Abstract
Synthetic feline facial pheromone (FFP) (Feliway; Ceva Animal Health) was assessed for the management of cats with recurrent feline idiopathic cystitis (FIC). Nine of 12 cats completed the randomised, double-blinded, placebo-controlled, crossover pilot study. They had their environment treated daily with either FFP or placebo for 2 months, after which time the treatment groups were reversed. Owners used visual analogue scales to define the severity of their cat's clinical signs and behavioural changes. Five (56%) of the owners stated that their cat's overall health was better when they were using FFP. Four (44%) of the owners noticed no difference between when using the FFP and when using the placebo. While there were no statistical differences between the two treatment groups there was a trend for the cats exposed to FFP to show fewer days with clinical signs of cystitis (FFP total, mean per cat±standard deviation, 30, 4.3±6.7; placebo 69, 9.9±19.1), a lower overall clinical score (1667, 238±476; 2009, 287±425), a reduced number of episodes of cystitis (9, 1.3±2.0; 10, 1.4±2.1) and reduced negative behavioural traits (e.g., less aggression and fear) (−128, −18.3±65.8; −73, −10.4±35.1).
Introduction
The term feline lower urinary tract disease (FLUTD) describes a collection of conditions that can affect the bladder and/or urethra of cats. However, the majority (55–69%) of cases are idiopathic (Barsanti et al., 1996; Buffington et al., 1997; Kruger et al., 1991; Lekcharoensuk et al., 2001) and research over the last 30 years has failed to find a consistent cause for the inflammation. A recent hypothesis suggests that feline idiopathic cystitis (FIC) may result, in part, from alterations in the nervous system of cats with FIC (Buffington and Pacak, 2001; Buffington, 2002; Westropp and Buffington, 2002a, b), and with their inability to cope with stress (Buffington and Pacak, 2001; Hague and Buffington, 2003). In this, and other respects, FIC has similarities to interstitial cystitis (IC) in humans (Buffington et al., 1997, 1999; Elbadawi, 1997; Rothrock et al., 2001).
Stress is believed to play an important role in triggering and/or exacerbating FIC (Cameron et al., 2001; Jones et al., 1997; Kalkstein et al., 1999); with suggested stressors including living in a multiple animal household (particularly when there is inter-cat conflict), and moving house. It has therefore been suggested that reducing stress may help to reduce the recurrence and/or severity of FIC.
Feline facial pheromone fraction F3 (FFP: Feliway; Ceva Animal Health) is a synthetic feline pheromone that mimics the natural marking that cats undertake by rubbing their faces on domestic objects (Pageat, 1996). It was developed as a possible treatment for anxiety-related behaviours in cats. Non-sexual urine spraying has been studied extensively as it is believed to be associated with stress and anxiety (Beaver, 1992; Overall, 1998). When FFP is applied to surfaces within a spraying cat's environment, or delivered by a continuous vaporisation process, it results in a significant (74–94%) reduction of spraying behaviour (Frank et al., 1999; Hunthausen, 2000; Mills and Mills, 2001; Ogata and Takeuchi, 2001; Pageat, 1996; White and Mills, 1997). In some cats this positive effect can result in long-term behavioural change (Mills and White, 2000). The application of FFP can also help to reduce stress incurred by hospitalisation or transport. Applying FFP to the cages of cats during hospitalisation results in increased self-grooming and interest in food (Griffith et al., 2000), while its application to cat carriers before a long car journey results in less crying, agitation, salivation, vomiting, urination and defecation (Gaultier et al., 1998).
The aim of this study was to determine whether or not the application of FFP to the home environment could reduce the severity and/or recurrence of clinical signs in cats with FIC.
Material and methods
Cats with a history of recurrent dysuria, pollakiuria, and haematuria were recruited through the Feline Clinic of the University of Edinburgh Small Animal Hospital. For inclusion, each cat had to have experienced a minimum of two episodes of FLUTD within the preceding 6 months. A diagnosis of FIC was made on the history of clinical signs of FLUTD, where physical examination, routine urinalysis and culture, survey abdominal radiography, contrast bladder radiography and/or ultrasound examination had ruled out all other causes of FLUTD. For inclusion in the trial any treatment that the cats were receiving could not have been changed in the preceding 4 weeks. The need to change treatment during the trial resulted in the cat's removal from the trial.
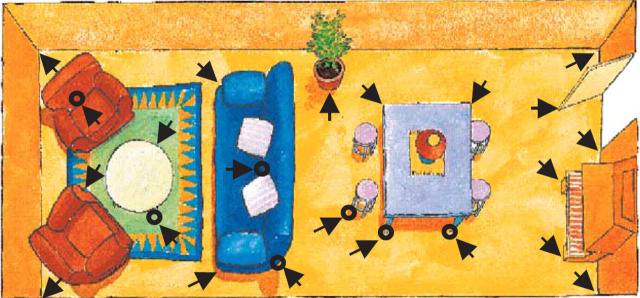
This pilot study was designed to compare an aerosol spray containing synthetic FFP (Feliway: Ceva Animal Health) with a placebo spray. The latter contained the same alcohol carrier as used in Feliway, but without the FFP. The study was performed as a randomised, double-blinded, placebo-controlled, crossover trial. Twelve cats with FIC were recruited and randomly assigned to the double-blinded treatment groups. Six cats were exposed to FFP for 2 months then placebo for 2 months, the other six were exposed to placebo then FFP. The owner's were directed to apply the sprays to any objects that protruded into the areas where the cat(s) walked (particularly the locations where the cat rubbed its face and applied its own facial pheromones) (see Fig. 1which was shown in the ‘Owner consent form’). At each site one depression of the spray nozzle was to be applied about 10 cm from the object and 20 cm from the floor. All of the rooms where the cat spent significant periods of time were to be treated. Where there were one or two cats in the household the spray was to be applied once daily. Where there were three cats or more in the household the spray was applied twice daily (morning and evening).
Figure 1.
Places within a room where the spray (feline facial pheromone or placebo) should be applied (marked by arrows).
The owners were asked to complete a diary; confirming that the environment was sprayed daily and recording when their cat showed signs of cystitis. Using linear visual analogue scales they were asked to define the severity of their cat's clinical signs (0 to 10 cm): (i) increased frequency of urination, (ii) straining while urinating, (iii) crying out while urinating, (iv) the presence of blood in the urine (macroscopic haematuria), (v) urination outside the litter box and (vi) increased grooming around the perineum. At the end of each week the owners were asked to assess whether their cat's behaviour had changed that week. This was achieved by means of a number of visual analogue scales (this time defined as −5 to +5 cm): (i) increase or decrease in negative behaviours (which had to be defined, e.g., aggression/fear/hiding), (ii) increase or decrease in positive behaviours (e.g., friendliness), (iii) increase or decrease in spraying in the house, and (iv) increase or decrease in eating.
At the end of the study, before the blinding code was broken, the owners were also asked to state whether they felt that their cat's overall health was better when they were using the FFP or the placebo. The results were analysed using Wilcoxon signed-rank tests, with significance determined at P≤0.05.
Results
Of the 12 cats recruited, eight (67%) were domestic short-haired cats, three (25%) were domestic long-haired cats, and one (8%) was a Persian. Six (50%) were neutered males and six (50%) were neutered females. The mean age of the cats was 8 years (range 3–13 years). Eleven (92%) of the cats lived in multiple cat households (median of three cats, range 2–8 cats).
Three of the cats failed to complete both stages of the study. In one case the cat developed acute renal failure and was euthanased, while the owners' of the other two cats underwent significant personal changes (in one case the birth of a baby, in the other, moving house). For the nine cats that completed the trial five (56%) of the owners stated that their cat's overall health was better when they were using FFP. Four (44%) of the owners noticed no difference between the FFP and the placebo. None felt that the placebo was better than the FFP. The owner's of two of the cats that completed the trial lost their diaries, so no data was available from their visual analogue scales.
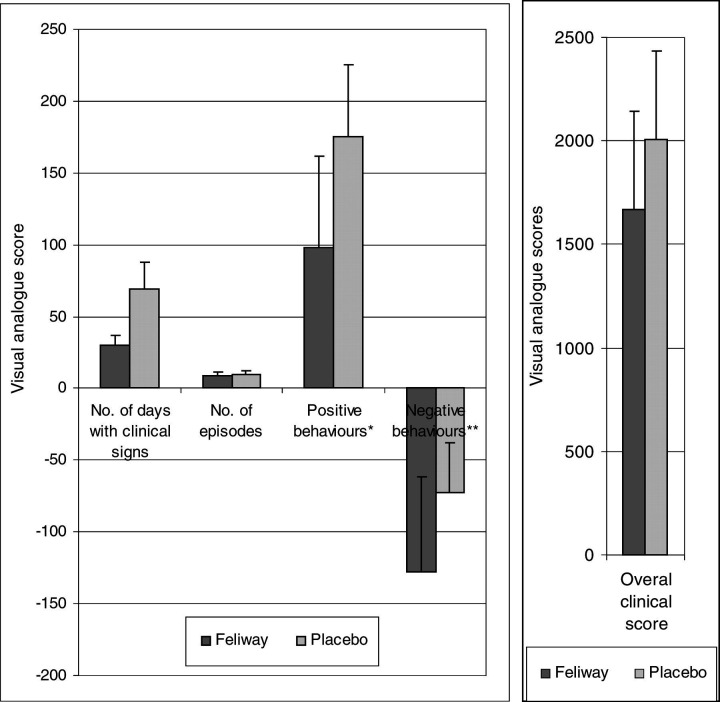
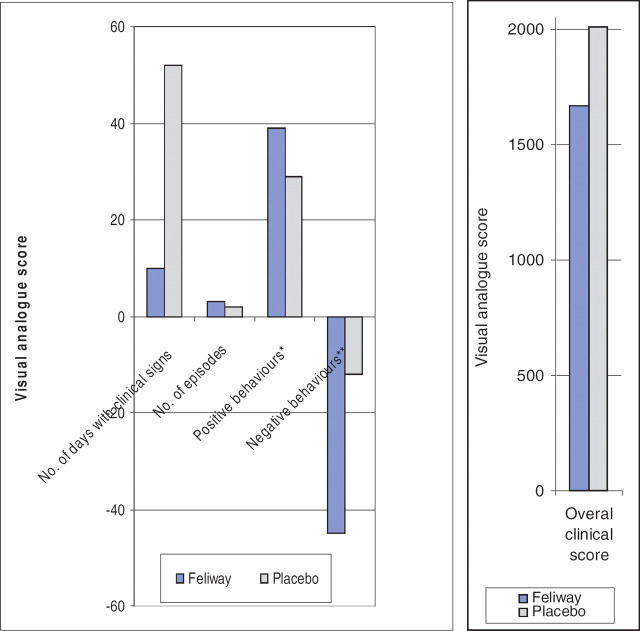
There was a trend for the cats exposed to FFP to show fewer days with clinical signs of cystitis (FFP total, mean±standard deviation, 30, 4.3±6.7; placebo 69, 9.9±19.1), a lower overall clinical score (1667, 238±476; 2009, 287±425), and reduced negative behavioural traits (e.g., less aggression and fear) (−128, −18.3±65.8; −73, −10.4±35.1) (Fig. 2). However, as with the number of episodes of cystitis (9, 1.3±2.0; 10, 1.4±2.1), the differences were not statistically significant. When looking at the most severely affected cat the differences between the two treatments was very marked (Fig. 3). As expected, when all of the cats were assessed, FFP appeared to be associated with an increased appetite (145, 20.7±50.3; 27, 3.9±25.5). Interestingly, it also appeared to be associated with less positive behaviour (e.g., less friendliness) (98, 14±64; 175, 25±69.7). However, the differences in the latter two cases were not statistically significant. The power of the analysis was ≈0.5 when looking to detect a difference of at least one times the standard deviation, using P=0.05, with n=7 cats in the analysis. Further episodes of cystitis were seen in four of the seven cats (57%) during the 4 months of the study.
Figure 2.
Graphs comparing the results of the visual analogue scores for the cats when exposed to FFP compared to when exposed to placebo. Error bars=1× standard deviation. ∗Positive behaviours, e.g., friendliness. ∗∗Negative behaviours, e.g., aggression, fear, hiding.
Figure 3.
Graphs for cat 3, the cat most severely affected with FIC, comparing the results of the visual analogue scores when exposed to FFP to when exposed to placebo. ∗Positive behaviours, e.g., friendliness. ∗∗Negative behaviours, e.g., aggression, fear, hiding.
Discussion
While there were no statistical differences between the two treatment groups there was a trend for the cats exposed to FFP to have less severe episodes and fewer recurrences of FIC. The preliminary findings of this small study indicate that this type of intervention warrants further investigation.
The suggested positive effect of FFP at reducing the signs of FIC supports the theory that stress and anxiety play a role in the induction and/or maintenance of FIC. This is because the application of FFP to the environment will reduce signs of stress in many cats. It has been shown to help reduce the signs of stress associated with hospitalisation and car transport (Gaultier et al., 1998; Griffith et al., 2000) and, more importantly, to decrease anxiety-related non-sexual spraying (Frank et al., 1999; Hunthausen, 2000; Mills and White, 2000; Mills and Mills, 2001; Ogata and Takeuchi, 2001; Pageat, 1996; White and Mills, 1997). Interestingly, in some of the studies on non-sexual urine spraying the cats were known to have physical urogenital tract disease, as well as apparent behavioural problems. Despite this, the application of FFP still reduced their clinical signs (Frank et al., 1999; Mills and Mills, 2001). This finding is perhaps not surprising when considering the interplay between physical and behavioural bladder disorders. In one study nearly 40% of cats referred for behavioural urinary problems were found to have a history of cystitis (Horwitz, 1997), and most cases referred for recurrent inappropriate urination are found to have FIC (Buffington et al., 1997, 1999).
Support for the importance of stress and exaggerated arousal in the induction and/or maintenance of FIC comes from a number of studies that have shown that affected cats respond to stress very differently from normal cats. Normal cats, when exposed to stressful situations, show signs of fear, aggression, hiding, anorexia, self-mutilation and weight change (McCune, 1995). Physiologically, in normal cats, stress results in activation of the hypothalamic–pituitary–adrenal axis. This is seen as increased activity in the locus coeruleus (an area of the brain that deals with vigilance and autonomic activity), increased plasma catecholamine concentrations (Jacobs, 1990), enhanced adrenal sensitivity to adrenocorticotropic hormone (ACTH), increased secretion of glucocorticoids from theadrenal cortex, and increased urine cortisol concentrations (Carlstead et al., 1993). The role of glucocorticoids and other α 2-adrenoceptor agonists is very complex. However, one of their essential functions is to provide negative feedback to control the stress response, which they do by inhibiting further transmission of noxious signals to the brain (Pertovaara, 1993).
Cats with FIC, when stressed, display more displacement activity than normal cats. This is seen as increased eating, drinking, grooming and urinating (Hague and Buffington, 2003). Interestingly, while they do show increased activity in their locus coeruleus and increased sympathetic activity (Reche-Junior and Buffington, 1998), they do not have increased plasma ACTH and cortisol concentrations (Buffington and Pacak, 2001; Westroppand Buffington, 2002b). This uncoupling of the hypothalamic–pituitary–adrenal axis is also seen in some chronic pain syndromes in humans (Clauw and Chrousos, 1997) and is believed to result from desensitisation of the α 2-adrenoceptors secondary to chronic stimulation (Pertovaara, 1993). While a recent study has shown that cats with FIC have multiple abnormalities in their α 2-adrenoceptor mediated signal transduction pathway (Westropp and Buffington, 2002a) it is still unclear whether this represents adaptation to living with chronic stress, or indicates that these cats have an innate defect in their ability to cope with stress.
Since cats with FIC appear to cope poorly with stress it is important that we try to identify and correct any potential stressful factors that occur within their environment. When this is not possible, the application of FFP may help to reduce the cat's overall sense of anxiety, and so reduce its FIC.
Acknowledgments
D. Gunn-Moore's lectureship is funded by Nestlé Purina. The authors would like to thank all members of the University of Edinburgh Hospital for Small Animals for their assistance with the cases, the referring veterinary surgeons for the cases and the owners for taking part in the study.
References
- Barsanti J.A., Brown J., Marks A., Reece L., Greene C.E., Finco D.R. Relationship of lower urinary tract signs to seropositivity for feline immunodeficiency virus in cats, Journal of Veterinary Internal Medicine, 10 (1, 1996, 34–38. [DOI] [PubMed] [Google Scholar]
- Beaver B.V. Feline communicative behavior, Feline Behavior: A Guide for Veterinarians, 1992, WB Saunders: Philadelphia, 63–85. [Google Scholar]
- Buffington C.A.T. External and internal influences on disease risk in cats, Journal of the American Veterinary Medicine Association, 220 (7, 2002, 994–1001. [DOI] [PubMed] [Google Scholar]
- Buffington C.A.T., Chew D.J., Kendall M., Scrivani P.V., Thompson S.B., Blaisdell J.L., Woodworth B.E. Clinical evaluation of cats with non-obstructive urinary tract disease, Journal of the American Veterinary Medicine Association, 210 (1, 1997, 46–50. [PubMed] [Google Scholar]
- Buffington C.A.T., Chew D.J., Woodworth A. Feline interstitial cystitis, Journal of the American Veterinary Medicine Association, 215 (5, 1999, 682–687. [PubMed] [Google Scholar]
- Buffington C.A.T., Pacak K. Increased plasma norepinephrine concentration in cats with interstitial cystitis, Journal of Urology, 165, 2001, 2051–2054. [DOI] [PubMed] [Google Scholar]
- Cameron M.E., Casey R.A., Bradshaw J.W.S., Waran N.K., Gunn-Moore D.A., 2001. Inappropriate urination: A study of the environmental and behavioural factors involved inthe triggering of idiopathic cystitis. Proceedings of BSAVA Congress, Birmingham, UK, p. 507.
- Carlstead K., Brown J.L., Strawn W. Behavioral and physiological correlates of stress in laboratory cats, Applied Animal Behavioural Science, 38, 1993, 143–158. [Google Scholar]
- Clauw D.J., Chrousos G.P. Chronic pain and fatigue syndromes: overlapping clinical and neuroendocrine features and potential pathogenic mechanisms, Neuroimmunomodulation, 4, 1997, 134. [DOI] [PubMed] [Google Scholar]
- Elbadawi A. Interstitial cystitis: A critique of current concepts with a new proposal for pathologic diagnosis and pathogenesis, Urology, 49 (Supplement 5A, 1997, 14–40. [DOI] [PubMed] [Google Scholar]
- Frank D.F., Erb H.N., Houpt K.A. Urine spraying in cats: presence of concurrent disease and effects of a pheromone treatment, Applied Animal Behaviour Science, 61, 1999, 263–272. [Google Scholar]
- Gaultier E., Pageat P., Tessier Y., 1998. Effect of a feline appeasing pheromone analogue on manifestations of stress in cats during transport. Proceedings of the 32nd Congress of the International Society for Applied Ethology, Clermont-Ferrand, p. 198.
- Griffith C., Steigerwald E.S., Buffington C.A.T. Effects of a synthetic facial pheromone on behavior of cats, Journal of the American Veterinary Medicine Association, 217 (8, 2000, 1154–1156. [DOI] [PubMed] [Google Scholar]
- Hague D., Buffington C.A.T., 2003. Effects of feline interstitial cystitis on behavior of cats exposed to a novel cage environment. Proceedings of the 20th American College of Veterinary Internal Medicine, Dallas, Texas, p. 810.
- Horwitz D.F. Behavioral and environmental factors associated with elimination behavior problems in cats: a retrospective study, Applied Animal Behavioral Science, 52, 1997, 129–137. [Google Scholar]
- Hunthausen W. Evaluating a feline facial pheromone analogue to control urine spraying, Veterinary Medicine, 95, 2000, 151–155. [Google Scholar]
- Jacobs B.L. Locus coeruleus neuronal activity in behaving animals. Heal D.J., Marsden C.A. The Pharmacology of Noradrenaline in the Central Nervous System, 1990, Oxford University Press: New York, 248. [Google Scholar]
- Jones B.R., Sanson R.L., Morris R.S. Elucidating the risk factors of feline lower urinary tract disease, New Zealand Veterinary Journal, 45, 1997, 100–108. [DOI] [PubMed] [Google Scholar]
- Kalkstein T.S., Kruger J.M., Osborne C.A. Feline idiopathic lower urinary tract disease. Part II. Potential causes, Compendium of Continuing Education for the Practising Veterinarian, 21 (2, 1999, 148–154. [Google Scholar]
- Kruger J.M., Osborne C.A., Goyal S.M., Wickstrom S.L., Johnston G.R., Fletcher T.F., Brown P.A. Clinical evaluation of cats with lower urinary tract disease, Journal of the American Veterinary Medicine Association, 199, 1991, 211–216. [PubMed] [Google Scholar]
- Lekcharoensuk C., Osborne C., Lulich J. Epidemiological study of risk factors for lower urinary tract diseases in cats, Journal of the American Veterinary Medicine Association, 218 (9, 2001, 1429–1435. [DOI] [PubMed] [Google Scholar]
- McCune S., 1995. Environmental enrichment for cats—a review. Proceedings of the Second International Conference of Environmental Enrichment, pp. 103–117.
- Mills D.S., Mills C.B. Evaluation of a novel method for delivering a synthetic feline facial pheromone to control urine spraying by cats, Veterinary Record, 149, 2001, 197–199. [DOI] [PubMed] [Google Scholar]
- Mills D.S., White J.C. Long-term follow up of the effect of a pheromone therapy on feline spraying behaviour, Veterinary Record, 147, 2000, 746–747. [PubMed] [Google Scholar]
- Ogata N., Takeuchi Y. Clinical trial of a feline pheromone analogue for feline urine marking, Journal of Veterinary Medicine and Science, 63 (2, 2001, 157–161. [DOI] [PubMed] [Google Scholar]
- Overall K.L. Tracing the roots of feline elimination disorders to aggression, Veterinary Medicine, 93, 1998, 363–366. [Google Scholar]
- Pageat P. Functions and use of the facial pheromones in the treatment of urine marking in the cat. Interest of a structural analogue. Johnston D., Waner T. Proceedings and Abstracts of the XXIst Congress of the World Small Animal Veterinary Association, Jerusalem, Israel, 1996, 197–198. [Google Scholar]
- Pertovaara A. Antinociception induced by alpha-2-adrenoceptor agonists, with special emphasis on medetomidine studies, Progress in Neurobiology, 40, 1993, 691–709. [DOI] [PubMed] [Google Scholar]
- Reche-Junior A., Buffington C.A.T. Increased tyrosine hydroxylase immunoreactivity in the locus coeruleus of cats with interstitial cystitis, Journal of Urology, 159 (3, 1998, 1045–1048. [DOI] [PubMed] [Google Scholar]
- Rothrock N.E., Lutgendorf S.K., Kreder K.J., Ratliff T., Zimmerman B. Stress and symptoms in patients with interstitial cystitis: a life stress model, Urology, 57 (3, 2001, 422–427. [DOI] [PubMed] [Google Scholar]
- Westropp J.L., Buffington C.A.T., 2002a. Effects of feline interstitial cystitis on α 2-adrenoceptor-mediated signal transduction pathways. Proceedings of the 20th American College of Veterinary Internal Medicine, Dallas, Texas, p. 810.
- Westropp J.L., Buffington C.A.T., 2002b. Uncoupling of the noradrenergic-hypothalamic-pituitary-adrenal axis in stressed cats with interstitial cystitis. Proceedings of the 20th American College of Veterinary Internal Medicine, Dallas, Texas, p. 813.
- White J.C., Mills D.S. Efficacy of synthetic feline facial pheromone (F3) analogue (Feliway) for the treatment of chronic non-sexual urine spraying by the domestic cat. Mills D.S., Heath S.E., Harrington L.J. Proceedings of the First International Conference on Veterinary Behavioural Medicine, 1997, Universities Federation for Animal Welfare: Potters Bar, 242. [Google Scholar]