Abstract
Itraconazole (Icz) has been known to increase the cyclosporine (CsA) trough level in human transplant patients. However, the interaction of Icz with CsA has not been reported in cats. In this study, the effect of multiple dosing of Icz on the pharmacokinetics of CsA in three healthy cats was investigated. The treatments included CsA 5 mg/kg alone and CsA 5 mg/kg+multiple-dose of Icz 10 mg/kg. Co-administration of Icz with CsA resulted in significant increases of oral bioavailability of CsA. The results of our study suggest that administration of multiple therapeutic doses of Icz may decrease the required CsA dosage in CsA-based immunosuppressive therapy used for renal transplantation in cats.
Renal transplantation has been recognised as one of the therapeutic options for the end stage kidney failure in cats. Cyclosporine (CsA) has been used as a core immunosuppressive drug in preventing acute allograft rejection in cats. 1,2 CsA is known as a substrate of cytochrome P450 (CYP) 3A and P-glycoprotein in humans. 3–5 In feline renal transplant patients, ketoconazole (Kcz) reduces the dosage of CsA, 6 which suggests that CsA metabolism is mediated by inhibition of both CYP3A and P-glycoprotein in cats as in humans. However, the use of Kcz leads to other problems associated with the side effects of Kcz, the main problem being its hepatotoxicity. If hepatotoxicity develops, Kcz needs to be removed from the immunosuppressive protocol, which may result in acute allograft rejection. It is known that itraconazole (Icz) can also inhibit CYP3A and P-glycoprotein, and it has less toxicity than Kcz. It has been reported that Icz significantly increases CsA bioavailability and may lower the daily drug cost in human renal transplant patients treated with CsA-based immunosuppressive therapy. 7 In cats, however, it has not been reported whether Icz affects the oral bioavailability of CsA. The aim of this study was to evaluate the effect of Icz on the CsA blood level in cats. We investigated the effects of multiple oral administration of Icz at therapeutic doses on the pharmacokinetics of CsA in healthy cats.
Three healthy male cats were used in this study. Their body weights ranged from 3.4 to 5.9 kg and their ages ranged from 1 to 2 years old. Prior to this study, all cats were confirmed to be healthy based on physical examination, complete blood count, biochemical profile and urinalysis. The cats were allocated to two treatment groups and the study was conducted by a crossover design. In a clinical setting, CsA is administered at a dosage of 3–5 mg/kg twice daily for renal transplantation in cats. 8 Therefore, the dose of CsA (Cicporal; Nichi-iko, Toyama, Japan) was adjusted to approximately 5 mg/kg (range, 4.9–5.9 mg/kg, according to capsule strength and cat weight; mean dose 5.5 mg/kg). In treatment group A, cats received oral CsA alone. In treatment group B, cats were given about 10 mg/kg (therapeutic dose level) of Icz (Toracona; Nichi-iko, Toyama, Japan; range, 8.5–11.1 mg/kg; mean dose 9.9 mg/kg) once daily for 3 weeks (day 1 to day 21) and CsA 2 h after the Icz treatment on day 21. The duration of Icz treatment was determined according to a previous report that steady state blood concentrations of Icz were achieved at 14–21 days in cats. 9 The washout times of each treatment were more than 1 month.
Whole blood samples were drawn through the cephalic vein catheter at 0.5, 1, 2, 4, 6, 8, 12 and 24 h after CsA administration and were collected in tubes containing ethylenediaminetetraacetic acid (EDTA). The cephalic vein catheter was flushed with heparinised saline (0.9% NaCl) solution after each sample collection. Blood samples were stored at −80°C until measurements. Measurement of the whole blood CsA concentration was performed using a fluorescence polarisation immunoassay (FPIA) using TDx Cyclosporine A Dynapack kit (Abbott, Tokyo, Japan). Documentation provided with the kit indicates that this FPIA method is sensitive within a CsA concentration range of 25–1500 ng/ml. When the sample contained CsA concentration above the upper limit of detection of this test kit (>1500 ng/ml), it was measured after diluting the blood samples two times with calibration solution A of the X Systems Cyclosporine and Metabolites Whole Blood Calibrators kit (Abbott, Tokyo, Japan).
The maximum blood concentration (Cmax) and its corresponding time (t1/2) were determined for each cat by observation of the blood CsA concentration versus time profile. The area under the curve from 0 to 24 h (AUC0–24) after a CsA administration was calculated by the linear trapezoidal method. The terminal elimination rate constant (k) was calculated by linear least squares regression analysis using the last three measurement points in the log-linear terminal phase. The tmax was estimated as 0.693/k. The area under the first moment curve from 0 to 24 h (AUMC0–24) after a CsA administration was also calculated by the linear trapezoidal method. The mean residence time (MRT) was calculated as AUMC0–24/AUC0–24.
Differences in the pharmacokinetic parameters between each treatment were analysed using the paired t-test and were regarded to be statistically significant at P<0.05. Each value is shown as the mean±standard error (SE).
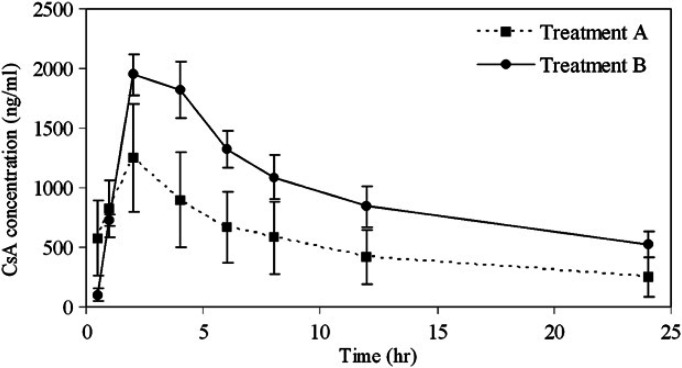
The blood concentration–time curves after the two treatments are shown in Fig. 1. The AUC0–24 of cats with Icz treatment (treatments B) was significantly higher than that of cats treated with CsA alone (treatment A) (P=0.04). The average CsA blood concentrations of cats treated with CsA–multiple oral dosing of Icz were almost two times higher than in cats treated with CsA alone at 12 and 24 h (P=0.02 and 0.04, respectively). In this study, one cat showed the CsA blood concentrations below the limit of quantification at 0.5 h with both treatments A and B. In this case, the lower limit value, 25 ng/ml, was used for the calculation of AUC0–24. Pre-administration of multiple doses of Icz did not significantly affect Cmax, tmax, t1/2 and MRT. The pharmacokinetic parameters of CsA with or without Icz are listed in Table 1.
Fig 1.
Mean CsA blood concentration–time curves of the three healthy cats following treatment group A (CsA alone), treatment group B (CsA+multiple therapeutic doses of Icz). Values are presented as means±SE.
Table 1.
Effects of multiple doses of oral Icz on the pharmacokinetic parameters of CsA in three healthy cats
| Parameters | AUC0–24 (ngh/ml) | Cmax (ng/ml) | tmax (h) | t1/2 (h) | C12 (ng/ml) | C24 (ng/ml) | AUMC0–24 (ngh2/ml) | MRT (h) |
|---|---|---|---|---|---|---|---|---|
| Treatment A | 12531±5506 | 1395±389 | 1.33±0.33 | 12.15±2.19 | 421±227 | 252±163 | 110318±60468 | 8.02±0.92 |
| Treatment B | 22923±3282*, a | 2075±195 | 3.33±0.67 | 15.43±0.83 | 840±170*, b | 522±111* c | 218803±40126* d | 9.45±0.37 |
Values are presented as means±SE. Treatment A=CsA alone. Treatment B=CsA+multiple therapeutic doses of Icz. C12 and C24=CsA blood concentrations at 12 h and 24 h after CsA intake, respectively.
*,a–dStatistically different from treatment group A (P=0.04, 0.02, 0.04 and 0.04, respectively).
Although hepatic metabolism is considered to be of prime importance for drug absorption, intestinal metabolism may play a much greater role in the pharmacokinetics of orally administered drugs than previously thought. Metabolism of CsA is mediated primarily by CYP, in particular CYP3A, in the liver and small intestine in humans. 3,5 P-glycoprotein is also associated with excretion of CsA, acting as a drug efflux pump that actively transports CsA back into the intestinal lumen. 5,10 It was reported that as much as 50% of oral CsA metabolism may be attributed to intestinal metabolism in humans. 4,5 Icz inhibits both hepatic CYP3A and P-glycoprotein. 11 Based on the results of our study, administration of multiple oral dosing of Icz could increase the blood concentration and AUC0–24 of CsA compared to CsA alone. In addition, with regard to t1/2 and MRT, there were no statistical differences between CsA alone and CsA with Icz. This suggests that administration of multiple doses of Icz may increase oral bioavailability of CsA in cats, mainly by decreasing the first-pass effect that is possibly associated with both CYP3A and P-glycoprotein activities.
Multiple therapeutic doses of Icz (treatment B) significantly increased the CsA blood concentrations at 12 h and 24 h compared with CsA alone (treatment A), and kept the CsA blood concentration within therapeutic ranges (400–600 ng/ml by FPIA) at 24 h after CsA intake. The use of Icz will, therefore, reduce the dosage as well as the administration frequency of CsA. This would be of clinical importance to make renal transplantation clinically acceptable for the owners. It has been reported that Icz is well tolerated with no apparent adverse effects such as hepatotoxicity in cats when administered 10 mg/kg of Icz twice daily for 6 weeks. 9 Icz may become an alternative option to Kcz for feline renal transplant patients needing continuous life-long immunosuppression.
In this study, FPIA using TDx was used to measure the CsA blood concentration because TDx is commercially available and provides the test results quickly. McAnulty and Lensmeyer reported that FPIA method which cross-reacted with not only the parent CsA but also its metabolites showed about two times higher values than the high performance liquid chromatography (HPLC) assay detecting the parent CsA in cats. 12 Therefore, the CsA blood concentrations measured in this study by FPIA might have been overestimated to some degree.
In conclusion, the results of our study showed that Icz significantly increases the oral bioavailability of CsA in healthy cats and suggest that co-administration of CsA with multiple oral dosing of Icz may replace the dose frequency from a twice a day schedule to once a day. Further studies on the effects of long-term treatment are needed before applying co-administration of Icz and CsA to feline renal transplant patients.
References
- 1.Katayama M., McAnulty J. Renal transplantation in cats: patient selection and preoperative management, Compend Cont Edu Pract Vet 24, 2002, 868–873. [Google Scholar]
- 2.Katayama M., McAnulty J. Renal transplantation in cats: techniques, Complications, and immunosuppression, Compend Cont Edu Pract Vet 24, 2002, 874–882. [Google Scholar]
- 3.Whalen R.D., Tata P.N., Burckart G.J., Venkataramanan R. Species differences in the hepatic and intestinal metabolism of cyclosporine, Xenobiotica 29, 1999, 3–9. [DOI] [PubMed] [Google Scholar]
- 4.Kolars J.C., Awni W.M., Merion R.M., Watkins P.B. First-pass metabolism of cyclosporin by the gut, Lancet 338, 1991, 1488–1490. [DOI] [PubMed] [Google Scholar]
- 5.Hebert M.F. Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery, Adv Drug Deliv Rev 27, 1997, 201–214. [DOI] [PubMed] [Google Scholar]
- 6.McAnulty J.F., Lensmeyer G.L. The effects of ketoconazole on the pharmacokinetics of cyclosporine A in cats, Vet Surg 28, 1999, 448–455. [DOI] [PubMed] [Google Scholar]
- 7.Florea N.R., Capitano B., Nightingale C.H., Hull D., Leitz G.J., Nicolau D.P. Beneficial pharmacokinetic interaction between cyclosporine and itraconazole in renal transplant recipients, Transplant Proc 35, 2003, 2873–2877. [DOI] [PubMed] [Google Scholar]
- 8.Gregory C., Bernsteen L. Organ transplantation in clinical veterinary practice. Slatter D. Textbook of small animal surgery, 3rd edn, 2003, Saunders: Philadelphia, 112–136. [Google Scholar]
- 9.Boothe D.M., Herring I., Calvin J., Way N., Dvorak J. Itraconazole disposition after single oral and intravenous and multiple oral dosing in healthy cats, Am J Vet Res 58, 1997, 872–877. [PubMed] [Google Scholar]
- 10.Lown K.S., Mayo R.R., Leichtman A.B., et al. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine, Clin Pharmacol Ther 62, 1997, 248–260. [DOI] [PubMed] [Google Scholar]
- 11.Sakaeda T., Iwaki K., Kakumoto M., et al. Effect of micafungin on cytochrome P450 3A4 and multidrug resistance protein 1 activities, and its comparison with azole antifungal drugs, J Pharm Pharmacol 57, 2005, 759–764. [DOI] [PubMed] [Google Scholar]
- 12.McAnulty J.F., Lensmeyer G.L. Comparison of high performance liquid chromatography and immunoassay methods for measurement of cyclosporine A blood concentrations after feline kidney transplantation, Vet Surg 27, 1998, 589–595. [DOI] [PubMed] [Google Scholar]