Abstract
A novel endoscopic approach for the removal of nasal polyps from a cat with upper respiratory obstruction is described. The cat's small oral cavity prevented polyp removal via traditional nasopharyngoscopy and the owner declined rhinotomy because of concerns about postoperative morbidity. Access to the nasopharynx was achieved by introducing an endoscope via gastrotomy and passing the instrument orad through the esophagus into the nasopharynx. Compared with traditional endoscopic approaches, this approach provided superior exposure of the nasopharynx and facilitated use of a larger scope and instruments than would have been possible using retroflexed nasopharyngoscopy. Multiple polyps were readily removed using this approach. The procedure was well tolerated, with minimal surgical morbidity.
A 5-year-old female spayed domestic shorthair cat was evaluated at another veterinary facility for nasal discharge. Empiric treatments with oral antibiotics, oral dexamethasone, a topical ophthalmic solution, and a compounded intranasal solution containing enrofloxacin and an unspecified steroid were unsuccessful. The cat was referred to the Kansas State University Veterinary Medical Teaching Hospital.
Physical examination revealed a thin cat (3.2 kg, body condition score 1.5/5), with a slight fever (103.3 °F), mild nasal discharge and stertorous breathing. A small mass protruded from the left naris. Routine blood tests showed mild changes including hemoconcentration (49.2%; reference range 30–45%), lymphopenia (1400/ul; reference range 2000–7000/ul), hypoalbuminemia (3.1 g/dl; reference range 3.2–4.7 g/dl) and hyperglobulinemia (4.9 g/dl; reference range 2.8–4.8 g/dl). Urinalysis was unremarkable. Cryptococcus species serology was negative. Cytologic analysis of the nasal exudate showed non-degenerate and degenerate neutrophils (>85% of total cells), macrophages, and numerous intra- and extracellular bacteria. Computed tomography of the nasal passages and skull showed increased density throughout the left frontal sinus and nasal cavity; the septum was pushed towards the right but no bony changes were evident. The bullae and external ear canals appeared normal. The nasopharynx could not be visualized well by retraction of the soft palate. A large mass, which nearly filled the caudal nasopharynx, was visualized by endoscopic nasopharyngoscopy but multiple attempts to remove or debulk the mass using endoscopic instruments were unsuccessful because the small size of the cat's oral cavity provided a limited working space. The mass protruding from the naris was removed using a small alligator forcep. An esophageal feeding tube was placed to facilitate feeding. Recovery from anesthesia was uneventful.
Histologic examination revealed the mass to be an inflammatory polyp that was covered with stratified squamous keratinized epithelium and had submucosal infiltration that consisted primarily of plasma cells, with fewer lymphocytes and occasional neutrophils. Rhinotomy was recommended to remove the larger mass but was declined by the owner. Feeding from the esophageal tube began the following day, and feeding amounts were increased over a 4-day period to meet maintenance requirements. The cat was discharged with a guarded prognosis.
The patient presented 2 months later with nasal discharge, stertorous breathing, and erythema and swelling at the esophagostomy tube site. The esophagostomy tube was removed. Rhinotomy was again declined, in part because the owner was concerned about postoperative complications. A novel approach was conceived whereby an endoscope introduced via gastrotomy would be passed orad through the esophagus to visualize the nasopharynx. We believed that this approach would cause minimal postoperative morbidity and would also ameliorate the difficulties associated with the cat's small size that were encountered during the previous endoscopy. The owner agreed to allow the new procedure.
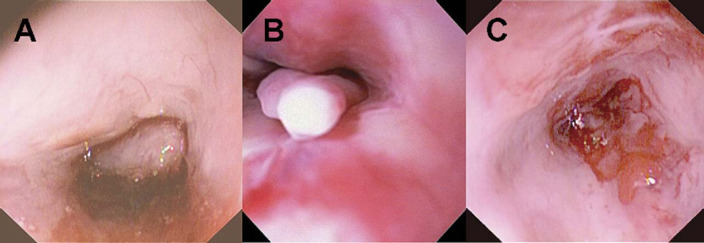
The anesthetized cat was placed in dorsal recumbency and prepared with routine surgical scrub. The abdomen was opened using a standard ventral midline incision. The stomach was exteriorized and isolated with laparotomy sponges. A 2 cm gastrotomy was performed through a non-vascular area in the fundus. A sterile endoscope was inserted into the gastrotomy incision and passed orad through the cardia into the esophagus until the oral cavity was reached. A large polyp that occluded the entire caudal nasopharynx was seen (Fig 1). The base of the polyp was identified and grasped with alligator-style endoscopic biopsy forceps (Fig 2). The polyp was pulled free with gentle traction and the endoscope, forceps, and polyp were withdrawn as a unit into the esophagus and out of the gastrotomy incision. The endoscope was rinsed with sterile saline, wiped with alcohol and re-introduced into the gastrotomy incision. Several smaller polyps now visible were removed and attempts were made to resect as much tissue as possible from the areas where the polyps had been attached. Throughout the procedure, hemostasis was controlled with topical iced saline and dilute phenylepinephrine solution infused via the endoscope. When the nasal cavity was cleared, the endoscope was withdrawn and the gastrotomy site was closed in two layers with a simple continuous pattern oversewn with a continuous Cushing pattern. Routine abdominal closure was performed after copious saline flushing.
Fig 1.
Endoscopic view of the nasopharynx. Panel A – large mass filling the nasopharynx. Panel B – smaller masses revealed after the large mass was removed (close-up view). Panel C – nasopharynx at the completion of mass removal and debulking.
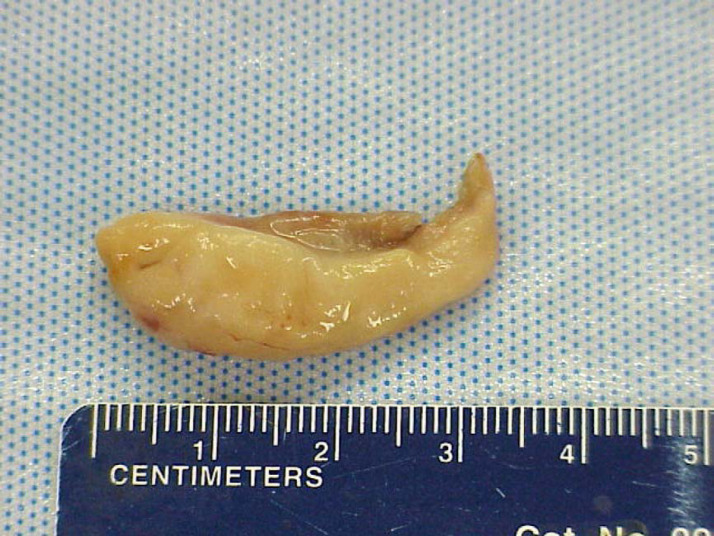
Fig 2.
The large polyp removed from nasopharynx using the described approach.
An esophageal feeding tube was replaced before recovery from anesthesia. Afterwards, the cat received acepromazine (0.025 mg IV once) and buprenorphine (0.026 mg q 6 h) for sedation and pain control. Amoxicillin–clavulanic acid (62.5 mg PO q 12 h), and cefazolin (72 mg IV q 8 h) were initiated. Mild epistaxis and a fever (104.9 °F) were noted postoperatively; both resolved within 24 h. The cat was discharged 2 days after the surgical procedure. The results of histologic examination of the removed tissue were consistent with an inflammatory polyp.
Nasopharyngeal polyps are a well documented cause of upper respiratory signs in young cats. However, recent studies indicate that middle aged to older cats occasionally develop nasopharyngeal polyps (Veir et al 2002) The etiology is unknown but has been linked to chronic inflammation and upper respiratory tract infection (Parker and Binnington 1985, Pope 1995). Other suggested causes include congenital abnormality of the fetal branchial arches (Baker 1982) and otitis media (Pope 1995).
Diagnosis of nasopharyngeal polyps is based on history and physical examination and confirmed with diagnostic imaging, endoscopic evaluation, and histopathologic examination. Cats with nasopharyngeal disease are likely to exhibit stertor, phonation change, and occasionally, nasal discharge (Allen et al 1999). A mass may be detected by oral examination and digital palpation of the soft palate (Allen et al 1999). Cats with otic polyps commonly present with otorrhea, otitis, or a mass protruding from the external canal (Pope 1995). Imaging the tympanic bulla with radiography, computed tomography (CT) and magnetic resonance imaging (MRI) is also helpful for determining the presence of middle ear disease and for determination of the proper treatment regimen (Allgoewer et al 2000). CT of the nasal cavity, bullae and the external ear canal is a sensitive technique for detecting early and subtle lesions (Seitz et al 1996, Muilenburg and Fry 2002). MRI detected bulla pathology in two cats, one with a nasopharyngeal polyp and one with an external ear canal polyp that was not detected by radiography (Allgoewer et al 2000).
Removal of nasopharyngeal polyps may be performed by traction avulsion, ventral bulla osteotomy (VBO), or total ear canal ablation (TECA) with lateral bulla osteotomy (LBO). Traction avulsion may be a viable option if bulla disease is not detected (Muilenburg and Fry 2002). Two recent studies support traction avulsion as a first line of treatment for nasopharyngeal polyps. Of 14 cats with nasopharyngeal polyps treated with traction avulsion, only five had recurrent polyps (Veir et al 2002). In another study, nasopharyngeal polyps recurred in only 11% of cats after traction removal and no recurrence occurred in cats receiving anti-inflammatory doses of prednisolone (Anderson et al 2000). Horner's syndrome, which affected 43% of cats after traction removal in one study (Anderson et al 2000), did not develop in our patient.
In this case, because CT evaluation had not revealed evidence of bullae involvement, it was decided that traction would be an appropriate method of polyp removal. Multiple attempts to remove the polyp with the endoscope held in the maximally retroflexed position failed. By performing a simple gastrotomy and passing the endoscope orad though the esophagus (retrograde esophagoscopy) into the nasopharynx, it was possible to view the polyp, locate its base, and apply the necessary traction to remove it. The use of the described approach had several advantages over the traditional endoscopic approach. The retrograde approach gave exposure to the nasopharynx that was superior to that obtained with nasopharyngoscopy. With the cat in dorsal recumbency and the head extended, the nasopharynx was directly in line with the tip of the endoscope where it emerged from the esophagus. This allowed the instruments to be advanced directly into the nasopharynx. It was also easier to pass endoscopic instruments out of the channel opening into the working field because the scope was extended rather than flexed. The new approach allowed the use of a larger endoscope and larger instruments than would have been possible with retroflex nasopharyngoscopy. Because the endoscope was not retroflexed, the instruments were easily manipulated, which allowed better control. A clear field of view was easy to maintain because the flush solution drained forward, out of the mouth and nose, and away from the scope rather than back towards the operator as it often does with the scope in the retroflexed view.
The described approach has limitations. Despite excellent surgical exposure, it was difficult to completely remove all abnormal tissue using endoscopic instruments. Laparotomy and gastrotomy could be associated with increased patient morbidity relative to less-invasive approaches, such as palate retraction or retroflexed nasopharyngoscopy, but with proper patient selection, surgical morbidity should be minimal (and less than that associated with rhinotomy). Finally, the described approach is more difficult and costly than non-invasive approaches because the procedure must be performed with a sterile endoscope and in the operating room.
We conclude that the retrograde esophageal approach facilitates endoscopic removal of nasopharyngeal polyps. While routine use of this approach is not recommended, it should be considered when traditional approaches to the nasopharynx are not possible or contraindicated.
References
- Allen H.S., Broussard J., Noone K. Nasopharyngeal diseases in cats: a retrospective study of 53 cases (1991–1998), Journal American Animal Hospital Association 35 (6), 1999, 457–461. [DOI] [PubMed] [Google Scholar]
- Allgoewer I., Lucas S., Schmitz S.A. Magnetic resonance imaging of the normal and diseased feline middle ear, Veterinary Radiology and Ultrasound 41 (5), 2000, 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D.M., Robinson R.K., White R.A. Management of inflammatory polyps in 37 cats, Veterinary Record 147 (24), 2000, 684–687. [PubMed] [Google Scholar]
- Baker G. Nasopharyngeal polyps in cats, Veterinary Record 111, 1982, 43. [DOI] [PubMed] [Google Scholar]
- Muilenburg R.K., Fry T.R. Feline nasopharyngeal polyps, Veterinary Clinics of North America: Small Animal Practice 32, 2002, 839–849. [DOI] [PubMed] [Google Scholar]
- Parker N.R., Binnington A.G. Nasopharyngeal polyps in cats: three case reports and a review of the literature, Journal American Animal Hospital Association 21, 1985, 473–478. [Google Scholar]
- Pope E.R. Feline inflammatory polyps, Seminars Veterinary Medicine and Surgery (Small Animal) 10 (2), 1995, 87–93. [PubMed] [Google Scholar]
- Seitz S.E., Losonsky J.M., Manfra-Marretta S. Computed tomographic appearance of inflammatory polyps in three cats, Veterinary Radiology and Ultrasound 37 (2), 1996, 99–104. [Google Scholar]
- Veir J.K., Lappin M.R., Foley J.E., Getzy D.M. Feline inflammatory polyps: historical, clinical, and PCR findings for feline calici virus and feline herpes virus-1 in 28 cases, Journal Feline Medicine and Surgery 4 (4), 2002, 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]