Abstract
A 14-year-old domestic short-hair cat presented with a history of intermittent malaise and increased drinking. A diagnosis of hyperthyroidism and cholelithiasis was made by a combination of blood testing, radiography and ultrasonography. After medical management of hyperthyroidism, thyroidectomy and cholecystectomy were successfully performed. Removed choleliths were comprised of calcium carbonate and bilirubinate. Histopathological analysis of tissue suggested low grade pancreatic and hepatobiliary disease, as well as hyperthyroidism, might have contributed to stone formation.
A14-year-old female neutered domestic short-hair cat presented with a 4 week history of intermittent malaise characterised by anorexia, lethargy and a tendency to sit in a hunched position with pilierection. Between episodes the cat was reported as bright, with some increased drinking. Physical examination revealed a bright, alert, fractious cat in sub-optimal body condition (condition score 2.5/5, weight 4.65 kg). There was a firm mobile nodule, approximately 1 cm in diameter, in the cervical region lateral to the trachea on the left. The heart rate was 200 beats per minute and pulse strength was good. No murmurs or gallop rhythms were detected.
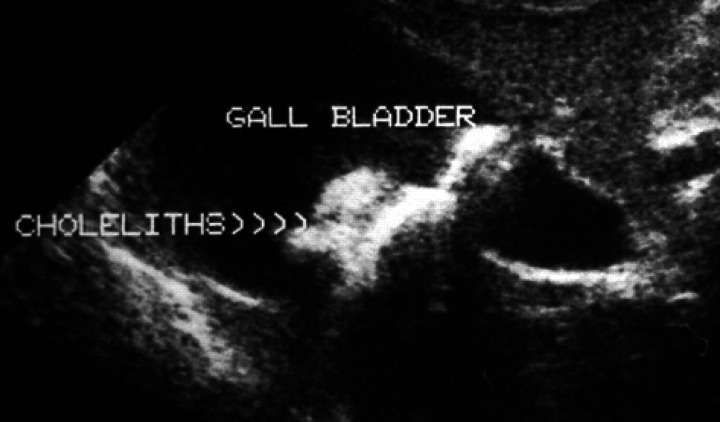
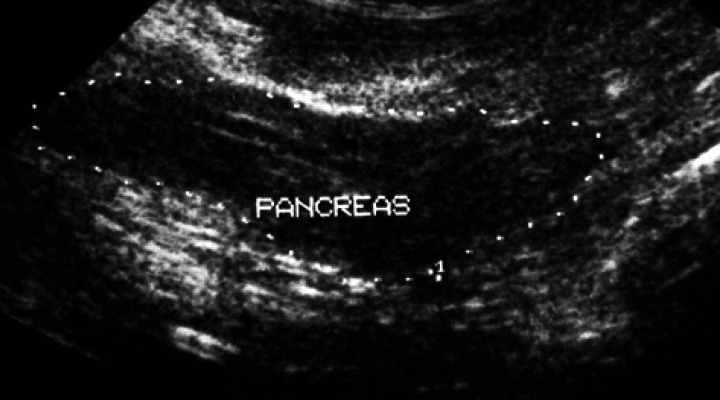
Clinical pathology results are summarised (Table 1). An electrocardiogram showed a sinus tachycardia (240 beats per minute) with normal complex sizes, a notched QRS and a mean electrical axis of −120°, consistent with conduction block. Lateral and dorsoventral thoracic radiographs showed a normal cardiac silhouette. Lateral and ventrodorsal abdominal radiographs [Fig 1 (a, b)] showed a cluster of polygonal mineral densities in the right anterior abdomen in a position consistent with the gall-bladder. Echocardiography showed left atrial enlargement (left atrial to aortic root ratio 1.51, normal <1.3). Non-invasive blood pressure determination was not available. Abdominal ultrasonographic examination revealed numerous convex, completely echogenic bodies within the gallbladder (Fig 2). The right lobe of the pancreas was considered relatively hypoechoic (Fig 3). No cells or crystals were seen on cytological examination of bile aspirated under ultrasound guidance and bacterial culture grew no organisms. Serum feline trypsin-like immunoreactivity (TLI) was increased (Table 1). A diagnosis of hyperthyroidism with cholelithiasis and possible pancreatic disease was made.
Table 1.
Laboratory findings and reference values at the time of presentation in a cat with cholelithiasis and hyperthyroidism
| Day 1 | Control range | |
|---|---|---|
| Blood chemistry | ||
| Sodium | 157 | 145–165 mmol/l |
| Potassium | 3.4 | 3.6–5.8 mmol/l |
| Urea | 12.6 | 5–10 mmol/l |
| Chloride | 121 | 112–129 mmol/l |
| Calcium | 2.64 | 2.1–2.9 mmol/l |
| Phosphate | 1.91 | 1.1–2.8 mmol/l |
| Glucose | 4.0 | 3.5–6.5 mmol/l |
| Total protein | 73 | 60–82 g/l |
| Albumin | 35 | 25–39 g/l |
| Globulin | 38 | 26–50 g/l |
| Bilirubin | 6 | 0–6.8 μmol/l |
| Alkaline phosphatase | 112 | <100 IU/l |
| Alanine aminotransferase | 109 | <75 IU/l |
| Creatinine | 99 | 40–150 μmol/l |
| Cholesterol | 3.5 | 1.5–6.0 |
| Haematology | ||
| Red blood cells | 8.63 | 5–11×1012/l |
| Haemoglobin | 14.0 | 8–15 g/dl |
| Haematocrit | 0.377 | 0.26–0.46 |
| Mean cell volume | 43.6 | 37–49 fl |
| Mean cell haemoglobin concentration | 37.1 | 32–35 g/dl |
| Platelets | 371 | 150–400×109/l |
| White blood cells | 9.7 | 5.5–19.5×109/l |
| Neutrophils | 6.5 | 2.5–12.5×109/l |
| Lymphocytes | 2.4 | 1.5–7.0×109/l |
| Monocytes | 0.1 | 0–0.9×109/l |
| Eosinophils | 0.7 | 0.1–1.5×109/l |
| Urinalysis | ||
| Source | Cystocentesis | |
| Specific gravity | 1.050 | |
| pH | 6.0 | |
| Protein | Trace | |
| Nitrite | — | |
| Bilirubin | — | |
| Blood | — | |
| Ketones | — | |
| Glucose | — | |
| Serology | ||
| FeLV antigen | Negative | |
| FIV antibody | Negative | |
| Thyroxine | 206 | 19–65 nmol/l |
| Feline TLI | 132 | 12–82 μg/l |
Fig 1.
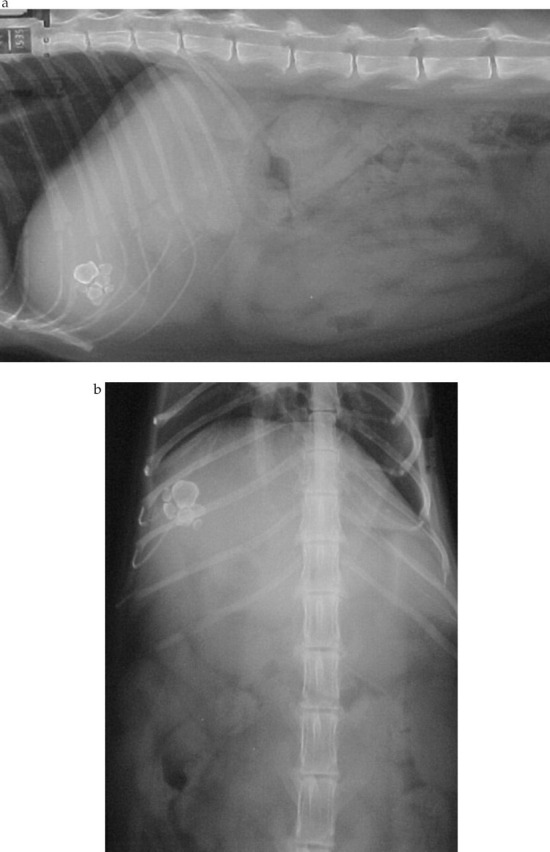
(a) Lateral and (b) ventrodorsal abdominal radiographs showing radio-opaque polygonal mineral densities in the right anterior abdomen consistent with cholelithiasis
Fig 2.
Ultrasound image of the gall-bladder showing numerous completely echogenic choleliths
Fig 3.
Ultrasound image of the right lobe of the pancreas which was considered relatively hypoechoic, suggestive of pancreatic pathology
Serum thyroxine was reduced by administration of the carbimazole (1 mg/kg TID, Neo-Mercazole, Roche, Welwyn Garden City, UK) to 5.8 nmol/l before surgery was performed 5 weeks later. At the time of surgery serum potassium was normal at 4.4 mmol/l.
At surgery, a cholecystectomy was performed as previously described (Martin 1993). The gallbladder was dissected from its hepatic fossa using a combination of sharp and blunt dissection. A 3–0 polypropylene suture (Prolene; Ethicon, Edinburgh, UK) was placed in the fundus of the gall-bladder and the dissection was performed from the base of the fundus working towards the cystic duct. Once the gall-bladder was completely mobilized from the hepatic parenchyma, the cystic duct was clamped and ligated with a 3–0 polypropylene ligature. Care was taken to ensure that the hepatic ducts were not included during the ligation of the cystic duct. The cystic artery was electrocoagulated following the removal of the gall-bladder distal to the ligation on the cystic duct. Haemorrhage from the hepatic fossa following the removal of the gall-bladder was minimal and required no intervention. A single representative liver biopsy was obtained from the quadrate lobe of the liver at surgery. At surgery the pancreas appeared normal but a pancreatic biopsy was obtained by open surgical dissection from the tip of the right limb because of the ultrasonographic appearance and the raised TLI. Abdominal closure was routinely performed. A bilateral thyroidectomy was performed using an extra-capsular dissection on the left side and a modified intra-capsular technique on the right as previously described (Flanders 1999). Post-operative recovery was routine and the cat was behaving normally 14 days post-operatively. At 18 months post-operatively, however, the cat was euthanased because of renal failure, blood samples showed no increase in liver enzymes.
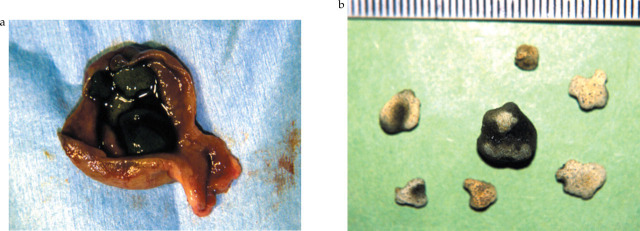
Numerous choleliths were found in the gallbladder (Fig 4). Analysis of the removed choleliths showed them to consist of 50% calcium carbonate and 50% calcium bilirubinate.
Fig 4.
(a) Resected and opened gall-bladder containing choleliths and (b) removed calcium carbonate and calcium bilirubinate containing choleliths (scale in mm)
Histopathological examination revealed moderate, diffuse, lymphoplasmacytic inflammation within the submucosa of the gall-bladder. In the liver there was diffuse, moderate cloudy swelling of hepatocytes with multiple small foci of hepatocytes containing slight to moderate green/gold pigment consistent with retained bile. There was slight to moderate hepatic periportal inflammation consisting of lymphocytes and plasma cells. In the pancreas there were slight focal accumulations of lymphocytes adjacent to large pancreatic ducts consistent with chronic (lymphocytic) periductal inflammation. Examination of thyroid tissue confirmed the presence of a single thyroid adenoma on the left.
Discussion
Cholelithiasis is a common clinical problem in humans that has been described only rarely in cats. Clinical findings in humans include abdominal pain and obstructive jaundice. In previous reports of cats, choleliths were either incidental findings or associated with acute extra-hepatic bile duct obstruction (vomiting, diarrhoea, abdominal pain and icterus) (Gibson 1952, O'Brien & Mitchum 1970, Naus & Jones 1978, Wolf 1984, Heidner & Campbell 1985, Joseph & Matthiesen 1986, Jorgensen et al 1987). Septic peritonitis has also been reported without evidence of biliary tract rupture (Jorgensen et al 1987). In this case of cholelithiasis in a cat, the clinical signs referable to this problem were limited to malaise and signs interpreted as representing abdominal pain. It is possible that these signs were attributable to hepatic or pancreatic inflammation, not the presence of stones. The reported increased water intake and evidence of a fractious nature were attributed to the hyperthyroidism. This case thus demonstrates that the mere presence of cholelithiasis may not in itself be associated with permanent or severe clinical signs.
A number of abnormal blood chemistry results were found in this cat. The urine specific gravity of 1.050 suggests pre-renal conditions, such as dehydration or cardiac insufficiency due to thyrotoxicosis, were the most likely cause of the increased urea. Anorexia and decreased water intake as a result of malaise could account for this. Anorexia might have contributed to the mild hypokalemia, as might have occult renal disease. The slight increase in mean cell haemoglobin likely reflects the fact that this is a calculated value. Increases in alkaline phosphatase (ALP) might have been due to the histologically demonstrated hepatobiliary disease, but these enzymes are also increased in feline hyperthyroidism, so the association of these changes with hepatobiliary disease is uncertain (Peterson et al 1983). The increase in feline TLI supports the presence of pancreatitis (Steiner & Williams 1999). Although the histologic specimen showed only slight, periductal inflammation this does not rule out the possibility of more severe, focal involvement that was not reflected in the submitted sample. Whilst the specificity of increased serum TLI for the diagnosis of feline pancreatitis has been questioned, the ultrasonographic and histologic findings suggest that this was present in this cat (Swift et al 2000). The options for treatment of the changes identified, and proof of benefit of these treatments, are limited. Given that signs abated after surgery, no therapy for pancreatitis was initiated.
Diagnosis of cholelithiasis may be made by radiography if stones are radio-opaque; ultrasonography is considered a sensitive means of detection in humans (Wachsberg 1995). In this case, ultrasound proved to be a useful technique that allowed demonstration of the stones and evaluation of associated structures, as well as guided aspiration of bile for cytology and culture.
In humans with asymptomatic cholesterol gallstone disease medical therapy with litholytic agents such as chenodeoxycholic acid and ursodeoxycholic acid is considered; stones containing significant amounts of calcium or pigment are treated surgically (Cooper 1993). Medical treatment is not likely to have been of use in this cat given the mineralised nature of the stones and their chemical composition. In previous reports of feline cholelithiasis, surgical removal of stones and biliary diversion procedures or cholecystectomy have been successful treatments (Naus & Jones 1978, Wolf 1984, Jorgensen et al 1987). Surgical removal of the gall-bladder is believed to prevent further bile stasis and stone formation and is a common treatment in humans. In this cat cholecystectomy appeared to prevent recurrence as has previously been reported, whereas cholecystojejunostomy was associated with recurrence in a single case report (Jorgensen et al 1987). No reports are available to determine whether surgery is indicated in asymptomatic cats with cholelithiasis. The decision to surgically treat this cat was based on the apparent signs of abdominal pain, the lack of knowledge of stone composition and the perceived risks of extra-hepatic biliary obstruction if the condition was left untreated.
The majority (∼80%) of human gallstones in the Western world are predominantly comprised of cholesterol, with the remaining 20% of stones being either bilirubin salt based (‘black stones’) or of mixed composition as a result of bacterial degradation of biliary matter (‘brown stones’) (Donovan 1999). Much less is understood about the pathogenesis of pigment stone formation in man compared to the more common cholesterol stones (Ko & Lee 1999). Previously reported feline choleliths have been either cholesterol and/or bilirubin based with calcium (Gibson 1952, O'Brien & Mitchum 1970, Naus & Jones 1978, Wolf 1984). Because of the rarity of feline cholelithiasis, the relative incidence of different stone types and factors leading to their formation is not known. The composition of the choleliths found in this cat were most consistent with so-called ‘black pigment’ stones found in humans, although the possibility that these were ‘brown pigment’ stone type could not be excluded, despite negative bacterial cultures.
Choleliths form when bile is supersaturated with chemical and there is a nidus for crystallisation (Ko & Lee 1999). Factors predisposing to black pigment stone formation in humans include abnormal gall-bladder motility and emptying, biliary tract infection, decreased bile acidity, decreased bile salt concentration and excess gallbladder mucin production (Ko & Lee 1999). Systemic factors such as hypercalcaemia and hyperbilirubinaemia might also predispose to ‘black pigment’ pathogenesis in man, and these abnormalities have been associated with haemolysis and ileal disease (Donovan 1999). In this cat, evidence of pancreatitis, cholecystitis and cholangitis suggests that chronic hepatobiliary disease might have been a predisposing factor to stone formation via factors such as altered gallbladder motility, excess mucin production and ascending infection. This complex has been associated with feline inflammatory bowel disease that was not specifically looked for in this cat but could have been an additional predisposing cause (Weiss et al 1996). The cholecystitis could be related to predisposing disease or might be a consequence of chronic local irritation from choleliths.
Hyperthyroidism and cholelithiasis have not previously been reported in the same cat but a direct association is possible. In rats, hyperthyroidism decreases the ratio of di- to mono-conjugated bilirubin in bile and increases bilirubin output (Van Steenbergen et al 1988, Van Steenbergen et al 1989). Monoconjugated bilirubin is less soluble in bile (Donovan 1999). In hyperthyroid humans, there is a reduced output of bile acids and an increased proportion of dihydroxy bile salts (Miller et al 1980, Pauletski et al 1989, Wiley et al 1978). Bilirubin becomes less soluble as bile salt concentrations decrease (Ko & Lee 1999). Hyperthyroidism can perturb gastrointestinal motor function in cats, and might have altered gall-bladder motility, which can be a predisposition to stone formation (Papasouliotis et al 1993, Ko & Lee 1999). It is possible, therefore, that altered bile chemistry (decreased bile salts concentration, decreased proportion of bilirubin diconjugate) leading to decreased bilirubin solubility, as well as abnormal gall-bladder motility, predisposed to cholelith formation in this case.
In summary, a cat with calcium bilirubinate cholelithiasis and hyperthyroidism was successfully treated by a combination of medical and surgical therapy. This is a previously unreported disease combination and a variety of factors associated with hepatobiliary disease, pancreatic disease and hyperthyroidism might have contributed to stone formation.
Acknowledgements
The authors would like to acknowledge the Minnesota Urolith Center and The Urolith Analysis Service of Hills Pet Nutrition Ltd. (Watford, UK), for analysis of cholelith composition.
References
- Cooper AD. (1993) Epidemiology, pathogenesis, natural history, and medical therapy of gallstones. In: Gastrointestinal Disease (5th edn) Sleisinger BF, Fordtran M. (eds). WB Saunders, Philadelphia, pp 1788–1804. [Google Scholar]
- Donovan JM. (1999) Physical and metabolic factors in gallstone pathogenesis. Gastroenterology Clinics of North America 28, 75–97. [DOI] [PubMed] [Google Scholar]
- Flanders JA. (1999) Surgical options for the treatment of hyperthyroidism in the cat. Journal of Feline Medicine and Surgery 1, 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KS. (1952) Cholelithiasis and choledocholithiasis in a cat. Journal of the American Veterinary Medical Association 121, 288–289. [PubMed] [Google Scholar]
- Heidner GL, Campbell KL. (1985) Cholelithiasis in a cat. Journal of the American Veterinary Medical Association 186, 176–177. [PubMed] [Google Scholar]
- Jorgensen LS, Pentlarge VW, Flanders JA, et al. (1987) Recurrent cholelithiasis in a cat. Compendium on Continuing Education for the Practising Veterinarian 9, 265–270. [Google Scholar]
- Joseph RJ, Matthiesen DT. (1986) What is your diagnosis? Journal of the American Veterinary Medical Association 188, 879–880. [Google Scholar]
- Ko CW, Lee SP. (1999) Gallstone formation. Local factors. Gastroenterology Clinics of North America 28, 99–115. [DOI] [PubMed] [Google Scholar]
- Martin RA. (1993) Liver and biliary system. In: Textbook of Small Animal Surgery. (2nd edn) Slatter D. (ed.) WB Saunders Company, Philadelphia, p. 653. [Google Scholar]
- Miller LJ, Owyang C, Malagelada JR, et al. (1980) Gastric, pancreatic and biliary responses to meals in hyperthyroidism. Gut 21, 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naus MJA, Jones BR. (1978) Cholelithiasis and choledocholithiasis in a cat. New Zealand Veterinary Journal 26, 160–161. [DOI] [PubMed] [Google Scholar]
- O'Brien TR, Mitchum GD. (1970) Cholelithiasis in a cat. Journal of the American Veterinary Medical Association 156, 1015–1017. [PubMed] [Google Scholar]
- Papasouliotis K, Muir P, Gruffyd-Jones TJ, et al. (1993) Decreased orocaecal transit time, as measured by the exhalation of hydrogen, in hyperthyroid cats. Research in Veterinary Science 55, 115–118. [DOI] [PubMed] [Google Scholar]
- Pauletzki J, Stellaard F, Paumgartner G. (1989) Bile acid metabolism in human hyperthyroidism. Hepatology 9, 852–855. [DOI] [PubMed] [Google Scholar]
- Peterson ME, Kintzer PP, Cavanagh PG, et al. (1983) Feline hyperthyroidism: pretreatment clinical and laboratory evaluation of 131 cases. Journal of the American Veterinary Medical Association 183, 103–110. [PubMed] [Google Scholar]
- Steiner JM, Williams DA. (1999) Feline exocrine pancreatic disorders. Veterinary Clinics of North America: Small Animal 29, 551–575. [PubMed] [Google Scholar]
- Swift NC, Marks SL, MacLachlan NJ, et al. (2000) Evaluation of serum feline trypsin-like immunoreactivity for the diagnosis of pancreatitis in cats. Journal of the American Veterinary Medical Association 217, 37–42. [DOI] [PubMed] [Google Scholar]
- Van Steenbergen W, Fevery J, De Groote J. (1988) Thyroid hormones and the hepatic handling of bilirubin. II. Effects of hypothyroidism and hyperthyroidism on the apparent maximal biliary secretion of bilirubin in the Wistar rat. Journal of Hepatology 7, 229–238. [DOI] [PubMed] [Google Scholar]
- Van Steenbergen W, Fevery J, De Vos R, et al. (1989) Thyroid hormones and the hepatic handling of bilirubin. I. Effects of hypothyroidism and hyperthyroidism on the hepatic transport of bilirubin mono- and diconjugates in the Wistar rat. Hepatology 9, 314–321. [DOI] [PubMed] [Google Scholar]
- Wachsberg RH. (1995) Sonographic evaluation of patients before laparoscopic cholecystectomy: imaging findings. American Journal of Radiology 164, 1419–1423. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Gagne JM, Armstrong PJ. (1996) Relationship between inflammatory hepatic disease, and inflammatory bowel disease, pancreatitis, and nephritis in cats. Journal of the American Veterinary Medical Association 209, 1114–1116. [PubMed] [Google Scholar]
- Wiley ZD, Lavigne ME, Liu KM, et al. (1978) The effect of hyperthyroidism on gastric emptying rates and pancreatic exocrine and biliary secretion in man. American Journal of Digestive Diseases 23, 1003–1008. [DOI] [PubMed] [Google Scholar]
- Wolf AM. (1984) Obstructive jaundice in a cat resulting from choledocholithiasis. Journal of the American Veterinary Medical Association 185, 85–87. [PubMed] [Google Scholar]