Abstract
Phaeochromocytomas are catecholamine-secreting tumours of the adrenal glands and are rare in cats. Plasma metanephrine levels are widely considered the diagnostic test of choice for phaeochromocytoma in people but have not been investigated in cats. In this study plasma free normetanephrine and metanephrine levels were measured using high-pressure liquid chromatography in healthy cats, sick cats with non-adrenal disease and in a cat with a suspected phaeochromocytoma. Plasma normetanephrine was significantly higher in sick cats with non-adrenal disease compared to healthy cats (P<0.05) and markedly higher in the cat with a suspected phaeochromocytoma when compared to either group. Plasma metanephrine was not significantly different in any of the groups. This study establishes a first-line guide reference range for plasma metanephrine and normetanephrine levels in healthy cats and cats with non-adrenal disease. These results provide rationale for further studies to establish the use of plasma normetanephrine levels as a potential diagnostic test for phaeochromocytoma in the cat.
Phaeochromocytomas are catecholamine-secreting tumours arising from chromaffin cells in the adrenal glands. Catecholamine-secreting tumours can also arise from extra-adrenal sites such as sympathetic paraganglia, where they are referred to as paraganglioma. Phaeochromocytomas can be benign but are often malignant. 1,2 Phaeochromocytomas are considered rare tumours in cats; only three cases of phaeochromocytoma are reported in the peer-reviewed literature. 3–5 Phaeochromocytomas are also considered rare in dogs. 1,2,6 Clinical signs of phaeochromocytoma which occur secondary to catecholamine-induced hypertension include, collapse, open-mouth breathing, muscle tremors, agitation and restlessness, polydipsia and polyuria. These signs can be subtle, may be episodic and are not pathognomonic. Signs may be present days to years before presentation. 7 Phaeochromocytomas can also cause a significant mass effect and may invade local anatomical structures including the caudal vena cava. 8
Making an ante-mortem diagnosis of phaeochromocytomas is a clinical challenge. Routine haematology, biochemistry and urine analysis are non-specific and detection of an adrenal mass via abdominal ultrasonography is neither sensitive nor specific. 2 Given the vague signs of phaeochromocytoma, an adrenal mass may be an incidental finding during an investigation of another problem. Abdominal ultrasonography is, however, recommended as part of the staging process in cats suspected of having a phaeochromocytoma. 1 The percentage of phaeochromocytomas diagnosed in cats post mortem are unknown. In dogs, approximately 44–48% of phaeochromocytomas are diagnosed incidentally at surgery or post mortem rather than clinically. 1,2 In which is similar to that in people a large proportion of phaeochromocytomas are diagnosed incidentally at surgery or post mortem. 9,10 Various techniques for detecting excessive catecholamines are available but their use in veterinary medicine has been very limited. In people, the test of choice for phaeochromocytoma is plasma metanephrine levels. One recent study has examined various assays for measuring plasma metanephrines in healthy dogs 11 but there are no reports describing measurement of feline plasma metanephrines. This study examines plasma free metanephrine and normetanephrine levels in healthy cats, cats with non-adrenal disease and in a cat with a suspected phaeochromocytoma.
Materials and methods
Animals
The study cohort consisted of 13 cats. Of these, seven were healthy cats, five were cats that presented to the Small Animal Specialist Hospital for other non-adrenal illnesses, and one was a cat with a suspected phaeochromocytoma. Of the seven healthy cats, five were castrated males and two were spayed females and all were domestic shorthair cats with a median age of 3 years (range 1–6). These cats were all owned by staff of the Small Animal Specialist Hospital and were regular blood donors for the hospital. They were presented for routine blood donation, which included a general health check and phlebotomy under general anaesthesia. These cats were judged to be healthy based on information provided by the detailed history obtained from their owners, physical examination and the unremarkable results of pre-anaesthetic haematology and biochemistry profiles.
Of the five sick cats with non-adrenal diseases, four were castrated males and one was a spayed female. These cats had a median age of 12 years (range 7–20) and included one Burmese, two domestic shorthair cats and two domestic longhair cats. These cats were client owned and presented for illness and evaluation, which included routine biochemistry, haematology, urinalysis and endocrine testing. All cats had abdominal imaging and their adrenal glands were determined to be of normal size, shape and echogenicity. One of these cats was presented after being hit by a car 48 h previously and was diagnosed with a ruptured bladder and post-renal azotaemia, one had International Renal Interest Society (IRIS) stage 3 chronic renal disease, diabetes mellitus and diabetic ketoaciodosis, one had stage 3 chronic renal disease with hypertension, one had hyperthyroidism, and suspected inflammatory bowel disease with infiltrative gastrointestinal neoplasia and one had acute pancreatitis, diabetes mellitus and diabetic ketoacidosis.
The cat with a suspected phaeochromocytoma was an 11-year-old female spayed domestic shorthair that presented for severe ocular haemorrhage and was diagnosed with a non-invasive left adrenal mass suspected to be a phaeochromocytoma. A lung lobe mass was also detected and was diagnosed by histopathology as a bronchoalveolar carcinoma.
Sample collection
Blood was collected as soon as practical after each subject was presented to the hospital. Firstly, blood was collected from the healthy cats at the time of collection for a pre-anaesthetic profile. These cats were then anaesthetised and routine phlebotomy for blood donation was performed. Secondly, blood was collected from the sick cats with non-adrenal disease at the time of collection to investigate their condition at their initial presentation. Blood was collected from the cat with a suspected phaeochromocytoma at three different times: first after the adrenalectomy and prior to the thoracotomy, second after the lung lobectomy and third after the first cycle of chemotherapy. All owners signed detailed forms giving informed consent for the collection of blood. Blood was collected from each cat using jugular venepuncture on the first attempt. Each cat was held by a senior veterinary nurse using minimal restraint. The blood was collected into a lithium heparin tube and centrifuged immediately. The plasma supernatant was collected and frozen at −20°C. Transportation of the samples to the laboratory was on dry ice and the samples remained frozen at −20°C until analysis.
Assay
Plasma free metanephrine levels were measured using high-pressure liquid chromatography (HPLC) using a method that has been previously described. 12
Statistical analysis
Data were analysed using Microsoft Excel 2003. Unpaired t-tests were used to determine difference between the groups. Values of P<0.05 were considered significant.
Results
Plasma free metanephrine and normetanephrine values in healthy cats compared to sick cats with non-adrenal disease
The plasma metanephrine range in healthy cats was 250–3300 ρmol/l (407–1799 ρmol/l with a 95% confidence interval - CI) and a mean of 1103 ρmol/l. The plasma metanephrine range in sick cats with non-adrenal disease was 710–4110 ρmol/l (286–2810 ρmol/l with a 95% CI) and a mean of 1548/l. The values of plasma metanephrine in sick cats with non-adrenal disease were not significantly different to those in healthy cats (P=0.58).
The plasma normetanephrine range in healthy cats was 1160–6280 ρmol/l (2472–4550 ρmol/l with a 95% confidence interval CI) and a mean of 3511 ρmol/l. The plasma normetanephrine range in sick cats with non-adrenal disease was 3250–11860 ρmol/l (4904–10420 ρmol/l with a 95% CI) with a mean of 7662 ρmol/l. The values of plasma normetanephrine in sick cats with non-adrenal disease were determined to be significantly elevated compared to those in healthy cats (P<0.05).
Cat with a suspected phaeochromocytoma
The cat with a suspected phaeochromocytoma initially presented for agitation, tachypnoea and a recent history of transient anterior chamber hyphaema. On examination there was inconsistent menace response and retinal examination revealed focal areas of retinal detachment, haemorrhage and oedema. Systolic arterial blood pressure (SAP) measured by the Doppler method was repeatedly 360 mmHg. Routine biochemistry, haematology and total T4 were unremarkable and urine specific gravity was 1.014 g/l. A coagulation study including prothrombin time, activated partial thromboplastin time, thrombin time and fibrinogen was unremarkable. An abdominal ultrasound examination revealed a large heterogeneous left adrenal mass. There was no evidence of vascular invasion. The right adrenal gland was unremarkable. On thoracic radiographs there was a mass in the left caudal lung lobe. The owners declined further investigation of this mass at the time but elected to proceed with adrenalectomy via exploratory laparotomy. Pre-operatively the cat was managed with atenolol (Tenormin; Astra Zeneca) 6.25 mg per os (PO) q24 h, phenoxybenzamine 2.5 mg PO q12 h (Dibenlyline; Golshield Healthcare, Australia) and amlodipine (Norvasc; Pfizer) 0.625 mg PO q24 h and the SAP dropped to 230 mmHg. An exploratory laparotomy was performed and the left adrenal mass was removed. Postoperatively the cat was managed with a hydrocortisone infusion due to the possibility that the tumour was a functional adrenocortical tumour. The SAP dropped further to 115 mmHg over the following 2 days; the cat was discharged on a tapering course of prednisolone. Histopathology of the mass was consistent with a neuroendocrine tumour and an adrenocortical tumour or phaeochromocytoma were both considered possible. At the 7-day postoperative recheck the owner reported that the cat had been anxious and physical examination revealed hyphaema of the left eye. The heart rate was 200 bpm and the SAP was 220 mmHg. The owners agreed to evaluate the thoracic mass which was investigated further with a thoracic computed tomography (CT) scan. This confirmed the presence of a single left caudal lung lobe mass; there were no signs of other metastasis. A repeat abdominal ultrasound was unremarkable. Plasma metanephrine was measured and was 1600 ρmol/l and normetanephrine was 15430 ρmol/l. Therapy with amlodipine 0.625 mg PO q24 h and phenoxybenzamine 2.5 mg PO q24 h was restarted and the clinical signs resolved with the SAP dropping to 150 mmHg. A thoracotomy was performed and the left caudal lung lobe was removed. Histopathology was consistent with a bronchoalveolar carcinoma. The cat was discharged from hospital uneventfully after 2 days on no medication. At the 7-day postoperative recheck, the cat was assessed to be recovering very well and the owner reported that all the signs of agitation had resolved. The SAP was measured in hospital to be 120–150 mmHg. At this time the plasma metanephrine level was 1220 ρmol/l and normetanephrine was 15200 ρmol/l. One month after surgery, chemotherapy with vincristine (Oncovin, Aspen Pharmacare Australia) 0.15 mg intravenous (IV) and cyclophosphamide (Cytoxan, Bristol-Myers Squibb) 50 mg PO was initiated every 2 weeks for four treatments but was discontinued due to mild lethargy and a poor appetite associated with the chemotherapy. After the first cycle of chemotherapy, plasma metanephrine was 1390 ρmol/l and normetanephrine was 13410 ρmol/l. During treatment with chemotherapy the cat developed clinical signs of hypertension again and therapy with amlodipine 0.625 mg PO q24 h and phenoxybenzamine 2.5 mg PO q24 h was restarted and the clinical signs again resolved. The cat remains alive and well 18 months after presentation with a SAP of 140–160 mmHg on phenoxybenzamine 2.5 mg PO q24 h and amlodipine PO q24 h. No restaging has been performed.
Plasma free metanephrine and normetanephrine values compared to that in a cat with a suspected phaeochromocytoma
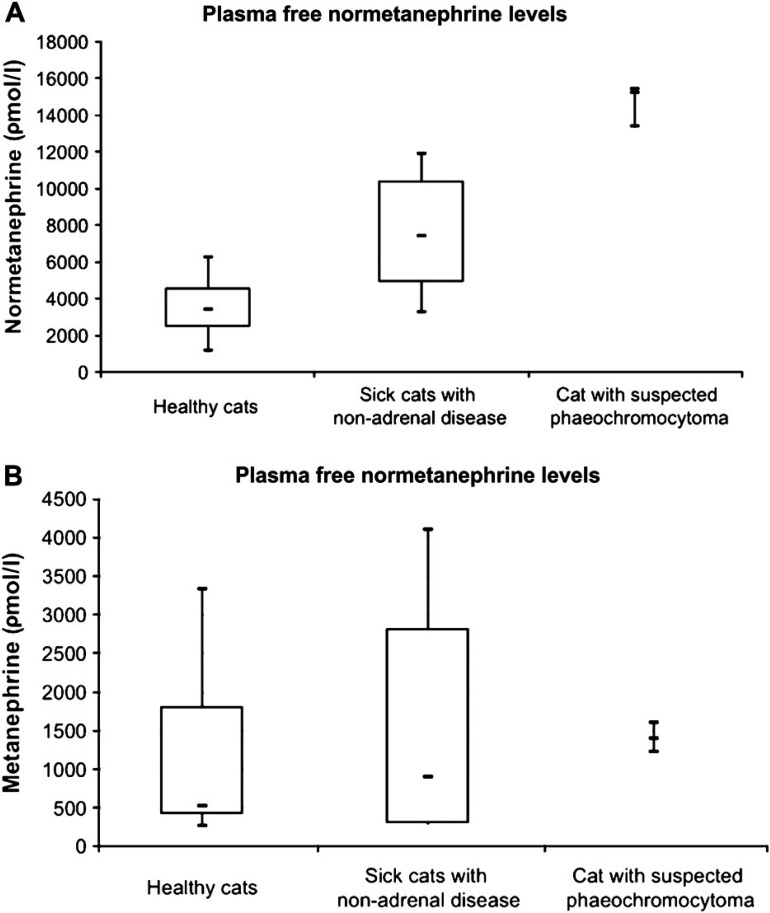
Plasma normetanephrine levels in the cat with a suspected phaeochromocytoma were significantly higher (outside the 95% CI) than plasma normetanephrine levels in both healthy cats, and sick cats with non-adrenal disease (Fig. 1A).
Fig 1.
(A). Normetanephrine levels in healthy cats, sick cats with non-adrenal disease and in the cat with phaeochromocytoma. (B). Metanephrine levels in healthy cats, sick cats with non-adrenal disease and in the cat with a suspected phaeochromocytoma.
There was significant overlap in the levels of plasma metanephrine in the cat with the phaeochromocytoma and the levels in healthy cats and sick cats with non-adrenal disease (Fig. 1B).
Discussion
In people, plasma metanephrine and normetanephrine levels are largely considered the best test for phaeochromocytoma 13–18 and phaeochromocytoma is considered likely if metanephrine is >500 ρmol/l or normetanephrine level is >1400 ρmol/l. Although plasma free metanephrines are more difficult to measure than plasma total metanephrines, free metanephrines are considered superior in the diagnosis of phaeochromocytoma in people. 14,15 Metanephrines can be measured via HPLC (gold standard), tandem mass spectroscopy, radioimmunoassay (RIA), or enzyme immunoassay (EIA). Other biochemical tests available include plasma and urine 24 h epinephrine, norepinephrine and vanillymandelic acid, plasma chromogranin A, clonidine and phentolamine suppression tests and histamine, tyramine, metoclopramide and glucagon provocation tests. Some studies have found urine 24-h metanephrine levels to be a superior test for detecting phaeochromocytoma in people. 19,20 In people, plasma metanephrine levels have been shown to be affected by body position during sample collection, food or caffeine prior to sampling, exercise prior to sampling, prolonged storage at room temperature or delayed centrifugation of a whole blood sample. 21
In veterinary patients, catecholamines and their metabolites are rarely measured. The only study examining plasma metanephrine levels has been in normal dogs breathing room air and an admixture of low oxygen content and compared levels measured via HPLC and a more expedient and economic RIA. These results demonstrated that RIA could be used to measure canine metanephrines but further work is required to validate the test. 11 Feline tissue metanephrine levels have been reported in the brain, heart, spleen, kidney and liver 22 but to our knowledge, feline plasma metanephrine levels have not been reported in the peer-reviewed literature.
Reasons for the lack of plasma and urinary catecholamine measurement in veterinary patients include the cost of the assays, impracticality of 24-h urine collection and because the effect of the stress associated with restraint and sample collection on plasma catecholamine levels renders this test unreliable. 6 A recent study evaluating spot urine metanephrine to creatinine ratios in normal dogs found that familiarity with the hospital and sample collection influenced the results significantly and concluded that samples should be collected at home once the dog has adapted to sample collection. 23 Metanephrines secreted by phaeochromocytomas, however, are less affected by short-term variations including paroxysmal excretion of catecholamines and short-term stressors such as sample collection. In this study, sample collection most likely had some influence on plasma metanephrine and normetanephrine levels, however despite this, plasma normetanephrine levels in healthy cats were significantly lower than those of sick cats with non-adrenal disease. Furthermore the plasma normetanephrine levels in the cat with a suspected phaeochromocytoma were all higher than any of the healthy or sick cats with non-adrenal disease.
In this study, plasma metanephrine levels were not significantly different in healthy cats, sick cats with non-adrenal disease or the cat with the suspected phaeochromocytoma. There was a trend towards higher plasma metanephrine levels in the cat with a suspected phaeochromocytoma compared with both healthy cats and sick cats with non-adrenal disease, and there was a trend towards higher plasma metanephrine levels in sick cats with non-adrenal disease compared with healthy cats. However, this was not statistically significant. The plasma metanephrine levels exhibited wide variance at the upper end of the range so that the levels of plasma metanephrine in the cat with phaeochromocytoma overlapped significantly with the ranges in healthy cats and sick cats with non-adrenal disease. The reasons why plasma metanephrine levels were of limited value in this case, yet plasma normetanephrine levels were significantly different between the groups of cats remain unknown. It is possible that metanephrine levels are affected relatively more by sympathetic tone associated with sampling than normetanephrine in cats. Alternatively, it may be because cats have a higher proportion of norepinephrine in their adrenal medulla than other species including people. 24 Epinephrine is metabolised by catechol-O-methyltransferase to metanephrine while norepinephrine is metabolised to normetanephrine. 6 It has also been shown that the amount of parent catecholamine present influences the amount of a particular metabolite produced. 25 It is possible that this particular tumour was secreting far higher concentrations of norepinephrine compared to epinephrine.
In the case described, despite undergoing surgery to remove the phaeochromocytoma and postoperative chemotherapy, the cat had markedly elevated normetanephrine levels. This is possibly due to the presence of micro-metastases unresponsive to the chemotherapy used. Furthermore, phaeochromocytomas are a heterogeneous group of tumours and response to treatment is complex. The location, size and malignancy of a particular phaeochromocytoma may also affect biochemical properties and the responses to surgery and chemotherapy with respect to plasma metanephrine, norepinephrine levels. 26
Limitations of this study include the relatively small sample size of each group, the fact that the healthy control cats were not age-matched and that only one cat with phaeochromocytoma was available for comparison. Increasing these patient numbers may improve the increasing trend of plasma metanephrine levels in sick cats with non-adrenal disease and in cats with phaeochromocytoma to become statistically significant. The cat with phaeochromocytoma did not undergo a full endocrine work-up to exclude other types of adrenal tumours such as corticosteroid or mineralocorticoid secreting tumours prior to surgery and plasma metanephrines were not assayed prior to surgery. Also, although histopathology of the adrenal tumour was consistent with a neuroendocrine tumour, a specific histogenesis could not be determined on histopathology. As such the authors cannot be completely sure that the adrenal mass was a phaeochromocytoma. However, the clinical presentation and biochemical findings were consistent with phaeochromocytoma. The pulmonary mass was initially presumed to be a metastasis of the phaeochromocytoma, however it was classified histologically as a bronchoalveolar carcinoma and there was no noticeable change in plasma metanephrine or normetanephrine levels following its removal, suggesting that it was unlikely to be secreting catecholamines. Further investigation with immunohistochemisty including antibodies to support phaeochromocytoma such as chromogranin A and synaptophysin or adrenocortical tumour markers such as vimentin, S100 and cytokeratin would be warranted. It is interesting to note that the SAP was more easily controlled following surgical removal of both the adrenal and lung tumours. This might be explained by higher physiological stress because of the presence of the lung mass or simply coincidence as the cat's normetanephrine levels did remained unchanged. In people, clinical signs of phaeochromocytoma improve with resection of gross tumours metastases despite the presence of micrometastases. 27
In conclusion, the results of this study give first-line guide reference ranges for plasma metanephrine levels in healthy cats and sick cats with non-adrenal disease. The study also demonstrates elevated normetanephrine levels in a cat with a suspected phaeochromocytoma. Further investigation into plasma metanephrine levels in larger numbers of healthy cats, sick cats with non-adrenal disease and cats with histologically confirmed phaeochromocytoma are required to develop a cutoff level to diagnose phaeochromocytoma. Investigation into the use of RIA and EIA to measure plasma metanephrine levels in cats and the measurement of spot urinary metanephrine to creatinine levels in cats as diagnostic tests for phaeochromocytoma would also be warranted.
Acknowledgements
The authors would like to thank Dr Kate Patterson for her editorial input and Dr Julia Crawford for referring the case to the Small Animal Specialist Hospital.
References
- 1.Gilson S.D., Withrow S.J., Wheeler S.L., Twedt D.C. Pheochromocytoma in 50 dogs, J Vet Intern Med 8, 1994, 228–232. [DOI] [PubMed] [Google Scholar]
- 2.Barthez P.Y., Marks S.L., Woo J., Feldman E.C., Matteucci M. Pheochromocytoma in dogs: 61 cases (1984–1995), J Vet Intern Med 11, 1997, 272–278. [DOI] [PubMed] [Google Scholar]
- 3.Patnaik A.K., Erlandson R.A., Lieberman P.H., Welches C.D., Marretta S.M. Extra-adrenal pheochromocytoma (paraganglioma) in a cat, J Am Vet Med Assoc. 197, 1990, 104–106. [PubMed] [Google Scholar]
- 4.Henry C.J., Brewer W.G., Jr., Montgomery R.D., Groth A.H., Jr., Cartee R.E., Griffin K.S. Clinical vignette. Adrenal pheochromocytoma, J Vet Intern Med 7, 1993, 199–201. [DOI] [PubMed] [Google Scholar]
- 5.Chun R., Jakovljevic S., Morrison W.B., DeNicola D.B., Cornell K.K. Apocrine gland adenocarcinoma and pheochromocytoma in a cat, J Am Anim Hosp Assoc 33, 1997, 33–36. [DOI] [PubMed] [Google Scholar]
- 6.Maher E.R., Jr., McNiel E.A. Pheochromocytoma in dogs and cats, Vet Clin North Am Small Anim Pract 27, 1997, 359–380. [DOI] [PubMed] [Google Scholar]
- 7.Twedt D.C., Wheeler S.L. Pheochromocytoma in the dog, Vet Clin North Am Small Anim Pract 14, 1984, 767–782. [DOI] [PubMed] [Google Scholar]
- 8.Bouayad H., Feeney D.A., Caywood D.D., Hayden D.W. Pheochromocytoma in dogs: 13 cases (1980–1985), J Am Vet Med Assoc 191, 1987, 1610–1615. [PubMed] [Google Scholar]
- 9.Werbel S.S., Ober K.P. Pheochromocytoma. Update on diagnosis, localization, and management, Med Clin North Am 79, 1995, 131–153. [DOI] [PubMed] [Google Scholar]
- 10.Manger W.M., Gifford R.W., Jr. Pheochromocytoma: current diagnosis and management, Cleve Clin J Med 60, 1993, 365–378. [DOI] [PubMed] [Google Scholar]
- 11.Francis R.C., Pickerodt P.A., Salewski L., Boemke W., Hohne C. Detection of catecholamines and metanephrines by radio-immunoassay in canine plasma, Vet J, 2008. Nov 19, 2008 Nov 19 [DOI] [PubMed]
- 12.Urinary Metanephrines by HPLC. Bio Rad Laboratories [Instruction Manual], Richmond, California: 94804. 1995; 956: 101–4. [Google Scholar]
- 13.Hickman P.E., Leong M., Chang J., Wilson S.R., McWhinney B. Plasma free metanephrines are superior to urine and plasma catecholamines and urine catecholamine metabolites for the investigation of phaeochromocytoma, Pathology 41, 2009, 173–177. [DOI] [PubMed] [Google Scholar]
- 14.Pacak K.E.G., Ahlman H., Bornstein S.R., et al. Phaeochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005, Nat Clin Pract Endocrinol Metab 3, 2007, 92–102. [DOI] [PubMed] [Google Scholar]
- 15.Lenders J.W., Pacak K., Walther M.M., et al. Biochemical diagnosis of pheochromocytoma: which test is best?, JAMA 287, 2002, 1427–1434. [DOI] [PubMed] [Google Scholar]
- 16.Lenders J.W., Keiser H.R., Goldstein D.S., et al. Plasma metanephrines in the diagnosis of pheochromocytoma, Ann Intern Med 123, 1995, 101–109. [DOI] [PubMed] [Google Scholar]
- 17.Raber W., Raffesberg W., Bischof M., et al. Diagnostic efficacy of unconjugated plasma metanephrines for the detection of pheochromocytoma, Arch Intern Med 160, 2000, 2957–2963. [DOI] [PubMed] [Google Scholar]
- 18.Unger N., Pitt C., Schmidt I.L., et al. Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass, Eur J Endocrinol 154, 2006, 409–417. [DOI] [PubMed] [Google Scholar]
- 19.d'Herbomez M., Forzy G., Bauters C., et al. An analysis of the biochemical diagnosis of 66 pheochromocytomas, Eur J Endocrinol 156, 2007, 569–575. [DOI] [PubMed] [Google Scholar]
- 20.Boyle J.G., Davidson D.F., Perry C.G., Connell J.M. Comparison of diagnostic accuracy of urinary free metanephrines, vanillyl mandelic acid, and catecholamines and plasma catecholamines for diagnosis of pheochromocytoma, J Clin Endocrinol Metab 92, 2007, 4602–4608. [DOI] [PubMed] [Google Scholar]
- 21.Deutschbein T., Jager A., Mann K., Petersenn S. Confounding variables for plasma metanephrines and normetanephrines may influence the diagnosis of chaeochromocytomas Endocrine Abstracts, 2009. April 25–29, European Congress of Endocrinology: Istanbul, Turkey, England, 31.
- 22.Anton A.H., Sayre D.F. Distribution of metanephrine and normetanephrine in various animals and their analysis in diverse biologic material, J Pharmacol Exp Ther 153, 1966, 15–29. [PubMed] [Google Scholar]
- 23.Kook P.H., Boretti F.S., Hersberger M., Glaus T.M., Reusch C.E. Urinary catecholamine and metanephrine to creatinine ratios in healthy dogs at home and in a hospital environment and in 2 dogs with pheochromocytoma, J Vet Intern Med 21, 2007, 388–393. [DOI] [PubMed] [Google Scholar]
- 24.Buffington C.A., Pacak K. Increased plasma norepinephrine concentration in cats with interstitial cystitis, J Urol 165 (6 Pt 1), 2001, 2051–2054. [DOI] [PubMed] [Google Scholar]
- 25.Yaworsky D.C., Wu A.H., Hill D.W. The use of plasma metanephrine to normetanephrine ratio to determine epinephrine poisoning, Clin Chim Acta 353, 2005, 31–44. [DOI] [PubMed] [Google Scholar]
- 26.Averbuch S.D., Steakley C.S., Young R.C., et al. Malignant pheochromocytoma: effective treatment with a combination of cyclophosphamide, vincristine and dacarbazine, Ann Intern Med 109, 1988, 267–273. [DOI] [PubMed] [Google Scholar]
- 27.Wan W.H., Tan K.Y., Ng C., et al. Metastatic malignant phaeochromocytoma: a rare entity that underlies a therapeutic quandary, Asian J Surg 29, 2006, 294–302. [DOI] [PubMed] [Google Scholar]