Abstract
Upper respiratory tract infection (URI) propagates readily within cats in shelters and often results in euthanasia of affected cats. In a case-control evaluation of 573 cats in eight shelters in California in 2001 and 2002, the prevalence of feline calicivirus (FCV) was from 13 to 36%, feline herpesvirus (FHV) was from 3 to 38%, and prevalence of Bordetella bronchiseptica, Chlamydophila felis, and Mycoplasma species was from 2 to 14%. Cats with URI tended to be housed in isolation, dehydrated, and younger than cats without URI, and infected with FHV, Mycoplasma species, FCV, or C felis. Shelters differed in the prevalence of pathogens and many cats appeared positive for infection after about 1 week of sheltering. It is helpful for shelters to understand the risk factors associated with URI in order to evaluate the costs and benefits of treatment and improve their procedures to decrease the incidence of URI within their facilities. Antiherpetics and antimycoplasmal drugs may be beneficial for individual animal care. Results document the utility of comprehensive URI surveillance and herd management for specific pathogens typical in that shelter.
Upper respiratory infection (URI) in an individual cat is common and often manageable. However URI propagates readily within shelters (Fisk and Soave 1973, Snyder et al 1973, Pedersen 1988, August 1990, Gaskell and Dawson 1994, McArdle et al 1994) and is often considered a ‘fatal disease’ because of risks to other shelter cats, difficulties managing affected cats, and the likelihood that affected cats will not be adopted. Cats harboring latent or pre-clinical URI often require veterinary care within days of adoption, incurring cost and frustration by adopters and further eroding public confidence in the shelter, in turn decreasing adoption. Upper respiratory infection in cats is the second leading cause of euthanasia in shelters after over-crowding (Foley and Bannasch 2004). Lack of data has impeded progress in managing URI and represents an important research objective. Shelters manage URI with isolation, vaccination, and antibacterial or antiherpetic treatment. Better understanding of risk factors associated with URI would assist cost–benefit evaluation of treatment and decrease the incidence of URI. Shelter personnel might also be better able to prevent URI in an individual animal and mitigate spread of disease to the rest of the herd.
In previous studies, at least five pathogens have been implicated as causative agents of feline URI: feline calicivirus (FCV), feline herpesvirus type 1 (FHV), Mycoplasma species, Chlamydophila felis and Bordetella bronchiseptica (Harbour et al 1991, Coutts et al 1996, Foster et al 1998, Binns et al 2000, Cai et al 2002, Foley et al 2002). This present study was conducted to identify the roles of these pathogens individually and in combination in URI and to identify associated risk factors for these infections in sheltered cat populations.
Materials and methods
Cat populations
Samples were collected from eight shelters over one year. Shelters were classified as ‘traditional’ or ‘no kill’. In traditional shelters, animals were brought to the shelter by animal control officers or relinquished by the public. Animals were held at least 6 days as mandated by California law and euthanasia was performed because of illness or injury, behavioral unsuitability for adoption, or insufficient available space. At ‘no kill’ shelters, animals were accepted or denied admission from the public or rescue organization based on criteria established by the shelter. Cats in ‘no kill’ shelters were not euthanased because of space.
Shelters A, B, D and H are traditional with no quarantine for incoming cats but with isolation for cats with URI in separate rooms within the main shelter. Shelters C, E and F are ‘no kill’ shelters with 5–7 day quarantine for incoming cats (although new cats were continuously introduced into quarantine), and a separate isolation room for cats with URI. Shelter G is a large traditional shelter with URI isolation in a separate annex building. Feral cats were held in individual feral cat cages, in separate rooms from all other cats with low public and staff traffic. None of the feral cats were vaccinated. Most were evaluated after 3 days in the shelter; the vast majority was deemed behaviorally unsuitable for adoption and humanely euthanased. All shelters, except for shelter G, used high passage modified live vaccines (MLV); A and B used Eclipse® 3 (Schering-Plough, Kenilworth, NJ), shelters C, D, E, and H used Fel-O-Guard (Fort Dodge Overland Park, KS) and shelter F used Protex-3 (Intervet, Boxmeer, The Netherlands). Shelter G used Felo-O-Guard for at least 3 months before two sampling events and a killed vaccine (Fel-O-Vax PCT, Fort Dodge, Overland Park, KS) for 3 months before one sampling event. Additional information about the shelters is given in Table 1.
Table 1.
Management characteristics of shelters participating in a URI epidemiological study
| Shelter | Shelter type | Mean daily census | Total cat population (2001) | Housing method | Cats and dogs separated | Air exchanges | Quarantine on entry | URI cats isolated from general population |
|---|---|---|---|---|---|---|---|---|
| A | Traditional | 125 | 10,064 | Individual a | No | Natural large bay door | No | Yes c |
| B | Traditional | 75 | 5403 | Individual a | Yes | Natural and mechanical 8–10 ex/hour | No | Yes c |
| C | No kill | 55 | 600 | Individual a , some group housing | Yes | Mechanical 10–12 ex/hour | Yes b | Yes c |
| D | Traditional | 120 | 1829 | Individual a | Yes | Mechanical 10–12 ex/hour | No | No |
| E | No kill | 50 | 376 | Individual a , some group housing | Yes | Mechanical 10–12 ex/hour | Yes b | Yes |
| F | No kill | 55 | 611 | Individual a , some group housing | Yes | Mechanical 10–12 ex/hour | Yes b | Yes |
| G | Traditional | 175 | 14,430 | Individual a | Yes | Mechanical <8 ex/hour | No | Yes c |
| H | Traditional | 150 | 10,594 | Individual a | Yes | Mechanical <8 ex/hour | No | Yes c |
Cats housed in stainless steel cages.
Cats are housed separately for 5–7 days prior to their introduction into the general population.
Occasional delay in identifying and moving URI cats into isolation.
Case definition and clinical descriptions
All cats were examined by a veterinarian or a registered veterinary technician at the time of sampling. Body condition was scored from 1 to 9 (Laflamme et al 1994), and hydration was assessed by skin turgor, mucous membrane moisture and ocular globe position. For eyes, notation was made of conjunctivitis, anterior uveitis or keratitis and serous or purulent discharge. Nasal discharge was characterized as serous or purulent and sneezing was noted if observed. Gingivitis, calculus and plaque, oral vesicles and faucitis were recorded. Scores for lesions as 0 (lesions not present), 1 (mild lesions), or 2 (moderate to severe lesions) were assigned. Case definition and severity were as given in Table 2.
Table 2.
Case definition and severity score for upper respiratory tract infection (URI) in cats in animal shelters
| 0 (No URI) | 1 (Mild URI) | 2 (Moderate to severe URI) |
|---|---|---|
| No clinical signs present. | Mild clinical signs (score of 1) in more than one site (eyes nose, oropharynx). | Mild purulent ocular or nasal discharge and a score ≥1 in one additional site. |
| or | or | or |
| Mild serous ocular or nasal discharge and no other signs. | Mild purulent ocular or nasal discharge and no additional signs. | Severe clinical signs (score of 2) in one site in addition to purulent ocular or nasal discharge. |
| or | or | or |
| Mild clinical signs present in only one site (eye, nose, mouth). | Severe clinical signs present in only one site. | Severe clinical signs in two or more sites. Mild clinical signs (score of 1) in one or more sites and actively sneezing during examination. |
The length of time the cat had been in the shelter, sex, and age were noted from shelter records; age was also estimated based on body size and dentition using age categories: 0–3 months, 4–6 months, 7–11 months, 12–72 months, 72–96 months, 96+ months. If data were not available, this cat was excluded from only the specific part of the analysis for which data were missing.
Sample collection
At each collection event, shelter personnel identified all cats with signs of URI (cases). After case numbers were determined, an equal number of controls were selected randomly from the general cat population. Unfortunately, after evaluating many of the ‘case’ and ‘control’ cats, it was determined that some ‘cases’ did not in fact meet the definition for URI cases, while some ‘controls’ had clinical URI; thus analysis was performed only after cats were appropriately assigned to the case group. Cats were placed on a stainless steel treatment table for sampling; the table was disinfected after each cat with 1:32 bleach. Conjunctival samples were obtained by reflecting the eyelid and rolling two Dacron swabs across the conjunctiva. One swab was placed into 250 μl Hanks balanced salt solution (HBSS) for culture of FCV and FHV, and the other was placed in 250 μl of 0.9% NaCl for polymerase chain reaction (PCR) for Mycoplasma species and C felis. An oropharyngeal sample was collected by Dacron swab in the fauces; this swab was also placed in HBSS for viral culture. The last sample was a BBL CultureSwab Plus (Becton-Dickinson, Franklin Lakes, NJ) placed deeply into the cat's oropharynx for isolation of B bronchiseptica. Samples were kept cool, brought immediately to the laboratory, and processed within 8 h, except for samples in saline, which were kept at −20 °C until extraction for PCR.
Bacterial and viral culture
Swabs for bacterial culture were streaked on to MacConkey agar plates and incubated at 37 °C overnight in room air. Isolates were identified as B bronchiseptica based on growth on MacConkeys agar with no utilization of lactose, growth on triple-sugar–iron medium with alkaline slant/alkaline butt, no gas, and no H2S, presence of oxidase, lack of indole, motility, hydrolysis of urea, and lack of fermentation or oxidation of glucose (Jang et al 2000).
Viral swabs were cultured in Crandell feline kidney (CrFK) cells at 37 °C with 5% CO2 and moisture in a 1:1 ratio of Leibovitz's L15 medium and minimal essential medium, with Earle's salts, L-glutamine, 10% fetal bovine serum, 100 u/ml penicillin, and 100 μg/ml streptomycin. Identification of FCV and FHV was made on the basis of characteristic cytopathic effect. DNA was extracted from swabs using a kit (Qiagen, Valencia, CA) and PCR was performed for C felis (Sykes et al 1999b) and Mycoplasma species (van Kuppeveld et al 1994) in a thermal cycler (MJ Research, Waltham, MA). Products were visualized in 1.5% agarose gels stained with ethidium bromide.
RNA was selected from 27 calicivirus-positive samples for sequencing. RNA was extracted from tissue culture fluid and reverse transcriptase-nested PCR was performed to amplify a 235 bp region of the capsid gene (Radford et al 1998). Amplicons were sequenced by big dye terminator cycle DNA sequencing (ABI Prism, Foster City, CA) using PCR primers after purification with a kit (Microcon, Millipore, Bedford, MA). Electropherograms were analyzed with Chromas (Technelysium, Helensvale, Australia) and sequences were evaluated using the BLAST algorithm from the National Center for Biotechnology Information and SeqWeb 2.1 (Accelrys, San Diego, CA).
Experimental design
An unmatched case control study design was used. Data were maintained in Excel 2002 (Microsoft, Redmond, WA) and analyzed in ‘R’ (The R-Development Core Team, http://www.r-project.org). Prevalence was calculated as the number of confirmed positive cats identified at the sampling visit divided by all cats observed at the time of sampling, which was possible because all cats with URI were counted in each shelter. Because faucitis, ocular and nasal discharge, sneezing, gingivitis and other classical signs of URI were part of the case definition, statistical testing to assess the association of URI with clinical variables was only performed for dehydration and body condition score using χ 2. Univariate evaluation of possible risk factors was performed by calculating odds ratios and confidence intervals (function ‘odds’ in ‘R’). To perform the logistic regression, age, location in shelter, and pathogen risk factors were included because they were statistically significantly associated with URI by univariate analysis. Age was dichotomized into two categories: older or younger than 12 months. Logistic regression was performed stepwise in both directions with the significant univariate risk factors plus FCV and B bronchiseptica (the latter two were included despite not being associated with URI on univariate analysis because of a possibility of synergism with other pathogens to increase URI clinical disease severity). The optimal model was chosen to minimize the Akaike Information Criterion (AIC). For all statistical tests P=0.05.
Results
Of 573 cats evaluated at eight animal shelters, 259 (45.2%) had a URI score of zero and 314 (54.8%) had mild to severe clinical URI (URI score of 1 or 2) (Table 3). Cats with URI were more dehydrated than cats without URI (P<0.001), but body condition scores were not significantly associated with URI (P=0.35). Among feral cats (housed separately), 48% had URI while 52% did not. Of 174 cats in isolation, 72% had URI, while 28% did not at the time of observation. However, 46% of cats in the general adoptable population did have URI.
Table 3.
Number of cats with various clinical manifestations and percentage (in parenthesis) of cats with the clinical sign and their associated URI status
| No URI | Mild URI | Severe URI | All URI | Total | |
|---|---|---|---|---|---|
| Gingivitis | 79 (30.5) | 59 (33.5) | 63 (45.6) | 122 (38.8) | 201 (35.1) |
| Vesicles | 12 (4.6) | 28 (15.9) | 35 (25.4) | 63 (20.1) | 75 (13.1) |
| Faucitis | 7 (2.7) | 22 (12.5) | 14 (10.1) | 36 (11.5) | 43 (7.5) |
| All ocular/nasal discharge | 20 (7.7) | 62 (35.2) | 110 (79.7) | 172 (54.8) | 192 (33.5) |
| Serous ocular discharge | 40 (15.4) | 40 (22.7) | 45 (32.6) | 85 (27.0) | 125 (21.8) |
| Purulent ocular discharge | 3 (1.2) | 62 (35.2) | 84 (60.9) | 146 (46.5) | 149 (26.0) |
| Serous nasal discharge | 19 (7.3) | 22 (12.5) | 13 (9.4) | 35 (11.1) | 44 (7.7) |
| Purulent nasal discharge | 2 (0.7) | 29 (16.5) | 68 (49.3) | 97 (30.9) | 99 (17.3) |
| Blepharitis | 74 (28.6) | 45 (25.6) | 47 (34.0) | 93 (29.6) | 167 (29.1) |
| Sneezing | 14 (5.4) | 40 (22.7) | 81 (58.7) | 121 (38.5) | 135 (23.5) |
| Dehydration | 28 (10.8) | 35 (19.9) | 31 (22.5) | 66 (21.0) | 94 (16.4) |
| Body score<4 | 15 (5.8) | 10 (5.7) | 16 (11.6) | 26 (8.2) | 41 (7.1) |
| All cats | 259 | 176 | 138 | 314 | 573 |
Pathogen detection
Feline calicivirus and FHV were the most prevalent infectious agents, followed by Mycoplasma species and B bronchiseptica (Table 4). The prevalence of FCV was between 0 and 67% among the shelters, although the very high prevalence of 67% in shelter C was based on a small sample size of six cats. FHV prevalence varied from 0 to 41%, and rates of B bronchiseptica, C felis, and Mycoplasma species ranged from 0 to 31%, 15%, and 50%, respectively. No infectious pathogens were identified in 38.4% of cats.
Table 4.
Prevalence (percent and confidence intervals) of URI and URI-associated pathogens in eight animal shelters
| Shelter | URI | Feline calicivirus | Feline herpesvirus | Mycoplasma species | B bronchiseptica | C felis | Animals sampled |
|---|---|---|---|---|---|---|---|
| A | 57 (49.2–64.3) | 37.3 (30.2–45.0) | 20.7 (15.1–27.6) | 14.9 (10.2–21.3) | 5.7 (3.0–10.6) | 2.9 (1.1–6.9) | 174 |
| B | 57 (47.7–65.9) | 34.7 (26.4–44.0) | 19.0 (12.7–27.4) | 13.2 (0.08–0.21) | 31.4 (23.4–40.6) | 2.5 (0.01–0.08) | 121 |
| C | 60 (24.1–94.0) | 66.7 (24.1–94.0) | 0 (0.0–48.3) | 0 (0.0–48.3) | 0 (0.0–48.3) | 0 (0.0–48.3) | 6 |
| D | 50 (9.5–90.5) | 0 (0.0–0.80) | 0 (0–80.2) | 50 (9.4–90.5) | 0 (0.0–0.80) | 0 (0.0–0.80) | 2 |
| E | 42 (24.0–62.8) | 0 (0.0–16.0) | 7.7 (1.3–26.6) | 15.4 (5.1–35.7) | 0 (0.0–16.0) | 15.4 (5.1–35.7) | 26 |
| F | 50 (18.8–81.2) | 33 (7.3–83.0) | 0 (0.0–53.7) | 33 (7.3–83.0) | 0 (0.0–53.7) | 0 (0.0–53.7) | 6 |
| G | 60 (52.3–67.2) | 16.0 (11.1–22.5) | 40.6 (33.3–48.3) | 19.4 (4.0–26.2) | 4.0 (1.8–8.4) | 1.1 (0.2–4.5) | 175 |
| H | 37 (21.7–43.3) | 31.8 (21.2–44.6) | 4.5 (1.2–13.6) | 0 (0.0–7.9) | 0 (0.0–7.9) | 3.0 (0.5–11.5) | 66 |
| All shelters | 54.7 (50.5–58.8) | 28.1 (24.5–32.0) | 23.4 (20.1–27.1) | 14.4 (11.7–17.6) | 9.5 (7.3–12.3) | 2.8 (1.6–4.6) | 576 |
Characteristics of sheltered cats with URI
There was no effect of gender on risk of URI (Table 5). Age was a significant risk factor, with increased risk in 0- to 3-month-old and 7- to 11-month-old kittens and reduced risk in cats greater than 12 months. Shelter H had significantly less URI than expected (P=0.002). When prevalence of URI and each pathogen was examined separately by regression on mean daily feline population per shelter, only FHV prevalence was significantly associated with shelter size, with R 2=0.58 (P=0.02). Cats in isolation were three times more likely to have URI than cats in the general population (P<0.001). Significant univariate pathogen risk factors were FHV, Mycoplasma species and C felis, but not B bronchiseptica or FCV. When risk factors for URI were examined together with a logistic regression model, the AIC was minimized with the model URI∼Age+Shelter+Location+Calici+Herpes+Chlamydia+Bordetella+Mycoplasma+(Calici×Bordetella).
Table 5.
Univariate associations of URI with various risk factors
| All cats | URI (%) | No URI (%) | Odds ratio | 95% C limits for the odds ratio (L–U) | P | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 60 | 31 (52) | 29 (48) | 1.00 | 0.52–1.93 | 1.00 |
| Female | 87 | 45 (52) | 42 (48) | 1.00 | 0.52–1.93 | 1.00 |
| Age (in months) | ||||||
| 0–3 | 152 | 100 (66) | 52 (34) | 1.85 | 1.25–2.74 | 2.50×10−03 |
| 4–6 | 62 | 29 (47) | 33 (53) | 0.67 | 0.40–1.15 | 0.17 |
| 7–11 | 40 | 30 (75) | 10 (25) | 2.58 | 1.24–5.41 | 0.01 |
| 12+ | 244 | 118 (48) | 126 (52) | 0.58 | 0.41–0.82 | 2.50×10−03 |
| 72–96 | 5 | 1 (20) | 4 (80) | 0.20 | 0.02–1.79 | 0.18 |
| 96+ | 8 | 5 (63) | 3 (37) | 1.34 | 0.32–5.71 | 0.74 |
| Location in shelter | ||||||
| General population | 331 | 153 (46) | 178 (54) | 0.41 | 0.29–0.58 | 6.47×10−07 |
| Feral | 45 | 22 (49) | 23 (51) | 0.77 | 0.42–1.42 | 0.44 |
| Foster | 11 | 7 (64) | 4 (36) | 1.45 | 0.42–5.01 | 0.76 |
| Isolation | 165 | 120 (73) | 45 (27) | 2.97 | 2.00–4.42 | 3.00×10−08 |
| Days in shelter | ||||||
| 0–5 | 188 | 96 (51) | 92 (49) | 0.81 | 0.56–1.19 | 0.29 |
| 6–12 | 149 | 84 (56) | 65 (44) | 1.08 | 0.74–1.58 | 0.70 |
| 13–20 | 47 | 29 (62) | 19 (40) | 1.29 | 0.70–2.38 | 0.44 |
| 21+ | 54 | 27 (50) | 27 (50) | 0.83 | 0.47–1.47 | 0.56 |
| Pathogen | ||||||
| Feline calicivirus | 150 | 89 (59) | 71 (44) | 1.04 | 0.72–1.51 | 0.85 |
| Feline herpesvirus | 134 | 92 (69) | 42 (31) | 2.15 | 1.42–3.24 | 2.2×10−04 |
| B bronchiseptica | 55 | 30 (55) | 25 (45) | 0.99 | 0.56–1.72 | 1.00 |
| Mycoplasma species | 80 | 65 (81) | 15 (19) | 4.26 | 2.36–7.68 | 1.88×10−07 |
| C felis | 15 | 14 (93) | 1 (7) | 12.02 | 1.57–92.06 | 2.46×10−03 |
Characteristics of cats with URI pathogens
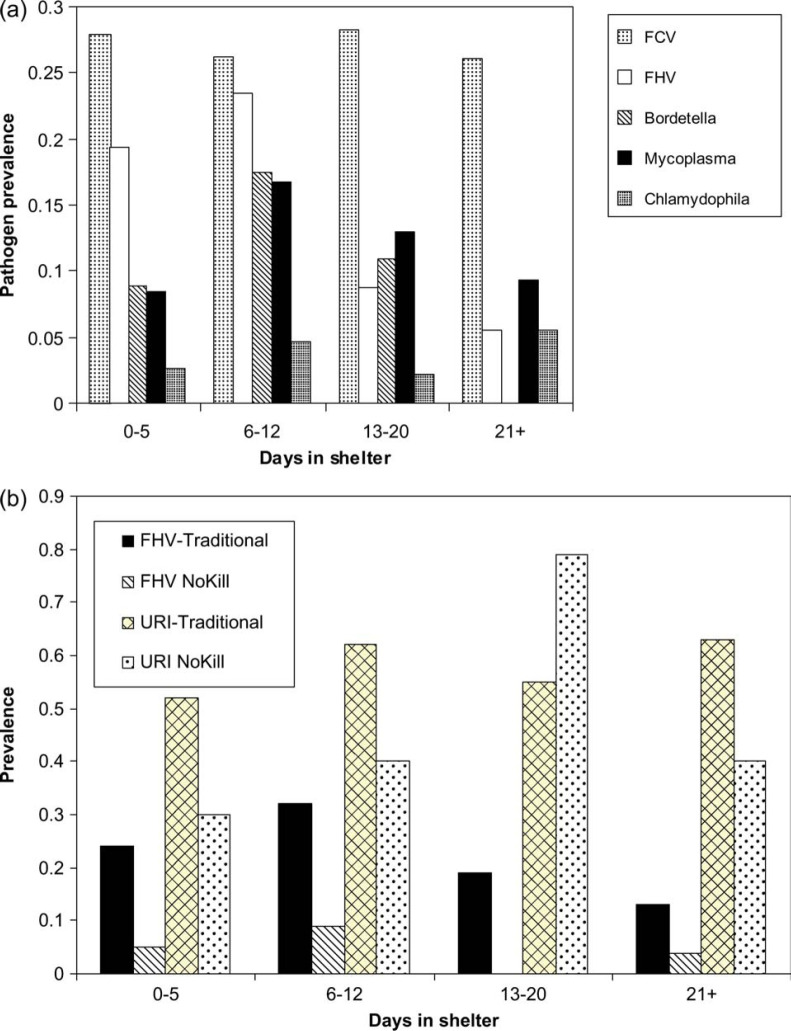
Clinical and diagnostic characteristics varied among cats with URI depending on the infections: values are given with confidence intervals although differences in presentation among pathogens were not evaluated statistically (Table 6). Cats infected with C felis tended to have ocular and nasal discharge and conjunctivitis, while cats with FCV and FHV had ocular and nasal discharge and sneezing. Cats with Mycoplasma species had more severe ocular discharge, conjunctivitis and nasal discharge than cats without. With the exception of FCV and C felis (for which maximum recovery of pathogen occurred evenly regardless of how many days the cats had been at the shelter), the maximum date of pathogen recovery was not uniform (Fig 1). Rather, for FHV and Mycoplasma species there was less pathogen recovery in cats recently introduced into the shelter, maximal recovery in cats at the shelter 6–12 days, and reduced recovery in cats kept in the shelter longer than 12 days. These differences, which were significant for FHV and B bronchiseptica (P=0.0009 and 0.0004, respectively), suggest in-shelter infection or shelter-associated reactivation of infection. A similar curve for Mycoplasma species was not statistically significant (P=0.33). When ‘no kill’ shelter patterns were compared with patterns in traditional shelters where euthanasia was performed, this pattern of maximum FHV recovery was observed in traditional shelters but could not be evaluated in ‘no kill’ shelters (because of low overall rates of FHV recovery); URI rates overall tended to increase and stay elevated in traditional shelters while they peaked until 13–20 days and then declined in ‘no kill’ shelters (Fig 1b).
Table 6.
Percentage (and 95% confidence intervals) of clinical signs and characteristics in cats infected with various URI-associated pathogens
| Pathogen | Ocular discharge | Conjunctivitis | Nasal discharge | Sneezing | URI |
| Feline calicivirus | 48 (40–56) | 20 (15–27) | 34 (27–43) | 31 (23–40) | 56 (48–56) |
| Feline herpesvirus | 57 (47–66) | 40 (31–49) | 40 (31–49) | 35 (27–44) | 70 (61–78) |
| Both FCV and FHV | 82 (56–95) | 42 (16–71) | 53 (29–76) | 53 (29–76) | 58 (33–81) |
| B bronchiseptica | 45 (32–58) | 22 (12–35) | 31 (20–45) | 22 (12–35) | 57 (43–70) |
| C felis | 81 (54–95) | 69 (41–88) | 63 (36–84) | 38 (16–64) | 82 (71–89) |
| Mycoplasma species | 63 (52–74) | 43 (32–54) | 58 (47–65) | 49 (38–61) | 94 (68–100) |
| No pathogens | 39 (32–46) | 29 (23–35) | 23 (17–29) | 16 (12–22) | 43 (37–50) |
Fig 1.
(a) Non-uniform prevalence of URI-associated pathogens as a function of length of stay in shelter, with significantly increased FHV and B bronchiseptica prevalence after 6–12 days. (b) Prevalence of URI and FHV in ‘no kill’, compared with traditional (practicing euthanasia) shelters.
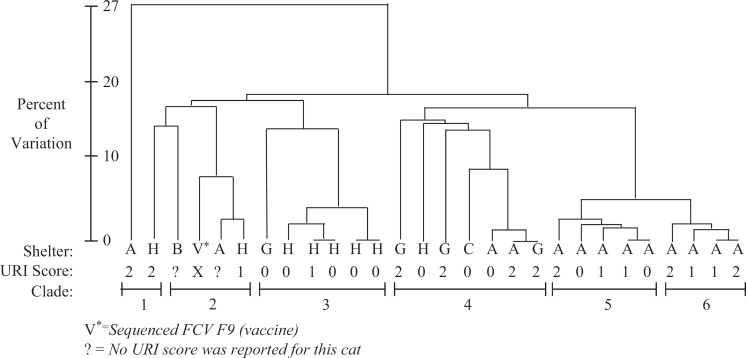
Calicivirus sequencing
A cladogram constructed of 27 FCV isolates showed 2–4 isolates clustered closely to the vaccine strain, clade 2 (Fig 2). Two of these cats had URI. The remaining isolates clustered into five distinct clades, all but one comprising multiple isolates. Clades 5 and 6 contained only isolates from shelter A. The majority of shelter H isolates clustered in clade 2. Furthermore, although these isolates were 24–27% different from vaccine strain, and the FCV prevalence (30.3%) in shelter H was similar to other shelters, shelter H was the only shelter in this study with a decreased risk of URI.
Fig 2.
Cladogram of FCV isolates from cats in shelters.
Discussion
Cats in animal shelters are predisposed to URI, are infected with primary pathogens and opportunists that increase their likelihood of developing URI, and are subject to management strategies that fail to manage or prevent URI. Previous studies evaluating a single or two pathogens at a time or studying cats in small groups have contributed valuable information but fail to provide sufficient guidance needed by shelter veterinarians to successfully treat herd URI problems in shelter cats. Risks, pathogen prevalence, and their interactions are unique to the typical shelter environment and must be studied in that environment as a single integrated problem comprising multiple host–pathogen–environmental factors.
Shelters are environments in which cats are highly predisposed to URI because of high turnover in the cat population, density, stress, and a proportion of the population with poor previous medical care, inadequate nutrition and concurrent medical problems. Shelters are notorious for providing environments that induce stress in cats such as single housing of individual cats, litter trays maintained in close proximity to caged cats, and in many cases, holding areas in close proximity to barking dogs and heavy foot traffic.
In this study, shelters were chosen to include diverse population dynamics and management philosophies. Shelters in this study ranged from private, limited admission shelters responsible for the care of fewer than 400 cats annually with an adoption success rate approaching 100%, to large inner city shelters with cat populations exceeding 10,000 annually and adoption rates below 15%. Irrespective of the shelter's cat population or management philosophy, a lack of adequate isolation of symptomatic cats was a consistent problem throughout this investigation as it is in the majority of sheltering organizations.
The 55% overall URI prevalence, including 24% of sheltered cats showing severe URI, represents a high rate of disease but was not homogeneous across the shelters studied, as would be expected. Variability is partly attributable to the variability in such things as vaccination, housing design, and number of animal care staff and other resources. The major risk factors for URI were cat age, number of days in the shelter and the particular shelter. Cats housed in close proximity to dogs, held in the shelter in excess of 6 days, or under the age of 12 months were at higher risk of URI. The prevalence of URI and each pathogen separately was not associated with shelter population size, with the exception of FHV. However, previously, increased population density has been previously shown to increase the prevalence of URI (Fisk and Soave 1973, Snyder et al 1973, Pedersen 1988, August 1990, Gaskell and Dawson 1994, McArdle et al 1994, Pedersen and Hawkins 1995). It is not clear why this was not observed in the present study, although it is possible that almost all of the shelters in the present study were large enough to exceed some threshold for endemic virus persistence. Interestingly, in shelters that practice euthanasia, URI prevalence over time could either decline or stay the same if cats with URI specifically were targeted for euthanasia. In contrast, declining URI prevalence might not be as likely in ‘no kill’ shelters. In fact, there was no reduction in URI in animal control facilities compared with ‘no kill’ shelters observed in Fig 1b, suggesting that euthanasia and removal from the shelter of cats with URI was only one of several possible mechanisms associated with reduced URI over time. There was a seemingly low prevalence of URI in shelter H, considering its size, low air turnover and lack of quarantine. We do not know how to account for this, although it is interesting that most of the reduced URI seems to be associated with relatively low levels of FHV. In this facility, cats were housed in at least three different, small, one-room buildings with about 6–10 cats in each. Weather permitting, the doors to the buildings were kept open. Additionally, this facility had a full-time veterinarian with a relatively low veterinarian:cat ratio. Cleaning protocols, a low stress environment, cage design, and other factors may have contributed to the low rates of URI in this facility.
Of the five pathogens examined, the most important contributors to URI were FHV, Mycoplasma species and C felis. While FHV is a well-known important pathogen in feline URI (Pedersen 1988, Binns et al 2000), the 23% rate of FHV isolation in this study, represented a four-fold increase over previous results (Harbour et al 1991, Binns et al 2000). FHV often is a latent infection in the nervous system and is shed sporadically especially during times of stress (Gaskell et al 1985), thus cats in this study may have experienced recrudescent rather than newly acquired infection.
Previous investigations of the role of Mycoplasma species have suggested that these bacteria are a part of the normal flora of the oropharynx in one third of cats, while isolation of Mycoplasma species from the lower respiratory tract has been correlated with disease (Randolph et al 1993, Foster et al 1998). In other species such as songbirds, Mycoplasma species infecting the upper respiratory tract is an important source of morbidity. In our study, the isolation of Mycoplasma species from oropharyngeal swabs in cats was significantly associated with URI. While we did not examine these cats for pulmonary disease, our results indicate that the recovery of Mycoplasma species in shelter cats is a significant finding. Irrespective of whether Mycoplasma species acts as a primary pathogen or an opportunistic secondary pathogen, the use of antimycoplasmal drugs may be warranted when treating URI in sheltered cats.
It was interesting that univariate statistical analysis did not implicate FCV as a significant cause of URI in the present study, although the multivariate model did include FCV to minimize the AIC. The overall isolation rate of FCV of 28% is consistent with isolation rates previously reported (Gaskell and Povey 1973, Wardley et al 1974, Gaskell and Dawson 1994, Binns et al 2000). The failure of univariate analysis to implicate FCV in shelter URI was probably because of the high frequency of asymptomatic cats shedding vaccine-like FCV strains. Calicivirus is a non-enveloped RNA virus shed at very high frequency by carriers and recently infected cats. Vaccination for FCV does not consistently prevent infection although in many instances, the vaccine mitigates signs of severe disease (Orr et al 1978). There was a large amount of genetic variation in the FCV isolates in the cat shelters, with some isolates clustering genetically with the vaccine strain as reported previously (Radford et al 1998). FCV infection can result in mild or severe disease depending on the strain as well as the individual cat's immune system's response to infection. Unfortunately, FCV vaccination also is associated with mild oculonasal clinical signs. Thus the genetic analysis in this study documented cats with very mild signs and vaccine-like FCV isolates (which were consistent with vaccine-induced morbidity), as well as more severe signs, possibly representing vaccine failure. The possibility of very mild vaccine-associated signs was the original justification in this study for such an elaborate case definition in which URI did not include mild or serous oculonasal discharge. Often FHV and FCV are reported concurrently in cats. Hoover and Kahn (1975) found that 80% of feline URI was associated with FHV and FCV. Our data showed that cats with concurrent FCV-FHV infection were at high risk of developing URI. FCV infection may also have allowed for the proliferation of secondary bacterial infection with B bronchiseptica and other bacteria.
B bronchiseptica was not significantly associated with URI in shelter cats, which may be because this bacterium in cats acts often as an opportunist. Interestingly, cats in shelter B (the only facility that houses cats in direct proximity to dogs) had a very elevated prevalence of B bronchiseptica, although the confidence intervals of the prevalence intersected those in several shelters with low sample size. Nevertheless, this suggests that cross-species infection may contribute to the spread of B bronchiseptica in the shelter environment. Previous studies have described the potential for cross-species transmission of B bronchiseptica between dogs and cats (Binns et al 1998, Dawson et al 2000, Foley et al 2002); cats with B bronchiseptica may act as reservoirs of infectious tracheobronchitis to the dog population in an animal shelter setting.
The prevalence of C felis in the feline population has been estimated previously between 0 and 32% (Sykes et al 1999a), compared with only 2.8% prevalence of C felis in this study. However, all but one shelter cat with C felis had severe URI. Overall, C felis detection in cats with URI comprised the smallest subset of all cases of URI; however, it appears that cats with C felis had the most severe clinical presentation of all URI cases.
Data in this paper suggest that appropriate use of antiherpetics and antimycoplasmal drugs may be beneficial for individual animal care. Preventive management should focus on minimizing stress, which has been described in the literature as contributing to herpesvirus recrudescence (Gaskell et al 1985) and preventing in-shelter transmission of infection. Additional studies regarding the utility of quarantine and isolation may be a logical next step, particularly if coupled with comprehensive diagnostic testing for common infectious causes of URI in shelter cats.
Acknowledgments
The Maddie's Shelter Medicine Program UC Davis and the Center for Companion Animal Health supported this research. We would like to thank Brad Case, Niki Drazenovich, Dr Kate Hurley, Amy Poland, for their invaluable assistance and all shelter staff who made this study possible.
References
- August J. The control and eradication of feline upper respiratory infections in cluster populations, Veterinary Medicine 9, 1990, 1002–1006. [Google Scholar]
- Binns S.H., Dawson S., Speakman A.J., Cuevas L.E., Hart C.A., Gaskell C.J., Morgan K.L., Gaskell R.M. A study of feline upper respiratory tract disease with reference to prevalence and risk factors for infection with feline calicivirus and feline herpesvirus, Journal of Feline Medicine and Surgery 2, 2000, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns S.H., Speakman A.J., Dawson S., Bennett M., Gaskell R.M., Hart C.A. The use of pulsed-field gel electrophoresis to examine the epidemiology of Bordetella bronchiseptica isolated from cats and other species, Epidemiology of Infections 120, 1998, 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Fukushi H., Koyasu S., Kuroda E., Yamaguchi T., Hirai K. An etiological investigation of domestic cats with conjunctivitis and upper respiratory tract disease in Japan, Journal of Veterinary Medical Science 64, 2002, 215–219. [DOI] [PubMed] [Google Scholar]
- Coutts A.J., Dawson S., Binns S., Hart C.A., Gaskell C.J., Gaskell R.M. Studies on natural transmission of Bordetella bronchiseptica in cats, Veterinary Microbiology 48, 1996, 19–27. [DOI] [PubMed] [Google Scholar]
- Dawson S., Jones D., McCracken C.M., Gaskell R.M., Hart C.A., Gaskell C.J. Bordetella bronchiseptica infection in cats following contact with infected dogs, Veterinary Record 146, 2000, 46–48. [DOI] [PubMed] [Google Scholar]
- Fisk S.K., Soave O.A. Bordetella bronchiseptica in laboratory cats from central California, Laboratory Animal Science 23, 1973, 33–35. [PubMed] [Google Scholar]
- Foley J., Bannasch M. Infectious diseases of dogs and cats. Miller L., Zawistowski S. Shelter Medicine for Veterinarians and Staff, 2004, Iowa University Press: Ames, Iowa, 235–284. [Google Scholar]
- Foley J.E., Rand C., Bannasch M.J., Norris C.R., Milan J. Molecular epidemiology of feline bordetellosis in two animal shelters in California, USA, Previews in Veterinary Medicine 54, 2002, 141–156. [DOI] [PubMed] [Google Scholar]
- Foster S.F., Barrs V.R., Martin P., Malik R. Pneumonia associated with Mycoplasma spp in three cats, Australian Veterinary Journal 76, 1998, 460–464. [DOI] [PubMed] [Google Scholar]
- Gaskell C.J., Dawson S. Viral-induced upper respiratory tract disease. Chandler E., Gaskell C.J., Gaskell R. Feline Medicine and Therapeutics, 2nd edn, 1994, Blackwell Scientific Publications: Oxford, 453–472. [Google Scholar]
- Gaskell R., Povey R. Re-excretion of feline viral rhinotracheitis virus following corticosteriod treatment, Veterinary Record 93, 1973, 204–205. [DOI] [PubMed] [Google Scholar]
- Gaskell R.M., Dennis P.E., Goddard L.E., Cocker F.M., Wills J.M. Isolation of felid herpesvirus I from the trigeminal ganglia of latently infected cats, Journal of General Virology 66, 1985, 391–394. [DOI] [PubMed] [Google Scholar]
- Harbour D.A., Howard P.E., Gaskell R.M. Isolation of feline calicivirus and feline herpesvirus from domestic cats 1980 to 1989, Veterinary Record 128, 1991, 77–80. [DOI] [PubMed] [Google Scholar]
- Hoover E.A., Kahn D.E. Experimentally induced feline calicivirus infection: clinical signs and lesions, Journal of the American Veterinary Medical Association 166, 1975, 463–468. [PubMed] [Google Scholar]
- Jang S.S., Biberstein E., Hirsh D.C. A Diagnostic Manual of Veterinary Clinical Bacteriology and Mycology, 2000, University of California; [Google Scholar]
- van Kuppeveld F.J., Johansson K.E., Galama J.M., Kissing J., Bolske G., van der Logt J.T., Melchers W.J. Detection of mycoplasma contamination in cell cultures by a mycoplasma group-specific PCR, Applied Environmental Microbiology 60, 1994, 149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme D., Kelly R.D., Schmidt D.A. Estimation of body fat by body condition scoring, Journal of Veterinary Internal Medicine 8, 1994, 154. [Google Scholar]
- McArdle H.C., Dawson S., Coutts A.J., Bennett M., Hart C.A., Ryvar R., Gaskell R.M. Seroprevalence and isolation rate of Bordetella bronchiseptica in cats in the UK, Veterinary Record 135, 1994, 506–507. [DOI] [PubMed] [Google Scholar]
- Orr C.M., Gaskell C.J., Gaskell R.M. Interaction of a combined feline viral rhinotracheitis-feline calicivirus vaccine and the FVR carrier state, Veterinary Record 103, 1978, 200–202. [DOI] [PubMed] [Google Scholar]
- Pedersen N. Feline respiratory disease. Greene C. Infectious Diseases of the Dog and Cat, 1988, WB Saunders: Philadelphia, 346–357. [Google Scholar]
- Pedersen N.C., Hawkins K.F. Mechanisms for persistence of acute and chronic feline calicivirus infections in the face of vaccination, Veterinary Microbiology 47, 1995, 141–156. [DOI] [PubMed] [Google Scholar]
- Radford A.D., Turner P.C., Bennett M., McArdle F., Dawson S., Glenn M.A., Williams R.A., Gaskell R.M. Quasispecies evolution of a hypervariable region of the feline calicivirus capsid gene in cell culture and in persistently infected cats, Journal of General Virology 79, 1998, 1–10. [DOI] [PubMed] [Google Scholar]
- Randolph J.F., Moise N.S., Scarlett J.M., Shin S.J., Blue J.T., Bookbinder P.R. Prevalence of mycoplasmal and ureaplasmal recovery from tracheobronchial lavages and prevalence of mycoplasmal recovery from pharyngeal swab specimens in dogs with or without pulmonary disease, American Journal of Veterinary Research 54, 1993, 387–391. [PubMed] [Google Scholar]
- Snyder S.B., Fisk S.K., Fox J.G., Soave O.A. Respiratory tract disease associated with Bordetella bronchiseptica infection in cats, Journal of the American Veterinary Medical Association 163, 1973, 293–294. [PubMed] [Google Scholar]
- Sykes J.E., Anderson G.A., Studdert V.P., Browning G.F. Prevalence of feline Chlamydia psittaci and feline herpesvirus 1 in cats with upper respiratory tract disease, Journal of Veterinary Internal Medicine 13, 1999a, 153–162. [DOI] [PubMed] [Google Scholar]
- Sykes J.E., Studdert V.P., Browning G.F. Comparison of the polymerase chain reaction and culture for the detection of feline Chlamydia psittaci in untreated and doxycycline-treated experimentally infected cats, Journal of Veterinary Internal Medicine 13, 1999b, 146–152. [DOI] [PubMed] [Google Scholar]
- Wardley R.C., Gaskell R.M., Povey R.C. Feline respiratory viruses–their prevalence in clinically healthy cats, Journal of Small Animal Practice 15, 1974, 579–586. [DOI] [PubMed] [Google Scholar]