Abstract
Exhaled breath condensate has been collected in other species and used as a non-invasive method of evaluating airway disease by measurement of various markers in the fluid, including hydrogen peroxide, nitric oxide, leukotrienes and prostaglandins. We describe a novel technique for the collection of exhaled breath condensate from cats, which enabled collection of fluid and measurement of its hydrogen peroxide concentration. Further studies will be needed to establish the value of this technique in the investigation of feline respiratory disease.
In man (Antczak and Gorski, 2002) and most recently in horses (Deaton et al., 2001), collection of exhaled breath condensate (EBC) has been established as a novel and non-invasive means of assessing lower airway inflammation, and as a means of monitoring response to interventional therapy (Hunt, 2002). In numerous studies, various indicators of airway inflammation, or markers of oxidative stress, have been evaluated, including hydrogen peroxide and thiobarbituric acid-reactive products (Antczak et al., 1997; Loukides et al., 2002), nitric oxide (Corradi et al., 2003), 8-isoprostane (Baraldi et al., 2003), leukotrienes and prostaglandins (Montuschi et al., 2003), and interleukins (Carpagnano et al., 2003). Assay of hydrogen peroxide (H2O2) in EBC has been one of the most widely reported and common applications of this technique, and there is clear evidence that EBC H2O2 is elevated in human pulmonary inflammatory conditions such as asthma, adult respiratory distress syndrome (ARDS) and human chronic obstructive pulmonary disease (COPD) (Antczak et al., 1997, 1999; Dekhuijzen et al., 1996; Kietzmann et al., 1993; Nowak et al., 1999). In addition, EBC H2O2 has also been shown to be increased following cigarette smoking (Nowak et al., 1996), during acute upper and lower respiratory tract infections (Dohlman et al., 1993), in bronchiectasis (Loukides et al., 1998), and sepsis (Sznajder et al., 1989). It appears that the H2O2 present in EBC is derived mainly from polymorphonuclear leucocytes, pulmonary macrophages and type II pneumocytes, and its concentration may reflect phagocyte activation (Loukides et al., 2002; Szkudlarek et al., 2003).
The purpose of this paper is to describe a non-invasive system we have developed to collect EBC from cats, and the successful measurement of H2O2 in the samples collected.
Previously, a system has been described for the analysis of hydrogen in exhaled breath of cats, using an acrylic plastic chamber through which air is drawn at a constant rate, with the hydrogen concentration being measured in the air drawn out from the chamber (Muir et al., 1991). Although breath hydrogen concentration can also be measured in expired air collected in a bag through the use of a close fitting face mask and a one-way valve (German et al., 1998), this technique is not always successful in cats, and we anticipated that the length of time required to collect a sufficient volume of EBC would preclude the use of this technique.
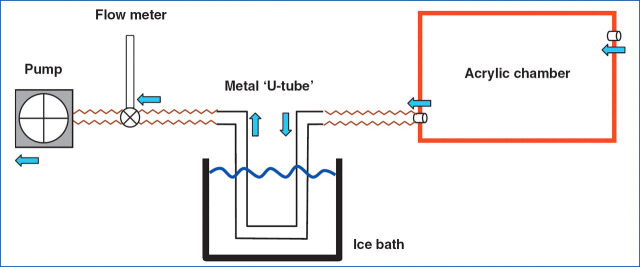
A collection system was therefore designed based around an acrylic plastic chamber formed from individual 5 mm sheets with a hinged lid measuring 40×25×25 cm (internal dimensions; internal volume equivalent to 25 litres; Figs. 1 and 2). A strip of rubber was placed between the lid and the box to form an air-tight seal when it was closed. Two steel flexible toggle latches were placed opposite the hinges on the front of the box to ensure a tight seal between the lid and box. On one of the ends of the box (i.e. 25×25 cm ends), a 25 mm diameter inlet was drilled towards the top of the box to allow air to flow into the chamber, and at the opposite end of the box an outlet of the same size was provided, except that this was positioned towards the bottom of the box. Both inlet and outlet were fitted with adaptors so that the PVC tubing could be attached to the chamber. The outlet from the chamber was connected to a 75 cm length of PVC tubing (28 mm OD, 25 mm ID), which was then connected to a ‘U-shaped’ welded stainless steel condensing tube (22 mm OD, 18 mm ID, and 85 cm total length). The condensing tube was immersed in an ice-water bath at ∼4 °C. A second 75cm length of PVC tubing connected the condensing tube to the inlet of a flowmeter (0–13 l/min; Influx UK), and a third length of PVC tubing (35 cm) connected the flowmeter outlet to a multi-stage pump (model 4MS8, ACI Ltd, Chard, Somerset, UK). Unloaded, the pump was capable of moving air at a rate of 70 m3/h. The speed of the pump was controlled by a rheostat, and the flow of air through the box was set to 900 ml/kg/min (using the pump and flow meter) to ensure that it exceeded the animals estimated minute ventilation volume by at least three-fold to prevent re-breathing of expired gases.
Figure 1.
Diagrammatic representation of the system used for breath condensate collection (blue arrows indicate flow of air).
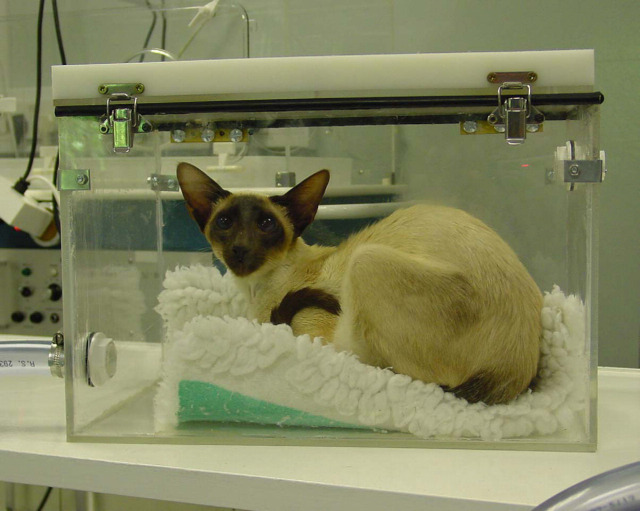
Figure 2.
Siamese cat with lower respiratory disease in the acrylic chamber having breath condensate collected.
During preliminary experiments, it was determined that in order to collect a volume of breath condensate of approximately 1.0 ml required the cat to be in the chamber for approximately 20–30 min (at room temperature and with the box bias flow being drawn from the room). The EBC sample collected in the condensing tube was decanted into a sterile plastic cuvette and analysed for H2O2 concentration within 10 min of collection. Between each EBC sample collection, the condensing tube was cleaned thoroughly by flushing through with distilled water followed by 100% ethanol to facilitate drying.
This EBC collection procedure was performed in six healthy adult cats (four females of which three were entire and two males both neutered). All cats tolerated being in the collection box and showed no signs of distress. A 30 min collection period resulted in the recovery of 0.9 to 1.2 ml of EBC. The concentration of H2O2 in the EBC was determined using a spectrophotometric assay based on the oxidation of tetramethylbenzidine by hydrogen peroxide and catalysed by horse radish peroxidase adapted from Gallati and Pracht (1985). Two hundred and fifty μl of 840 μM 3′3′5′5′-tetramethylbenzidine in 0.42 M citrate buffer pH 3.8 and 50 μl of 52.5 U/ml horse radish peroxidase were added to 500 μl breath condensate and the peak absorbance read at 370 nm after approximately 10 s. The limit of detection was 0.1 μmol/l with an intra-assay coefficient of variation <4% (calculated from pooled samples). In the six healthy cats, EBC H2O2 concentration ranged from <0.1 to 2.1 μmol/l (median 1.0 μmol/l, mean 0.9 μmol/l).
Mean EBC H2O2 in healthy human control subjects from a number of different studies have ranged from 0.01 to 0.72 μmol/l (Antczak et al., 1999; Dekhuijzen et al., 1996; Loukides et al., 2002; Nowak et al., 1999; Sznajder et al., 1989), and similarly in healthy horses, EBC H2O2 has been reported to be in the range of 0.5 to 1.0 μmol/l (Deaton et al., 2001). Therefore, the EBC H2O2 concentrations measured in these healthy cats are comparable to those previously reported in man and horses.
This study demonstrates that, using the described system, EBC can be successfully collected from cats in a safe, non-invasive and very well-tolerated way. Furthermore the EBC recovered can be subjected to analyses that have been used for evaluation of lower airway disease in other species. However, further work will be necessary to establish normal ranges for values of inflammatory markers in larger numbers of cats, and to investigate changes associated with disease.
Acknowledgements
The authors would like to thank Steve Vale for constructing the collection system.
References
- Antczak A., Gorski P. Markers of pulmonary diseases in exhaled breath condensate, International Journal of Occupational Medical and Environmental Health, 15, 2002, 317–323. [PubMed] [Google Scholar]
- Antczak A., Nowak D., Bialasiewicz P., Kasielski M. Hydrogen peroxide in expired air condensate correlates positively with early steps of peripheral neutrophil activation in asthmatic patients, Archivum immunologiae et therapiae experimentalis, 47, 1999, 119–126. [PubMed] [Google Scholar]
- Antczak A., Nowak D., Shariati B., Krol M., Piasecka G., Kurmanowska Z. Increased hydrogen peroxide and thiobarbituric acid-reactive products in expired breath condensate of asthmatic patients, European Respiratory Journal, 10, 1997, 1235–1241. [DOI] [PubMed] [Google Scholar]
- Baraldi E., Ghiro L., Piovan V., Carraro S., Ciabattoni G., Barnes P.J., Montuschi P. Increased exhaled 8-isoprostane in childhood asthma, Chest, 124, 2003, 25–31. [DOI] [PubMed] [Google Scholar]
- Carpagnano G.E., Barnes P.J., Geddes D.M., Hodson M.E., Khartinov S.A. Increased leukotrience B4 and interleukin-6 in exhaled breath condensate in cystic fibrosis, American Journal of Critical Care Medicine, 167, 2003, 1109–1112. [DOI] [PubMed] [Google Scholar]
- Corradi M., Pesci A., Casana R., Alinovi R., Goldoni M., Vettori M.V., Cuomo A. Nitrate in exhaled breath condensate of patients with different airway diseases, Nitric Oxide, 8, 2003, 26–30. [DOI] [PubMed] [Google Scholar]
- Deaton C.M., Marlin D.J., Roberts C.A., Smith N., Harris P.A., Schroter R.C., 2001. Monitoring airway inflammation by expired breath condensate. Proceedings of the World Equine Airways Symposium, Edinburgh, 19th–23rd July, 2001, p. 15.
- Dekhuijzen P.N., Aben K.K., Dekker I., Aarts L.P., Wielders P.L., van Herwaarden C.L., Bast A. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease, American Journal of Critical Care Medicine, 154, 1996, 813–816. [DOI] [PubMed] [Google Scholar]
- Dohlman A.W., Black H.R., Royall J.A. Expired breath hydrogen peroxide is a marker of acute airway inflammation in pediatric patients with asthma, American Review of Respiratory Disease, 148, 1993, 955–960. [DOI] [PubMed] [Google Scholar]
- Gallati H., Pracht I. Horseradish peroxidase: Kinetic studies and optimization of peroxidase activity determination using the substrates H2O2 and 3,3′,5,5′-tetramethylbenzidine, Journal of Clinical Chemistry and Clinical Biochemistry, 23, 1985, 453–460. [PubMed] [Google Scholar]
- German A.J., Martin M.A., Papasouliotis K., Hall E.J. Assessment of a breath collection technique and portable breath hydrogen monitor for use in dogs and cats, Research in Veterinary Science, 65, 1998, 173–175. [DOI] [PubMed] [Google Scholar]
- Hunt J. Exhaled breath condensate: An evolving tool for non-invasive evaluation of lung disease, Journal of Allergy and Clinical Immunology, 110, 2002, 28–34. [DOI] [PubMed] [Google Scholar]
- Kietzmann D., Kahl R., Muller M., Burchardi H., Kettler D. Hydrogen peroxide in expired breath condensate of patients with acute respiratory failure and with ARDS, Intensive Care Medicine, 19, 1993, 78–81. [DOI] [PubMed] [Google Scholar]
- Loukides S., Bouros D., Papatheodorou G., Panagou P., Siafakas N.M. The relationships among hydrogen peroxide in expired breath condensate, airway inflammation, and asthma severity, Chest, 121, 2002, 338–346. [DOI] [PubMed] [Google Scholar]
- Loukides S., Horvath I., Wodehouse T., Cole P.J., Barnes P.J. Elevated levels of expired breath hydrogen peroxide in bronchiectasis, American Journal of Critical Care Medicine, 158, 1998, 991–994. [DOI] [PubMed] [Google Scholar]
- Montuschi P., Kharitonov S.A., Ciabattoni G., Barnes P.J. Exhaled leukotrienes and prostaglandins in COPD, Thorax, 58, 2003, 585–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir P., Pappasouliotis K., Gruffydd-Jones T.J., Cripps P.J., Harbour D.A. Evaluation of carbohydrate malassimilation and intestinal transit time in cats by measuring breath hydrogen excretion, American Journal of Veteirnary Research, 52, 1991, 1104–1109. [PubMed] [Google Scholar]
- Nowak D., Antczak A., Krol M., Pietras T., Shariati B., Bialasiewicz P., Jeczkowski K., Kula P. Increased content of hydrogen peroxide in the expired breath of cigarette smokers, European Respiratory Journal, 9, 1996, 652–657. [DOI] [PubMed] [Google Scholar]
- Nowak D., Kasielski M., Antczak A., Pietras T., Bialasiewicz P. Increased content of thiobarbituric acid-reactive substances and hydrogen peroxide in the expired breath condensate of patients with stable chronic obstructive pulmonary disease: No significant effect of cigarette smoking, Respiratory Medicine, 93, 1999, 389–396. [DOI] [PubMed] [Google Scholar]
- Szkudlarek U., Maria L., Kaisielski M., Kaucka S., Nowak D. Exhaled hydrogen peroxide correlates with the release of reactive oxygen species by blood phagocytes in healthy subjects, Respiratory Medicine, 97, 2003, 718–725. [DOI] [PubMed] [Google Scholar]
- Sznajder J.I., Fraiman A., Hall J.B., Sanders W., Schmidt G., Crawford G., Nahum A., Factor P., Wood L.D. Increased hydrogen peroxide in the expired breath of patients with acute hypoxemic respiratory failure, Chest, 96, 1989, 606–612. [DOI] [PubMed] [Google Scholar]