Abstract
This report describes the clinical presentation, diagnosis and treatment of a cat with vegetative valvular endocarditis temporally associated with natural infection with Bartonella henselae. Lethargy, abnormal gait and weakness were the main clinical signs that resulted in referral for diagnostic evaluation. Using a novel and sensitive culture approach, B henselae was isolated from the blood. Following antibiotic therapy there was total resolution of clinical signs, the heart murmur, the valvular lesion by echocardiography, and no Bartonella species was isolated or amplified from a post-treatment blood culture. In conjunction with previous case reports, infective endocarditis can be associated with natural B henselae infection in cats; however, early diagnosis and treatment may result in a better prognosis than previously reported.
A 6.8 kg, 3-year-old male castrated cat was referred to the Veterinary Teaching Hospital, College of Veterinary Medicine, North Carolina State University (NCSU-VTH) for evaluation of a heart murmur and possible aortic thromboembolism. During the previous 24 h, the owners noted progressive lethargy and weakness in the hind limbs. The cat lived exclusively indoors, with another cat and a dog, both of which were reportedly healthy. Past medical history included a heart murmur (unknown duration) and idiopathic megacolon. Vaccinations were current but the cat had not been tested for feline leukemia virus (FeLV) or feline immunodeficiency virus (FIV).
At the time of referral, physical examination revealed a grade III/VI systolic heart murmur with no gallop sounds or arrhythmias. Femoral pulses were weak bilaterally. Vital signs and indirect blood pressure measurements were normal (systolic arterial pressure 155 mmHg, Doppler). Poorly localized pain was elicited on palpation of the pelvic region. Neurological and orthopedic examinations were unremarkable. Hematological and serum biochemical abnormalities included leukocytosis (white blood cell count 22.36×103/l; reference range (RR) 4.28–14.3×103/l) characterized by neutrophilia (20.348×103/l [2.773–6.975×103/l]), and a regenerative left shift (bands 447×103/l) associated with mild toxicity. Albumin was mildly decreased (2.9 g/dl; RR 3.0–4.2 g/dl) and creatine kinase (CK) activity was increased (2275 IU; RR 72–480). The cat was hyperfibrinogenemic (500 mg/dl; RR 50–300 mg/dl). Urinalysis was unremarkable. The cat was seronegative for FIV and FeLV.
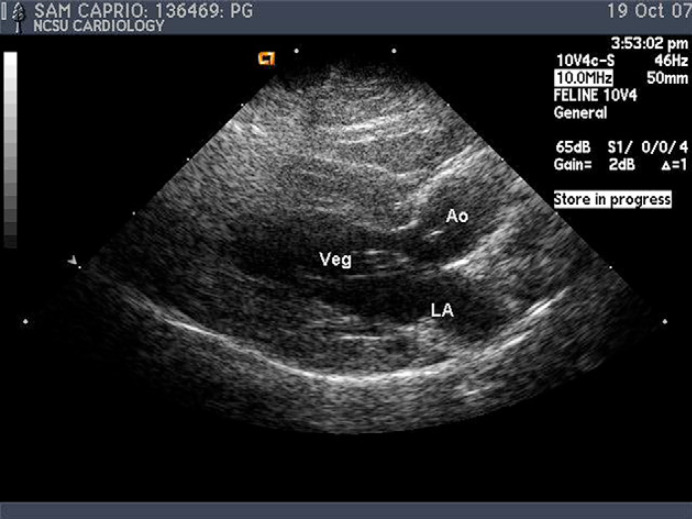
Mild generalized cardiomegaly was noted on thoracic radiographs, although no signs of congestive heart failure were identified. Abdominal and pelvic radiographs were unremarkable. A resting electrocardiogram was within normal limits, disclosing sinus rhythm. Two-dimensional echocardiography demonstrated an independently oscillating vegetation attached to the anterior mitral valve leaflet (Fig. 1). There was mild mitral valve regurgitation on color-flow Doppler examination. No other valvular lesions or functional abnormalities were noted. Based on these findings, a presumptive diagnosis of mitral valve endocarditis was made. In light of the cat's weakness, poorly localized pain, bilateral weak pulses, and increased CK value, aortic embolization was also suspected.
Fig 1.
Left parasternal long axis view. An echogenic vegetative lesion (Veg) associated with the anterior mitral leaflet was noted to move independently of the leaflet. LA=left atrium. Ao=Aorta.
Three 3 ml blood samples were collected aseptically at 60 min intervals from the right jugular vein and submitted for standard aerobic and anaerobic culture. To enhance the molecular detection and microbiological isolation of Bartonella species in animal and human patients our research group developed a diagnostic platform that incorporates enrichment culture of patient samples in an optimized insect cell culture medium (Bartonella alpha Proteobacteria growth medium, BAPGM). Therefore, Bartonella species polymerase chain reaction (PCR) was performed directly from the blood sample, and at the same time 2 ml of Ethylenediaminetetraacetic acid (EDTA)-anti-coagulated blood were inoculated into liquid BAPGM and incubated at 35°C in 5% CO2, water-saturated atmosphere. After a 7-day incubation period, 1 ml of liquid culture was sub-inoculated onto 10% sheep blood-agar plates and incubated for 14 days. Bartonella species-like colonies (too numerous to count) were pooled from the plate for DNA extraction and PCR amplification. DNA sequencing of partial 16S–23S intergenic spacer (ITS) region amplification products obtained directly from the blood sample, the BAPGM enrichment culture and isolate from the subculture, all identified Bartonella henselae (a Houston-1-like strain). Standard aerobic and anaerobic blood cultures were negative. Bartonella species serology was not initially requested.
Antibiotic therapy was initiated after obtaining blood cultures, which included amoxicillin–clavulanate acid (22 mg/kg per os [PO] q24 h), marbofloxacin (5 mg/kg PO q24 h for 6 weeks) and azithromycin (10 mg/kg PO q24 h for 7 days followed by every other day administration for 6 weeks). Clopidogrel (1/4 of a 75 mg tablet PO q24 h for 6 weeks) was also prescribed as an anticoagulant, in an attempt to prevent further systemic embolization. Treatment with amoxicillin–clavulanate was discontinued in 3 days due to vomiting associated with its administration.
On re-evaluation 2 weeks later, the cat's attitude, physical status, and gait had improved. A repeat echocardiogram revealed persistent thickening of the mitral valve, but the previously observed vegetative lesion had resolved. Three weeks after completing administration of antibiotic therapy (9 weeks after the initial echocardiogram) the cat was reportedly healthy and the heart murmur could no longer be auscultated. Repeat echocardiography revealed resolution of the mitral valvular lesion (Fig. 2). A post-treatment BAPGM enrichment blood culture and PCR failed to isolate or detect B henselae DNA. Also at this time, 1 month after finishing the antibiotic therapy, the cat was B henselae seroreactive by indirect immunofluorescent antibody (IFA) testing at a titer of 1:1024. During a recheck evaluation 20 months after endocarditis was diagnosed, the cat had reportedly remained healthy, no murmur could be auscultated and the B henselae titer was 1:256.
Fig 2.
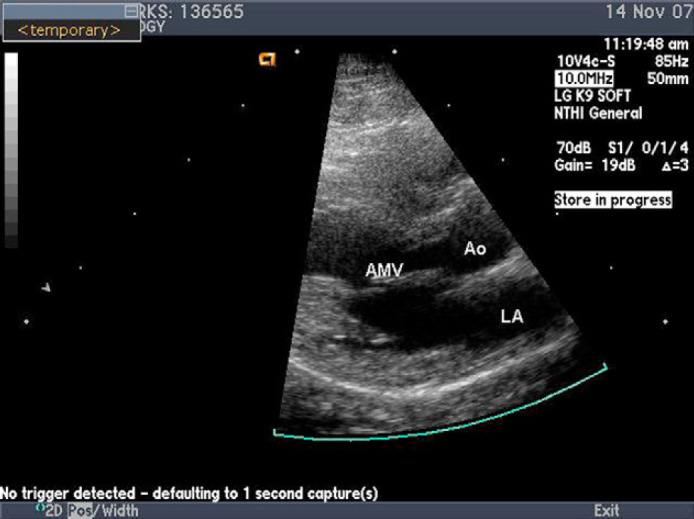
Left parasternal long axis view. Image was obtained 9 weeks after the image in Fig. 1. The vegetative mitral valve lesion is no longer present.
Bartonella species are fastidious, hemotropic, arthropod-transmitted bacteria that have emerged as important pathogens in domestic animals and people. Bartonella species has been reported to have tropism for cardiac valves and are recognized as a causative agent of endocarditis in domestic animals (cats, cows, dogs and sea otters) and human patients. 1,2 Bacterial endocarditis of any cause has been infrequently reported in cats; to our knowledge, less than 20 cases of vegetative endocarditis have been reported in the USA and Australia. 3,4 In a case series involving six cats, Bartonella species were isolated from two cats with aortic valve endocarditis, although due to the high prevalence of occult B henselae bacteremia, direct causality was not proven. 4 In this series, all six cats were presented for signs of heart failure and outcome was poor, with only two cats surviving longer than 5 months. A fatal case of B henselae endocarditis was reported in a cat in 2003 and a second case was recently reported. 3,5 Both of these cats were diagnosed at necropsy by amplifying B henselae genotype I from the affected heart valve. To our knowledge, this is the first case of Bartonella species infection in a domestic animal in which antimicrobial administration resulted in resolution of endocarditis. The BAPGM enrichment culture platform was used to document the initial infection, but more importantly, this highly sensitive approach supported therapeutic elimination of B henselae infection after antibiotic treatment. Return to a healthy state, in conjunction with resolution of the heart murmur and the vegetative valvular lesion, supported the negative post-antibiotic culture results.
Bartonella species were first reported to cause endocarditis in humans in 1993, when cases caused by Bartonella quintana, Bartonella elizabethae and B henselae were reported. 6–10 In humans at least eight species have been associated with endocarditis, including B henselae, B quintana, Bartonella rochalimae, Bartonella elizabethae, Bartonella vinsonii subspecies berkhoffii, B vinsonii subspecies arupensis, Bartonella koehlerae, Bartonella alsatica and Bartonella washoensis. 6–11 B vinsonii subspecies berkhoffii was originally isolated from a dog with aortic endocarditis in 1993. During the ensuing years, numerous Bartonella species have been identified and these bacteria are now considered an important cause of blood culture-negative endocarditis in dogs and human patients. 8–10,12–14
Domestic cats are a major natural reservoir host for B henselae, Bartonella clarridgeiae and B koehlerae. Cats are considered healthy carriers of these bacteria. As a reservoir host, cats can transmit these bacteria to humans. 1–3 As B henselae bacteremia has been reported in 8–56% of healthy cats in various study populations, 15 one way to support a diagnosis of Bartonella species endocarditis is by demonstrating the organism in valvular lesions. In two previous reports B henselae DNA was amplified and sequenced directly from cat heart valves obtained at necropsy. 4,6 Although this approach is supportive of a diagnosis of B henselae endocarditis, immunohistochemistry or DNA hybridization techniques that localize large numbers of these bacteria within the heart valves would better support direct causation by a Bartonella species in a reservoir-adapted host, such as a cat. In our case, conventional blood cultures were negative, whereas the same 16S–23S ITS strain of B henselae was amplified and sequenced by direct PCR from the blood sample, from the BAPGM enrichment culture and from a subculture isolate at the time endocarditis was diagnosed. Although B henselae can often be isolated effectively when culturing cat blood samples using standard blood-agar plates in a high CO2 incubator, the BAPGM diagnostic approach was chosen due to its high sensitivity. 16,17 In this cat, all three PCR components of the BAPGM diagnostic platform were negative following antibiotic treatment. Clinical signs also resolved completely following antibiotic therapy, in conjunction with echocardiographic resolution of the vegetative lesion. Collectively, our findings, in conjunction with previous reports, are supportive of B henselae as a cause of ‘culture-negative’ endocarditis in cats. The term ‘culture-negative’ refers to lack of growth when using standard aerobic and anaerobic blood culture techniques and conditions, as specialized microbiologic approaches are almost always required for isolation of Bartonella species.
Treatment with azithromycin for at least 6 weeks has been recommended for Bartonella species infections, although various antibiotics appear to be effective, based on in vitro testing and outcomes from human and veterinary case reports. Unfortunately, data from controlled efficacy studies with long-term follow-ups are lacking. Treatment failures have been reported, which suggests that therapeutic elimination of Bartonella species infections remains a challenge in some cases. Recently, antibiotic resistance genes in B henselae have been characterized, which suggests that alternative treatment options may be required in some patients (EB Breitschwerdt, unpublished data).
The high B henselae antibody titer 1 month after therapy was an expected finding in this case. Although not uniformly true, dogs and humans with Bartonella species endocarditis generally have very high antibody titers, likely due to recurrent bouts of bacteremia originating from infected valves. 18–20 Of diagnostic importance, a relapsing bacteremia and fluctuating IgG antibody titers can persist in experimentally infected cats for up to 18 months. 19,20 Based on retrospective studies in humans and dogs, serological testing after therapy may be useful when attempting to support therapeutic elimination of Bartonella species infections, as clinical improvement is usually accompanied by a decrease in antibodies. 21,22 In our case, the cat remained B henselae seroreactive 20 months after diagnosis of endocarditis; however, there was a fourfold decrease in antibody titer.
This case illustrates that feline endocarditis associated with natural infection with B henselae may be a treatable condition if recognized early, and might carry a better prognosis than previously described. In addition, enrichment Bartonella species culture and PCR would be recommended for the laboratory evaluation of cats with vegetative valvular lesions on echocardiography.
Acknowledgements
This research was supported in part by the State of North Carolina and a grant from the American College of Veterinary Internal Medicine Foundation.
References
- 1.Anderson B.E., Neuman M.A. Bartonella spp as emerging human pathogens, Clin Microbiol Rev 10, 1997, 203–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chomel B.B., Kasten R.W., Williams C., et al. Bartonella endocarditis: a pathology shared by animal reservoirs and patients, Rickettsiology and rickettsial diseases – fifth international conference. Ann N Y Acad Sci 1166, 2009, 120–126. [DOI] [PubMed] [Google Scholar]
- 3.Chomel B.B., Boulouis H.J., Maruyama S., Breitschwerdt E.B. Bartonella spp in pets and effect on human health, Emerg Infect Dis 12, 2006, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik R., Barrs V.R., Church D.B., et al. Vegetative endocarditis in six cats, J Feline Med Surg 1, 1999, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chomel B.B., Wey A.C., Kasten R.W., Stacy B.A., Labelle P. Fatal case of endocarditis associated with Bartonella henselae type I infection in a domestic cat, J Clin Microbiol 41, 2003, 5337–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raoult D., Fournier P.E., Drancourt M., et al. Diagnosis of 22 new cases of Bartonella endocarditis, Ann Intern Med 125, 1997, 646–652. [DOI] [PubMed] [Google Scholar]
- 7.Raoult D., Fournier P.E., Vandenesch F., et al. Outcome and treatment of Bartonella endocarditis, Arch Intern Med 163, 2003, 226–230. [DOI] [PubMed] [Google Scholar]
- 8.Roux V., Eykyn S.J., Wyllie S., Raoult D. Bartonella vinsonii subsp berkhoffii as an agent of afebrile blood culture-negative endocarditis in a human, J Clin Microbiol 38, 2000, 1698–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avidor B., Graidy M., Efrat G., et al. Bartonella koehlerae, a new cat-associated agent of culture-negative human endocarditis, J Clin Microbiol 42, 2004, 3462–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Scola B., Raoult D. Culture of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients, Medicine (Baltimore) 80, 1999, 245–251. [DOI] [PubMed] [Google Scholar]
- 11.Kosoy M., Murray M., Gilmore R.D., Jr., Bai Y., Gage K.L. Bartonella strains from ground squirrels are identical to Bartonella washoensis isolated from a human patient, J Clin Microbiol 41, 2003, 645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breitschwerdt E.B., Kordick D.L., Malarkey D.E., Keene B., Hadfield Tl, Wilson K. Endocarditis in a dog due to infection with a novel Bartonella subspecies, J Clin Microbiol 33, 1995, 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kordick D.L., Swaminathon B., Greene C.E., et al. Bartonella vinsonii subsp berkhoffii subsp nov isolated from dogs; Bartonella vinsonii subsp vinsonii; and emended description of Bartonella vinsonii, Int J Syst Bacteriol 46, 1996, 704–709. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald K.A., Chomel B.B., Kittleson M.D., Kasten R.W., Thomas W.P., Pesavento P. A prospective study of canine infective endocarditis in northern California (1999–2001): emergence of Bartonella as a prevalent etiologic agent, J Vet Intern Med 18, 2004, 56–64. [DOI] [PubMed] [Google Scholar]
- 15.Chomel B.B., Kasten R.W., Floyd-Hawkins K.A., Kikuchi Y., Koehler J.E., Pedersen N.C. Experimental and natural infection with Bartonella henselae in domestic cats, Comp Immunol Microbiol Infect Dis 20, 1997, 41–51. [DOI] [PubMed] [Google Scholar]
- 16.Duncan A.W., Maggi R.G., Breitschwerdt E.B. A combined approach for the enhanced detection and isolation of Bartonella species in dog blood samples: pre-enrichment liquid culture followed by PCR and subculture onto agar plates, J Microbiol Methods 69, 2007, 273–281. [DOI] [PubMed] [Google Scholar]
- 17.Maggi R.G., Duncan A.W., Breitschwerdt E.B. Novel chemically modified liquid medium that will support the growth of seven Bartonella species, J Clin Microbiol 43, 2005, 2651–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulein R., Seubert A., Gille C., et al. Invasion and persistent intracellular colonization of erythrocytes. A unique parasitic strategy of the emerging pathogen Bartonella, J Exp Med 193, 2001, 1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Reilly K.L., Bauer R.W., Freeland R.L., et al. Acute clinical disease in cats following infection with a pathogenic strain of Bartonella henselae (LSU16), Infect Immun 67, 1999, 3066–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breitschwerdt E.B. Feline bartonellosis and cat scratch disease, Vet Immunol Immunopathol 123, 2008, 167–171. [DOI] [PubMed] [Google Scholar]
- 21.Breitschwerdt E.B., Blann K.R., Stebbins M.E., et al. Clinicopathological abnormalities and treatment response in 24 dogs seroreactive to Bartonella vinsonii (berkhoffii) antigens, J Am Anim Hosp Assoc 40, 2004, 92–101. [DOI] [PubMed] [Google Scholar]
- 22.Regnery R.L., Rooney J.A., Johnson A.M., et al. Experimentally induced Bartonella henselae infections followed by challenge exposure and antimicrobial therapy in cats, Am J Vet Res 58, 1997, 803. [PubMed] [Google Scholar]