Abstract
Congestive heart failure and atrial fibrillation were diagnosed in a 4-year-old castrated Birman cat with progressive signs of dyspnea, tachypnea, and lethargy. Echocardiography revealed massive right-sided heart dilatation with ascites and hydrothorax. Electrocardiogram recording showed atrial fibrillation. Medical therapy with diuretics, benazepril, and antithrombotic agents was unsuccessful. The owner requested euthanasia. In post-mortem examination, changes associated with myocardial fibro-fatty infiltration were confirmed. Changes were most marked in the right ventricular wall but with left ventricular involvement was detected.
A 4-year-old Birman castrated male cat weighing 3.8 kg was referred to the Veterinary Teaching Hospital, University of Helsinki, Finland, because of progressive signs of dyspnea and lethargy. The owner had noticed the signs after the cat had returned home from a 2-week visit to a cattery. In the previous 6 months, the cat had lost weight (800 g), but had shown no respiratory signs. The referring veterinarian had diagnosed hydrothorax, and treatment had been initiated with methylprednisolone sodium succinate (Solu Medrol; Pfizer, 2 mg/kg IV). On presentation, the cat appeared depressed and thin (body score 2/5). Mucous membranes were pale and slightly icteric, and capillary refill time was 4 s. The metatarsal pulse was weak. Respiration was rapid (rate varied between 36 and 48/min), and lung sounds were muffled. The cat had mild hypothermia (37.7°C) and was dehydrated (7%). Heart was arrhythmic in auscultation. A systolic 2/6 grade murmur was detected on both sides of the sternum. Thoracocentesis yielded 147 ml of serous fluid, and the next day thoracic suction yielded a further 88 ml of modified transudate confirmed by biochemistry and cytology. Slight neutrophilia (neutrophil count 10.5×109/l; reference interval (RI) 1.1–10×109/l) and slightly elevated hemoglobin (156 g/l; RI 80–150 g/l) were the only hematological abnormalities. Feline leukemia virus (FeLV) antigen and feline immunodeficiency virus (FIV) antibody serology (SNAP Combo plus, IC Laboratories) were negative. Serum biochemistry revealed elevated levels of alkaline phosphatase (216 U/l; RI 20–120 U/l), alanine aminotransferase (385 U/l; RI 30–125 U/l), total bilirubin (14.1 μmol/l; RI 2.5–8.5 μmol/l), creatinine (238 μmol/l; RI 110–167 μmol/l), and urea (19.8 mmol/l; RI 4.0–15.5 mmol) and a slightly reduced concentration of albumin (28 g/l; RI 34–42 g/l). Right and left lateral and ventrodorsal thoracic radiographs showed soft tissue density ventrally, and the heart silhouette was invisible, indicating free fluid in the thorax. The trachea was elevated towards the thoracic spine. Standard six-lead electrocardiography (Electrocardiogram PC USB version, Eickemeyer Veterinary Equipment) showed atrial fibrillation and electrical alternans. The average heart rate was 200 beats/min during a 15-min recording.
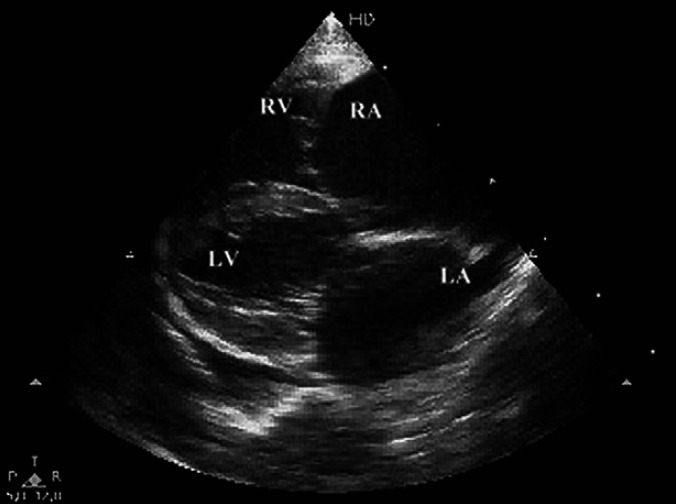
The main findings in 2D echocardiography using a 12 MHz transducer (Philips EnVisor, Philips Ultrasound, Bothell, WA) demonstrated a markedly dilated right atrium and ventricle (end diastole 14.6 mm). The left atrium was markedly dilated (left atrium to aorta ratio 2:1) and the left ventricle slightly dilated (end diastole 15.8 mm) and the left ventricular chamber appeared hypokinetic (Fig 1). Mitral and tricuspid regurgitations were observed in color flow Doppler studies and quantified (1.2 m/s and 4.5 m/s, respectively), but no dysplastic changes were demonstrated.
Fig 1.
2D right parasternal long-axis echocardiographic view of a cat with ARVC. Massive right atrial and ventricular enlargement with moderate left atrial and ventricular increases, can be observed. Pleural effusion is evident. LA=left atrium, RA=right atrium, LV=left ventricle, RV=right ventricle.
Abdominal ultrasonography (Philips iU22 C5-8 curvilinear transducer, Philips Ultrasound, Bothell, WA) revealed free fluid and hepatic venous distension suggesting congestion, and both kidneys were hyperechoic with decreased corticomedullary definition. Pyelectasia and a nephrolith were found in the left kidney. The urinary bladder contained a small amount of sediment. A large amount of pleural free fluid and partially atelectatic and patchy lungs was detected. The sternal and mediastinal lymph nodes were enlarged and clearly visible. Echogenic intraluminal content was found in the bifurcation of the carotid artery.
The cat was medicated with furosemide (Furesis; Orion Pharma) 1 mg/kg intramuscularly in emergency unit continued by 2 mg/kg intravenously twice daily, 0.5 mg/kg benazepril hydrochloride (Fortekor; Novartis) orally once daily, 0.01 mg/kg buprenorphine (Temgesic; Schering-Plough) intramuscularly once daily, a 25 μg/h transdermal fentanyl patch (Durogesic; Janssen-Cilag), 10 mg/kg ampicillin (A-pen, Orion Pharma) intravenously once daily, and intravenous fluids (Ringer-Acetat; Baxter) at a rate of 7.6 ml/h. Oxygen supplementation was used to relieve the hypoxia and antithrombotic treatment 12.5 mg/kg acetylsalicylic acid (Disperin; Orion Pharma) orally every 3rd day.
Despite the medication and the chest tube constantly collecting fluid, the cat's general condition did not improve. At this point, the owner refused further treatment because of poor prognosis and requested euthanasia.
A complete post-mortem examination was performed, revealing a markedly enlarged and rounded heart. The right side of the heart was flaccid, with severely dilated right ventricle and atrium. The free wall was thin and multifocally translucent. Inside the ventricle, the trabecular pattern was flattened. The valves were normal. The left side of the heart was slightly dilated.
Changes indicating cardiac insufficiency were observed. The liver was firm and the edges were rounded due to chronic congestion. The lungs were congested, heavy, and edematous, with a small amount of fluid in the thoracic cavity. Organ samples fixed in 10% buffered formalin solution were processed for histology and stained with hematoxylin and eosin (HE) and Masson's Trichrome for collagen. Histopathological examination of the heart showed severe myocardial atrophy in the wall of the right ventricle. The myocardial fibers were replaced by abundant fibrosis and fat tissue infiltration with scattered lymphocytes (Fig 2). The residual myofibers were thin and disorganized. The lesion was most remarkable beneath the epicardium and extended out multifocally through the whole thickness of the wall. Similar but milder and more focal changes were seen in the interventricular septum and the left ventricular wall. In addition, histological examination showed centrilobular fibrosis consistent with chronic hepatic congestion and severe pulmonary edema.
Fig 2.
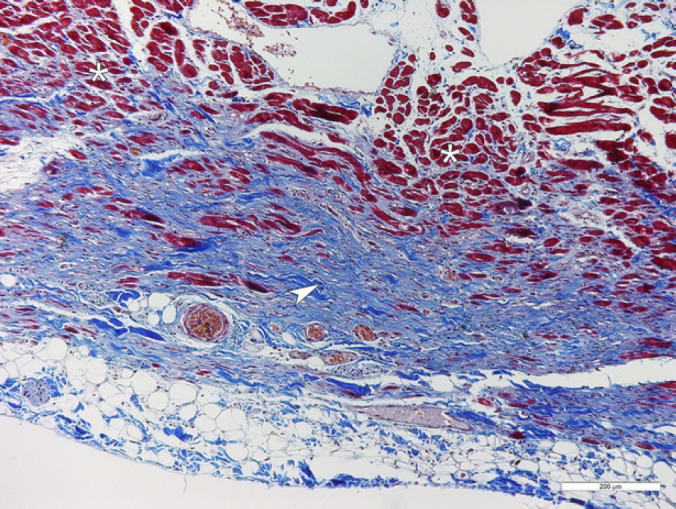
A section from the right ventricular wall of the heart. Myocardial fibers (asterisk, stained red) are replaced by collagen-rich fibrous tissue (arrowhead, stained blue). Masson's Trichrome stain, bar=200 μm.
Congestive heart failure (CHV) and atrial fibrillation described in this case possessed features of arrhythmogenic right ventricular cardiomyopathy (ARVC). ARVC is a progressive atrophy of the right ventricular myocardium and replacement by fibro-fatty or fatty infiltration of unknown etiology; it has been described in cats, dogs, and humans. Myocardial cell injury and death may be the initiating event in ARVC. 1–3 The major clinical findings in ARVC in humans are ECG abnormalities and sudden death. 3 In dogs, the syndrome is seen in Boxers and one case in English Bulldog, causing signs such as ventricular arrhythmias, CHF, and sudden death. 4–6 In cats, the clinical presentation of ARVC may be only lethargy or anorexia. 2 A unique feature in this case was that in addition to signs of right-sided CHF (ascites, congestion of liver, hydrothorax) typical for ARVC, lung edema combined with hydrothorax referred to left-sided CHF. 2,7 In electrocardiography, ARVC can manifest as a variety of abnormalities, including atrial fibrillation, ventricular tachycardia, supraventricular tachycardia, premature ventricular complexes, right bundle-branch-block, or first-degree atrioventricular block. 2,7 A 24-h ECG recording is recommended to diagnose especially ventricular ectopy. In this case, the short electrocardiography revealed atrial fibrillation, but was unable to demonstrate other arrhythmias. Careful cardiac echocardiography is the most suitable means of diagnosing ARVC excluding other myocardial disorders, pulmonary hypertension and congenital diseases such as tricuspid dysplasia, atrial septal defect, anomalous pulmonary venous drainage. 2,8 The magnetic resonance imaging and computed tomography methods used in human ARVC diagnostics may also be used in future in veterinary patients. 3,8,9
In cats, ARVC has been reported in three breeds; domestic shorthair, Burmese, and Birman cats. 2,7,8 Familial forms with autosomal dominant inheritance patterns have been demonstrated in humans. 1–3 In Boxers, the ARVC gene has also been suggested. 10 The genetic background of ARVC in cats is still unknown, although the overpresentation of Birman cats suggests a hereditary nature of the disease. 2
The pathological findings were similar to those described previously in feline ARVC cases: dilatation of the right ventricle and atrium and a thin, translucent ventricular wall. 2,7,8 The histological examination showed myocardial atrophy in the right ventricular wall and replacement by fibrous and adipose tissue. Only very few inflammatory cells in the myocardium were detected, although such findings are reported in most cases of human or feline ARVC. 2,3,8 In feline ARVC, changes may appear in the left ventricle, as in this case. 8
The prognosis of ARVC for cats is very poor. The median survival time is 1 month, and therapy with various drugs, such as furosemide, angiotensin-converting enzyme inhibitors (ACE-inhibitors), or antiarrhythmic drugs, has been unrewarding. 2,7
Acknowledgement
The authors thank Professor Marjatta Snellman for encouragement and support in writing this manuscript.
References
- 1.Basso C., Thiene G., Corrado D., Angelini A., Nava A., Valente M. Arrhythmogenic right ventricular cardiomyopathy, dysplasia, dystrophy, or myocarditis?, Circulation 94, 1996, 983–991. [DOI] [PubMed] [Google Scholar]
- 2.Fox P.R., Maron B.J., Basso C., Liu S.-K., Thiene G. Spontaneously occurring arrhythmogenic right ventricular cardiomyopathy in the domestic cat: a new model similar to the human disease, Circulation 102 (15), 2000, 1863–1870. [DOI] [PubMed] [Google Scholar]
- 3.Thiene G., Basso C. Arrhythmogenic right ventricular cardiomyopathy: an update, Cardiovasc Pathol 10, 2001, 109–117. [DOI] [PubMed] [Google Scholar]
- 4.del Palacio M.J. Fernández, Bernal L.J., Bayón A., Bernabé A., de Oca R. Montes, Seva J. Arrhythmogenic right ventricular dysplasia/cardiomyopathy in a Siberian Husky, J Small Anim Pract 42 (3), 2001, 137–142. [DOI] [PubMed] [Google Scholar]
- 5.Basso C., Fox P.R., Meurs K.M., et al. Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in Boxer dogs: a new animal model of human disease, Circulation 109, 2004, 1180–1185. [DOI] [PubMed] [Google Scholar]
- 6.Santilli R.A., Bontempi L.V., Perego M., Fornai L., Basso Cristina. Outflow tract segmental arrhythmogenic right ventricular cardiomyopathy in an English Bulldog, J Vet Cardiol 11, 2009, 47–51. [DOI] [PubMed] [Google Scholar]
- 7.Harvey A.M., Battersby I.A., Faena M., Fews D., Darke P.G.G., Ferasin L. Arrhythmogenic right ventricular cardiomyopathy in two cats, J Small Anim Pract 46, 2005, 151–156. [DOI] [PubMed] [Google Scholar]
- 8.Ciaramella P., Basso C., Di Loria Antonio, Piantedosi D. Arrhythmogenic right ventricular cardiomyopathy associated with severe left ventricular involvement in a cat, J Vet Cardiol 11, 2009, 41–45. [DOI] [PubMed] [Google Scholar]
- 9.Tandri H., Saranathan M., Rodriquez E.R., et al. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging, J Am Coll Cardiol 45, 2005, 98–103. [DOI] [PubMed] [Google Scholar]
- 10.Meurs K.M., Mauceli E., Acland G.M., Lindblad-Toh K. Genome-wide association identifies a mutation for arrhythmogenic right ventricular cardiomyopathy in the boxer dog, J Vet Intern Med 23, 2009, 687–688, [abstract] [Google Scholar]