2001, 175–203. [DOI] [PubMed] [Google Scholar]
- Robertson S.A., Richter M., Martinez S., 1995. Comparison of two injectable anesthetic regimens for onychectomy in cats. Annual Meeting of the American College of Veterinary Anesthesiologists, Atlanta, Georgia.
- Robertson S.A., Taylor P.M., Davies W., Dixon M. The effect of lidocaine and ketamine on thermal thresholds in cats, Veterinary Anaesthesia and Analgesia, 29, 2001, 95–96. [DOI] [PubMed] [Google Scholar]
- Robertson S., Lascelles B.D., Taylor P. Effect of low dose ketamine on thermal thresholds in cats, Veterinary Anaesthesia and Analgesia, 30, 2003a, 110. [DOI] [PubMed] [Google Scholar]
- Robertson S.A., Lascelles B.D.X., Taylor P. Effect of 0.1, 0.2, 0.4 and 0.8 mg/kg of IV butorphanol on thermal antinociception in cats, Veterinary Anaesthesia and Analgesia, 30, 2003b, 108. [DOI] [PubMed] [Google Scholar]
- Robertson S.A., Taylor P.M., Dixon M.J. Analgesia in the cat: Effects of buprenorphine, butorphanol and morphine on thermal thresholds, Veterinary Record, 153, 2003c, 462–465. [DOI] [PubMed] [Google Scholar]
- Robertson S.A., Taylor P.M., Sear J.W. Systemic uptake of buprenorphine after oral mucosal administration in cats, Veterinary Record, 152, 2003d, 675–678. [DOI] [PubMed] [Google Scholar]
- Robinson T., Kruse-Elliott K., Markel M., Pluhar G., Massa K., Bjorling D. A comparison of transdermal fentanyl versus epidural morphine for analgesia in dogs undergoing major orthopedic surgery, Journal of the American Animal Hospital Association, 35, 1999, 95–100. [DOI] [PubMed] [Google Scholar]
- Runk A., Kyles A., Downs M. Duodenal perforation in a cat following the administration of nonsteriodal anti-inflammatory medication, Journal of the American Animal Hospital Association, 35, 1999, 52–55. [DOI] [PubMed] [Google Scholar]
- Sawyer D., Rech R. Analgesia and behavioral effects of Butorphanol, Nalbuphine, and Pentazocine in the cat, Journal of the American Animal Hospital Association, 23, 1987, 438–446. [Google Scholar]
- Sawyer D.C.D., Rech R.H.P., Durham R.A.B. Does ketamine provide adequate visceral analgesia when used alone or in combination with acepromazine, diazepam, or butorphanol in cats, Journal of the American Animal Hospital Association, 29, 1993, 257–263. [Google Scholar]
- Sawynok J. Topical and peripherally acting analgesics, Pharmacological Reviews, 55, 2003, 1–20. [DOI] [PubMed] [Google Scholar]
- Scherk-Nixon M. A study of the use of a transdermal fentanyl patch in cats, Journal of the American Animal Hospital Association, 32, 1996, 19–24. [DOI] [PubMed] [Google Scholar]
- Slingsby L., Lane E., Mears E., Shanson M., Waterman-Pearson A. Postoperative pain after ovariohysterectomy in the cat: A comparison of two anaesthetic regimes, Veterinary Record, 143, 1998, 589–590. [DOI] [PubMed] [Google Scholar]
- Slingsby L., Waterman-Pearson A.E. Comparison of pethidine, buprenorphine and ketoprofen for postoperative analgesia after ovariohysterectomy in the cat, Veterinary Record, 143, 1998, 185–189. [DOI] [PubMed] [Google Scholar]
- Slingsby L., Waterman-Pearson A. The post-operative analgesic effects of ketamine after canine ovariohysterectomy—a comparison between pre- or post-operative administration, Research in Veterinary Science, 69, 2000a, 147–152. [DOI] [PubMed] [Google Scholar]
- Slingsby L., Waterman-Pearson A.E. Postoperative analgesia in the cat after ovariohysterectomy by use of carprofen, ketoprofen, meloxicam or tolfenamic acid, Journal of Small Animal Practice, 41, 2000b, 447–450. [DOI] [PubMed] [Google Scholar]
- Slingsby L., Jones A., Waterman-Pearson A.E. Use of a new finger-mounted device to compare mechanical nociceptive thresholds in cats given pethidine or no medication after castration, Research in Veterinary Science, 70, 2001, 243–246. [DOI] [PubMed] [Google Scholar]
- Slingsby L., Waterman-Pearson A.E. Comparison between meloxicam and carprofen for postoperative analgesia after feline ovariohysterectomy, Journal of Small Animal Practice, 43, 2002, 286–289. [DOI] [PubMed] [Google Scholar]
- Smith L.J., Krugner-Higby L., Clark M., Wendland A., Heath T. Liposome-encapsulated oxymoprhone prevents hyperalgesia for 7 days in a rat model of neuropathic pain, Veterinary Anaesthesia and Analgesia, 30, 2003, 116. [DOI] [PubMed] [Google Scholar]
- Stanway G., Taylor P., Brodbelt D. A preliminary investigation comparing pre-operative morphine and buprenorphine for postoperative analgesia and sedation in cats, Veterinary Anaesthesia and Analgesia, 29, 2002, 29–35. [DOI] [PubMed] [Google Scholar]
- Sysel A.M., Pleasant R.S., Jacobson J.D., Moll H.D., Warnick L.D., Sponenberg D.P., Eyre P. Systemic and local effects associated with long-term epidural catheterization and morphine-detomidine administration in horses, Veterinary Surgery, 26, 1997, 141–149. [DOI] [PubMed] [Google Scholar]
- Taylor P., Delatour P., Landoni F., Deal C., Pickett C., Shojaee Aliabadi F., Foot R., Lees P. Pharmacodynamics and enantioselective pharmacokinetics of carprofen in the cat, Research in Veterinary Science, 60, 1996, 144–151. [DOI] [PubMed] [Google Scholar]
- Taylor P. Newer analgesics. Nonsteroid anti-inflammatory drugs, opioids, and combinations, Veterinary Clinics of North America: Small Animal Practice, 29, 1999, 719–735. [DOI] [PubMed] [Google Scholar]
- Taylor P.M., Lees P., Reynoldson J., Stodulski G., Jefferies R. Pharmacodynamics and pharmacokinetics of flunixin in the cat: A preliminary study, Veterinary Record, 128, 1991, 258. [DOI] [PubMed] [Google Scholar]
- Taylor P.M., Winnard J.G., Jefferies R., Lees P. Flunixin in the cat: A pharmacodynamic, pharmacokinetic and toxicological study, British Veterinary Journal, 150, 1994, 253–262. [DOI] [PubMed] [Google Scholar]
- Taylor P.M., Robertson S.A., Dixon M.J., Sear J.W., Lascelles B.D.X., Waters C., Bloomfield M. Morphine, pethidine and buprenorphine disposition in the cat, Journal of Pharmacology and Therapeutics, 24, 2001, 391–398. [DOI] [PubMed] [Google Scholar]
- Troncy E., Junot S., Keroack S., Sammut V., Pibarot P., Genevois J.P., Cuvelliez S. Results of preemptive epidural administration of morphine with or without bupivacaine in dogs and cats undergoing surgery: 265 cases (1997–1999), Journal of the American Veterinary Medical Association, 221, 2002, 666–672. [DOI] [PubMed] [Google Scholar]
- Tung A., Yaksh T. The antinociceptive effects of epidural opiates in the cat: Studies of the pharmacology and the effects of lipophilicity in spinal analgesia, Pain, 12, 1982, 343–356. [DOI] [PubMed] [Google Scholar]
- Valverde A., Doherty T.J., Hernandez J., Davies W. Effect of intravenous lidocaine on isoflurane MAC in dogs, Veterinary Anaesthesia and Analgesia, 30, 2002, 105. [DOI] [PubMed] [Google Scholar]
- Wagner A., Walton J., Hellyer P., Gaynor J., Mama K. Use of low doses of ketamine administered by constant rate infusion as an adjunct for postoperative analgesia in dogs, Journal of the Americal Veterinary Medical Association, 221, 2002, 72–75. [DOI] [PubMed] [Google Scholar]
- Wagner A.E. Is butorphanol analgesic in dogs and cats?, Veterinary Medicine, 94, 1999, 346–351. [Google Scholar]
- Watson A., Nicholson A., Church D., Pearson M. Use of anti-inflammatory and analgesic drugs in dogs and cats, Australian Veterinary Journal, 74, 1996, 203–210. [DOI] [PubMed] [Google Scholar]
- Wegner K., Robertson S.A. Evaluation of the side effects and thermal threshold antinociceptive effects of intravenous hydromoprhone in cats, Veterinary Anaesthesia and Analgesia, 30, 2003, 101. [DOI] [PubMed] [Google Scholar]
- Welsh E., Nolan A. The effect of abdominal surgery on thresholds to thermal and mechanical stimulation in sheep, Pain, 60, 1995, 159–166. [DOI] [PubMed] [Google Scholar]
- Yaksh T., Provencher J., Rathbun M., Kohn F. Pharmacokinetics and efficacy of epidurally delivered sustained-release encapsulated morphine in dogs, Anesthesiology, 90, 1999, 1402–1412. [DOI] [PubMed] [Google Scholar]