Abstract
Four new cases of sarcoptic mange in cats are described. Two cats resided in areas known to be frequented by foxes, another cohabited with a dog recently diagnosed with sarcoptic mange, while the final cat lived with a mixed breed dog that had been treated for sarcoptic mange 7 months previously. Three cases were diagnosed on the basis of characteristic mite size and morphology in skin scraping from representative lesions, situated on the head (two cases) or head and distal hind limbs (one case). Mites were highly mobile and abundant in all instances, and easily detected also in skin biopsy specimens procured from two cases. Eosinophilic inflammation, hyperkeratosis and parakeratosis were prominent in the tissue sections. In the remaining case, the diagnosis was presumptive, based on characteristic lesions, cohabitation with a canine scabies patient and positive response to scabicide therapy. Pruritus was not a prominent clinical feature in any patient and was considered to be absent in three of the four cases. Lesions in three cats with long-standing disease were reminiscent of crusted scabies (synonym: Norwegian scabies, parakeratotic scabies) as seen in human patients. In three cases, in-contact human carriers developed itchy cutaneous papular lesions. Two cases responded promptly to therapy with systemic avermectin drugs, while one responded to topical treatment with lime sulphur and the remaining cat received both a lime sulphur rinse and ivermectin. Sarcoptic mange should be considered in the differential diagnosis of cats with non-pruritic crusting skin diseases, especially when there is contact with foxes or dogs, and when owners have itchy papular lesions.
Mites from the family Sarcoptidae include both the genera Sarcoptes and Notoedres. Notoedres cati is the most common mite associated with feline scabies, and is well described in texts and the published literature (Muller et al 1989b). It primarily infests cats, producing a highly pruritic crusting dermatitis, but can also infest foxes, dogs and rabbits.
Sarcoptes scabiei is an astigmatid mite that causes scabies in people and mammals. It is an important emerging disease of wildlife throughout the world. The mite is said to originate from an ancestor parasitic on humans (S scabiei var hominis) which is thought to have spread to domestic and then free-living animals. Based on the recent emergence of sarcoptic mange in Australian wildlife and aboriginal populations, it is thought that S scabiei was introduced to Australia by the Europeans and their animals (Andrews 1983, Skerratt 2005).
S scabiei var canis and S scabiei var vulpes have a host preference for dogs, wolves, coyotes, dingoes and foxes, but can infest also a wide variety of other hosts including lynx, hare, horses, ungulates and cats (Muller et al 1989a, Morner 1992). Both mites have a predilection for sparsely haired regions of the integument, such as the edges of the pinnae, the elbows, hocks and ventral abdomen. Both Sarcoptes and Notoedres species can produce transient lesions in humans.
Sarcoptic mange (sarcoptic acariasis) due to S scabiei has been reported infrequently in the cat. Indeed, there are only five well-documented individual patients (Lindquist and Cash 1973, Bussieras 1984, Hawkins et al 1987, Huang et al 1998, Kontos et al 1998) and an ‘epidemic’ of 25 cats in a single household (Bornstein et al 2004) in searchable electronic databases. S scabiei is easily differentiated from N cati because S scabiei mites are substantially larger, have longer limb stalks and a terminal (rather than a dorsal) anus (Muller et al 1989a, b).
The present study has assembled four new cases, and tabulated the five cases and one ‘epidemic’ reported previously, in an attempt to provide a consensus picture of S scabiei infestation in the cat. The most remarkable finding is that in this species, the interplay between the mites and the immunological response of the host generally results in the development of crusted parakeratotic lesions associated with minimal or absent pruritus, a picture reminiscent of Norwegian scabies in certain human patients (Robinson 1985, Walton et al 2004). Usually cats had a history of contact with infested Canidae, either dogs or foxes, and owners typically developed pruritic papular skin lesions concurrently. The disease is contrasted to sarcoptic mange in other species and feline notoedric mange.
Case Report
Case 1
A 14-year-old neutered male domestic crossbred cat living in Mittagong was presented in January 2003 for crusting of the ear, apparently of acute onset. Mittagong is a small town in the southern highlands of New South Wales (Australia) approximately 100 km from Sydney and is an area with high endemicity for sarcoptic mange in feral foxes, owned dogs and wombats.
The dorsal margin of the right pinna and its inside surface were covered by dry cream-coloured crusted plaques. The lesions somewhat resembled equine dermatophilosis, and bled when scraped. Lesions were not pruritic according to the cat's owner and in support of this there were no obvious secondary lesions referable to self-trauma. No definitive diagnosis was made and topical antibiotic and antifungal formulations were prescribed.
The cat was re-examined 40 days later. There had been no response to therapy, and indeed more extensive lesions were now present on both ears (Fig 1A). The dorsal surfaces of both pinnae were deeply fissured, especially towards the pinnal margins (Fig 1B). The abnormal skin felt cold to the touch, and was clearly demarked from the more normal adjacent integument. Lesions extended across the preauricular area of alopecia of the right forehead (Fig 1C).
Fig 1.
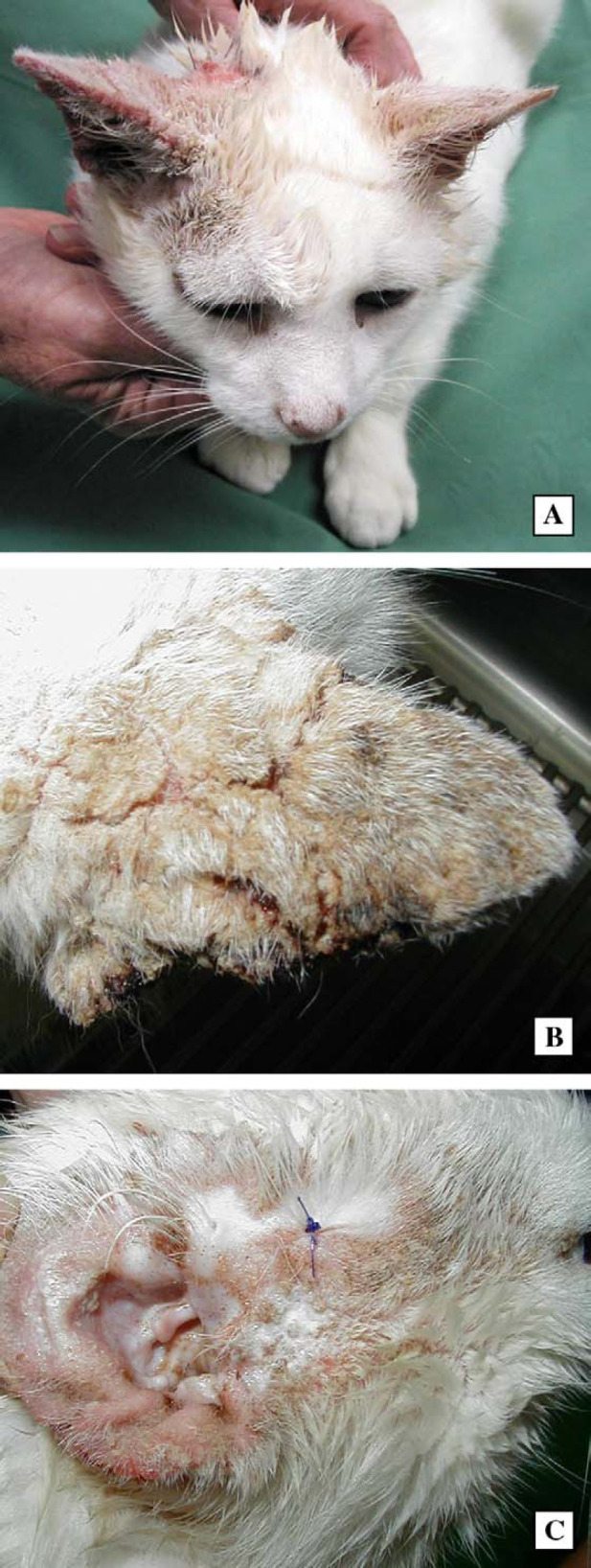
Photographs demonstrating the lesions present in case 1. The most conspicuous lesion is the crusting fissured dorsal surface of the pinna (A), seen most clearly in the second photograph (B). Similar lesions, but of lesser severity, are evident on the ventral surface of the pinna and on the periorbital area of alopecia (C).
Using a pathoanatomic classification scheme, the causes for severe crusting skin diseases could be inflammatory (infectious, allergic or immune-mediated), degenerative (toxic, traumatic) or due to a disorder of growth (cornification/keratinisation defects, neoplasia). From a clinical perspective, the differential diagnosis would thus include allergic dermatitis (food allergy, flea allergy, atopic dermatitis), pemphigus foliaceus, lupus, dermatophytosis, dermatophilosis, demodecosis, notoedric mange, sarcoptic mange, ear mite infestation, a drug eruption, atypical squamous cell carcinoma and squamous cell carcinoma in situ.
Under heavy sedation, the crusted material was scraped away, collected and examined microscopically at low power (Fig 2). Enormous numbers of highly mobile, round-bodied mites (200–400 μm) were observed amongst the hairs and crusty keratinous debris. Adult mites of both sexes, nymphs, hexapod larvae (Fig 3) and eggs were observed in abundance. The adult mites had a terminal anus, and were substantially larger than Notoedres cati mites, which are distinguished also by their dorsal anus and shorter limb stalks. The identification of the mites was confirmed as Sarcoptes scabiei at a reference laboratory (Menzies School of Health Research, Casurina Northern Territory).
Fig 2.
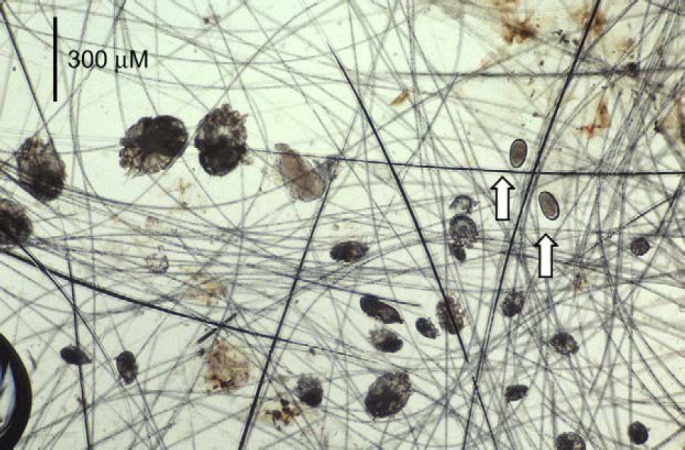
Low power photomicrograph from skin scrapings obtained from case 1. Note the presence of mites of both sexes and at various stages of development, from eggs (arrows), hexapod larvae, nymphs, to adult male and female mites. Original magnification −25×.
Fig 3.
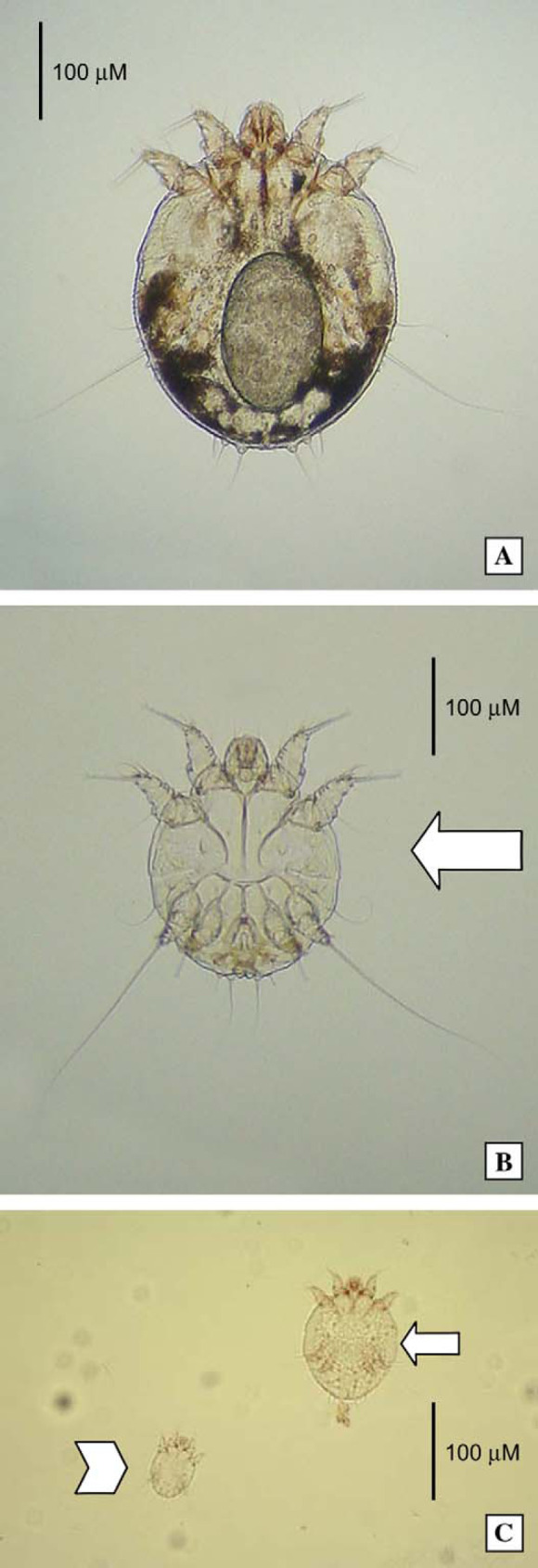
Photomicrographs of sarcoptic mites from case 1, at higher magnification. A female mite, containing an egg, is present in the top micrograph (A). A male is identified by the large arrow in the middle photograph (B), while a nymph (small arrow) and hexapod larva (arrow head) is seen in the lower most image (C). (A) and (B) original magnification − 100×; (C) original magnification − 50×.
A skin biopsy was collected from the preauricular area of alopecia (Fig 1C). Histologically, there was a generalised, mild to marked, epidermal hyperplasia with mild to marked hyperkeratosis that was predominantly parakeratotic in focal areas. In some sections with particularly marked hyperkeratosis, ‘tunnel’ formation was evident subcorneally within the epidermis, with sections of mites present in many tunnels (Fig 4A). In the superficial dermis there was a mild to moderate infiltrate, predominantly of eosinophils (some degranulating), in a perivascular pattern (Fig 4B). There were approximately 30–40 mast cells per high power field (400×) and scattered lymphocytes. Epidermal transmigration (exocytosis) was evident with mild to moderate numbers of eosinophils present in the hyperkeratotic crust. Infiltration of the superficial dermis with eosinophils and mast cells is consistent with an inflammatory response with a prominent type I hypersensitivity component.
Fig 4.
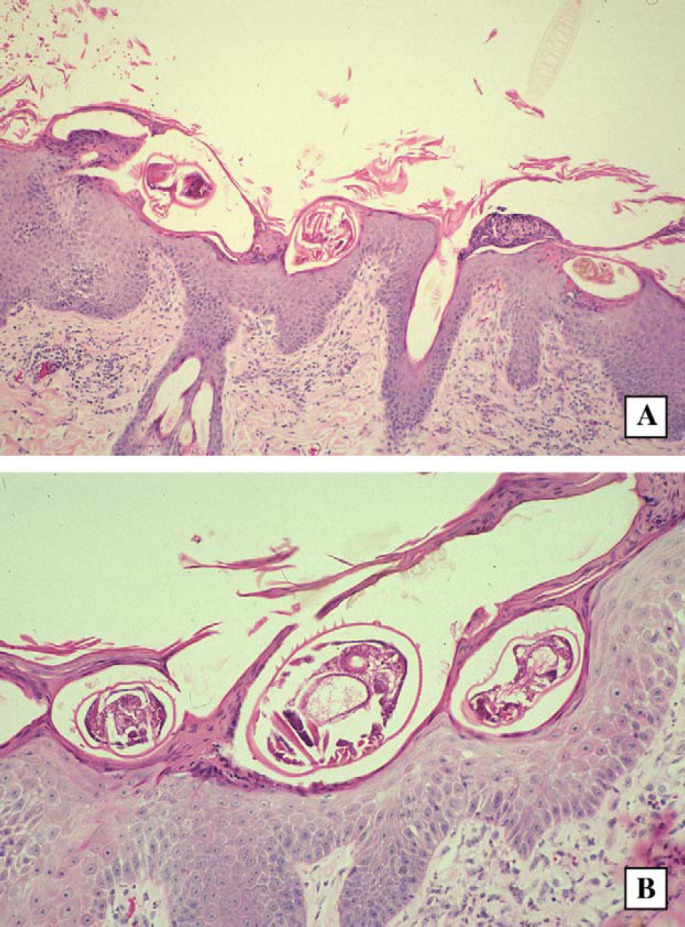
Histological sections from case 1, a cat with crusted scabies. Note the parakeratosis, hyperkeratosis, presence of mites in epidermal ‘tunnels’ or burrows and mild inflammatory infiltrate in the dermis. H&E; original magnification − 40× and 80×, respectively.
The presence of a crusting non-pruritic skin disease affecting the ears, with deep fissuring, marked hyperkeratosis and enormous numbers of mites was compatible with a diagnosis of crusted (or Norwegian) scabies. Accordingly, a more detailed history was obtained to search for a cause for the infestation and to explore the possibilities of immune suppression or defective grooming ability. Firstly, erythematous papules were present on the forearms of the cat's owners (Fig 6A). Secondly, the cat was said to spend much time in a paddock opposite the owner's house, an area known to be frequented by foxes and ‘mangy’ wombats. As crusted scabies is often associated with defective cell-mediated immunity in human patients, the cat was screened for the presence of FIV antibodies and FeLV antigen; it tested negative for both.
Fig 6.
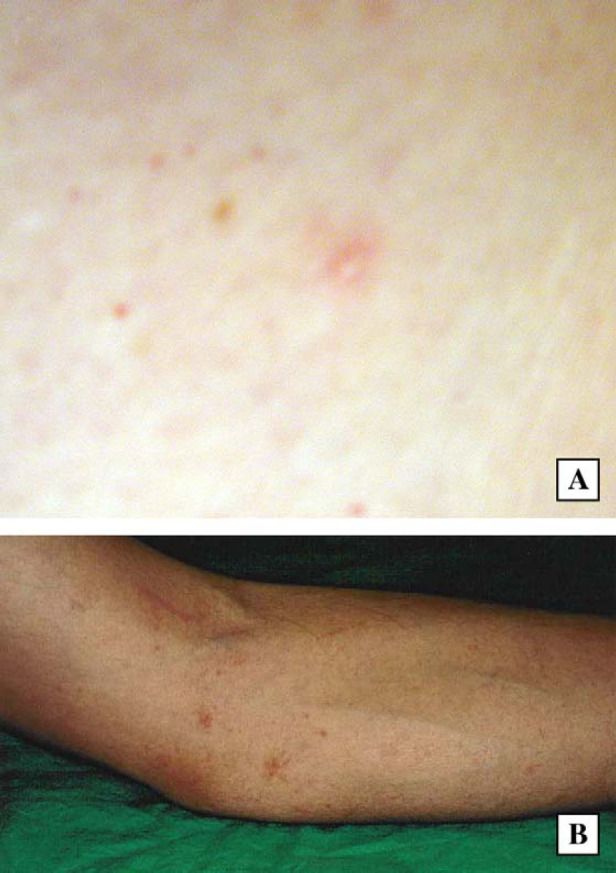
Erythematous papules present on the forearm of the owners of case 1 (A) and case 2 (B), respectively.
Treatment of crusted scabies requires physical removal of keratinous debris known to harbour the enormous numbers of mites, followed by systemic and/or topical antiparasitic therapy (Hadfield-Law 2000, Walton et al 2004). In this case, selamectin was used as a scabicide (Six et al 2000), and also to prevent environmental reinfestation. A 5–10 kg canine size Revolution applicator (120 mg/ml; 0.5 ml) was used to administer 60 mg selamectin in the recommended manner (http://www.revolutionpet.com/default.html) every 2 weeks for 2 months; then once monthly. Following therapy, residual scale and crusts fell off, revealing slightly pink skin. Skin scrapings became negative and all lesions resolved.
Selamectin was applied monthly at the recommended feline dose (60 mg/ml; 0.75 ml per cat; approx. 6 mg/kg) to prevent the infestation from recurring. The cat subsequently developed ultraviolet-related squamous cell carcinoma of both pinnae, which were surgically resected. Hyperthyroidism was diagnosed clinically and confirmed by an elevated thyroxine value approximately 21 months after the cat was initially presented for scabies. The owners elected not to treat the endocrinopathy and it remained in reasonable condition for another 9 months until it developed an abdominal mass and was euthanased. A necropsy was not performed.
Case 2
A 7-year-old, castrated male domestic shorthaired cat (4.9 kg), domiciled in London (England), was presented in May 2000 for abrupt onset of a non-pruritic scaling crusting dermatosis affecting the head for 1 week's duration (Fig 5). The cat was otherwise well. There was no evidence of otitis externa, ear mites were not detected on otoscopic examination and there was no evidence of self-trauma. The owner had had pruritic lesions on the arms (Fig 6B) and torso for approximately 2 months.
Fig 5.
Photograph of the early lesions and scale in case 2. This case was diagnosed and treated in a timely manner, prior to development of extensive crusty lesions.
Scale from the lesions was examined microscopically, clear sticky-tape preparations were obtained and skin scrapings were taken from the lesions. Scrapings demonstrated numerous, actively mobile, round-bodied mites. Mites were not evident in the scale. These were identified as a Sarcoptes species based on size (300–400 μm), terminal anus and cuticular spines (Dr Anne Baker, Entomology Unit, Natural History Museum, London).
Upon further questioning, it was established that urban foxes visited the owner's backyard. The cat had been castrated as an adult, and upon testing was found to be FeLV-negative, but FIV-positive using an in house ELISA test.
The cat was treated with subcutaneous ivermectin (200 μg/kg, ie, 1 mg) (Yazwinski et al 1981). Skin scrapings 7 days after treatment were negative for mites, and the skin condition resolved subsequently. The owner's lesions likewise cleared. The cat presented 1 year later (July 2001) with marked weight loss (2.9 kg), polyuria, polydipsia, dehydration and massive irregular kidneys. It was likely the cat had renal lymphoma, however, the owner declined further investigation and the cat was lost to follow-up.
Case 3
A 2-year-old neutered male Himalayan cat (3.6 kg) was presented for a second opinion following a 4 month history of a crusting dermatitis (Sousa 1999). The cat was kept entirely indoors, and lived with a mixed breed dog in northern California. The skin condition began with scaling on the hind legs and progressed to involve other areas of the integument. The cat was only mildly pruritic. Skin scrapings performed by another veterinarian were reported to be negative. The cat failed to respond to a single injection of methylprednisolone acetate (30 mg intramuscularly), cephalexin, amoxycillin and a miconazole bath.
Physical examination revealed poor body condition, partial alopecia and thick crusts on the medial aspects of the pinnae (Fig 7A) and the distal portions of the pelvic limbs, being most severe on the hocks and peri-pad areas (Fig 7B). Scattered crusts were observed also on the top of the head. Peripheral lymphadenomegaly was present and the owner complained of pruritic ‘bites’ on her trunk.
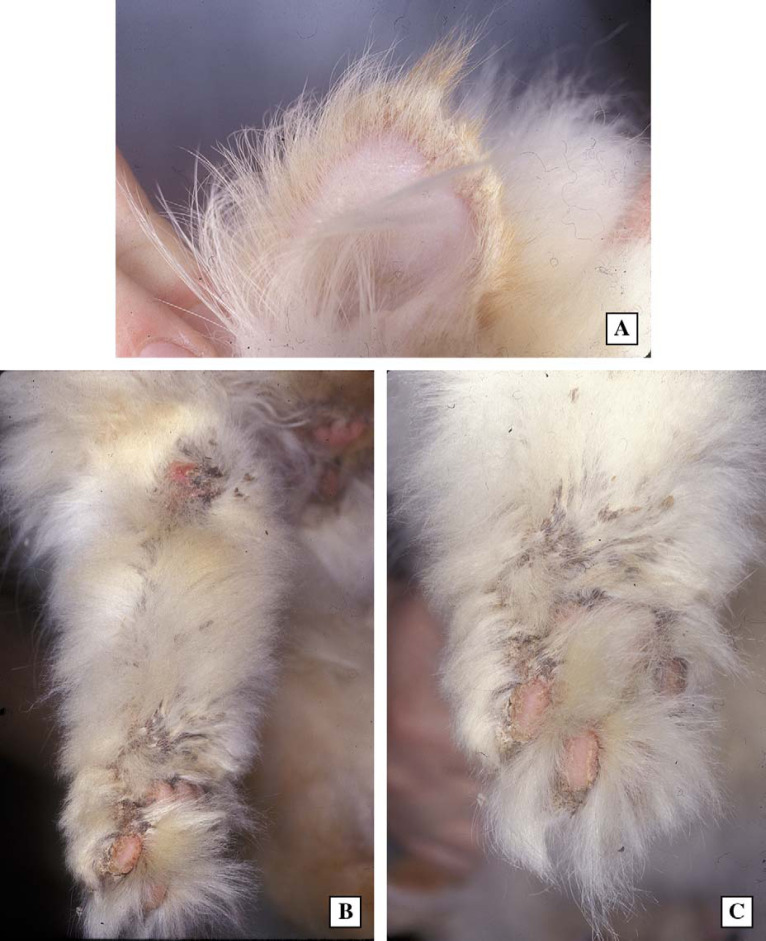
Fig 7.
Skin and hair on the medial aspect of the pinnae (upper photo; A) and distal hind limb (lower photos B and C) of case 3. Note the prominent crusts and partial alopecia.
Results of a complete blood count indicated eosinophilia (5445/μl, reference range 0–1500/μl) and basophilia (165/μl, reference range 0/μl). Serum chemistries were within reference ranges. An FeLV antigen test was negative. Microscopy of a skin biopsy revealed a hyperplastic, spongiotic, crusting eosinophilic/pleocellular dermatitis, with mites visible in ‘tunnels’ in many sections. A skin scraping demonstrated numerous mobile sarcoptic mites. Further history revealed that the dog in the household had been treated for sarcoptic mange about 7 months prior to presentation of the cat. On re-examination, the dog was found to be normal (Fig 8).
Fig 8.
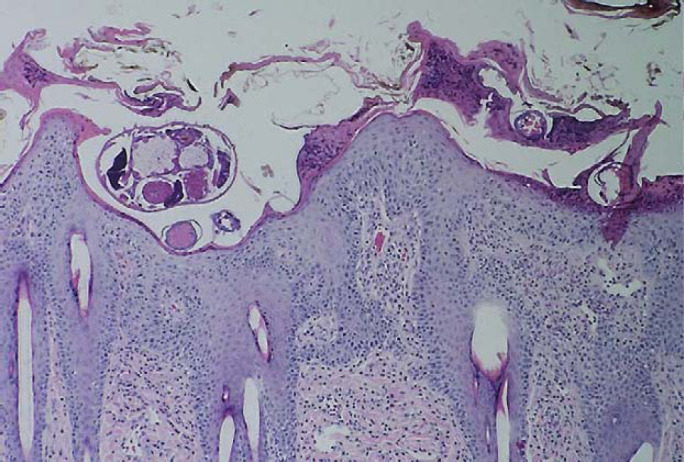
Photomicrograph of skin biopsy specimen from case 3 showing hyperplastic spongiotic crusting with numerous eosinophils. A cross section of a Sarcoptes scabiei mite is evident in the superficial epidermis. H&E; original magnification − 40×.
The cat was rinsed with 2% lime sulphur and given ivermectin (250 μg/kg subcutaneously; repeated after 14 days). Within 2 h of the lime sulphur rinse a second skin scraping revealed only dead mites. The skin and hair coat of the cat were normal 1 month after presentation and the lesions on the owner had resolved.
Case 4
A case of canine scabies was diagnosed in a multi-animal household in northern California. S scabiei mites were detected in skin scrapings from the index dog, a fairly typical canine scabies case. The owners mentioned that another dog in the household was similarly affected and an in-contact cat had a crusty face and ears and ‘thickening’ of the facial skin, but was not pruritic. The owners lived at some distance from the referral centre and declined to bring the cat in for investigation. However, both canine patients and the cat responded to topical acaricidal therapy, an organophosphate rinse (Paramite dip; Vet-Kem) for the dogs and lime sulphur rinse for the cat. The owner reported that the crusts on the cat's face and ears had fallen off, and that new hair was growing in at 1 month after treatment.
Discussion
In human patients, sarcoptic mange is caused by S scabiei var hominis (McCarthy et al 2004, Walton et al 2004). Human scabies is typically an intensely pruritic papulovesicular dermatitis. The ‘burrow’ of the mite (in the horny layer of the skin) is the hallmark clinical feature, and can usually be located using magnification, ancillary aids (staining, etc) and diligent technique. The fertilised female mite lays eggs in the burrows as well as depositing faecal matter in the tunnel behind her. Burrows may be linear or serpentine, and usually end with a small surface vesicle over a black speck, the location of the female mite. Lesions are most commonly found on the hands, interdigital areas, wrist, elbows and ankles, tending to occur in areas with little body hair. In hosts of normal immune status, usually 3–50 mites (average number 11) account for the clinical signs, which are probably related to both physical irritation caused by burrowing and hypersensitivity reactions to mite excretory antigens (Mellanby 1944, Robinson 1985, Walton et al 2004).
Canine scabies is conceptually similar to human scabies, although the burrow is rarely recognized. As in man, the disease is extremely pruritic, but mites are difficult and sometimes impossible to find, so called scabies in cognito (Muller et al 1989a). As in man, lesions in dogs usually start in less densely haired regions of the integument, such as the ear margin and elbows, although secondary hair loss due to excoriation allows the mites to spread to an increasing proportion of the integument (Morris and Dunstan 1996, Scott et al 2001). Puppies and older debilitated dogs may be at increased risk for developing severe infestations and the disease is very contagious within populations of dogs (Walton et al 2004). Contact with foxes is often an important clue in the history, and accounts for the disease occurring more commonly in certain geographical regions (Stone et al 1976, Morner 1992). For example, sarcoptic mange due to S scabiei var vulpes became prevalent in red foxes in Sweden from 1975 to 1984, spilling over into other wildlife species (eg, arctic fox, lynx, pine marten, wolf, mountain hare) and domestic species (dog, horse, cat). Similarly in Switzerland, lynx developed sarcoptic mange referable to either N cati (probably crossover from cats) and/or S scabiei (from red foxes) (Ryser Degiorgis et al 2002). In relation to the critical role of foxes in the epidemiology of scabies, it is pertinent to note that in many regions there is either an increase in fox numbers, or an alteration in their behaviour or habitat, with increased proximity to villages and the outskirts of towns and cities. In Europe, the diagnosis of sarcoptic mange is facilitated by the availability of two commercial ELISA tests that detect antibodies against mite antigens in the patient's serum (Bornstein and Zakrisson 1993, Lower et al 2001). In practice, a tentative diagnosis of sarcoptic mange is confirmed in many cases by a convincing response to scabicide therapy, rather than by direct visualisation of mites.
In all our cases, and in at least two cases in the literature, the development of scabies was possibly linked to exposure to dogs or foxes with proven or suspected infestations (Table 1). Interestingly, the contact could be as long as 7 or even 15 months earlier. It is, therefore, critical, when taking a history from cats with crusting skin disease, to enquire as to lesions on owners and also in-contact animals, including cats, dogs and potential wildlife reservoirs.
Table 1.
Feline scabies cases caused by S scabiei reported previously and in the current case series
| Reference or case number | Age | Breed | Gender | Contact with Canidae | Lesions | Pruritus | Number of mites | Crusts | FIV/FeLV |
|---|---|---|---|---|---|---|---|---|---|
| Lindquist and Cash 1973 | 8–10 weeks | DSH | Female | Ears, neck, head, abdomen, tail; lesions on in-contact people | No | Abundant | Skin scaly, thickened, wrinkled; euthanased | NT | |
| Bussieras 1984 | 1 year old | Persian | Female | In-contact dogs (but treated with acaricide); in-contact cavies with pruritus (but no mites) | Head, neck, distal limbs (but had concurrent dermatophytosis) | Yes | Numerous, with multiple stages of development | Diffuse alopecia and crusts; multiple papules also | NT |
| Hawkins et al 1987 | >6 years | DSH | Female | Rural domicile; contact with dogs | Ears, head, neck, tail, thigh, feet, nail beds; lesions on in- contact people | Yes | Abundant | Thick (1 cm) tightly adherent yellow/grey crusts; cat died despite therapy | NT/− |
| Huang et al 1998 | 1 year | Persian | Male | Four paws, tail; 3 month history; lesions on in-contact people | No | Abundant | Haemorrhagic crusts; hyperkeratinisation of the skin of the tail | −/− | |
| Kontos et al 1998 | 6 months | Persian | Female | Limbs, tail, head; 4 week history; lesions on in-contact people | Yes | Abundant; all stages seen | Yellow/gray crusts and fissures; cat died despite appropriate therapy | NT | |
| Morner 1992 | N/A | Domestic cat | N/A | N/A | N/A | N/A | N/A | NT | |
| Bornstein et al 2004 | N/A | Domestic cats | N/A | One in-contact dog with scabies 15 months prior | N/A | N/A | Abundant | N/A | −/− in 21 cats tested |
| Case 1 | 14 years | Domestic cat | Male | Foxes and wombats | Ears, head; lesions on in-contact people | No | Abundant | Fissures and crusts | −/− |
| Case 2 | 7 years | Domestic cat | Male | Foxes | Head; lesions on in-contact people | No | Abundant | Scale only | +/− |
| Case 3 | 2 years | Himalayan | Male | One in-contact dog with scabies 7 months prior | Distal hind limbs, ears, widespread lesions | Mild | Abundant | Scale, fissures, thick crusts, partial alopecia | NT/− |
| Case 4 | N/A | Domestic cat | N/A | Two in-contact dogs with scabies | Face | No | N/A | Crusts, thickening of facial skin | NT |
DSH = domestic short haired cat; N/A = not available; NT = not tested; FeLV = feline leukaemia virus; FIV = feline immunodeficiency virus.
In the elegant investigations of Bornstein et al (2004), mites harvested from cats had identical DNA sequences to mites collected from naturally infected dogs and wildlife. This provided compelling evidence that S scabiei is not absolutely specific in relation to host species, and similar observations have been made in relation to wombat scabies in Australia (Skerratt et al 1998, 1999, Skerratt 2003) and wildlife scabies in Sweden (Morner 1992). The molecular evidence concerning whether there are indeed different host-specific varieties of S scabiei is contradictory (Zahler et al 1999, Skerrat et al 2002, Morrison et al 2003), although recent molecular studies indicate consistent gene sequence differences between human and other animal S scabiei strains (Walton et al 2004, Heukelbach et al 2005). However, the ability of mites to cross the species barrier and establish patent infections seems well established (Goldman and Feldman 1949, Walton et al 1997, 1999).
The weight of evidence in all mammalian species studied indicates that sarcoptic acariasis is a complex hypersensitivity reaction involving both humoral and cell-mediated components, and circulating immune complexes (Bornstein and Zakrisson 1993, Lower et al 2001, Scott et al 2001, Arlian et al 2004). Dogs with clinical signs of sarcoptic mange seroconvert to various mite antigens 4–5 weeks post infection (Bornstein and Zakrisson 1993). ELISA assays show IgG response to scabies antigens (Lower et al 2001), T-lymphocytes (CD3 epsilon +), CD11c+, MHC class II+, and CD1a+ cells in the dermis play a major role in a successful immune response to scabies mites in experimentally infected dogs (Stemmer et al 1996). Most of the clinical features that characterise sarcoptic acariasis in all species, including extreme pruritus, are probably associated with hypersensitivity to mite antigens, including proteins in the cuticle, saliva and faeces. In the normal situation, an infested individual mounts a cell-mediated immune response to mite antigens, and this acquired immunity limits the spread of mites, preventing overwhelming infestation (Table 2).
Table 2.
Some features of scabies in different mammalian species
| Species | Infestation | Abundance of mites | Extent of pruritus | Presence of crusts and scale |
|---|---|---|---|---|
| Man (Caucasian) Robinson 1985 | Sarcoptes scabiei var hominis | + | ++++ | 0 to + |
| Man crusted (aboriginal) Roberts et al 2005 | Sarcoptes scabiei var hominis | ++ to ++++ | 0 to + | + to ++++ |
| Dog (domestic) | Sarcoptes scabiei var canis | + | ++++ | + |
| Fox | Sarcoptes scabiei var vulpes | ++++ | ++ | |
| Cat | Notoedres cati | ++++ | ++++ | ++ to +++ |
| Cat | Sarcoptes scabiei | ++++ | 0 to + | ++++ |
| Pig Davies 1995 | Sarcoptes scabiei var suis | + to ++ | + to ++ | ++ to +++ |
| Wombat (Vombatus ursinus and Lasiorhinus latifrons) Skerratt 2005 | Sarcoptes scabiei var wombati | ++ to ++++ | ++ to ++++ | +++ to ++++ |
| Gibbon Goldman and Feldman 1949 | Sarcoptes scabiei | +++ to ++++ | + to ++ | +++ to ++++ (and spread to human contacts) |
Variation in the intensity of the immune response probably in part explains the variability in severity of clinical signs from individual to individual, the development of asymptomatic carriers, and a reduction in mite numbers during disease progression. Interestingly, some dermatologists have documented scabies mites on asymptomatic dogs living in households with dogs with clinically apparent disease (Peter Ihrke, personal observations), and in populations of pigs, a minority of individuals harbour a large proportion of the mites (Davies 1995). Dogs with compromised immune systems, due to co-existent immunosuppressive disease or therapy with corticosteroids or cytotoxic drugs, may be infested by much larger populations of mites, ie, Norwegian scabies (Anderson 1981).
Crusted scabies is a term used to refer to a syndrome seen in a subset of human scabies patients (Walton et al 2004). It was first described in 1848 by Danielssen and Boeck in Norwegian patients with leprosy. The syndrome came to be known by the moniker ‘Norwegian scabies’, and ‘Norwegian people have been afflicted by jokes and innuendos ever since’ (Robinson 1985). Crusted scabies tends to occur in patients with impaired cell-mediated immunity, for example as a result of immunosuppressive therapy for immune-mediated disease, transplantation and in the setting of HIV/AIDS (Schlesinger et al 1994, Walton et al 1999). It is also encountered in neurologically impaired people who are unable to properly interpret or respond to the itch sensation by scratching, and in certain indigenous populations, including Australian Aborigines, in which no overt immunologic risk factors can be identified (Walton et al 2004, Roberts et al 2005).
In crusted scabies, mites multiply unchecked and reach extremely large numbers in skin and scale. The skin reacts to the infestation by increasing keratinocyte turnover, which causes cracks and fissures to develop, giving rise to adherent crusts. Because of the enormous numbers of mites present within lesions and the immediate environment, people (and animals) with crusted scabies are highly infectious to other individuals. Pruritus is conspicuously absent in up to 50% of cases, although in the remainder it can be mildly irritating or sometimes quite pronounced. This is somewhat counterintuitive, as human patients with crusted scabies generally have prominent eosinophilia and markedly elevated IgE levels (Roberts et al 2005). In terms of comparative parasitology, infestation with S scabiei in certain species, such as the wombat, is conceptually similar to Norwegian scabies in terms of the lesion histology and the number of mites, but different in relation to the extent of irritation (ie, wombats generally suffer from pruritus) (Skerratt et al 1999). Feline notoedric mange due to Notoedres cati is likewise associated with crust, scale, abundant mites, and contagious to other cats and in-contact humans, but invariably in association with extreme pruritus.
In at least three of the present cats of this series (cases 1, 2 and 3), all of which had long-standing signs (at least 40 days, likely 2 months and 7 months, respectively), gross lesions and symptomatology were remarkably similar to human patients with crusted scabies, as was the presumptive case. Why the lesions in these patients were minimally or not pruritic remains a mystery, especially in the light of heavy infiltration of the affected skin with mast cells and eosinophils. Only a limited number of feline cases with sarcoptic mange have been recorded (Table 1), so it is difficult to make firm generalisations. However, it would seem that most cats reported in the literature had advanced lesions reminiscent of crusted scabies with abundant mites in skin scrapings and evidence of contagion. For example, the stray kitten described by Hawkins et al (1987) had thick, wrinkled, scaly skin with extensive hair loss over the ears, head, neck, abdomen, proximal limb and tail, and of six in-contact people, four developed skin lesions. In other cats, however, as in case 2 of the present report, lesions were subtler, perhaps because these cats were presented at an earlier stage of pathogenesis.
We speculate that when S scabiei is well established in natural animal populations, individuals with strong acquired cutaneous immunity to ectoparasites are favoured by natural selection. Such individuals are likely to have a more severe inflammatory reaction, greater pruritus and less likelihood of harbouring large infestations. In contrast, when sarcoptic mites are introduced to a ‘new’ host species, either a naive population of the same species, or a completely different species, there is less effective systemic and local immunity, and less pruritus; this favours the establishment of heavier infestations, the phenomenon of secondary thick, adherent crusting and the development of crusted scabies.
The lesions in virtually all reported cases of feline sarcoptic mange, including the four new patients recorded here, were similar to feline notoedric mange in that the cats had abundant mites present in the lesions. They differed, however, in not having the intense pruritus so typical of Notoedres species infestations. Knowledge about the host specificity of the scabies mite is incomplete, although physiological and genetic differences have been described for different strain variants. When a host-adapted mite infects a non-permissive host it generally fails to feed successfully, and thus does not develop and reproduce. This is the case, for example, with the self-limiting infections in humans exposed to dogs or cats with sarcoptic mange. In some instances, however, the mite can produce severe and self-perpetuating infestations in non-target species: this would appear to be the case in the present instance, and also in wombats that develop life-threatening sarcoptic mange following infection by Sarcoptes scabiei from foxes and the situation in Sweden where there is a obvious spill-over from mangy red foxes to other native and domestic species. In Australia, scabies has also spilled over into other native animals such as the ring-tailed possum, agile wallaby and koala (Skerratt et al 1998).
The severity of the lesions in case 1 may have been attributable to an underlying immunodeficiency, although no obvious comorbidities were evident when the cat was initially presented, and the patient tested retrovirus negative. The later development of solar keratitis induced squamous cell carcinoma may have been associated with reduced local immunity, or the colonisation of an abnormal site. There is no need to implicate such a pathomechanism, however, as scabies mites have a clear predilection for cool relatively poorly haired regions such as the pinnae. Another possibility was that the terminal cancer to which the cat succumbed, possibly abdominal lymphoma on the basis of probabilities, may have been associated with an underlying deficit in cell-mediated immunity which pre-dated overt signs of the malignancy. Likewise in case 2, the development of scabies may have been, in part, due to defective cell-mediated immunity referable to FIV infection, which may have predisposed also to the development of the patient's terminal malignancy. The extent and severity of lesions in case 3 could well have been a reflection of the effect of methylprednisolone on the host immune response, or the chronic (in excess of 4 months) nature of the infestation.
The diagnosis and management of these four cases was straightforward. The initial plan was to obtain deep skin scrapings under heavy sedation to obtain representative material from lesions. In cases 1 and 3, skin biopsies were harvested also during a single procedure, because skin scraping had not been performed prior to sedation or anaesthesia. This afforded the opportunity of observing the pathological response of the host to such a heavy mite infestation. Sedation afforded the opportunity of debulking the lesions of crusts and scurf in cases 1 and 3. Removal of crust and scale represents a cornerstone in the management of this condition, as such keratinous material harbours a huge number of mites. This, and subsequent application of an effective acaricide (selamectin or lime sulphur rinse), rendered cats non-infective to in-contact animals and humans.
Insufficient cases of feline scabies have been treated to make firm recommendations concerning therapy, however, based on information extrapolated from human (Orkin and Maibach 1993) and canine cases, systemic therapy with avermectin compounds is likely to be more convenient and at least as effective as topical therapy with lime sulphur or other insecticides (Walton et al 2000). Synthetic pyrethroids, which are widely used for topical therapy in man and dogs, are far too toxic to be useful in the feline patient (Volmer et al 1998). The safety and convenience of selamectin (Six et al 2000) (Revolution; Pfizer) which can be used ‘on label’ at the manufacturer's recommended dose for routine parasitic prophylaxis, afforded an advantage over other effective alternatives such as ivermectin (Yazwinski et al 1981), milbemycin and fipronil. Ivermectin was used in case 2 because selamectin had not been released in the UK when this case was treated, but it should be borne in mind that some cats (especially kittens) can show transient signs of neurological dysfunction after its use (Frischke and Hunt 1991), which is off label at this dose rate. Moxidectin would likely have been effective also, although we could find no precedence for use of this drug in this setting. The cat responded promptly to therapy, and ongoing prophylactic treatment with selamectin prevented further lesions developing despite continued exposure from an at risk geographical location.
When treating cats or dogs with S scabiei infestation, it may be prudent to treat all in-contact companion animals to guard against the possibility of contagion and ongoing infestations. Anecdotally, canine and feline scabies appears to be increasing in frequency in geographic regions where modern insect-specific parasiticides (eg, imidacloprid) that do not kill acarids are used for flea control. Perhaps the simplest way to prevent feline scabies due to N cati and S scabiei is to use ectoparasite preventative therapies that are effective against mites as well as fleas (eg, selamectin, fipronil).
References
- Andrews J.R.H. The origin and evolution of host associations of Sarcoptes scabiei and the subfamily Sarcoptinae Murray, Aracologica 24, 1983, 85–94. [PubMed] [Google Scholar]
- Anderson R.K. Norwegian scabies in a dog: a case report, Journal of the American Animal Hospital Association 17, 1981, 101–104. [Google Scholar]
- Arlian L.G., Morgan M.S., Estes S.A., Walton S.F., Kemp D.J., Currie B.J. Circulating IgE in patients with ordinary and crusted scabies, Journal of Medical Entomology 41 (1), 2004, 74–77. [DOI] [PubMed] [Google Scholar]
- Bornstein S., Gidlund K., Karlstam E., Bergstrom K., Zakrisson G., Nikkila T., Bergevall K., Renstrom L., Mattsson J.G. Sarcoptic mange epidemic in a cat population, Veterinary Dermatology 15 (Suppl. 1), 2004, 34, (FC-42) [Google Scholar]
- Bornstein S., Zakrisson G. Humoral antibody response to experimental Sarcoptes scabiei var vulpes infection in the dog, Veterinary Dermatology 4, 1993, 107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussieras J. A rare case of sarcoptic mange in a cat, Pratique Medicale and Chirurgicale de l'Animal de Compagnie 19, 1984, 375–377. [Google Scholar]
- Davies P.R. Sarcoptic mange and production of swine: a review of the literature and studies of associations between mite infestation, growth rate and measures of mange severity in growing pigs, Veterinary Parasitology 60, 1995, 249–264. [DOI] [PubMed] [Google Scholar]
- Frischke H., Hunt L. Suspected ivermectin toxicity in kittens, Canadian Veterinary Journal 32, 1991, 245. [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Feldman M.D. Human infestation with scabies of monkeys, Archives of Dermatology and Syphilology 59, 1949, 175–178. [DOI] [PubMed] [Google Scholar]
- Hadfield-Law L. Scratching the itch: management of scabies in A&E, Accident and Emergency Nursing 8, 2000, 230–232. [DOI] [PubMed] [Google Scholar]
- Hawkins J.A., McDonald R.K., Woody B.J. Sarcoptes scabiei infestation in cats, Journal of the American Veterinary Medical Association 190, 1987, 1572–1573. [PubMed] [Google Scholar]
- Heukelbach J., Walton S.F., Feldmeier H. Ectoparasitic infestations, Current Infectious Diseases Reports 7, 2005, 373–380. [DOI] [PubMed] [Google Scholar]
- Huang H.P., Liang S.L., Yang H.L., Chen K.Y. Sarcoptes scabiei infestation in a cat, Feline Practice 26 (2), 1998, 10–12. [Google Scholar]
- Kontos V., Sotiraki S., Himonas C. Two rare disorders in the cat: demodectic otitis externa and sarcoptic mange, Feline Practice 26, 1998, 18–20. [Google Scholar]
- Lindquist W.D., Cash W.C. Sarcoptic mange in a cat, Journal of the American Veterinary Medical Association 162, 1973, 639–640. [PubMed] [Google Scholar]
- Lower K.S., Medleau L.M., Hnilica K., Bigler B. Evaluation of an enzyme-linked immunosorbent assay (ELISA) for the serological diagnosis of sarcoptic mange in dogs, Veterinary Dermatology 12 (6), 2001, 315–320. [DOI] [PubMed] [Google Scholar]
- McCarthy J.S., Kemp J.S., Walton S.F., Currie B.J. Scabies: more than just an irritation, Postgraduate Medical Journal 80 (945), 2004, 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby K. The development of symptoms, parasitic infection and immunity in human scabies, Parasitology 35, 1944, 197–206. [Google Scholar]
- Morner T. Sarcoptic mange in Swedish wildlife, Reviews in Science and Technology 11, 1992, 1115–1121. [PubMed] [Google Scholar]
- Morris D.O., Dunstan R.W. A histomorphological study of sarcoptic acariasis in the dog: 19 cases, Journal of the American Animal Hospital Association 32, 1996, 119–124. [DOI] [PubMed] [Google Scholar]
- Morrison D.A., Ljunggren E.L., Mattson J.G. The origin of Sarcoptes scabiei in wombats, Parasitology Research 91, 2003, 497–499. [DOI] [PubMed] [Google Scholar]
- Muller G.H., Kirk R.W., Scott D.W. Canine scabies (Sarcoptic mange), Cutaneous Parasitology (4th edn) Small Animal Dermatology, 1989a, pp. 396–404 (Chapter 8).
- Muller G.H., Kirk R.W., Scott D.W. Feline scabies (Notoedric mange), Cutaneous Parasitology (4th edn) Small Animal Dermatology, 1989b, pp. 404–407 (Chapter 8)
- Orkin M., Maibach H.I. Scabies therapy-1993, Seminars in Dermatology 12, 1993, 22–25. [PubMed] [Google Scholar]
- Roberts L.J., Huffam S.E., Walton S.F., Currie B.J. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature, Journal of Infection 50, 2005, 375–381. [DOI] [PubMed] [Google Scholar]
- Robinson R. Fight the mite, and ditch the itch, Parasitology Today 1, 1985, 140–142. [DOI] [PubMed] [Google Scholar]
- Ryser-Degiorgis M.P., Ryser A., Bacciarini L.N., Angst C., Gottstein B., Janovsky M., Breitenmoster U. Notoedric and sarcoptic mange in free-ranging lynx from Switzerland, Journal of Wildlife Diseases 38, 2002, 228–232. [DOI] [PubMed] [Google Scholar]
- Schlesinger I., Oelrich D.M., Tyring S.K. Crusted (Norwegian) scabies in patients with AIDS: the range of clinical presentations, Southern Medical Journal 87, 1994, 352–356. [DOI] [PubMed] [Google Scholar]
- Scott D.W., Miller W.H., Griffin C.E. Muller & Kirk's Small Animal Dermatology, 6th edn, 2001, WB Saunders Co.: Philadelphia, pp. 476–483 [Google Scholar]
- Six R.H., Clemence R.G., Thomas C.A., Behan S., Boy M.G., Watson P., Benchaoui H.A., Clements P.J.M., Rowan T.G., Jernigan A.D. Efficacy and safety of selamectin against Sarcoptes scabiei on dogs and Otodectes cynotis on dogs and cats presented as veterinary patients, Veterinary Parasitology 91, 2000, 291–309. [DOI] [PubMed] [Google Scholar]
- Skerratt L.F. Clinical responses of captive common wombats (Vombatus ursinus) infected with Sarcoptes scabiei var wombati, Journal of Wildlife Diseases 39, 2003, 179–192. [DOI] [PubMed] [Google Scholar]
- Skerratt L.F. Sarcoptes scabiei: an important exotic pathogen of wombats, Microbiology Australia, June 2005, 79–81.
- Skerratt L.F., Martin R.W., Handasyde K.A. Sarcoptic mange in wombats, Australian Veterinary Journal 76, 1998, 408–410. [DOI] [PubMed] [Google Scholar]
- Skerratt L.F., Middleton D.J., Beveridge I. Distribution of the life cycle stages of Sarcoptes scabiei var wombati and effects of severe mange on common wombats in Victoria, Journal of Wildlife Diseases 35, 1999, 633–646. [DOI] [PubMed] [Google Scholar]
- Skerratt L.F., Campbell N.J.H., Murrel A., Walton S., Kemp D., Barker S.C. The mitochondrial 12S gene is a suitable marker for populations of Sarcoptes scabiei from wombats, dogs and humans in Australia, Parasitology Research 88, 2002, 376–379. [DOI] [PubMed] [Google Scholar]
- Sousa CA. (1999) Sarcoptes scabiei infestation in a cat. Challenging cases in dermatology and parasitology. A Pfizer symposium held in conjunction with the 24th Congress of the World Small Animal Veterinary Association. Lyon, France.
- Stone W.B., Roscoe D.E., Weber B.L. Spontaneous and experimental transfer of sarcoptic mange mites from red foxes to humans, New York Fish and Game Journal 23, 1976, 183–184. [Google Scholar]
- Stemmer B.L., Arlian L.G., Morgan M.S., Rapp C.M., Moore P.F. Characterization of antigen presenting cells and T-cells in progressing scabietic skin lesions, Veterinary Parasitology 67 (3–4), 1996, 247–258. [DOI] [PubMed] [Google Scholar]
- Volmer P.A., Kahn S.A., Knight M.W., Hansen S.R. Warning against the use of some permethrin products in cats, Journal of the American Veterinary Medical Association 213, 1998, 800. [PubMed] [Google Scholar]
- Walton S.F., Choy J.L., Bonson A., Valle A., McBroom J., Taplin D., Arlian L., Mathews J.D., Currie B., Kemp D.J. Genetically distinct dog-derived and human-derived Sarcoptes scabiei in scabies-endemic communities in northern Australia, American Journal of Tropical Medicine and Hygiene 61, 1999, 542–547. [DOI] [PubMed] [Google Scholar]
- Walton S.F., Currie B.J., Kemp D.J. A DNA fingerprinting system for the ectoparasite Sarcoptes scabiei, Molecular and Biochemical Parasitology 85, 1997, 187–196. [DOI] [PubMed] [Google Scholar]
- Walton S.F., Dougall A., Pizzutto S., Holt D., Taplin D., Arlian L.G., Morgan M., Currie B.J., Kemp D.J. Genetic epidemiology of Sarcoptes scabiei (Acari: Sarcoptidae) in northern Australia, International Journal of Parasitology 34, 2004, 839–849. [DOI] [PubMed] [Google Scholar]
- Walton S.F., Holt D.C., Currie B.J., Kemp D.J. Scabies: new future for a neglected disease, Advances in Parasitology 57, 2004, 309–376. [DOI] [PubMed] [Google Scholar]
- Walton S.F., McBroom J., Mathews J.D., Kemp D.J., Currie B.J. Crusted scabies: a molecular analysis of Sarcoptes scabiei variety hominis populations from patients with repeated infestations, Clinical Infectious Diseases 29, 1999, 1226–1230. [DOI] [PubMed] [Google Scholar]
- Walton S.F., Myerscough M.R., Currie B.J. Studies in vitro on the relative efficacy of current acaricides for Sarcoptes scabiei var hominis, Transactions of the Royal Society of Tropical Medicine and Hygiene 94, 2000, 92–96. [DOI] [PubMed] [Google Scholar]
- Yazwinski T.A., et al. Efficacy of ivermectin against Sarcoptes scabiei and Otodectes cynotis infestation in dogs, Veterinary Medicine/Small Animal Clinician 76, 1981, 1749–1751. [PubMed] [Google Scholar]
- Zahler M., Essig A., Gothe R., Rinder H. Molecular analyses suggest monospecificity of the genus Sarcoptes (Acari: Sarcoptidae), International Journal of Parasitology 29, 1999, 759–766. [DOI] [PubMed] [Google Scholar]