Abstract
A 10.5-year-old domestic shorthair presented with a history of progressive inappetence, lethargy and elevated respiratory rate. Clinical and diagnostic findings confirmed the presence of a chylothorax with evidence of a mass or collapsed lung within the right cranial thorax. Computed tomography, sternotomy and histopathology confirmed the presence of a right middle lung lobe torsion associated with a chylothorax. The torsion was successfully managed with surgical removal of the affected lung lobe, and the patient continues to be asymptomatic 6 months postoperatively.
Lung lobe torsion (LLT) in cats is a rare occurrence in our veterinary population. Though the exact mechanism of LLT is not well understood, most often it is thought to be either spontaneous or secondary to pleural effusion (commonly chylothorax in canine), trauma, neoplasia or chronic respiratory disease. 1–3 An LLT is defined as an axial rotation of the lung lobe and the vascular pedicle. The rotation results in compression of the venous vasculature and lymphatics, but leaves the thick walled arteries partially patent. The continued influx of blood with lack of outflow leads to lobar congestion, edema, hemorrhage and necrosis. 1,2,4,5 Chylothorax has been associated with LLT in dogs, especially in the Afghan hound, but to the authors’ knowledge, there has been no published report of a case of spontaneous LLT associated with chylothorax in a clinical feline case. 6,7
A 10.5-year-old, male castrated domestic shorthair (DSH) was referred to our facility for diagnostic work-up of pleural effusion. The patient had previously presented to its regular veterinarian for suspected upper respiratory tract infection (URI), due to a short history of sneezing, hyporexia and elevated respiratory rate. Orthogonal thoracic radiographs performed at that clinic revealed moderate pleural effusion. There was no previous medical history of cardiac/respiratory disease or any known trauma to the thoracic cavity.
On presentation to our facility, the cat was quiet, alert and responsive. The patient had a body condition score of 9/9, a heart rate of 200 beats per minute (bpm) and was mildly tachypneic (40–50 breaths/min). Diminished lung sounds in the ventral lung fields and a grade 2/6 left parasternal heart murmur with a regular rhythm were auscultated. A therapeutic thoracocentesis was performed that yielded 90 ml of white, opaque fluid.
Serum biochemistry performed on the day of presentation to our facility revealed no significant abnormalities except for elevated blood glucose and creatine kinase, most likely stress induced. Complete blood count revealed changes consistent with a stress leukogram. Thyroid level, urinalysis, and feline pancreatic lipase immunoreactivity were also within normal limits. Analysis of the pleural effusion revealed a majority of small mature lymphocytes along with triglycerides and cholesterol concentrations of the fluid being 1502 mg/dl (reference interval 20–90 mg/dl) and 98 mg/dl (82–218 mg/dl), respectively, all consistent with a diagnosis of chylous effusion (Table 1).
Table 1.
Fluid analysis.
| Results | ||
|---|---|---|
| Conventional units | SI units | |
| Source | Pleural | |
| Volume | 2.0 ml | |
| Appearance | White, milky | |
| Protein | 6.8 g/dl | 68 g/l |
| Red blood cell count | <106 cells/μl | <1012 cells/l |
| Nucleated cell count | 3.4×104 cells/μl | 3.4×1010 cells/l |
| Neutrophil | 14% | |
| Small mononuclear | 81% | |
| Large mononuclear | 5% | |
| Cholesterol | 98 mg/dl | 2.6 mmol/l |
| Triglyceride | 1502 mg/dl | 16.9 mmol/l |
Cytologic interpretation: the fluid contains many small mature lymphocytes and low numbers of neutrophils and foamy macrophages. It is a chylous effusion, compatible with a ruptured or leaking thoracic duct.
The radiographs performed by the referring hospital were reviewed and showed moderate pleural effusion and evidence of a soft tissue opacity in the cranial mediastinum. An echocardiogram was performed which revealed a structurally normal heart with normal wall thickness, left atrial size (LA: Ao=1.36, reference interval 1.25±0.18) 8 and valvular function. A hyperechoic mass/atelectatic lung lobe was identified by thoracic ultrasound in the right mid-cranial thorax. An abdominal ultrasound was performed which had no significant findings other than a mildly enlarged pancreas, consistent with acute/chronic pancreatitis. Top differentials at this time for the cause of the chylothorax included neoplasia of the right middle lung lobe (RMLL) or cranial mediastinum, granuloma, LLT or idiopathic chylous effusion. Thoracic computed tomography (CT) with ioversol contrast (Optiray 300; Mallinckrodt at a dose of 2 ml/kg) study was performed. The imaging revealed the presence of right middle lung mass/consolidation, a widened cranial mediastinum and enlarged sternal lymph nodes (Figs 1 and 2). Due to the CT findings, exploratory sternotomy was pursued.
Fig 1.
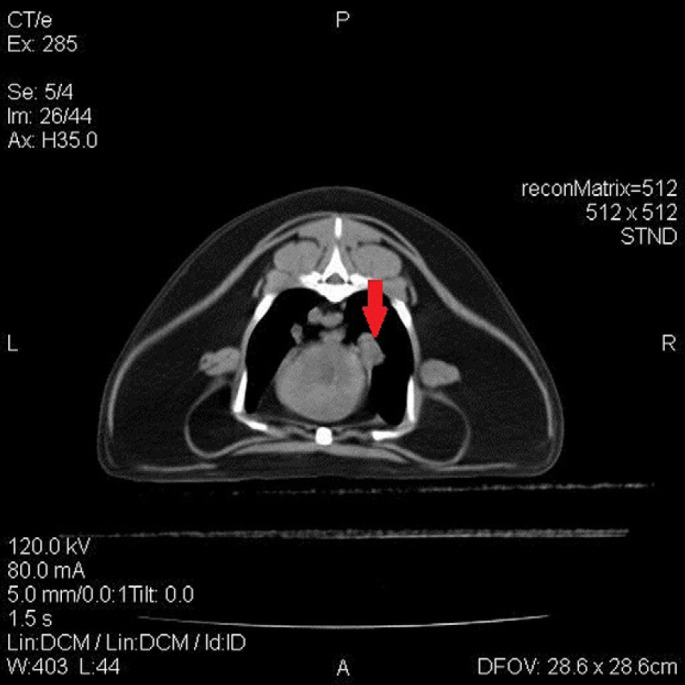
Transverse slice of thoracic cavity reveals a suspected parabronchial mass (see arrow); which has well-defined peripheral, rounded margination, located within right lung field adjacent to the heart.
Fig 2.
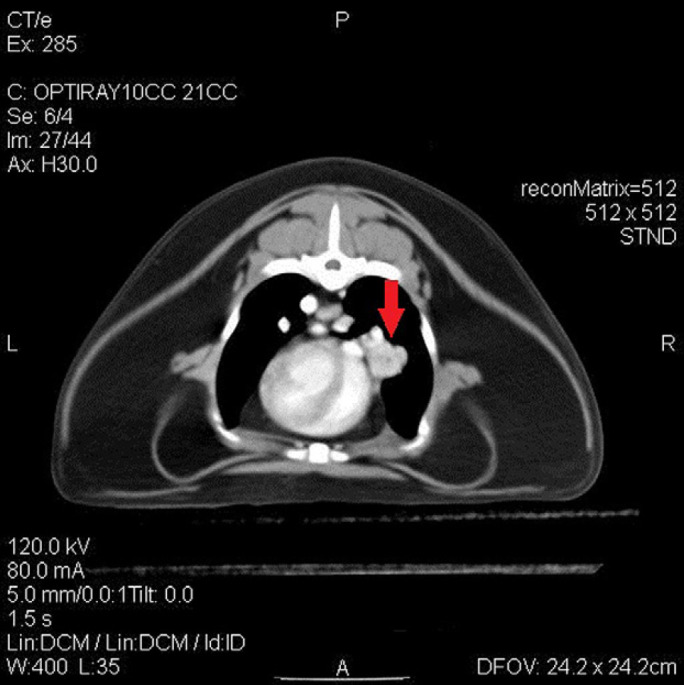
Lesion (see arrow) described in Fig 1 can be seen following administration of intravenous contrast, moderate contrast enhancement of lesion is noted.
The patient was premedicated with ketamine (Ketaset; Fort Dodge) 1 mg/kg IM and midazolam (Hospira) 0.2 mg/kg IM. Induction was performed with IV propofol (Propflo; Abbott) 4 mg/kg IV and fentanyl (Hospira) 3 μg/kg IV, and anesthesia was maintained with 1–2% isoflurane inhalant gas (VetOne) and a fentanyl (Hospira) constant rate infusion at 0.1–0.5 μg/kg/min. Peri-operative cefazolin (West-Ward) was administered at 22 mg/kg IV every 90 min. A routine median sternotomy was performed and upon entering the thoracic cavity a moderate amount of chylous effusion was noted along with thickened pleurae. A complete thoracic exploratory revealed a moderate amount of adipose tissue, explaining the wide appearance observed on CT, with enlarged sternal lymph nodes and a severely atelectatic RMLL, which failed to expand with positive pressure ventilation to 20 cm H2O. A sternal lymph node was removed and a right middle lung lobectomy without de-rotation was performed at the level of the hilus using a Thoracoabdominal V3 stapler, with the site testing negative for free air leakage. Both specimens were submitted for histopathologic examination. A 14 French thoracostomy tube was placed before closure. Following this a subcutaneous diffusion catheter was placed to facilitate postoperative analgesia of the sternotomy site and a mixture of 1 ml bupivacaine (Hospira) and 0.5 ml lidocaine (Hospira) was instilled every 6 h. Subcutaneous and skin layers were closed routinely. Recovery from anesthesia was uneventful. Postoperatively the patient was maintained on a fentanyl constant rate infusion at 5 μg/kg/min, maintenance intravenous fluids, and routine thoracostomy tube management. The patient did well throughout its hospital stay, aside from development of mild purulent nasal discharge and sneezing which was suspected to be secondary to a URI. After removal of both the diffusion catheter and thoracostomy tube, the patient was discharged from the hospital 3 days postoperatively with buprenorphine (Buprenorphine HCl; Bedford) 0.015 mg/kg sublingually q 12 h, Fluocinolone acetonide 0.01% and dimethyl sulfoxide 60% (Synotic Otic Solution; Fort Dodge) 1 drop intranasal q 12 h, 1 ml (250 mg) PO q 24 h l-lysine HCl (Enisyl-F; Vetoquinol) PO q 12 h, and amoxicillin trihydrate/clavulanate potassium (Clavamox drops) 62.5 mg/cat PO q 12 h, along with instructions for incision care and respiratory monitoring.
At the 2-week postoperative examination the owner reported that the patient's appetite and respiratory rate had improved significantly since the surgery. Re-check thoracic radiographs and ultrasound showed only trace pleural effusion with no parenchymal or cardiac abnormalities. A 6 months postoperative examination revealed complete resolution of previous clinical signs and re-check radiographs at this time revealed the same amount of pleural effusion.
Histopathologic exam of the submitted RMLL, showed extensive areas of collapse and marked congestion of alveolar capillaries. Also noted were multifocal areas of reactive fibroplasia, chronic inflammation and pleural fibrosis along the pleural surface. These findings were interpreted as severe atelectasis with marked congestion and pleuritis, consistent with a full/partial LLT. The resected lymph node histopathology results were consistent with lymphoid hyperplasia and lymphadenitis. No evidence of infection or neoplasia was noted in either sample.
LLT is an uncommon condition in both cats and dogs, with only eight cases reported in cats over the past 40 years. 3,4,9–11 The RMLL is the most commonly affected lobe in cats and deep/narrow chested dogs, but torsion of the left cranial lung lobe has been observed more commonly in small breed dogs such as Poodles and Pugs, there has been one report in canines of multiple lobes being affected. 1,2,4,5 It has been theorized that the RMLL is most commonly affected due to its shape and loose attachment to adjacent structures, which ultimately allows increased mobility compared to other lung lobes. Pleural effusion is a common sequelae to LLT secondary to the venous and lymphatic occlusion. Varying types of pleural effusion have been associated with LLT, such as: chylothorax, hemorrhagic, septic and neoplastic effusion. 4,9,10 In most cases it is difficult to determine if the pleural effusion occurred first, or if it was secondary to the LLT.
Clinical signs associated with LLT include lethargy, anorexia, hemoptysis and varying severities of respiratory distress. 1,3 Physical exam findings consistent with pleural effusion are commonly observed such as muffled heart and lung sounds. Blood work findings may show evidence of inflammation but are commonly within normal limits and thoracic radiographs can reveal pleural effusion accompanied by opacification of a lung lobe. 1,4 Occasionally, air bronchograms extending in an abnormal direction may be also observed in the affected lung lobe. Other diagnostic imaging techniques include thoracic ultrasound which can reveal evidence of pleural effusion, lung lobe consolidation, and/or lack of venous return or faint arterial pulsation. 12 Advanced imaging, such as CT or bronchoscopy, can also be utilized to support a diagnosis of LLT as well as to rule out other causes of pleural effusion. 11,13 Definitive diagnosis requires exploratory thoracotomy or thoracoscopy and treatment requires a partial/full lung lobectomy. 1,2 Recurrence of LLT has been reported in dogs, though it appears to be rare. 2
Of the reported LLTs in the feline population, observed co-morbidities include chylothorax, pyothorax, mediastinal lymphoma, asthma, or a combination of renal and cardiac diseases. 1–3,5,9 Of the two cases with chylothorax, one was diagnosed at necropsy, therefore, no treatment or follow-up data were available. 9 The second case involved a cat with both a pericardial peritoneal diaphragmatic hernia and left cranial LLT. This patients’ chylous effusion did not resolve after repair of the diaphragm and lung lobectomy, and was eventually diagnosed with idiopathic chylothorax. 10 To the authors’ knowledge this is the first case describing a feline patient with a spontaneous LLT and chylous effusion which has resolved clinically after lung lobectomy. Presently, the patient reported in this case report remains asymptomatic and is thriving at home.
References
- 1.Moon M., Fossum T.W. Lung lobe torsion. Bonagura J.D. Kirks current veterinary therapy, 12th edn, 2000, WB Saunders: Philadelphia, 919–921. [Google Scholar]
- 2.Nelson A.W., Monnet E. Lungs. Slatter D. Textbook of small animal surgery, 3rd edn, 2002, WB Saunders: Philadelphia, 880–888. [Google Scholar]
- 3.Dye T.L., Teague H.D., Poundstone M.L. Lung lobe torsion in a cat with chronic feline asthma, J Am Anim Hosp Assoc 34, 1998, 493–495. [DOI] [PubMed] [Google Scholar]
- 4.d’Anjou M.A., Tidwell A.S., Hecht S. Radiographic diagnosis of lung lobe torsion, Vet Radiol Ultrasound 46, 2005, 478–484. [DOI] [PubMed] [Google Scholar]
- 5.Neath P.J., Brockman D.J., King L.G. Lung lobe torsion in dogs: 22 cases (1981–1999), J Am Vet Med Assoc 217, 2000, 1041–1044. [DOI] [PubMed] [Google Scholar]
- 6.Williams J.H., Duncan N.M. Chylothorax with concurrent right cardiac lung lobe torsion in Afghan hound, J S Afr Vet Assoc 201, 1986, 599–602. [PubMed] [Google Scholar]
- 7.Johnston G.R., Feeney D.A., O’Brien T.D., et al. Recurring lung lobe torsion in three Afghan hounds, J Am Vet Med Assoc 184, 1984, 842–845. [PubMed] [Google Scholar]
- 8.Fox P.R. Feline cardiomyopathies. Fox P.R., Sisson D., Moise N.S. Textbook of canine and feline cardiology, 2nd edn, 1999, WB Saunders: Philadelphia, 621–678. [Google Scholar]
- 9.Brown N.O., Zontine W.J. Lung lobe torsion in the cat, J Am Vet Radiol Soc 17, 1976, 219–223. [Google Scholar]
- 10.Kerpsack S.K., McLoughlin M.A., Graves T.K., Smeak D.D., Biller D., Leake L. Chylothorax associated with lung lobe torsion and peritoneopericardial diaphragmatic hernia in a cat, J Am Anim Hosp Assoc 30, 1994, 351–354. [Google Scholar]
- 11.Millard R.P., Myers J.R., Novo R.E. Spontaneous lung lobe torsion in a cat, J Vet Intern Med 22, 2008, 671–673. [DOI] [PubMed] [Google Scholar]
- 12.Reichle J.K., Wisner E.R. Non-cardiac thoracic ultrasound in 75 feline and canine patients, Vet Radiol Ultrasound 41, 2000, 154–162. [DOI] [PubMed] [Google Scholar]
- 13.Schultz R.M., Peters J., Zwingenberger A. Radiography, computed tomography and virtual bronchoscopy in four dogs and two cats with lung lobe torsion, J Small Anim Pract 50, 2009, 360–363. [DOI] [PubMed] [Google Scholar]


