Abstract
In the past, feline leukaemia virus (FeLV) infection, and also latent FeLV infection, were commonly associated with lymphoma and leukaemia. In this study, the prevalence of FeLV provirus in tumour tissue and bone marrow in FeLV antigen-negative cats with these tumours was assessed. Seventy-seven diseased cats were surveyed (61 antigen-negative, 16 antigen-positive). Blood, bone marrow, and tumour samples were investigated by two polymerase chain reaction (PCR) assays detecting deoxyribonucleic acid (DNA) sequences of the long terminal repeats (LTR) and the envelope (env) region of the FeLV genome. Immunohistochemistry (IHC) was performed in bone marrow and tumour tissue. None of the antigen-negative cats with lymphoma was detectably infected with latent FeLV. The prevalence of FeLV viraemia in cats with lymphoma was 20.8%. This suggests that causes other than FeLV play a role in tumourgenesis, and that latent FeLV infection is unlikely to be responsible for most feline lymphomas and leukaemias.
Previous studies have identified haematopoietic tumours as the most common primary feline malignancy. Of these, about 90% are lymphomas. 1 Lymphomas and leukaemias account for about 30% of all feline tumours, which is the highest proportion recorded in any animal species. 1–5 The association between feline leukaemia virus (FeLV) and lymphomas in cats has been established in several ways. Lymphoma can be induced in kittens through experimental FeLV infection, 6–8 and cats naturally infected with FeLV have a higher risk of developing lymphoma than uninfected cats. 6,9 Some years ago, most cats with lymphoma were FeLV-positive in tests that detected infectious virus or FeLV antigen. Thus, up to 80% of feline lymphomas and leukaemias were considered FeLV-related. 10–16 However, while 54% of all FeLV-positive cats investigated had lymphoma or leukaemia at necropsy, 17 another study found that only 7% of cats with lymphoma were FeLV-positive. 18 The finding of lymphomas without detectable FeLV infection 19 indicates that the association of FeLV and lymphoma is not absolute. Yet data from epidemiological studies suggest that FeLV may also be involved in the development of some of these lymphoma cases in which FeLV is not readily detected by standard diagnostic methods. In multiple cat households with FeLV exposure, the risk for a FeLV-negative cat to develop lymphoma is increased up to 40 times. 5,6,20,21
The incidence of FeLV in naturally occurring lymphomas can best be examined using molecular methods. Polymerase chain reaction (PCR) enables the detection of latent virus infection and residues of proviral deoxyribonucleic acid (DNA).
The purpose of this study was to determine the prevalence of FeLV antigen-positive and antigen-negative (but provirus-positive) cats and to identify the role of latent FeLV infection in naturally occurring cases of lymphoma and leukaemia.
Materials and methods
Study groups
The population in this prospective study was made up of cats presented to the Clinic of Small Animal Medicine, LMU University of Munich, between 1996 and 2008. Cats with a histological diagnosis of lymphoma or a diagnosis of leukaemia (based on a bone marrow aspirate) were included into the study.
FeLV antigenaemia was determined in all cats by detection of p27 antigen in serum using an enzyme-linked immunosorbent assay (ELISA) (Feline Leukaemia Virus Antigen/Feline Immunodeficiency Virus Antibody Test Kit; Idexx, Westbrook, ME, USA). Only cats that had positive test results, twice in separate runs, were considered to have positive FeLV antigen test results. Cats were assigned to two groups based on FeLV status. The ‘FeLV-negative lymphoma group’ consisted of FeLV antigen-negative cats with a diagnosis of lymphoma or/and leukaemia. Inclusion into the group was based on (1) the presence of lymphoma and/or leukaemia and (2) two negative FeLV antigen tests. Of the 61 cats that met these criteria, 55 had lymphoma, two had leukaemia, and four had lymphoma and leukaemia. The ‘FeLV-positive lymphoma group’ was made up of 16 FeLV antigen-positive cats, 12 of these with lymphoma, three with leukaemia, and one with bilateral renal lymphoma and leukaemia. The ‘control group’ consisted of 41 FeLV antigen-negative cats without malignancies presented for various diseases. The absence of neoplastic disease in these cats was confirmed at necropsy.
T- and B-cell determination
Immunohistochemistry (IHC) was used to differentiate T-cell and B-cell antigens in tumour tissue. Immunohistochemical identification of B- and T-cells was performed in 28 cats of the FeLV-negative lymphoma group and in eight cats of the FeLV-positive lymphoma group (Table 1) by routine ABC immunostaining methods, using cross-reacting human antibodies against T cells (CD3: DakoCytomation, Hamburg, Germany) and B cells (CD20: Dunn Labortechnik GmbH, Ansbach, Germany).
Table 1.
T- and B-cell determination of the investigated (n=36) tumours (in numbers) (percentages in parentheses).
| FeLV-negative lymphoma group | FeLV-positive lymphoma group | |
|---|---|---|
| T-cell positive | 18 (64.3) | 4 (50.0) |
| B-cell positive | 8 (28.6) | 0 (0) |
| T- and B-cell negative | 2 (7.1) | 4 (50.0) |
| Total | 28 | 8 |
Detection of FeLV proviral DNA
Two different PCR assays (env and LTR) were performed to detect proviral FeLV DNA in blood, bone marrow, and tumour tissue. Samples were frozen and stored at −70°C prior to analysis. DNA isolation from blood was performed using the QIAamp Blood Kit (Qiagen, Valencia, CA, USA). For isolation of the genomic DNA from bone marrow samples and fresh tissue samples, the QIAamp Tissue Kit (Qiagen, Valencia, CA, USA) was used. In seven cats (one of the ‘FeLV-positive lymphoma group’ and six of the ‘FeLV-negative lymphoma group’), only paraffin-embedded tumour tissue was available. Paraffin-embedded tissue was incubated 20 min in xylol to remove paraffin and was then rehydrated with serial ethanol dilutions prior to DNA extraction.
The env PCR used in this study was obtained from Synbiotics (San Diego, CA, USA). This system detects a DNA sequence of 312 base pairs in the env region coding for the gp70 of the FeLV genome (6110–6421). The LTR U3-PCR used in this study has been described by Tandon et al. 22 It detects a sequence of the U3 region of the LTR specific for exogenous FeLV. Table 2 gives an overview of all samples investigated by PCR.
Table 2.
Results of the FeLV env PCR, LTR U3-PCR, and IHC in the 118 cats of the study.
| Method | FeLV-negative lymphoma group | FeLV-positive lymphoma group | Control group | |
|---|---|---|---|---|
| (n=61) | (n=16) | (n=41) | ||
| Blood env PCR | Tested | 17 | 3 | 0 |
| Negative | 17 | 0 | 0 | |
| Positive | 0 | 3 | 0 | |
| Blood LTR U3-PCR | Tested | 3 | 0 | 33 |
| Negative | 3 | 0 | 33 | |
| Positive | 0 | 0 | 0 | |
| Bone marrow env PCR | Tested | 0 | 0 | 0 |
| Negative | 0 | 0 | 0 | |
| Positive | 0 | 0 | 0 | |
| Bone marrow LTR U3-PCR | Tested | 10 | 3 | 41 |
| Negative | 10 | 0 | 41 | |
| Positive | 0 | 3 | 0 | |
| Tumour tissue env PCR | Tested | 49 | 10 | 0 |
| Negative | 49 | 0 | 0 | |
| Positive | 0 | 10 | 0 | |
| Tumour tissue LTR U3-PCR | Tested | 57 | 11 | 0 |
| Negative | 57 | 2 | 0 | |
| Positive | 0 | 9 | 0 | |
| Bone marrow IHC | Tested | 10 | 3 | 35 |
| Negative | 10 | 0 | 35 | |
| Positive | 0 | 3 | 0 | |
| Tumour tissue IHC | Tested | 39 | 12 | 0 |
| Negative | 37 | 0 | 0 | |
| Positive | 2 | 12 | 0 |
Env=envelope; PCR=polymerase chain reaction; LTR=long terminal repeat; IHC=immunohistochemistry.
Detection of intracellular FeLV p27 antigen
Intracellular FeLV p27 antigen in tumour tissue and bone marrow samples was detected by IHC. A polyclonal goat anti-FeLV p27 antibody (Biodesign International, Saco, ME, USA) (diluted 1:2000) was used as primary antibody, and a rabbit anti-goat immunoglobulin (IgG) couples with peroxidase (DAKO, Hamburg, Germany) (diluted 1:400) as a secondary antibody. A lymphnode of a FeLV antigen-negative cat was used as negative control. As positive control, a lymphnode of a FeLV antigen-positive cat was used. Table 2 gives an overview of the IHC performed in 99 bone marrow and tumour tissue samples. In all but eight cats, IHC was carried out of fresh tissue. Of the eight cats, one belonged to the ‘FeLV-positive lymphoma group’, six belonged to the ‘FeLV-negative lymphoma group’ and one belonged to the ‘control group’.
Statistical evaluation
Statistical analysis was performed using an exact binominal test for determination of confidence intervals (CIs). The binominal test was one-tailed and was used to prove that the prevalence of FeLV antigen was within the 95% CI. Discrepancies in FeLV antigen test results in cats with different tumour locations were statistically evaluated using the χ2 test. A P-value <0.05 was considered significant.
Results
Characteristics and prevalence of FeLV antigen in cats with lymphoma
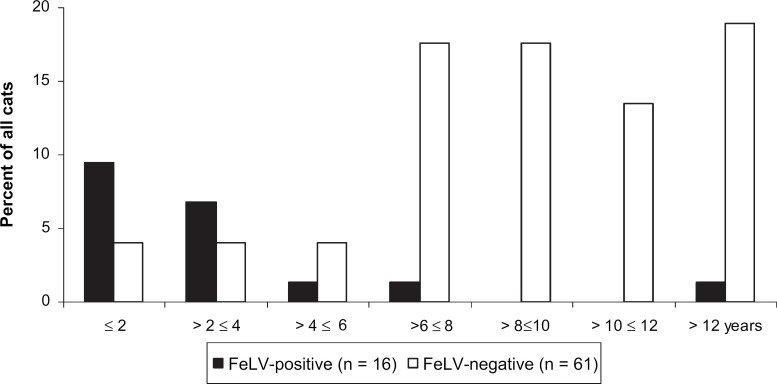
A total of 77 cats diagnosed with lymphoma or leukaemia were included in this study. The age distribution of these cats is shown in Fig 1. In the FeLV-positive lymphoma group, 7/16 (43.7%) cats were younger than 2 years and 12/16 (75.0%) cats were younger than 4 years, but only 1/16 (6.3%) cat was older than 10 years. In contrast, in the FeLV-negative lymphoma group, only 6/61 (9.8%) cats were younger than 4 years, but 24/61 (39.3%) cats were older than 10 years and 15/61 (24.6%) cats were older than 12 years. The prevalence of FeLV antigenaemia in cats with lymphoma or leukaemia was 20.8% (95% CI: 12.4–31.5). The anatomical distribution of tumours is depicted in Table 3. In the FeLV-positive lymphoma group, 4/16 (25.0%) cats had thymic lymphomas, whereas in the FeLV-negative lymphoma group, only 1/61 (1.6%) had thymic lymphoma. Tumour locations differed significantly between the FeLV-negative and -positive groups (P=0.002; five degrees of freedom [df]). Five of the 77 cats had both lymphoma and leukaemia. These cats were excluded in the comparison of antigen presence in individual tumour location sites. There was a statistically significant difference in the presence of FeLV antigen in the blood of cats with intestinal versus cats with other forms of lymphomas (χ2 test: P=0.006). There was also a statistically significant difference in the occurrence of T-cell and B-cell lymphomas between FeLV antigen-negative and -positive cats (P=0.009) (Table 1). Eight out of 28 (64.3%) FeLV antigen-negative cats had T-cell lymphoma and 8/28 (28.6%) had B-cell lymphomas as detected by IHC. Four out of 8 (50.0%) FeLV antigen-positive cats with lymphoma were negative for T- and B-cell markers.
Fig 1.
Age distribution of the 77 cats of the FeLV-negative lymphoma group and the FeLV-positive lymphoma group (in percent).
Table 3.
Distribution of tumour location (in numbers) in 77 cats with lymphoma and/or leukaemia.
| FeLV-negative lymphoma group | FeLV-positive lymphoma group | |
|---|---|---|
| Multi-centric | 15 | 4 |
| Intestinal | 30 | 2 |
| Thymic | 1 | 4 |
| Solitary organ | 9 | 2 |
| Leukaemia | 2 | 3 |
| Lymphoma and leukaemia | 4 | 1 |
| Total | 61 | 16 |
Detection of FeLV proviral DNA
In the FeLV-negative lymphoma group, all cats tested were negative for FeLV by PCR in blood (20/20) as well as in bone marrow (10/10). In addition, all tested tumour tissue samples were negative by env (49/49) and LTR U3-PCR (57/57) (Table 2). Likewise, all cats tested in the FeLV-positive lymphoma group were positive for FeLV by PCR in blood (3/3) and bone marrow (3/3). However, the tumour tissue samples of two cats in this group were negative for FeLV by PCR. In the control group, all cats were negative for FeLV in bone marrow (41/41) and blood (33/33).
Detection of intracellular FeLV p27 antigen
Thirty-seven of the 39 investigated tumour samples and all of 51 examined bone marrow samples of FeLV antigen-negative cats (FeLV-negative lymphoma group) were negative for FeLV p27 antigen by IHC. In two FeLV antigen-negative cats, a few tumour tissue cells and a few splenic cells, respectively, were FeLV p27-positive. In the FeLV-positive lymphoma group, all cats tested were positive on IHC of tumour tissue (12/12) and bone marrow (14/14) (Table 2).
Discussion
This study confirms the previously reported decrease in the prevalence of FeLV infection in cats with lymphoma or leukaemia as well as in the general population, 18,23–26 which may be associated with the observed shift in tumour causation in recent years. A past investigation examining cats in the same geographical region in Germany 25 as the present study, found that 59% of all cats with lymphoma or leukaemia were FeLV antigen-positive in the years from 1980 to 1995, consistent with reports on the prevalence of FeLV in other parts of Europe (60–70% in cats with lymphoma 14,15 ). In contrast, only 21% of cats with lymphoma and leukaemia were FeLV antigen-positive in the present study (1996–2008). One major reason for the low prevalence of FeLV among cats with these haematopoietic tumours seems to be the decreasing prevalence of FeLV infection in the overall cat population. Between 1988 and 1994, prevalence decreased by 50%, 25 and a very recent study examining the cat population of Southern Germany found only 2% FeLV-positive cats. 23 The decline in FeLV infection rates may be the result of extensive FeLV vaccination and testing and elimination programmes. 26
Prevalence of FeLV antigenaemia in cats with lymphomas is still higher than in the overall population and varies with tumour location. Thus, FeLV is found significantly less often in cats with intestinal lymphomas than in cats with other lymphomas or leukaemia. Most studies indicate that multi-centric and thymic lymphomas are mainly of T-cell origin, and that cats with these tumours are FeLV antigen-positive, while intestinal lymphomas are mostly ‘FeLV-negative’ and of B-cell origin. 5,11,27–29 Estimates on the prevalence of FeLV antigenaemia in cats with intestinal lymphomas range from 25 to 30%. 5,15,21,30,31 In the present study, however, only 6% of cats with intestinal lymphomas were FeLV-positive, which is only about twice the FeLV prevalence observed in the entire study population (2.9%). This is comparable to the results of two studies in which only 2/67 cats 32 and none of 21 cats with gastrointestinal lymphomas were FeLV-positive, respectively. 33 These results suggest that today other stimuli in the gastrointestinal tract of older cats (such as food components or inflammatory bowel disease) may act as more important predisposing factors in oncogenesis.
According to previous reports, 80–90% of cats with thymic lymphoma are FeLV-positive, 5,15,21 which coincides with findings of the current study, in which 4/5 (80%) of cats with thymic lymphoma were FeLV-positive. The high prevalence of FeLV infection in cats with thymic lymphoma can be explained by the course of FeLV infection. Most cats are infected with FeLV early in their lives. In young cats, virus replication in the thymus occurs at a very early stage of infection and, thus, predisposes to tumour development in this organ. In older cats, on the other hand, the thymus regresses and cats develop some age-resistance against FeLV. Thus, adult cats usually do not become persistently viraemic if in contact with FeLV. Consequently, the incidence of FeLV is higher in younger cats and cats with thymic lymphoma (Fig 1, Table 3).
Of the 28 FeLV antigen-negative cats in this study, 18 (64.3%) had T-cell lymphomas and eight (28.6%) had B-cell lymphomas as detected by IHC. Of the eight FeLV antigen-positive cats with lymphoma, four (50%) were negative for T- and B-cell markers. None of the investigated FeLV-positive tumours were of B-cell origin. This finding is in accordance with most reports indicating that ‘FeLV-positive lymphomas’ are mainly of T-cell origin and ‘FeLV-negative lymphomas’ mainly of B-cell origin. 5,11,26,28,29,34 Only one study by Jackson et al (1996) identified B-cell tumours as frequently as T-cell tumours in FeLV-positive cats. 35 FeLV transforms mature as well as immature T cells (prothymocytes), null cells, and possibly monocytes. As there is a lack of surface immunoglobulin expression in feline lymphoma cell lines and primary tumours, 36 transformation of mature B cells is not thought to occur. Consequently, the results of Jackson et al (1996) 35 are very difficult to explain and are in conflict with current concepts of FeLV-induced tumour pathogenesis.
According to past studies, FeLV is the major oncogen causing lymphoma and leukaemia in cats. 10–16 These studies suggested that FeLV may be responsible for all lymphomas originating in cats following exposure to the virus, regardless of whether cats remained FeLV-positive following infection. 5 Thus, Jackson et al (1993) as well as Gabor et al (2001) detected FeLV proviral DNA in lymphoma tissue of FeLV antigen-negative cats indicating that the virus may be associated with most if not all lymphomas independent of the antigenaemic status of cats. 37,38 Various studies have investigated the potential role of a so-called ‘latent’ FeLV infection in which re-activatable FeLV provirus remains integrated in the cellular genome of bone marrow and potentially other cells without release of infectious virus or antigens into the blood. Recently, a sensitive real-time PCR was able to show that even seemingly immune cats become provirus-positive after virus exposure. 22,39–41 Routine FeLV testing methods such as the ELISA 42 yield negative results 43 in these latently infected cats.
For the above reasons, FeLV has been considered by some to be responsible for all lymphomas irrespective of whether or not they produce infective virus. 5 However, more recent studies suggest that FeLV latency is not always present in malignancies classically associated with FeLV. 44 Thus, intestinal lymphomas have been described in specific pathogen-free (SPF) cats never exposed to FeLV. 45 Furthermore, lymphomas have developed in SPF cats following only infection with feline immunodeficiency virus (FIV). 46,47
In this study which investigated a large number of naturally occurring cases of lymphoma and leukaemia, FeLV provirus was not detected in any of the blood, tumour, or bone marrow samples of FeLV antigen-negative cats with lymphoma or leukaemia using two different PCR assays. Moreover, proviral DNA was not detected in tumour tissue of FeLV-negative cats with lymphoma. One possible explanation may be that FeLV is still responsible for tumourgenesis but that parts of the FeLV sequence are missing or have been altered by mutation resulting in a replication-defective virus. If this occurs in a region of the genome to which the primers bind, PCR would yield negative results. To reduce the likelihood of this occurrence, two different PCRs with primers binding to different regions of the FeLV genome were used in the present study. Another explanation for the failure to detect proviral FeLV DNA in the tumour tissue of FeLV-negative cats with lymphoma may be that FeLV is responsible for tumour development by inducing a cell clone, but that the virus is not persistently integrated into the genome of the neoplastic cell and, therefore, has been eliminated from tumour tissue by the time the tumour has reached a detectable size. As a third explanation, FeLV infection may be present in other cells, inducing oncogenesis via epigenetic mechanisms such as cytokine release or chronic immunostimulation. This explanation, however, is not supported by the results of the present study in which not only tumour tissue but also bone marrow was investigated and found negative for FeLV. Finally and most likely, tumourgenesis in FeLV-negative cats with lymphoma may be unrelated to FeLV and caused by other mechanisms. Tumour tissue samples and both tumour tissue and bone marrow samples of FeLV antigen-negative cats were negative by env and LTR U3-PCR, respectively, indicating that FeLV provirus was not present in these samples, even in an incomplete form. However, certain exclusion of the presence of parts of the FeLV genome would have required sequencing of the genome which was not performed in this study.
Although tumour tissue of all FeLV antigen-positive cats with lymphoma was positive by env PCR, two of 11 were negative by LTR U3-PCR. This result may have been due to a mutation in the LTR region (although it is highly conserved) of the FeLV strain in these cats. Another explanation could be the fact that the LTR U3-PCR sometimes misses FeLV-C-like LTR sequences. 39 Also, LTR U3-PCR may have yielded false-negative results due to low DNA copy-numbers, especially as in one of these cats the env PCR also only produced a weak signal. FeLV antigen, however, had repeatedly been identified in the blood of this cat.
Using IHC, intracellular FeLV p27 antigen was detected in the tumour tissue of all cats that were FeLV-positive in serum. Interestingly, FeLV p27 antigen was also detected in the tumour tissue of two of 29 FeLV antigen-negative cats. These results are difficult to interpret as the presence of antigen in cells requires the presence of corresponding sequences of the viral genome. It is conceivable that incomplete integration of provirus or the presence of gag sequence coding for p27 but absence or mutation of the sequences detected by the two PCR assays 48 could explain these unexpected findings. Alternatively, IHC can yield false positive results if the tissue is too dry at time of fixation or if fixation lasts too long or autolysis of the tissue has commenced. Finally, the discrepancy between IHC and ELISA could be a matter of sensitivity. IHC investigates single cells, and as only very few cells were positive in both cases, the antigen ELISA in blood may have not been sensitive enough to detect FeLV infection. However, this explanation appears unlikely, as both cats were also negative by PCR, which is considered the most sensitive diagnostic method.
A limitation of the study was that not every test was performed in all cats as samples could not always be obtained (eg, bone marrow). Additionally, PCR of bone marrow samples was performed using LTR U3-PCR only in all specimens. Although the two PCRs used in this study are very sensitive, it could be possible that they still were not sensitive enough to detect latent FeLV infections. This appears to be unlikely, however, as the LTR U3-PCR used had a detection limit of one proviral copy per 5 μl DNA solution typically corresponding to 50,000 blood leukocytes. In conclusion, this study clearly demonstrated that latent FeLV infection was not the cause of oncogenesis in the FeLV antigen-negative cats of the investigated population. It was also shown that parallel to the overall decreasing prevalence of FeLV infection the proportion of FeLV antigen-negative cats with lymphomas (versus FeLV antigen-positive cats with lymphomas) has significantly increased over the past 20 years. The role of FeLV in the oncogenesis seems to have been overestimated in the past and should be reassessed. The results suggest that other oncogens or epigenetic carcinogenesis are playing an increasingly important role in tumourgenesis of cats, and that FeLV should no longer be considered the major cause of feline tumours.
Acknowledgments
We thank The Pfizer Animal Health Group, Louvain-La-Nauve, France for supporting parts of the study.
References
- 1.Dorn C.R., Taylor D.O., Schneider R., Hibbard H.H., Klauber M.R. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County, J Natl Cancer Inst 40, 1968, 307–318. [PubMed] [Google Scholar]
- 2.Dorn C.R., Taylor D.O., Hibbard H.H. Epizootiologic characteristics of canine and feline leukemia and lymphoma, Am J Vet Res 28, 1967, 993–1001. [PubMed] [Google Scholar]
- 3.Hardy W.D., Jr. Epidemiology of primary neoplasms of lymphoid tissues in animals. Twomey J.J., Good R.A. Comprehensive immunology of lymphoreticular neoplasms, 1978, Plenum Medical Book Co: New York, 129–180. [Google Scholar]
- 4.Crighton G.W. Lymphosarcoma in the cat, Vet Rec 84, 1969, 329–331. [DOI] [PubMed] [Google Scholar]
- 5.Hardy W.D., Jr. Hematopoietic tumors of cats, J Am Anim Hosp Assoc 17, 1981, 921–940. [Google Scholar]
- 6.Hardy W.D., Jr., Hirshaut Y., Hess P. Detection of the feline leukemia virus and other mammalian oncornaviruses by immunofluorescence, Bibl Haematol 39, 1973, 778–799. [DOI] [PubMed] [Google Scholar]
- 7.Jarrett O., Laird H.M., Hay D. Determinants of the host range of feline leukaemia viruses, J Gen Virol 20, 1973, 169–175. [DOI] [PubMed] [Google Scholar]
- 8.Rickard C.G., Post J.E., Noronha F., Barr L.M. A transmissible virus-induced lymphocytic leukemia of the cat, J Natl Cancer Inst 42, 1969, 987–1014. [PubMed] [Google Scholar]
- 9.Essex M., Cotter S.M., Hardy W.D., Jr., et al. Feline oncornavirus-associated cell membrane antigen. IV. Antibody titers in cats with naturally occurring leukemia, lymphoma, and other diseases, J Natl Cancer Inst 55, 1975, 463–467. [PubMed] [Google Scholar]
- 10.Cotter S.M., Hardy W.D., Jr., Essex M. Association of feline leukemia virus with lymphosarcoma and other disorders in the cat, J Am Vet Med Assoc 166, 1975, 449–454. [PubMed] [Google Scholar]
- 11.Francis D.P., Cotter S.M., Hardy W.D., Jr., Essex M. Comparison of virus-positive and virus-negative cases of feline leukemia and lymphoma, Cancer Res 39, 1979, 3866–3870. [PubMed] [Google Scholar]
- 12.Francis D.P., Essex M., Hardy W.D., Jr. Excretion of feline leukaemia virus by naturally infected pet cats, Nature 269, 1977, 252–254. [DOI] [PubMed] [Google Scholar]
- 13.Hardy W.D., Jr., MacEwen E.G. Hematopoietic tumors. Withrow S.J., MacEwen E.G. Clinical veterinary oncology, 1989, JB Lippincott: Philadelphia, USA, 362–411. [Google Scholar]
- 14.Hardy W.D., Jr., McClelland A.J., Zuckerman E.E., et al. Development of virus non-producer lymphosarcomas in pet cats exposed to FeLV, Nature 288, 1980, 90–92. [DOI] [PubMed] [Google Scholar]
- 15.Reinacher M. Infektionen mit dem felinen Leukämie-virus (FeLV) bei sezierten Katzen, Kleintierprax 32, 1987, 65–72. [Google Scholar]
- 16.Shelton G.H., Grant C.K., Cotter S.M., Gardner M.B., Hardy W.D., Jr., DiGiacomo R.F. Feline immunodeficiency virus and feline leukemia virus infections and their relationships to lymphoid malignancies in cats: a retrospective study (1968–1988), J Acquir Immune Defic Syndr 3, 1990, 623–630. [PubMed] [Google Scholar]
- 17.Suntz M. Untersuchung zu Vorkommen und Bedeutung latenter Infektionen mit dem felinen Leukämievirus (FeLV) bei Sektionskatzen [Dissertation]. VVB Laufersweiler Verlag: Justus-Liebig-Universität Gießen, 2007.
- 18.Teske E., van Straten G., van Noort R., Rutteman G.R. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol, J Vet Intern Med 16, 2002, 179–186. [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Kyaw-Tanner M., Lee C., Robinson W.F. Characterisation of lymphosarcomas in Australian cats using polymerase chain reaction and immunohistochemical examination, Aust Vet J 79, 2001, 41–46. [DOI] [PubMed] [Google Scholar]
- 20.Francis D.P., Essex M., Cotter S.M., Gutensohn N., Jakowski R., Hardy W.D., Jr. Epidemiologic association between virus-negative feline leukemia and the horizontally transmitted feline leukemia virus, Cancer Lett 12, 1981, 37–42. [DOI] [PubMed] [Google Scholar]
- 21.Onions D. Animal models: lessons from feline and bovine leukaemia virus infections, Leuk Res 9, 1985, 709–711. [DOI] [PubMed] [Google Scholar]
- 22.Tandon R., Cattori V., Gomes-Keller M.A., et al. Quantitation of feline leukaemia virus viral and proviral loads by TaqMan real-time polymerase chain reaction, J Virol Methods 130, 2005, 124–132. [DOI] [PubMed] [Google Scholar]
- 23.Englert T., Lutz H., Hartmann K. Untersuchung des FeLV-Status der süddeutschen Katzenpopulation, Tierärztl Prax 37, 2009, A9. [Google Scholar]
- 24.Gleich S., Hartmann K. Hematology and serum biochemistry of feline immunodeficiency virus-infected and feline leukemia virus-infected cats, J Vet Intern Med 23, 2009, 552–558. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann K., Gerle K., Leutenegger C.M., Jarrett O. Feline leukemia virus, ESVIM Newsletter 9, 1999, 11. [Google Scholar]
- 26.Vail D.M., Moore A.S., Ogilvie G.K., Volk L.M. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats, J Vet Intern Med 12, 1998, 349–354. [DOI] [PubMed] [Google Scholar]
- 27.Pohlman L.M., Higginbotham M.L., Welles E.G., Johnson C.M. Immunophenotypic and histologic classification of 50 cases of feline gastrointestinal lymphoma, Vet Pathol 46, 2009, 259–268. [DOI] [PubMed] [Google Scholar]
- 28.Hardy W.D., Jr., Zuckerman E.E., MacEwen E.G., Hayes A.A., Essex M. A feline leukaemia virus- and sarcoma virus-induced tumour-specific antigen, Nature 270, 1977, 249–251. [DOI] [PubMed] [Google Scholar]
- 29.Neil J.C., Hughes D., McFarlane R., et al. Transduction and rearrangement of the myc gene by feline leukaemia virus in naturally occurring T-cell leukaemias, Nature 308, 1984, 814–820. [DOI] [PubMed] [Google Scholar]
- 30.Jeglum K.A., Whereat A., Young K. Chemotherapy of lymphoma in 75 cats, J Am Vet Med Assoc 190, 1987, 174–178. [PubMed] [Google Scholar]
- 31.Mahony O.M., Moore A.S., Cotter S.M., Engler S.J., Brown D., Penninck D.G. Alimentary lymphoma in cats: 28 cases (1988–1993), J Am Vet Med Assoc 207, 1995, 1593–1598. [PubMed] [Google Scholar]
- 32.Fondacaro J.V., Richter K.P., Carpenter J.L., Hart J.R., Hill S.L., Fettman M.J. Feline gastrointestinal lymphoma: 67 cases (1988–1996), Eur J Comp Gastroenterol 4, 1999, 5–11. [Google Scholar]
- 33.Zwahlen C.H., Lucroy M.D., Kraegel S.A., Madewell B.R. Results of chemotherapy for cats with alimentary malignant lymphoma: 21 cases (1993–1997), J Am Vet Med Assoc 213, 1998, 1144–1149. [PubMed] [Google Scholar]
- 34.Casey J.W., Roach A., Mullins J.I., et al. The U3 portion of feline leukemia virus DNA identifies horizontally acquired proviruses in leukemic cats, Proc Natl Acad Sci USA 78, 1981, 7778–7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson M.L., Wood S.L., Misra V., Haines D.M. Immunohistochemical identification of B and T lymphocytes in formalin-fixed, paraffin-embedded feline lymphosarcomas: relation to feline leukemia virus status, tumor site, and patient age, Can J Vet Res 60, 1996, 199–204. [PMC free article] [PubMed] [Google Scholar]
- 36.Rojko J.L., Kociba G.J., Abkowitz J.L., et al. Feline lymphomas: immunological and cytochemical characterization, Cancer Res 49, 1989, 345–351. [PubMed] [Google Scholar]
- 37.Jackson M.L., Haines D.M., Meric S.M., Misra V. Feline leukemia virus detection by immunohistochemistry and polymerase chain reaction in formalin-fixed, paraffin-embedded tumor tissue from cats with lymphosarcoma, Can J Vet Res 57, 1993, 269–276. [PMC free article] [PubMed] [Google Scholar]
- 38.Gabor L.J., Jackson M.L., Trask B., Malik R., Canfield P.J. Feline leukaemia virus status of Australian cats with lymphosarcoma, Aust Vet J 79, 2001, 476–481. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann-Lehmann R., Huder J.B., Gruber S., Boretti F., Sigrist B., Lutz H. Feline leukaemia provirus load during the course of experimental infection and in naturally infected cats, J Gen Virol 82, 2001, 1589–1596. [DOI] [PubMed] [Google Scholar]
- 40.Hofmann-Lehmann R., Tandon R., Boretti F.S., et al. Reassessment of feline leukaemia virus (FeLV) vaccines with novel sensitive molecular assays, Vaccine 24, 2006, 1087–1094. [DOI] [PubMed] [Google Scholar]
- 41.Torres A.N., Mathiason C.K., Hoover E.A. Re-examination of feline leukemia virus: host relationships using real-time PCR, Virol 332, 2005, 272–283. [DOI] [PubMed] [Google Scholar]
- 42.Lutz H., Pedersen N.C., Theilen G.H. Course of feline leukemia virus infection and its detection by enzyme-linked immunosorbent assay and monoclonal antibodies, Am J Vet Res 44, 1983, 2054–2059. [PubMed] [Google Scholar]
- 43.Hoover E.A., Mullins J.I. Feline leukemia virus infection and diseases, J Am Vet Med Assoc 199, 1991, 1287–1297. [PubMed] [Google Scholar]
- 44.Herring E.S., Troy G.C., Toth T.E., Forrester S.D., Weigt L.A., Herring I.P. Detection of feline leukaemia virus in blood and bone marrow of cats with varying suspicion of latent infection, J Feline Med Surg 3, 2001, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarrett O., Edney A.T., Toth S., Hay D. Feline leukaemia virus-free lymphosarcoma in a specific pathogen free cat, Vet Rec 115, 1984, 249–250. [DOI] [PubMed] [Google Scholar]
- 46.Poli A., Abramo F., Baldinotti F., Pistello M., Da Prato L., Bendinelli M. Malignant lymphoma associated with experimentally induced feline immunodeficiency virus infection, J Comp Pathol 110, 1994, 319–328. [DOI] [PubMed] [Google Scholar]
- 47.Terry A., Callanan J.J., Fulton R., Jarrett O., Neil J.C. Molecular analysis of tumours from feline immunodeficiency virus (FIV)-infected cats: an indirect role for FIV?, Int J Cancer 61, 1995, 227–232. [DOI] [PubMed] [Google Scholar]
- 48.Kipar A., Kremendahl J., Grant C.K., von Bothmer I., Reinacher M. Expression of viral proteins in feline leukemia virus-associated enteritis, Vet Pathol 37, 2000, 129–136. [DOI] [PubMed] [Google Scholar]