Abstract
The increased prevalence of obesity after neutering in cats is problematic in veterinary practice. Although many factors seem to be involved, the role of prolactin (PRL) and insulin-like growth factor-I (IGF-I), both implicated in adipose tissue development and glucose intolerance, should be considered. Seven male cats were castrated when 11 months old. Body weight was then recorded for 56 weeks and PRL, IGF-I and leptin assayed for 44 weeks. Body weight increased steadily but only significantly after 36 weeks. It stabilised after 44 weeks, and the cats then gained about 20% of their initial body weight. IGF-I increased rapidly and was significantly higher by week 3. PRL and leptin increased with initial peaks during the eighth and eleventh weeks, respectively. This study confirms that castration rapidly modifies the hormonal balance, partly explaining the body weight increase, and that hormonal changes precede this body weight increase. Hyperleptinaemia is apparently a consequence of excess weight.
The increased prevalence of obesity in cats is problematic in veterinary practice as obesity is associated with diabetes mellitus, hepatic lipidosis and many diseases of the urinary tract and skin. Neutering has been identified as one of the main risk factors. Allan et al (2000) studied the risk factors for obesity in an urban cat population in New Zealand. They found that 31% of the desexed cats were obese whereas none of the entire cats were overweight. Previous studies have demonstrated an increase in body weight after gonadectomy (Fettman et al 1997, Kanchuk et al 2003, Nguyen et al 2004). This could result from increased food intake, decreased metabolic rate or both. However, it was shown in neutered cats allowed free access to food (Martin et al 2001, Kanchuk et al 2003) that energy expenditure, on a lean body mass basis, did not decrease after gonadectomy. Nevertheless, food intake does increase rapidly after neutering, with neutered cats eating significantly more than entire ones (P<0.01) as early as 3 days after surgery (Kanchuk et al 2003).
However data, regarding the effect in cats of gonadectomy on release of the hormones implicated in gonadal function, are scarce. Testicular endocrine regulation in the male is complex and involves many neuroendocrine factors. Briefly, gonadal function is controlled by the pituitary gonadotrophins (LH and FSH) and prolactin (PRL). PRL stimulates testicular function in many species (Hair et al 2002) and a suppression of PRL secretion in man causes a decrease in testicular weight and spermatogenesis (Hair et al 2002). Evidence also suggests a strong implication of the somatotropic axis (Chandrashekar et al 2004). Growth hormone (GH) and insulin-like growth factor 1 (IGF-I) both stimulate gonadotropin release, GnRH secretion, gametogenesis and steroidic synthesis in the gonads. IGF-I seems to play a major role in reproductive system control (Adam et al 2000) and is a specific and strong stimulator of PRL release (Fruchtman et al 2002, Chandrashekar and Bartke 2003). Testicular steroidogenesis occurs in Leydig cells under the control of luteinising hormone (LH). The action of LH is also regulated by many other hormones such as PRL, GH and IGF-I (Huhtaniemi and Toppari 1995). Excess secretion of GH/IGF-I results in significant increase of LH receptors in the testis accompanied by enhanced plasma LH levels. On the other hand, the hyperprolactinaemia inhibits the pulsatile release of LH. In many species, especially rats and ferrets, neutering induces an increase in LH pulse frequency (Sisk and Desjardins 1986, Bakker and Baum 2000). Moreover, an increase of circulating PRL has been observed in castrated rats (Brar et al 1985) and hyperprolactinaemia significantly reduced the post-castration increase of LH (Park et al 1993, Grattan and Selmanoff 1994).
Thus in cats, brutal suppression of the testes may also have several effects on the secretion of circulating IGF-I and PRL. Moreover, IGF-I and PRL are strongly implicated in adipose tissue development and glucose homeostasis (Reis et al 1997, Kopelman 2000, Frystyk 2004). It has also been reported that appetite should be increased by PRL through mechanisms independent of gonadal hormones but probably by a direct stimulation of the brain (Sauve and Woodside 2000). It is thus apparent that chronic hyperprolactinaemia increases body weight through an effect on food intake. In addition, there are interactions between PRL and leptin secretion with leptin increasing PRL secretion but, conversely, PRL stimulating leptin secretion by white adipocytes in rats, but through an indirect mechanism (Casanueva and Dieguez 1999).
The purpose of our study was to identify the modifications occurring in circulating PRL and IGF-I after neutering. We hypothesised that gonadectomy in cats would bring about an increase of PRL and IGF-I that preceded the body weight modifications and could be involved in the increased food consumption and that an increase in circulating leptin would result from this.
Material and Methods
Animals
Seven post-pubertal male cats were castrated at the age of 11 months. Their body condition score was optimal (BCS=3) before gonadectomy. The cats were housed together and food and water were freely available before and after gonadectomy. Room lighting consisted of 12 h light and dark periods and the inside temperature ranged from 18°C to 21°C. The cats were able to go outside. Husbandry and use of the cats were in accordance with the international rules for experimental animals.
Methods
Blood samples for the determination of plasma hormones were collected by jugular venepuncture into heparin plasma tubes from all cats, weekly for 10 weeks after castration then twice a month until 20 weeks and monthly until 44 weeks and, centrifuged within 1 h. Plasma aliquots were frozen immediately at −20°C in plastic tubes until assayed.
Commercially available kits were used. All assays were previously validated for use in cats in our laboratory and the assay procedures were performed according to the manufacturer's instructions.
For the determination of total testosterone (TT) in cat plasma, the RIA kit used in humans (Spectria testosterone RIA, from Orion Diagnostica, Espoo, Finland) had to be modified. Differences in the sex-hormone-binding-globulins (SHBG) bound to testosterone in blood were encountered which interacted with the assay procedure. Satisfactory results were obtained by including an additional extraction step to dissociate the testosterone from SHBG before the TT assay. The testosterone was extracted from the plasma with an organic solvent composed of hexane and ethyl acetate.
IGF-I is highly conserved across species and presents a high degree of cross-reactivity especially with anti-human IGF-I antibodies. It was assayed with the human IGF-I RIA kit (Nichols Institute Diagnostics, California, USA) (Starkey et al 2004). Leptin was assayed with a RIA kit previously validated for cats according to the literature (Multi-species Leptin RIA Kit, Linco Research, Inc., USA) (Appleton et al 2002).
Analysis of the PRL sequence has shown significant similarities between species. For example, feline and bovine PRLs showed 86% similarity (Warren et al 1996) whereas cross-reactivity was observed between canine and bovine hormones (Knight et al 1977). A highly specific solid phase enzyme immunometric assay (EIA) from Milenia Biotec (Bad Nauheim, Germany) was, therefore, tested and validated for cats. Briefly, after incubation, a sandwich complex consisting of two monoclonal antibodies specific for canine PRL and of feline PRL is formed and the non-reactive sample components are removed during two washing steps. The mean PRL level in normal males and anoestrous females is <6 ng/mL and the lower detection limit for this test is 0.4 ng/mL.
Hormones were measured weekly for 10 weeks after castration then twice a month until 20 weeks and monthly until 44 weeks. Body weight was recorded weekly for 56 weeks.
Statistical analysis was performed with statistical software (Statview 4.1, SAS Abacus Concept, Inc., Berkeley, California, USA). All continuous data were expressed as mean±SEM. A repeated-measure analysis of variance (ANOVA) was used to examine the effect of time on the measured parameters (continuous). The Scheffe post-hoc test was applied to examine the differences between means as these latter were not normally distributed. Values of P<0.05 were considered significant in all the analyses.
Results
Body weight varied significantly during the study (P<0.0001). The mean initial body weight was 3.8±0.2 kg. Body weight decreased slightly during the first 3 weeks (approximately 200 g) then increased progressively and continuously for about 40 weeks (Fig 1). After 40 weeks, it seemed to plateau (4.5 kg) (SD±0.3) and the cats gained about +20% of their initial body weight and were overweight. Nevertheless, from a statistical point of view, the body weight was only significantly increased by the 36th week after castration (P=0.0072).
Fig 1.
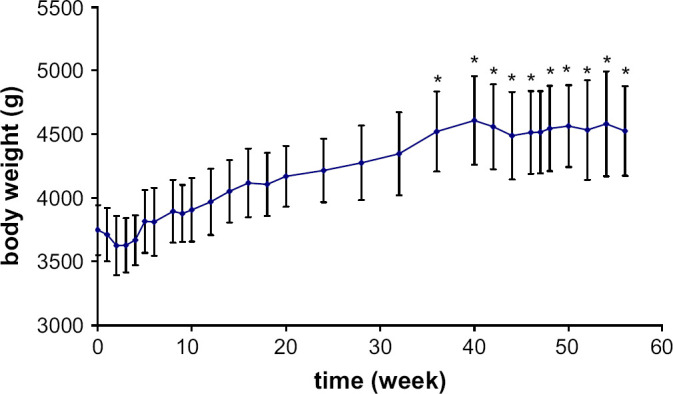
Mean body weight variations (±SE) following castration in seven male cats. Significantly (*) different from baseline concentration.
The testosterone concentration before gonadectomy was 16.6±3.9 nmol/L. The basal plasma concentration in intact male cats (11 months old), ranged from 8.3 to 31.3 nmol/L. One week after gonadectomy, the testosterone concentration decreased significantly to 2.7±0.5 nmol/L (P<0.0001) and ranged from 1.4 to 4.4 nmol/L (Fig 2).
Fig 2.
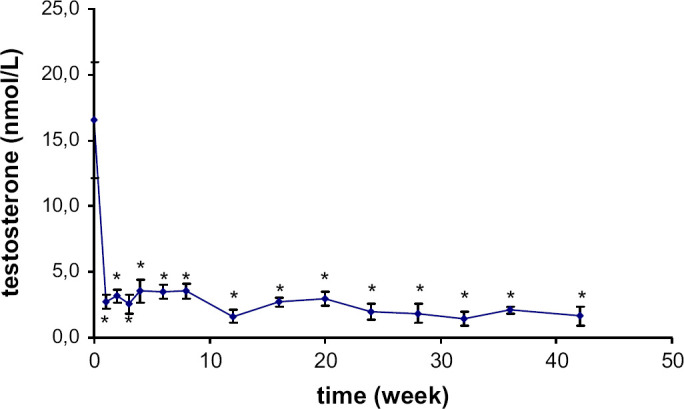
Mean plasma testosterone concentration variations (±SE) following castration in seven male cats. Significantly (*) different from baseline concentration.
The variations in IGF-I concentration were significant throughout the study (P=0.0084) (Fig 3). IGF-I increased rapidly after castration and was significantly higher as early as the second week (P=0.003). The mean basal plasma concentration was 271±35 ng/L and after 2 weeks, the concentration rose to 410±40 ng/L. The final concentration was significantly higher than the initial one (372±93 ng/l; P=0.008).
Fig 3.
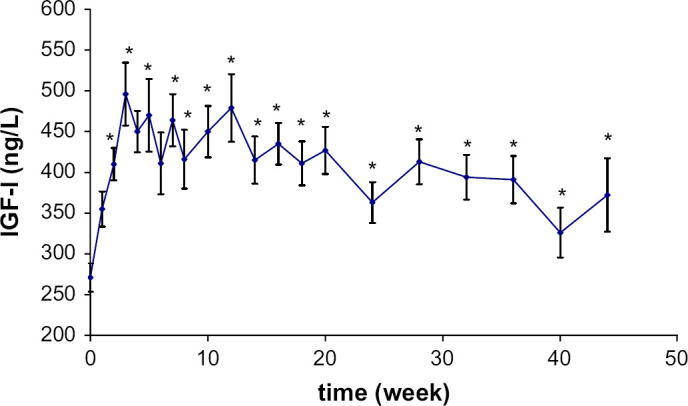
Mean plasma IGF-I concentration variations (±SE) following castration in seven male cats. Significantly (*) different from baseline concentration.
Prolactinaemia varied significantly during the study (P=0.0001) (Fig 4). PRL increased regularly after castration from 18±5 (range 5–41 ng/mL) to 91±9 ng/mL with an initial peak measured during the seventh week (P=0.027) and persistent hyperprolactinaemia after the twelfth week (P=0.0009).
Fig 4.
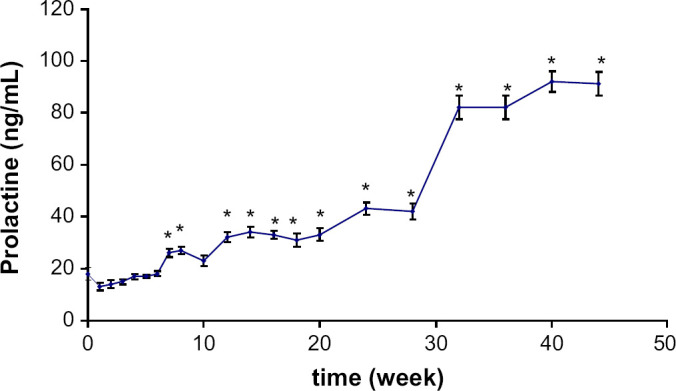
Mean plasma PRL concentration variations (±SE) following castration in seven male cats. Significantly (*) different from baseline concentration.
Leptin also increased significantly after castration from 1.1±0.6 to 5.8±0.6 ng/mL (P=0.0001) but hyperleptinaemia was only significant 11 weeks after castration (P=0.0042) (Fig 5).
Fig 5.
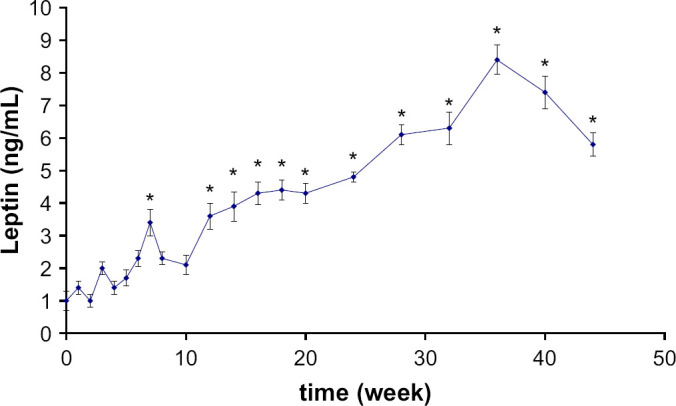
Mean plasma leptin concentration variations (±SE) following castration in seven male cats. Significantly (*) different from baseline concentration.
Leptinaemia and prolactinaemia were closely correlated to body weight increase (r2=0.86 and P<0.001 and r2=0.82 and P<0.001, respectively).
All these abnormalities were still apparent 2 years after castration. The mean IGF-I concentration was 384±56 ng/L, all cats still exhibited hyperprolactinaemia. Leptinaemia was still high: 10.5±2.6 ng/mL.
Discussion
The withdrawal of androgens by orchiectomy seems to have many consequences in healthy post-pubertal cats. This study confirms that certain hormonal changes precede the body weight increase and that these modifications concern the hormonal factors involved in adipose tissue growth and glucose homeostasis.
After gonadectomy, the body weight increased as previously reported in cats allowed free access to food (Martin et al 2001, Kanchuk et al 2003). Nevertheless, in this study, the body weight was significantly increased only 36 weeks after castration.
The testosterone concentrations before gonadectomy were in accordance with previous data (Tarttelin et al 1998) and decreased rapidly as early as the first week after orchiectomy, as previously demonstrated by Johnstone et al (1984). This brutal decrease possibly induced a physiological increase in LH as in other species (Park et al 1993, Grattan and Selmanoff 1994). Interestingly, it has been suggested that in ferrets, another induced- or reflex-ovulating species, testosterone regulates LH release because castration provokes a rapid increase in the frequency of LH pulses (Sisk and Desjardins 1986). It could be supposed that a similar mechanism is activated after neutering in cats.
Fascinatingly, the first observed hormonal change concerned IGF-I release which to our knowledge has never been evidenced in other species. Circulating IGF-I started to rise as early as the first week after castration and was significantly increased by 2 weeks post-castration. It was assumed that the increased secretion of IGF-I would be a consequence of the decrease in circulating testosterone. As yet, the mechanism behind this increase can only be hypothesised. If neutering in the cat induces increased LH secretion, this latter could also stimulate an increased concentration of IGF-I (Huhtaniemi and Toppari 1995). The rise in IGF-I could also be a response to an increase in body weight characterised by an enlargement in body fat. Indeed, although studies into the regulation of the somatotropic axis in obesity report contradictory results as to the secretion of IGF-I, receptors have been identified in pre-adipocyte and adipocyte cells lines (Louveau and Gondret 2004). Thus the increase in IGF-I secretion that follows neutering may have a primary role in the onset of obesity in the cat, since it promotes the multiplication and even enlargement of the adipocytes.
Although the mean prolactinaemia was elevated at the start of the study, the standard deviation between cats was considerable. Significant changes in PRL secretion occur with stress (Balcombe et al 2004) and handling and blood collection are manipulations that lead to stress-related responses. During the first week of blood collection, 5/7 cats showed elevated prolactinaemia (>6 ng/mL). Nevertheless, prolactinaemia increased significantly after castration. This observed rise following castration in cats was also shown in rats. However, the initial peak was apparent 8 weeks after castration and was not the first hormonal modification observed. Hyperprolactinaemia could also have a deleterious effect on glucose metabolism in the cat in both the short and long terms because of the role of PRL in the production and maintenance of adipose tissue (Flint et al 2003). PRL can also stimulate appetite (Bray 2000), promoting glucose intolerance, insulin resistance and hyperinsulinaemia (Reis et al 1997, Kopelman 2000).
In this study, hyperleptinaemia was the most belated hormonal modification observed and seemed only to be a consequence of the excess weight. It is probably only a reflection of the increased fat mass in castrated cats, as previously demonstrated (Martin et al 2001). At term, such hyperleptinaemia could have deleterious consequences for the cat. Indeed, Appleton et al (2002) demonstrated that leptin concentrations in the cat are correlated with the intensity of insulin resistance and involved in the pathogenesis of insulin resistance. Hyperleptinaemia could, therefore, be another key element controlling the onset of diabetes mellitus in the castrated cat.
Conclusion
Castration in male cats rapidly modifies the hormonal balance which could partly explain the body weight increase and the changes in food intake. Neutering, by modifying endocrine homeostasis, induces a new state of equilibrium in which the hormones involved in obesity and the dysregulation of glucose metabolism predominate. This situation explains the clinical consequences observed in the majority of castrated cats that are taken to the vet. The subsequent deleterious effects can only be limited by preventing obesity after neutering by reducing food allowance and monitoring body weight.
References
- Adam C.L., Gadd T.S., Findlay P.A., Wathes D.C. IGF-I stimulation of luteinizing hormone secretion, IGF-binding proteins (IGFBPs) and expression of mRNAs for IGFs, IGF receptors and IGFBPs in the ovine pituitary gland, Journal of Endocrinology 166, 2000, 247–254. [DOI] [PubMed] [Google Scholar]
- Allan F.J., Pfeiffer D.U., Jones B.R., Esslemont D.H., Wiseman M.S. Related A cross-sectional study of risk factors for obesity in cats in New Zealand, Preventive Veterinary Medicine 46, 2000, 183–196. [DOI] [PubMed] [Google Scholar]
- Appleton D.J., Rand J.S., Sunvold G.D. Plasma leptin concentrations are independently associated with insulin sensitivity in lean and overweight cats, Journal of Feline Medicine and Surgery 4, 2002, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J., Baum M.J. Effect of gonadal steroids on pituitary LH secretion and mediobasal hypothalamic GnRH mRNA in ferrets, Journal of Reproduction and Fertility 119, 2000, 315–321. [DOI] [PubMed] [Google Scholar]
- Balcombe J.P., Barnard N.D., Sandusky C. Laboratory routines cause animal stress, Contemporary Topics in Laboratory Animal Science 43, 2004, 42–51. [PubMed] [Google Scholar]
- Brar A.K., McNeilly A.S., Fink G. Effects of hyperprolactinaemia and testosterone on the release of LH-releasing hormone and the gonadotrophins in intact and castrated rats, Journal of Endocrinology 104, 1985, 35–43. [DOI] [PubMed] [Google Scholar]
- Bray G.A. Afferent signals regulating food intake, Proceedings of the Nutrition Society 59, 2000, 373–384. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V., Bartke A. The role of insulin-like growth factor-I in neuroendocrine function and the consequent effects on sexual maturation, inferences from animal models, Reproductive Biology 3, 2003, 7–28. [PubMed] [Google Scholar]
- Casanueva F.F., Dieguez C. Neuroendocrine regulation and actions of leptin, Frontiers in Neuroendocrinology 20, 1999, 317–363. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V., Zaczek D., Bartke A. The consequences of altered somatotropic system on reproduction, Biology of Reproduction 71, 2004, 17–27. [DOI] [PubMed] [Google Scholar]
- Fettman M.J., Stanton C.A., Banks L.L., Hamar D.W., Johnson D.E., Hegstad R.L., Johnston S. Effects of neutering on bodyweight, metabolic rate and glucose tolerance of domestic cats, Research in Veterinary Science 62, 1997, 131–136. [DOI] [PubMed] [Google Scholar]
- Flint D.J., Binart N., Kopchick J., Kelly P. Effects of growth hormone and prolactin on adipose tissue development and function, Pituitary 6, 2003, 97–102. [DOI] [PubMed] [Google Scholar]
- Fruchtman S., McVey D.C., Borski R.J. Characterization of pituitary IGF-I receptors, modulation of prolactin and growth hormone, American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 283, 2002, R468–R476. [DOI] [PubMed] [Google Scholar]
- Frystyk J. Free insulin-like growth factors – measurements and relationships to growth hormone secretion and glucose homeostasis, Growth Hormone and IGF Research 14, 2004, 337–375. [DOI] [PubMed] [Google Scholar]
- Grattan D.R., Selmanoff M. Prolactin- and testosterone-induced inhibition of LH secretion after orchidectomy, role of preoptic and tuberoinfundibular gamma-aminobutyric acidergic neurones, Journal of Endocrinology 143, 1994, 165–174. [DOI] [PubMed] [Google Scholar]
- Hair W.M., Gubbay O., Jabbour H.N., Lincoln G.A. Prolactin receptor expression in human testis and accessory tissues, localization and function, Molecular Human Reproduction 8, 2002, 606–611. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I., Toppari J. Endocrine, paracrine and autocrine regulation of testicular steroidogenesis, Advances in Experimental Medicine and Biology 377, 1995, 33–54. [DOI] [PubMed] [Google Scholar]
- Johnstone I.P., Bancroft B.J., McFarlane J.R. Testosterone and androstenedione profiles in the blood of domestic tom-cats, Animal Reproduction Science 7, 1984, 363–375. [Google Scholar]
- Kanchuk M.L., Backus R.C., Calvert C.C., Morris J.G., Rogers Q.R. Weight gain in gonadectomized normal and lipoprotein lipase-deficient male domestic cats results from increased food intake and not decreased energy expenditure, Journal of Nutrition 133, 2003, 1866–1874. [DOI] [PubMed] [Google Scholar]
- Knight P.J., Hamilton J.M., Scanes C.G. Homologous radioimmunoassay for canine prolactin, Acta Endocrinology 85, 1977, 736–743. [DOI] [PubMed] [Google Scholar]
- Kopelman P.G. Physiopathology of prolactin secretion in obesity, International Journal of Obesity and Related Metabolism Disorders 24 (Suppl 2), 2000, S104–S108. [DOI] [PubMed] [Google Scholar]
- Louveau I., Gondret F. Regulation of development and metabolism of adipose tissue by growth hormone and the insulin-like growth factor system, Domestic Animals Endocrinology 27, 2004, 241–255. [DOI] [PubMed] [Google Scholar]
- Martin L., Siliart B., Dumon H., Backus R., Biourge V., Nguyen P. Leptin, body fat content and energy expenditure in intact and gonadectomized adult cats, a preliminary study, Journal of Animal Physiology and Animal Nutrition 85, 2001, 195–199. [DOI] [PubMed] [Google Scholar]
- Nguyen P.G., Dumon H.J., Siliart B.S., Martin L.J., Sergheraert R., Biourge V.C. Effects of dietary fat and energy on body weight and composition after gonadectomy in cats, American Journal of Veterinary Research 65, 2004, 1708–1713. [DOI] [PubMed] [Google Scholar]
- Park S.K., Grattan D.R., Selmanoff M. Differential effects of adrenalectomy on the prolactin-induced suppression of LH and FSH secretion after castration in male rats, Journal of Reproduction and Fertility 99, 1993, 209–217. [DOI] [PubMed] [Google Scholar]
- Reis F.M., Reis A.M., Coimbra C.C. Effects of hyperprolactinaemia on glucose tolerance and insulin release in male and female rats, Journal of Endocrinology 153, 1997, 423–428. [DOI] [PubMed] [Google Scholar]
- Starkey S.R., Tan K., Church D.B. Investigation of serum IGF-I levels amongst diabetic and non-diabetic cats, Journal of Feline Medicine and Surgery 6, 2004, 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve D., Woodside B. Neuroanatomical specificity of prolactin-induced hyperphagia in virgin female rats, Brain Research 868, 2000, 306–314. [DOI] [PubMed] [Google Scholar]
- Sisk C.L., Desjardins C. Pulsatile release of luteinizing hormone and testosterone in male ferrets, Endocrinology 119, 1986, 1195–1203. [DOI] [PubMed] [Google Scholar]
- Tarttelin M.F., Hendriks W.H., Moughan P.J. Relationship between plasma testosterone and urinary felinine in the growing kitten, Physiology and Behaviour 65, 1998, 83–87. [DOI] [PubMed] [Google Scholar]
- Warren W.C., Bentle K.A., Bogosian G. Cloning of the cDNAs coding for cat growth hormone and prolactin, Gene 168, 1996, 247–249. [DOI] [PubMed] [Google Scholar]


