Abstract
Since insulin-like growth factor-I (IGF-I) was first discovered as a mediator of glucose homeostasis, it has been extensively investigated in diabetes research in humans, rodents and primates. To date, however, relatively little work has been carried out on this hormone in the cat, despite the pathophysiological similarities between human and feline diabetes mellitus, as well as the relatively common nature of the disease in cats. This study reports on the IGF-I concentrations of 42 insulin treated diabetic cats and 25 normal cats. Diabetic subjects were grouped according to length of insulin treatment as either short, medium or long term. Analysis of variance (ANOVA) and Fischer's pair-wise comparisons revealed that mean IGF-I levels in short-term diabetic cats were significantly lower than those in normal cats whilst mean levels in long-term diabetics were significantly higher. The direction and extent of these alterations may have implications for our understanding of the pathophysiology of feline diabetes mellitus and for the use of this hormone in the diagnosis of acromegaly in diabetic cats.
Introduction
Since 1963, when Froesch first reported that serum had more insulin-like activity than could be explained by insulin alone (Froesch et al., 1963), insulin-like growth factor-I (IGF-I) (then known as non-suppressible insulin-like activity [NSILA]) has been extensively investigated as a mediator of growth and glucose homeostasis. These investigations have considered the structure, function and regulation of production of IGF-I, mainly in humans, rodents and non-human primates. To this date, however, relatively little attention has been directed towards the study of IGF-I in diabetic cats, despite there being a number of clinical and pathophysiological similarities between feline and human diabetes mellitus (Struble and Nelson, 1997), and the fact that the condition is not uncommon in the domestic cat (Feldman and Nelson, 1996).
Some initial work has been performed by Lewitt et al. These workers measured IGF-I concentrations and IGF Binding protein (IGFBP) profiles in eight diabetic and eight normal cats (Lewitt et al., 2000). This work revealed a highly significant increase in IGF-I levels amongst non-acromegalic, diabetic cats. These findings were of interest for two reasons: firstly, in contrast to the diabetic cats in the Lewitt study, human type-I diabetic patients have been consistently reported to have decreased levels of IGF-I (Bismar et al., 1994; Dunger and Acerini, 1998) and human type-II diabetic subjects are reported to have variable changes in IGF-I, with levels reported to either decrease (Bang et al., 1994) or remain unchanged (Frystyk et al., 1999). Secondly, since growth hormone's anabolic effects are exerted by IGF-I, and serum IGF-I concentrations tend to reflect average 24 h growth hormone (GH) levels in humans (Clasey et al., 2001), some authors have suggested that IGF-I be used as an aid in the diagnosis of feline acromegaly (Norman and Mooney, 2000). However, since none of the diabetic cats in the Lewitt study had evidence of acromegaly and all showed elevated IGF-I levels, the authors felt that the use of IGF-I to screen for GH excess may be unreliable. Considering these important and unanticipated findings, we decided to further investigate feline IGF-I concentrations in the diabetic state through both a larger sample size and an analysis of the effect of length of disease.
Materials and methods
Subjects
Serum was collected from 42 insulin-treated diabetic cats (median age 11 years, range 4.5–18 years) presenting as clinical cases to the University of Sydney Veterinary Centre (UVC-S), and to various other veterinary hospitals across Australia. Whilst diagnostic procedures varied across the institutions, all cats admitted to the study displayed clinical evidence of diabetes mellitus, typically polyuria and polydipsia, as well as persistent hyperglycaemia and an appropriate response to insulin therapy. None of the animals included in this study showed clinical evidence of acromegaly at any time before or during the study period. We considered clinical evidence of acromegaly to include classical alterations of the flat bones and dentition as well as evidence of insulin resistance and or poorly controlled diabetes mellitus. In addition, no animal was enrolled into the study, which had evidence of other concurrent diseases at the time of serum collection. Concurrent diseases in these patients were ruled out on the basis of physical examination, blood biochemistry and pertinent medical history. Information was obtained on breed, age, sex and length of insulin treatment for all diabetic cats in the study.
Serum was also obtained from 25 normal, control cats (median age 8 years, range 2–17 years). These cats presented to the UVC-S and other Australian veterinary hospitals for routine medical and surgical procedures such as vaccination, dental prophylaxis and de-sexing. Such cats were considered normal if they were free of a recent history of disease and a physical examination revealed no grossly detectable signs of disease. For the purposes of this study, recent history was deemed to be a period of 12 months since the resolution of any condition, which required veterinary attention. All animals, both controls and diabetics, with a history of endocrine or metabolic disease were excluded from the study. Eighteen of the 25 normal cats had blood glucose concentrations determined prior to their enrollment in the study. Blood glucose levels were within normal limits for all 18 of these animals. Since blood glucose was not determined on the seven remaining cats, it is possible that one or more of these subjects could have had elevated blood glucose concentrations and may even have been early diabetic subjects. However, considering the lack of any historical or physical evidence to this effect, the probability of such an occurrence is anticipated to be very low and therefore these subjects were included in the study. Owners' permission was obtained prior to sample collection in all cases.
Prior to IGF-I determination and data analysis, diabetic cats were divided into three groups based on the length of insulin treatment. The first group was comprised of short-term diabetics who had been treated for 31 days or less (n=18). Subjects allocated to the second group had been treated for between 32 days and 14 months, inclusive (n=10). The third group contained long-term diabetics who had been treated for over 14 months (n=14). Although these groupings may seem somewhat arbitrary, they were chosen to approximate events often observed in the management of feline diabetes mellitus, as well as to allow us to investigate the effects of length of insulin treatment upon IGF-I concentrations. A period of 31 days was chosen to represent short-term diabetics. Evidence from our hospital, and reports in the literature, support the notion that cats tend to require a period of time, which averages approximately 1 month, to adjust to insulin regimens (Nelson, 1998). When considering long-term therapy, it has been documented that cats typically live for a period of 1 to 3 years after the initiation of insulin therapy. In one study of 104 insulin treated diabetic cats, the median age of survival was found to be 17 months, although a significant proportion survived only 12 months (Goossens et al., 1998). We therefore chose 14 months as the lower bound for our long-term diabetic cats as this point represents relatively long-term survival whilst still allowing sufficient numbers of subjects to be obtained during the study period. By default then, our medium-term subjects were those who had been treated for between 32 days and 14 months.
Sample handling
All serum samples were obtained after a fast of between 6 and 10 h to lessen the effects of post-prandial glucose increases and subsequent short-term hormone changes. Whole blood was collected by venipuncture and transferred to plain serum tubes, which were left at room temperature for a minimum of 45 min and a maximum of 1.5 h prior to centrifugation and serum harvesting. Serum collected at the university was refrigerated once harvested and analysed by radio immunoassay within 5 days of collection. Samples obtained from referring hospitals were collected in a similar manner, as directed by study personnel. All external samples were sent on ice via overnight courier to the university for immediate refrigeration. These samples were also analysed within 5 days of receipt.
IGF-I assay
IGF-I is a hormone, which is highly conserved across many different genera and species (Daughaday and Rotwein, 1989), thus there is a high degree of cross-reactivity of cat IGF-I with anti-human IGF-I antibodies. This cross reactivity allows established human IGF-I radioimmunoassays (RIAs) to be readily adapted to investigate IGF-I concentrations in the domestic cat (Church et al., 1994; Lewitt et al., 2000). In this study, IGF-I was obtained by acid–ethanol extraction of sera and then quantified by RIA using human IGF-I standard, anti-human IGF-I antibody and human IGF-I tracer as per Lewittet al., 2000(after Church et al., 1994). In their 1994 study, Church et al. confirmed the assay's specificity by the demonstrating parallel displacement curves for serial dilutions of feline samples and human IGF-I standards. They also determined the intra-assay variation to be 3.9% for pooled feline samples with low IGF-I concentrations and 4.3% for those with high IGF-I concentrations. Inter-assay variation was found to be 5.7% and 7.9%, respectively. From the time of the 1994 study until the current study, this assay was performed at the University of Sydney as a diagnostic test for acromegaly in feline patients. The same assay and apparatus was also used during the Lewitt et al. study of 2000. Prior to the commencement of the current study, specificity was reconfirmed by the demonstration of parallel displacement curves. Throughout the 10 runs in which the samples from this study were analysed, the sensitivity of the assay was found to be 1.8 nM. In addition, the interassay variation was 5.5% for pooled feline samples with low IGF-I levels and 8.7% for those with high IGF-I levels.
Statistics
To investigate statistical differences between the IGF-I concentrations of the four groups in this study, we initially performed a one-way analysis of variance (ANOVA). We then performed a series of Fischer's pair-wise comparisons to compare all group means and determine which were significantly different from one another. All statistical analyses were performed using the SAS statistical package release 8.02 (SAS Institute, Cary, NC, USA).
Results
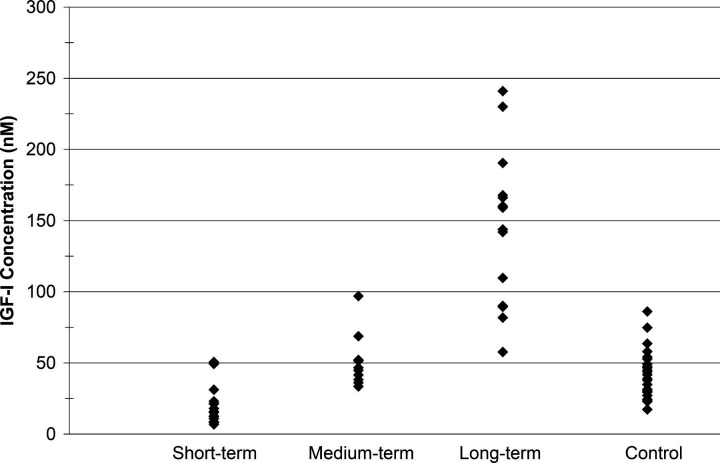
The mean IGF-I concentration of the control cats was found to be 42.4 nM (±16.6 nM SD), a value similar to that reported by other investigators(Lewitt et al., 2000). Amongst the diabetic cats, IGF-I concentrations were found to be: 22.1 nM (±14.62 nM), 51.0 nM (±19.0 nM) and 145 nM (±54.6 nM) for short-, medium- and long-term insulin treated diabetics, respectively. Figure 1 illustrates these results in the form of a dot plot.
Figure 1.
IGF-I concentration by group.
A one-way ANOVA revealed a highly significant P-value (P<0.0001), strongly indicating that at least one group mean was significantly different from the others. Subsequently, a series of Fischer's pair-wise comparisons indicated that the mean IGF-I concentration in the control cats and in the medium-term diabetic cats were not significantly different (at P=0.05). Significant differences existed between all other combinations of groups. That is, control cats had more IGF-I than short-term diabetics and less than long-term diabetics whereas short-term diabetic cats had less IGF-I than control cats and both medium and long-term diabetic cats.
Discussion
Our results indicate that cats that have received long-term insulin therapy for their diabetes have significantly higher levels of circulating IGF-I than short and medium-term insulin treated diabetic cats, as well as normal cats. To our knowledge, such changes have not been previously reported in the domestic cat. These findings have a number of important implications: firstly, as Lewitt et al., concluded, caution may be necessary when IGF-I levels are employed to diagnose acromegaly in diabetic cats. Our findings suggest that such caution is most warranted when a diagnosis of acromegaly is sought based on IGF-I levels in long-term insulin treated diabetic cats, since our study has indicated that such cats may have IGF-I levels significantly above normal, in the absence of clinical signs of acromegaly, including the absence of overt insulin resistance. Finally, as discussed below, our findings may also have implications for the understanding of the aetiology and pathophysiology of feline diabetes mellitus.
In mammals, nearly all circulating IGF-I is produced by the liver under the positive influence of pituitary derived GH (Wheelhouse et al., 1999). However, portal insulin is a critical permissive factor, without which the liver is unable to produce IGF-I (Hanaire-Broutin et al., 1996a; Russell-Jones et al., 1992). Exogenous insulin administered via the subcutaneous route fails to alter portal insulin levels in humans. In order to allow for portal insulin levels to rise and hepatic IGF-I production to continue, exogenous insulin must be administered via the intraperitoneal route (Hanaire-Broutin et al., 1996b). IGF-I acts as a major negative feedback inhibitor of pituitary GH production (Ghigo et al., 1999).
Type-I diabetes mellitus is characterised by β-cell destruction and an absolute requirement for exogenous insulin. Humans with type-I diabetes have been reported to have significantly lower IGF-I levels than normal subjects. This is due to the fact that such patients lack β-cell function and are typically maintained on subcutaneous insulin therapy, thus they lack portal insulinisation (Amiel et al., 1984; Batch et al., 1991). Human type-I diabetics also tend to have elevated GH secretion owing to reduced or absent IGF-I negative feedback (reviewed in Dunger et al., 2002).
Unlike type-I diabetes mellitus, the type-II form of the disease is defined as a condition of relative insulin deficiency seen with or without reduced β-cell function (Greco et al., 1995). Owing to the broad definition of the condition, type-II diabetes may have many different aetiologies and the condition thus represents a broader clinical syndrome than the type-I form of the disease. Considering the pathophysiological heterogeneity amongst human type-II patients, it is not surprising that conflicting reports have been generated regarding GH, insulin and IGF-I levels in such patients. Various studies have reported IGF-I levels to be reduced (Banget al., 1994; Tan and Baxter, 1986) or unchanged (Frystyk et al., 1999; Moses et al., 1996), whilst GH secretory frequency has been reported to increase (Barnes et al., 1985) or remain unchanged (Kjeldsen et al., 1975). Insulin levels are also variable amongst this group of patients, however, owing to insulin resistance many human type-II diabetics are hyperinsulinaemic (Krans, 1999). It is therefore likely that IGF-I levels in human type-II diabetics will vary depending on the prevailing hormonal balance of the individual patient. IGF-I levels will be a function of GH secretion in those with residual β-cell function. However, in those with hypoinsulinaemia, IGF-I levels will be dominated by the concomitant lack of the permissive factor, portal insulin.
Cats have been reported to suffer both type-I and type-II diabetes mellitus based on a number of factors including: variable responses to oral hypo-glycaemics, diet and response to insulin therapy as well as pathological findings and reported responses to insulin secretagogue tests (reviewed in Rand, 1999). However, the relative proportion of cats suffering from each type of the disease is often difficult to determine. Whilst the majority of cats may present in an insulin dependent state, this may not truly reflect the proportion of cats with type-I diabetes mellitus. It is likely that delays in diagnosis and the effects of chronic hyperglycaemia lead to glucose toxicity and β-cell exhaustion, thus increasing the apparent frequency of type-I diabetes mellitus (Link and Rand, 1996). Further support of a type-II aetiology in the cat is seen in the pathological findings of a study in which only a small proportion of diabetic cats showed histological lesions similar to those seen in type-I diabetic humans (Goossens et al., 1998).
Whilst considering the known state of the insulin/GH/IGF-I axis in humans, rodents and non-human primates, as well as the work of Link and Rand on the effects of hyperglycaemia in the cat, we feel that the changes in IGF-I levels seen amongst the groups of diabetic subjects in this study support the concept of glucose toxicity in the cat as well as the notion that the majority of feline diabetics have the type-II form of the disease. It is our contention that most newly diagnosed diabetic cats mimic type-I human diabetics in reference to an absolute insulin requirement as well as reduced IGF-I levels. We speculate that delays in diagnosis lead to long periods of hyperglycaemia, which ultimately reduce residual β-cell function and portal insulinisation. The low portal insulin levels then inhibit IGF-I production. Such inhibition of production is manifested as the significantly lower IGF-I concentrations seen amongst the short-term insulin treated diabetic cats in our study relative to the normal, control cats. As treatment is instituted, we hypothesise that the effects of hyperglycaemia on β-cell function decrease and subsequently endogenous insulin secretion recovers to a variable degree. Such a recovery may then allow for portal insulin levels to rise to levels sufficient for hepatic IGF-I to be produced again. This theory is supported by the normal levels of IGF-I seen amongst the medium-term diabetic cats in our study.
The explanation of the supra-physiological IGF-I levels seen in the long-term insulin treated diabetic cats in this study is somewhat more troublesome. As discussed above, improved β-cell function in the insulin treated diabetic cat may explain the normalisation of serum IGF-I levels. However, even if β-cell function fully recovered, it is unlikely that this alone would explain IGF-I concentrations exceeding those seen in normal cats. Since GH is the main positive regulator of IGF-I, it is possible that increases in GH secretion in the long-term insulin-treated diabetic cats could explain these findings, even in the absence of clinical acromegaly or insulin resistance. Perhaps the combined effects of the disease and insulin treatment lead to an adaptive process whereby GH secretion increases but the insulin antagonistic effects of GH are moderated. Such a hypothesis could be investigated by comparing the level of diabetic control amongst short-, medium- and long-term insulin treated diabetic cats and correlating this information with the subjects' GH and IGF-I levels. Such a comparison cannot be performed in the current study as we only have subjective data indicating that all cats were well controlled at the time of entry into the study, as opposed to more specific measures of glycaemic control such insulin dosages and glycosylatedhaemoglobin or fructosamine levels.
Currently the measurement of GH levels in domestic cats is limited to a small number of laboratories around the world, and as such GH levels were not obtained for the cats in this study. Future work in this field would benefit greatly from simultaneous measurement of GH, IGF-I and insulin levels to confirm our theories and extend our knowledge of the feline GH/IGF-I axis.
In conclusion, this study appears to confirm the suggestion of Lewitt et al., that caution may be warranted when employing IGF-I levels to diagnose acromegaly in diabetic cats. However, we qualify this suggestion by emphasising that such caution may be best reserved for long-term diabetic cats in whom IGF-I levels may be elevated in the absence of clinical evidence of acromegaly. In addition, we believe that the trend of IGF-I concentrations seen in the diabetic subjects in this research is further evidence that the majority of diabetic cats are indeed type-II diabetics. The effects of long-term hyperglycaemia and delays in diagnosis likely lead to β-cell burnout and initially low IGF-I levelsimmediately following diagnosis. The normal and subsequently supra-physiological IGF-I levels seen in medium and long-term insulin treated diabetic cats, respectively, occur once insulin therapy has improved β-cell function and allowed for a degree of portal insulinisation to return. Ultimately this study has increased our understanding of the feline GH/IGF-I axis whilst raising new questions for further research in this field.
Acknowledgments
This work was supported by a grant from The Petplan Charitable Trust. The authors would like to thank Professor D.R. Fraser of the University of Sydney for his assistance in reviewing this manuscript. Additional thanks also goes to Ms Dot Lewis of the University of Sydney for providing technical and logistical support during the course of this study.
References
- Amiel S.A., Sherwin R.S., Hintze R.L., Gertner I.M., Press M., Tamborlane W.V. The effects of diabetes and its control on insulin-like growth factors in the young subject with type 1 diabetes, Diabetes, 33, 1984, 1175–1179. [DOI] [PubMed] [Google Scholar]
- Bang P., Bismar K., Rosenfeld R.G., Hall K. Fasting affects serum insulin-like growth factor-I (IGFs) and IGF-binding proteins differently in patients with non-insulin-dependent diabetes mellitus versus healthy non-obese and obese subjects, Journal of Clinical Endocrinology and Metabolism, 78, 1994, 960–967. [DOI] [PubMed] [Google Scholar]
- Barnes A.J., Kohner E.M., Johnston D.G., Alberti K.G. Severe retinopathy and mild carbohydrate intolerance: possible role of insulin deficiency and elevated growth hormone, Lancet, 1, 1985, 1465–1468. [DOI] [PubMed] [Google Scholar]
- Batch J.A., Baxter R.C., Werther G. Abnormal regulation of insulin-like growth factor binding proteins in adolescents with insulin-dependant diabetes mellitus, Journal of Clinical Endocrinology and Metabolism, 73, 1991, 964–968. [DOI] [PubMed] [Google Scholar]
- Bismar K., Fernqvist-Forbes E., Wahren J., Hall K. The effects of insulin on the hepatic production of insulin-like growth factor binding protein-I (IGFBP-1), IGFBP-3, and IGF-I in insulin-dependent diabetes, Journal of Clinical Endocrinology & Metabolism, 79, 1994, 872–878. [DOI] [PubMed] [Google Scholar]
- Church D.B., Watson A.D., Emslie D.R., Middleton D.J., Tan K., Wong D. Effects of proligestone and megestrol on plasma adrenocorticotrophic hormone, insulin and insulin-like growth factor-1 concentrations in cats, Research in Veterinary Science, 56 (2, 1994, 175–178. [DOI] [PubMed] [Google Scholar]
- Clasey J.L., Weltman A., Patrie J., Weltman J.Y., Pezzoli S., Bouchard C., Thorner M.O., Hartman M.L. Abdominal visceral fat and fasting insulin are important predictors of 24-hour GH release independent of age, gender, and other physiological factors, Journal of Clinical Endocrinology and Metabolism, 86 (8, 2001, 3845–3852. [DOI] [PubMed] [Google Scholar]
- Daughaday W.H., Rotwein P. Insulin-like growth factors I and II: Peptide, messenger ribonucleic acid and gene structures, serum and tissue concentrations, Endocrine Reviews, 10, 1989, 68–91. [DOI] [PubMed] [Google Scholar]
- Dunger D.N., Acerini C.L. IGF-I and diabetes in adolescents, Diabetes and Metabolism, 24, 1998, 101–107. [PubMed] [Google Scholar]
- Dunger D.N., Ahmed L., Ong K. Growth and body composition in type 1 diabetes mellitus, Hormone Research, 58 (Suppl 1, 2002, 66–71. [DOI] [PubMed] [Google Scholar]
- Feldman E.C., Nelson R.W. Diabetes mellitus. Feldman E.C., Nelson R.W. Canine and Feline Endocrinology and Reproduction, second ed, 1996, W.B. Saunders: Philadelphia, 339–391. [Google Scholar]
- Froesch E.R., Burgi H., Ramseier E.B., Bally P., Labhart A. Antibody suppressible and non-suppressible insulin-like activities in human serum and their physiological significance. An insulin assay with adipose tissue of increased precision and specificity, Journal of Clinical Investigation, 43, 1963, 1816–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frystyk J., Skjaerbaek C., Vestbo E., Fisker S., Orskov I.I. Circulating levels of free insulin-like growth factors in obese subjects: the impact of type 2 diabetes, Diabetes/Metabolism Research and Reviews, 15, 1999, 314–322. [DOI] [PubMed] [Google Scholar]
- Ghigo E., Gianotti L., Aravat E., Ramunni J., Valetto M.R., Broglio F., Rolla M., Cavagnini F., Muller E.E. Effects of recombinant human insulin-like growth factor I administration on growth hormone (GH) secretion, both spontaneous and stimulated by GH-releasing hormone or hexarelin, a peptidyl GH secretagogue, in humans, Journal of Clinical Endocrinology and Metabolism, 84 (1, 1999, 285–290. [DOI] [PubMed] [Google Scholar]
- Goossens M.M.C., Nelson R.W., Feldman E.C., Griffey S.M. Response to treatment and survival in 104 cats with diabetes mellitus (1985–1995), Journal of Veterinary Internal Medicine, 12, 1998, 1–6. [DOI] [PubMed] [Google Scholar]
- Greco D.S., Broussard I.D., Peterson M.E. Insulin therapy, Veterinary Clinics of North America Small Animal Practice, 25, 1995, 252–257. [DOI] [PubMed] [Google Scholar]
- Hanaire-Broutin H., Sallerin-Caute B., Poncet M.F., Tauber M., Bastide R., Rosenfeld R., Tauber J.P. Insulin therapy and GH-IGF-I axis disorders in diabetes: impact of glycaemic control and hepatic insulinization, Diabetes and Metabolism, 22, 1996a, 245–250. [PubMed] [Google Scholar]
- Hanaire-Broutin H., Sallerin-Caute B., Poncet M.F., Tauber M., Bastide R., Chale J.J., Rosenfeld R., Tauber I.P. Effect of intraperitoneal insulin delivery on growth hormone binding protein, insulin-like growth factor (IGF)-I, and IGF-binding protein-3 in IDDM, Diabetologia, 39, 1996b, 1498–1504. [DOI] [PubMed] [Google Scholar]
- Kjeldsen H., Hansen A.P., Lundbaek K. Twenty-four-hour serum growth hormone levels in maturity-onset diabetics, Diabetes, 24, 1975, 977–982. [DOI] [PubMed] [Google Scholar]
- Krans H.M.J. Type 2 diabetes: overview and genetics. Turtle J.R., Kaneko T., Osato S. Diabetes in the New Millennium, 1999, The Endocrinology and Diabetes Research Foundation of the University of Sydney: Sydney. [Google Scholar]
- Lewitt M.S., Hazel S.J., Church D.B., Watson A.D.J., Powell S.E., Tan K. Regulation of insulin-like growth factor-binding protein-3 ternary complex in feline diabetes mellitus, Journal of Endocrinology, 166, 2000, 21–27. [DOI] [PubMed] [Google Scholar]
- Link K.R.S., Rand J.S. Glucose toxicity in cats [abstract], Journal of Veterinary Internal Medicine, 10, 1996, 185. [Google Scholar]
- Moses A.C., Young S.C.J., Morrow L.A., O'Brien M., Clemmons D.R. Recombinant human insulin-like growth factor-I increases insulin sensitivity and improves glycaemic control in type 2 diabetes, Diabetes, 45, 1996, 91–100. [DOI] [PubMed] [Google Scholar]
- Nelson R.W. Disorders of the endocrine pancreas. Nelson R.W., Cuto C.G. Small Animal Internal Medicine, second ed, 1998, Mosby: St. Louis, 734–774. [Google Scholar]
- Norman E.J., Mooney C.T. Diagnosis and management of diabetes mellitus in five cats with somatotrophic abnormalities, Journal of Feline Medicine and Surgery, 2 (4, 2000, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand J.S. Current understanding of feline diabetes mellitus: Part 1, Pathogenesis, Journal of Feline Medicine and Surgery, 1 (3, 1999, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell-Jones D.L., Rattray M., Wilson V.J., Jones R.H., Sonksen P.H., Thomas C.R. Intraperitoneal insulin is more potent than subcutaneous insulin at restoring hepatic insulin-like growth factor-I mRNA levels in the diabetic rat: a functional role for the portal vascular link, Joural of Molecular Endocrinology, 9 (3, 1992, 257–263. [DOI] [PubMed] [Google Scholar]
- Struble A.L., Nelson R.W. Non insulin-dependent diabetes mellitus in cats and humans, The Compendium on Continuing Education for the Practicing Veterinarian, 19, 1997, 935–945. [Google Scholar]
- Tan K., Baxter R.C. Serum IGF-I levels in adult diabetic patients: the effect of age, Journal of Clinical Endocrinology & Metabolism, 63, 1986, 651–655. [DOI] [PubMed] [Google Scholar]
- Wheelhouse N.M., Stubbs A.K., Lomax M.A., MacRae J.C., Hazlerigg D.G. Growth hormone and amino acid supply interact synergistically to control insulin-like growth factor-I production and gene expression in cultured ovine hepatocytes, Journal of Endocrinology, 163 (2, 1999, 353–361. [DOI] [PubMed] [Google Scholar]