Abstract
Parenteral administration of Crandell Rees feline kidney (CRFK) cell lysates or feline herpesvirus 1, calicivirus, and panleukopenia virus-containing vaccines (FVRCP) grown on CRFK cells induces antibodies against CRFK cells. These antibodies also react with feline renal cell extracts. The purpose of this study was to determine whether interstitial nephritis would be detected in cats that were immunologically sensitized with CRFK lysates, boosted with CRFK lysates, and then biopsied 2 weeks after the booster. Cats (2 per group) were immunologically sensitized against CRFK lysates by administering 10 μg, 50 μg, or 50 μg plus alum 13 times (12 times in the first 50 weeks) over 2 years. Two cats were inoculated three times, 4 weeks apart with an FVRCP vaccine for intranasal administration as kittens, boosted 50 and 102 weeks later, and then renal biopsies taken 2 weeks after the last booster. Neither of the cats vaccinated with the FVRCP for intranasal administration had detectable renal inflammation. One cat in each of the three CRFK lysate sensitization groups had lymphocytic–plasmacytic interstitial nephritis.
Lymphocytic–plasmacytic interstitial nephritis is a common histological lesion in cats with renal failure (Lulich et al 1992, Minkus et al 1994). Detection of lymphocytes and plasma cells in renal tissues is not specific for any one disease; there are a number of known causes including diet and some infectious diseases (DiBartola et al 1993, Minkus et al 1994, Kordick et al 1999). Many client-owned cats are inoculated parenterally with feline herpesvirus 1, calicivirus, and panleukopenia virus-containing vaccines (FVRCP) multiple times in their lives. Because the vaccine viruses in most of the commercially available FVRCP vaccines are grown on the Crandell Rees feline kidney (CRFK) cell line (Crandell et al 1973, Scott et al 1970, Lee et al 1969), we previously performed a study to determine whether vaccinated cats or cats immunologically sensitized with CRFK cell lysates developed antibody responses to the lysates and whether the antibodies reacted with feline renal tissue lysates. We also evaluated whether the cats would develop clinical, biochemical, or urinalysis evidence of renal disease, and whether the cats would develop histological evidence of renal disease (Lappin et al 2005). Cats (n=14) were inoculated SQ multiple times (12 times over 50 weeks) with varying concentrations of CRFK lysate or administered a FVRCP vaccine three times, 4 weeks apart as kittens and then boosted at 50 weeks. Prior to CRFK sensitization or vaccination and at week 56 of the study, renal biopsies were obtained for histopathological evaluation (Lappin et al 2005). Antibodies against CRFK lysates were detected in all cats that were sensitized with CRFK lysates, in five of six cats administered a commercially available FVRCP vaccine parenterally, but in neither of the cats administered a commercially available FVRCP vaccine intranasally (Feline UltraNasal; FVRCP Vaccine, Heska Corporation, Fort Collins, CO). Antibodies against renal cell lysates were detected in all cats that were sensitized with CRFK lysates, in six of six cats administered a commercially available FVRCP vaccine parenterally, but in neither of the cats administered a commercially available FVRCP vaccine intranasally. However, clinical, urinalysis, or biochemical panel abnormalities were not noted in any cat and histological changes in kidneys could not be attributed to vaccination or CRFK sensitization (Lappin et al 2005).
In the first study, renal biopsies were collected 6 weeks after the last vaccination or CRFK sensitization (Lappin et al 2005). It is possible that inflammation of renal tissues occurred but was transient and resolved by the time of biopsy. In the study described herein, we hypothesized that interstitial nephritis would be detected in cats sensitized with CRFK lysates, boosted with CRFK lysates, and then biopsied 2 weeks after the booster. The two cats that had been administered the intranasal FVRCP vaccine and the six cats that had been administered CRFK lysates in the previous study had been housed in isolation and had not been vaccinated or inoculated with CRFK lysates for 1 year. At the beginning of the current study, 10 μg of CRFK cell lysate, 50 μg of CRFK cell lysate, or 50 μg of CRFK cell lysate mixed thoroughly with an equal volume of alum (1 mg) was administered SC to two cats per group, using the same groupings as the previous study. These dosages were chosen to approximate the amount of CRFK lysates estimated to contaminate commercially available FVRCP vaccines. The two cats that previously were vaccinated with the intranasal FVCRP vaccine were also boosted at that time. Two weeks after the boosters, a renal biopsy was obtained from each cat and prepared for light microscopic examination as previously described (Lappin et al 2005). The slides were evaluated by one pathologist (RB) from the previous study who was unaware of the groupings.
Cats administered the intranasal FVRCP vaccine had no evidence of renal inflammation (Table 1). Of the six cats that were sensitized with CRFK lysates, three had histopathological evidence of interstitial nephritis. For one cat administered 10 μg of CRFK lysate, the inflammatory infiltrate was graded as marked (Fig 1). The cats were within the reference interval on biochemical evaluation and urinalysis.
Table 1.
Histopathological findings of cats vaccinated with a FVRCP vaccine for intranasal administration (IN) or sensitized with CRFK lysates and biopsied 2 weeks after the last booster
| Cats | Histological descriptions |
|---|---|
| IN vaccine cat 1 | No significant microscopic lesions. |
| IN vaccine cat 2 | Mild mesangial thickening and hypercellularity. No significant interstitial inflammation or fibrosis is present. |
| CRFK 10 μg cat 1 | Glomeruli are shrunken with marked mesangial and Bowman's capsule fibrosis. Interstitial infiltrates of mixed cells (lymphocytes, plasma cells, fewer macrophages with an occasional neutrophil) are marked. Inflammatory cells replace extensive segments of tubules and glomeruli. Interstitial fibrosis is moderate. |
| CRFK 10 μg cat 2 | No significant microscopic lesions. |
| CRFK 50 μg cat 1 | Glomeruli are normal. Multifocal interstitial infiltrates of mixed mononuclear cells (lymphocytes and plasma cells) are mild and replace segments of tubules. No significant fibrosis is present. |
| CRFK 50 μg cat 2 | Mesangial thickening and hypercellularity is mild but no interstitial inflammation or fibrosis is seen. |
| CRFK 50 μg cat 1 (with alum) | Glomeruli are normal. Multifocal interstitial infiltrates of mixed mononuclear cells (lymphocytes and plasma cells) are mild and replace segments of tubules. There is no significant fibrosis. |
| CRFK 50 μg cat 1 (with alum) | There are no significant microscopic lesions. |
Fig 1.
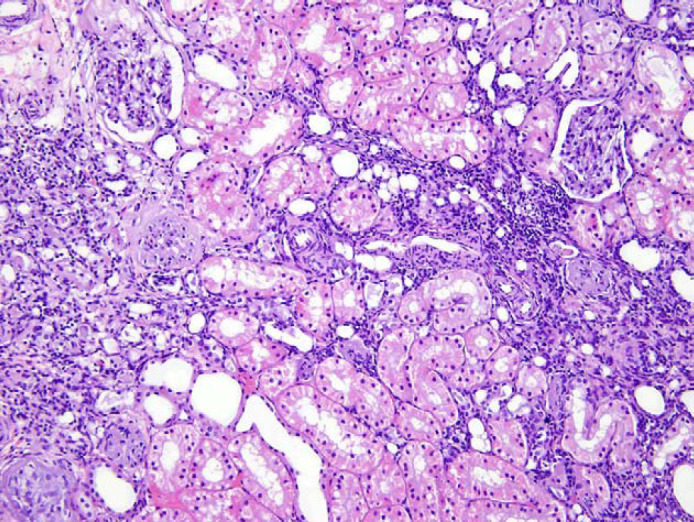
Renal tissues of the cat with marked interstitial nephritis described in Table 1. The cat had been sensitized with 13 inoculations of CRFK lysate over 2 years and biopsied 2 weeks after the last booster. Hematoxylin and eosin stain; 200× magnification.
When the kidney biopsies from these cats were assessed by the same pathologist (RB) 1 year previously, none had significant numbers of inflammatory cells detected (Lappin et al 2005). Thus, we believe the most likely explanation for the detection of mild to moderate interstitial nephritis in three of the cats in the current study was an immune-mediated reaction against the CRFK lysates administered 2 weeks prior to biopsy. If this assumption is true, our failure to see significant numbers of inflammatory cells in biopsies obtained 6 weeks post-booster may have related to timing of the biopsy (Lappin et al 2005). Alternately, the inflammation recognized in kidneys from the three cats described here may have occurred spontaneously or from another undetermined cause. However, as the last renal biopsy, the cats had been housed in isolation, were fed the same diet, and had not been administered any substance orally or by injection. Lastly, the potential for inflammation to occur post-CRFK administration may have increased over time.
In the previous study, cats that were sensitized with CRFK lysates and the majority of the cats vaccinated parenterally with FVRCP vaccines grown on the CRFK cell line developed antibodies against CRFK lysates (Lappin et al 2005). These findings were expected because parenteral inoculation of the lysates to immunocompetent cats would be expected to induce an immune response. The viruses used in the production of the FVRCP vaccine for intranasal administration are also grown on CRFK cells. However, while the viruses in the vaccine are alive, the CRFK cell line components that contaminate the vaccine are not. Thus, we believe the reason cats inoculated with this vaccine do not develop CRFK antibodies relates to immune exclusion of the CRFK cell line components by the mucosa or mucosal immune responses (Tizard 2004). This may also explain why interstitial nephritis was not found in the two cats vaccinated with the FVRCP vaccine for intranasal administration in the current study. However, this finding should be interpreted cautiously as only two cats were evaluated.
While a group of cats vaccinated parenterally with FVRCP vaccines contaminated with CRFK cell components was not used in this study, the concentration of CRFK cell component contamination of commercially available FVRCP vaccines is similar to the concentration of CRFK lysate used to sensitize the cats described herein. Thus, parenteral inoculation of cats with CRFK cell component-contaminated FVRCP vaccines may also induce transient renal inflammation. However, it is unknown whether this occurs with routine vaccination programs where the FVRCP vaccine is given every 1–3 years. The CRFK sensitized cats in this study were inoculated 13 times with CRFK proteins over the 2 years prior to documentation of renal inflammation. This aggressive sensitization schedule may also have influenced the results. The cats with detectable inflammation in this study came from each of the three sensitization groups and the most significant inflammatory response was detected in the cat administered the least amount of CRFK lysate (10 μg). Thus, the propensity to develop inflammation may relate to the individual cat, not just the amount of CRFK lysate or the presence of adjuvant. It is currently unknown whether CRFK antibodies induced by FVRCP vaccine administration or the potential for transient induction of renal inflammation by CRFK cell component inoculation is detrimental to cats. However, these findings should be considered when individualizing a vaccination protocol for each cat (Richards et al 2001).
Acknowledgment
This project was funded by a grant from Heska Corporation.
References
- Crandell R.A., Fabricant C.G., Nelson-Rees W.A. Development, characterization, and viral susceptibility of a feline (Felis catus) renal cell line (CRFK), In Vitro 9, 1973, 176–185. [DOI] [PubMed] [Google Scholar]
- DiBartola S.P., Buffington C.A., Chew D.J., McLoughlin M.A., Sparks R.A. Development of chronic renal disease in cats fed a commercial diet, Journal of the American Veterinary Medical Association 202, 1993, 744–751. [PubMed] [Google Scholar]
- Kordick D.L., Brown T.T., Shin K., Breitschwerdt E.B. Clinical and pathologic evaluation of chronic Bartonella henselae or Bartonella clarridgeiae infection in cats, Journal of Clinical Microbiology 37, 1999, 1536–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin M.R., Jensen W.A., Jensen T.D., Basaraba R.J., Brown C.A., Radecki S.V., Hawley J.R. Investigation of the induction of antibodies against Crandall Rees feline kidney cell lysates and feline renal cell lysates after parenteral administration of vaccines against feline viral rhinotracheitis, calicivirus, and panleukopenia in cats, American Journal of Veterinary Research 66, 2005, 506–511. [DOI] [PubMed] [Google Scholar]
- Lee K.M., Kniazeff A.J., Fabricant C.G., Gillespie J.H. Utilization of various cell culture systems for propagation of certain feline viruses and canine herpesvirus, Cornell Veterinarian 59, 1969, 539–547. [PubMed] [Google Scholar]
- Lulich J.P., O'Brien T.D., Osborne C.A., Polzin D.J. Feline renal failure: questions, answers, questions, Compendium on Continuing Education for the Practicing Veterinarian 14, 1992, 127–152. [Google Scholar]
- Minkus G., Reusch C., Horauf A., Breuer W., Darbes J., Kraft W., Hermanns W. Evaluation of renal biopsies in cats and dogs-histopathology in comparison with clinical data, Journal of Small Animal Practice 35, 1994, 465–472. [Google Scholar]
- Richards J., Rodan I., Elston T., Flemming D., Ford R., Henry S., Hustead D., Lappin M., Paul M., Rosen D., Scherk M., Scott F., Welborn L. Feline vaccine selection and administration, Compendium on Continuing Education for the Practicing Veterinarian 23, 2001, 71–80. [Google Scholar]
- Scott F.W., Csiza C.K., Gillespie J.H. Feline viruses. IV. Isolation and characterization of feline panleukopenia virus in tissue culture and comparison of cytopathogenicity with feline picornavirus, herpesvirus, and reovirus, Cornell Veterinarian 60, 1970, 165–183. [PubMed] [Google Scholar]
- Tizard I.R. Veterinary Immunology, 7th edn, 2004, Saunders: Philadelphia, 239–241. [Google Scholar]


