Abstract
Twenty-one lower respiratory tract infections diagnosed in cats at University of Sydney Veterinary Centre between 1995 and 2000 were identified retrospectively. Patient records were analysed to determine historical, clinical, clinicopathologic and radiographic features of lower respiratory tract infections. Response to therapy was also assessed. Infectious agents identified were Mycoplasma spp., Pasteurella spp., Bordetella bronchiseptica, Salmonella typhimurium, Pseudomonas sp., Mycobacterium thermoresistible, Cryptococcus neoformans, Toxoplasma gondii, Aelurostrongylus abstrusus and Eucoleus aerophilus. The study provides a detailed retrospective analysis of infectious lower respiratory tract disease in this population of cats.
Introduction
Lower respiratory tract (LRT) disease in cats may be caused by infectious agents (viruses, bacteria, fungi, parasites), cardiac disease, neoplasia, trauma, toxins or irritants. Most cats with LRT infection (LRTI) have pneumonia (inflammation of the lung parenchyma), although occasionally pathology is limited to the airways (Bart et al., 2000).
Common bacterial causes of feline pneumonia are reported to include Pasteurella multocida, Escherichia coli, Klebsiella pneumoniae, Bordetella bronchiseptica, and Streptococcus canis (Henik and Yeager, 1994). However, published reports of pneumonia are invariably restricted to case reports and a literature search revealed only one detailed retrospective study of infectious causes of pneumonia in cats (Bart et al., 2000). In that study, bacteria were isolated post-mortem from feline lungs in cases of bronchitis, pneumonia and septicaemia. The majority of cases were kittens and no clinical details were provided. The bacteria identified as causing bronchitis and pneumonia were B. bronchiseptica, Pasteurella spp., Mycoplasma spp., E. coli and Streptococcus spp. The bacteria isolated from lungs in cases of septicaemia were E. coli, Streptococcus spp. and Pasteurella spp. Other infectious agents identified as causing pneumonia in the study were viruses (herpesvirus), lungworm (Aelurostrongylus abstrusus), Toxoplasma gondii and fungi (Mucor sp. and Aspergillus sp.).
Viral causes of LRTI are unlikely to be diagnosed without lung histopathology and specific viral detection techniques. Parasitic, bacterial and fungal LRTIs can be diagnosed by routine investigation of LRT disease. The aim of this study was to provide clinical, clinicopathologic, radiographic and therapeutic details of 21 non-viral cases of feline LRTI identified at University of Sydney Veterinary Centre (UCVS) between 1995 and 2000.
Materials and methods
Nineteen cases of feline LRTI that presented to UVCS between 1995 and 2000 were identified from a retrospective study of bronchoalveolar lavage (BAL) cytology and microbiology (Foster et al., 2004a). In that study, pure culture of any bacterium or fungus was considered significant as was moderate to heavy growth of any microbe with minimal growth of oral contaminants. However, the infectious agents in cases with a positive microbial culture were only considered the aetiological agentsif historical, clinical, radiographic and cytologic findings were supportive and if there was an unambiguous response to appropriate antimicrobial therapy. Parasitic LRTIs were identified from unstained wet preparations of BAL fluid. Two other LRTIs were diagnosed during the same time by means other than BAL, both of which have been published previously (Foster et al., 1998a, 1999).
Signalment was analysed for the 21 cases. Statistical comparisons for data on sex and breed were performed using Statistix for Windows (Analytical software, Tallahassee, FL, USA). Breeds were classified as domestic, Siamese, Burmese, purebred shorthair (other than Burmese and Siamese) and purebred longhair for comparison with the hospital population of cats at the time of the study (Gabor et al., 1998).
Historical and clinical data were also analysed. Clinical data was recorded as peracute if signs had been present for 72 h or less, acute, if signs had been present for less than a month, and chronic, if signs had been present for a month or longer. Tachypnoea was defined as a respiratory rate greater than or equal to 60 breaths per min as effects of temperament, transport and ambient temperature could not be assessed retrospectively. Date of presentation and, if known, date of onset of clinical signs were also analysed. The seasonsin Sydney are defined as summer (December to February), autumn (March to May), winter (June to August) and spring (September to November).
Haematology, serum biochemistry and serological test results from commercial ELISA orimmunomigration kits for feline immunodeficiency virus (FIV) antibody, feline leukaemia virus (FeLV) antigen and heartworm antigen were analysed. Cytologic and microbiological findings for the 21 cases were also analysed. Differential cell counts for BAL cytology were classified by the predominant inflammatory cell type, if the cell type comprised at least 50% of the total white cells, or described as mixed, if no cell type comprised at least 50% of the total.
Radiographs for 19 cases were reviewed by a specialist radiologist who was blinded as to the clinical diagnosis (GA). For two cases, only the specialist reports from the original radiographs were available. Bronchial signs were defined as mild (first generation of bronchi visible), moderate (second generation visible) and severe (third generation visible). Alveolar patterns were defined as mild (isolated fluffy infiltrates), moderate (well defined with air bronchograms) and severe (lobar sign). Nodular interstitial patterns were recorded as nodular. Reticular interstitial patterns wererecorded as interstitial and defined as mild (interstitial framework visible but could be bronchial pattern), moderate (interstitial framework can be distinguished from bronchial) and severe (undisputed reticular interstitial pattern).
Therapeutic agents, response to therapy and long term follow-up in all survivors, were recorded.
Results
Signalment, history, physical findings
Nineteen LRTIs in 18 cats were diagnosed by BAL cytology and microbiology. Two further cases in two cats were diagnosed by other means: mycobacterial LRTI by ultrasound-guided fine-needle aspirate cytology and microbiology (Foster et al., 1999) and toxoplasmosis, by lung squash-preparation cytology and lung histology (Foster et al., 1998a). The LRTIs were due to Mycoplasma spp. (11), mycoplasmas and P. multocida (1), mycoplasmas and B. bronchiseptica (1), Pasteurella sp. and mixed anaerobes (1), Pasteurella sp. and an unidentified Gram negative bacterium (1), Salmonella typhimurium and A. abstrusus (2, one of which also had Pseudomonas sp. cultured), Mycobacterium thermoresistibile (1), T. gondii (1), Cryptococcus neoformans var. grubii (previously var. neoformans serotype A) (1) and Eucoleus aerophilus (previously Capillaria aerophila) (1). The cases are detailed in Table 1; cases 1, 4, 6, 11, 16, 18–21 have been published previously (Barrs et al., 1999, 2000;Foster et al., 1998a, b, 1999).
Table 1.
Case data for twenty-one cases of feline lower respiratory tract infection
| Case | Age | Sex | Breed | Main Complaint | Other Complaints | Physical examination findings | Culture / infectious agent | Outcome after specific therapy for aetiological agent |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 years | MN | Domestic | Chronic cough | Weight loss | Dyspnoea, sneezing, POD | Light to moderate mycoplasma | Treated with doxycycline and terbutaline. Resolved (3 year follow-up). |
| 2 | 1 year | MN | British shorthair | Peracute cough | Peracute dyspnoea | Cough, inspiratory stridor | Heavy mycoplasma, some mixed contaminants | Treated with doxycycline and theophylline. Resolved (3 year follow-up). |
| 3 | 10 years | MN | Australian Mist | Chronic cough | Inspiratory wheeze, epiphora, obesity | Light to moderate mycoplasma from lung FNA; heavy mycoplasma from BAL | Initially responsive to doxycycline. Recurrence of mycoplasmal LRTI 10 months later and doxycycline ineffective. Required azithromycin for complete resolution. | |
| 4 | 6 months | FN | Burmese | Chronic cough | Regurgitation | Cough, pyrexia, tachypnoea | Heavy mycoplasma | Treated with ciprofloxacin (and amoxycillin initially). Resolved but occasional cough; cat has megaoesophagus. |
| 5 | 4 years | MN | Abyssinian | Acute cough | Occasional vomiting | Not possible | Heavy mycoplasma | Treated with oral doxycycline and parenteral enrofloxacin. Resolved but occasional recurrence of self limiting cough. |
| 6 | 7 months | FN | Australian Mist | Chronic cough | Cough, wheezing, pyrexia | Heavy myoplasma | Treated with doxycycline. Resolved but occasional recurrence of self limiting cough. | |
| 7 | 11.5 years | MN | Oriental | Chronic cough | Weight loss, acute dyspnoea | Dyspnoea, tachypnoea, cyanosis, weakness, hypothermia | Heavy mycoplasma | Failed to respond to enrofloxacin, terbutaline and prednisolone. Resolved after doxycycline, terbutaline and prednisolone. Occasional terbutaline-responsive cough. |
| 8 | 15 years | MN | Domestic | Chronic cough | Nasal discharge, sneezing | Wheezing, expiratory grunt, bilateral nasal discharge, URT stertor, cardiac murmur (previously hyperthyroid) | Heavy mycoplasma | Treated with doxycycline. Resolved. Recurrent doxycycline (tylosin)-responsive coughing each winter. |
| 9 | middle- aged | MN | Domestic | Peracute dyspnoea | Inappetence, weight loss | Dyspnoea, POD, cardiac arrhythmia | Heavy mycoplasma, some mixed contaminants | Treated with doxycycline and terbutaline. Resolved but recurrent doxycycline- responsive cough, especially in cold weather. |
| 10 | 9 years | MN | Persian | Acute dyspnoea | Weight loss | Dyspnoea, tachypnoea, cyanosis, pyrexia, poor body condition, fleas | Heavy mycoplasma | Poor response to clindamycin and enrofloxacin. Responded to gentamicin. Oral enrofloxacin and nebulised gentamicin only partially successful. Good response to azithromycin and constant azithromycin required. |
| 11 | aged | MN | Domestic | Acute cough | Nasal discharge | Cough, POD, ocular discharge, nasal SCC | Heavy Mycoplasma, FIV | Treated with doxycycline and terbutaline. Resolved initially but diagnosed with FBD disease 16 months later. |
| 12 | 9 years | MN | Domestic | Peracute cough | Chronic sneezing | Cough, tachypnoea, pyrexia, tachycardia | Heavy mycoplasma, Bordetella bronchiseptica | Treated with enrofloxacin. Cough resolved. URT signs continued (15 month follow-up). |
| 13 | 16 years | FN | Domestic | Chronic cough | Occasional vomiting | Cough, dyspnoea, crackles, nasal discharge, hyperthyroidism | Heavy mycoplasma, moderate Pasteurella multocida | Treated with doxycycline. Resolved. Coughing recurred 8 months later but attributed to cardiac disease. |
| 14 | 11 years | FN | Siamese | Chronic cough | Anorexia | POD | Pasteurella sp., unidentified Gram negative bacterium | Treated with enrofloxacin. Resolved but had two further episodes of coughing: 8 months later (neutrophilic BAL and negative culture; resolved after amoxcillin-clavulanate and terbutaline) and 15 months later (resolved spontaneously). Died 6 months later due to unknown cause. |
| 15 | 2.5 years | MN | Domestic | Acute anorexia | Straining to defaecate | Dyspnoea, poor body condition | Pasteurella sp., mixed anaerobes | Treated with amoxycillin and enrofloxacin. Responded but died 9 days later due to potassium bromide-induced FBD. |
| 16 | 14 wks | M | Domestic | Peracute dyspnoea | Acute cough | Dyspnoea, tachypnoea, pyrexia | Salmonella typhimurium, Aelurostrongylus abstrusus | Treated with chest drainage, amoxycillin (initially), enrofloxacin and fenbendazole. Resolved. |
| 17 | 1.5 years | MN | Domestic | Chronic cough | Dyspnoea | Dyspnoea, tachypnoea, wheezing, expiratory grunt | Salmonella typhimurium, Pseudomonas sp., Aelurostrongylus abstrusus | Treated with enrofloxacin (initially) ciprofloxacin (when susceptibilities known). Still coughing 4.5 weeks later but BAL negative for bacteria and larvae. Terbutaline required for one month then resolved (2 y follow-up). |
| 18a | 12 years | MN | Domestic | Chronic cough | Sneezing | Dyspnoea, cough | Cryptococcus neoformans, FIV | Treated with itraconazole. Resolved. One year later second LRTI (see case 19). |
| 19a | 13 years | MN | Domestic | Chronic cough | Sneezing, ocular discharge | Cough, bilateral ocular discharge | Mixed contaminants, Eucoleus aerophilus, FIV | Treated with abamectin. Resolved. Euthanased two months later with mast cell neoplasia. |
| 20 | 1.3 years | FN | Domestic | Acute cough | Depression | Dyspnoea, tachypnoea, poor body condition | Mycobacterium thermoresistibile | Treated with rifampicin, clarithromycin and doxycycline. Resolved (12 month follow-up). |
| 21 | 8 years | FN | Domestic | Acute neurologic signs | Anorexia, weight loss | Neurologic signs, cardiac murmur, pale mucous membranes, dyspnoea, thin | Not cultured. Toxoplasma gondii, FIV | Euthanased. No treatment. |
Cases 18 and 19 were from the same cat on two different occasions.
Abbreviations: MN=desexed male; FN=desexed female; M=male; POD=periodontal disease; URT=upper respiratory tract; SCC=squamous cell carcinoma; FBD=feline bronchial disease; FNA=fine needle aspirate; BAL=bronchoalveolar lavage.
Historical complaints, main presenting complaints and physical examination findings are detailed in Table 1. The cases presented throughout the year: winter (10), summer (6), spring (2), autumn (3). The date of onset of clinical signs was only accurately recorded in 10 cases: four in summer, four in autumn, one in winter and one in spring. Clinical signs in the cats with mycoplasmal LRTIs commenced in autumn in 4/8 cases for which a date of onset was known with the others recorded as spring (1), winter (1) and summer (2).
The median age was 10 years. Fifteen cats were male and six were female. The sex difference was not significant (P=0.07 with Chi-square test) but using odds ratios (Martin et al., 1987), males were 2.4 times more likely to have LRTIs than females (95% confidence interval 0.9, 6.1). There were no significant differences between any of the breeds or between domestic and purebred cats. However, compared to the hospital population, purebred shorthair cats (other than Burmese and Siamese) were four times more likely to have LRTIs than domestic cats (95% confidence interval 1.2, 12.7).
Haematology, serum biochemistry and serology
Haematological and serum biochemical data are listed in Table 2. Seven cats had serum biochemistry performed; only the more commonly abnormal analytes were listed.
Table 2.
Haematological and serum biochemical data for twelve cases of feline lower respiratory tract infection
| Case | Infection | Analyte with reference range | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCV 0.30–0.45l/l | TPP59–78 g/l | WCC8–14×109/l | Neutrophils 3.8–10.1×109/l | Bands 0–0.4×109/l | Lymphocytes 1.6–7×109/l | Monocytes 0.1–0.6×109/l | Eosinophils 0.2–1.4×109/l | Globulin 26–51 g/l | ALT <60 U/l | CK <200 U/l | ||
| 2 | mycoplasma | N | N | |||||||||
| 5 | mycoplasma | 0.23 | N | 5.5 | 3.4 | 0.9 | 1.1 | N | N | |||
| 6 | myoplasma | N | N | 18.1 | 10.2 | N | N | 1.1 | N | N | 84 | N |
| 7 | mycoplasma | 0.25 | 95 | 28.3 | 23.8 | N | N | N | 60 | N | 2616 | |
| 9 | mycoplasma | 0.28 | 97 | 26.3 | 20 | N | N | N | 2.1 | 61 | N | N |
| 10 | mycoplasma | N | 93 | 16 | 14.2 | N | 1.1 | N | N | 52 | N | N |
| 12 | mycoplasma, Bordetella bronchiseptica | N | 88 | |||||||||
| 13 | mycoplasma, Pasteurella multocida | 0.28 | 96 | 32.4 | 24.3 | N | N | 1.6 | 1.9 | 71 | 166 | 223 |
| 14 | Pasteurella, Gram negative bacterium | N | 89 | 24.6 | 13.0 | N | 9.1 | N | 1.7 | |||
| 15 | Pasteurella, mixed anaerobes | N | 87 | N | 12.0 | N | 0.5 | 1.1 | N | N | 97 | 239 |
| 16 | Salmonella typhimurium, Aelurostrongylus abstrusus | N | N | 25.6 | 16.1 | N | N | N | 3.1 | |||
| 19 | Eucoleus aerophilus | N | 90 | 17.1 | 15.6 | N | 0.9 | N | N | 54 | 81 | 1020 |
Abbreviations: N=normal (value in reference range); PCV=packed cell volume; TPP=total plasma protein (measured by refractometry); WCC=white cell count; ALT=alanine aminotransferase; CK=creatine kinase.
Serological testing for FIV antibody was performed in 12 cases (3, 7, 10–12, 15–21) and was positive in four (11, 18, 19, 21). Serological testing for FeLV antigen in seven cases (7, 12, 15, 16, 18, 19, 20) was negative in each. Heartworm antigen tests in four cases (3, 10, 11, 15) were negative.
Radiology
Radiographic findings for each case are detailed in Table 3. Lung patterns were classed as bronchial (5), bronchoalveolar (4), alveolar (4, one with concurrent pneumothorax), bronchointerstitial (3, two of which also had right middle lung lobe consolidation) and mixed (5, four of which had one or more nodules). In total, 17 cats had bronchial changes and 14 had alveolar changes.
Table 3.
Thoracic radiographic features of 21 cases of lower respiratory tract infection
| Case | Infection | Radiographic features | ||||||
|---|---|---|---|---|---|---|---|---|
| Bronchial | Alveolar | Interstitial | Nodular | Cardiovascular | Pleural | Comments | ||
| 1 | mycoplasma | Severe | Improved after treatment. | |||||
| 2 | mycoplasma | Moderate | ||||||
| 3 | mycoplasma | Moderate | Mild, focal, R cranial lobe | 15 mm nodule in L cranial lobe | Changes persisted for 10 months. No radiographs after clinical cure. | |||
| 4 | mycoplasma | Moderate | Severe, focal, R cranial lobe | Moderate | Bronchointerstitial pattern resolved with treatment. R cranial lobe consolidation persisted. | |||
| 5 | mycoplasma | Moderate (diffuse) to severe (lobar) | Alveolar pattern resolved with treatment. Residual mild bronchial pattern. | |||||
| 6 | myoplasma | Moderate | Mild to severe multifocal. Consolidation cranial part of L cranial lobe | Improved with treatment to mild alveolar pattern but mild broncho-interstitial pattern developed 19 months later. | ||||
| 7 | mycoplasma | Moderate | Severe multifocal | Mild | 5 mm nodule in R cranial lobe | |||
| 8 | mycoplasma | Severe | Lobar sign R middle lobe | Severe | ||||
| 9 | mycoplasma | Severe | Lobar sign R middle lobe | Severe | Air trapping, overinflation evident. | |||
| 10 | mycoplasma | Severe diffuse | Alveolar pattern improved with treatment but severe bronchial pattern then evident. | |||||
| 11 | mycoplasma | Mild | Mild, focal, dorsocaudal margins of caudal lobes | Caudal lobar pulmonary artery dilation and tortuosity | Alveolar pattern resolved with treatment. Vascular changes increased in severity. | |||
| 12 | mycoplasma, Bordetella bronchiseptica | Mild | Mild | |||||
| 13 | mycoplasma, Pasteurella multocida | Severe | Cardiomegaly | Bronchial pattern improved after treatment. | ||||
| 14 | Pasteurella sp., unidentified Gram negative bacterium | Moderate | ||||||
| 15 | Pasteurella sp., mixed anaerobes | Severe | Mild diffuse | Mild broncho-interstitial pattern five months prior to this. | ||||
| 16 | Salmonella typhimurium, Aelurostrongylus abstrusus | Severe diffuse | Severe pneumo-thorax | Improved with treatment to severe interstitial and moderate bronchial pattern one month later. | ||||
| 17 | Salmonella typhimurium, Pseudomonas sp., Aelurostrongylus abstrusus | Severe | Patchy R middle lung lobe atelectasis | |||||
| 18 * | Cryptococcus neoformans | Moderate | Two focal nodules, 3–5 mm diameter | Nodules resolved with treatment. | ||||
| 19 * | Eucoleus aerophilus | Severe | ||||||
| 20 | Mycobacterium thermoresistibile | Severe diffuse | ||||||
| 21 | Toxoplasma gondii | Moderate | Mild | Six to ten nodules, 2–5 mm diameter | ||||
Abbreviations: R=right; L=left.
Cases 18 and 19 were from the same cat on two different occasions.
Cytology and microbiology
BAL cytology revealed large numbers of inflammatory cells in 18 cases and moderate numbers in one. Inflammation was neutrophilic in 17 cases (15 of which had 80% or more neutrophils) and histiocytic in two. The two cases with histiocytic inflammation were both from the same FIV-infected cat (Cases 18 and 19). Eosinophils comprised less than 20% of the cell population in all samples except one, in which certain areas of the smear had up to 34% of eosinophils. Infectious agents were observed cytologically except in the cases of mycoplasmal infections.
Two cats with persistent coughing despite therapy had BALs performed to check microbiological cure: Case 17 (salmonellosis) and Case 20 (mycobacteriosis). Both had many inflammatory cells and there was mixed inflammation (predominantly macrophages and neutrophils) in Case 17 and histiocytic inflammation in Case 20. Culture was negative in both cases.
Three cats had lung fine-needle aspirate cytology and culture. Ultrasound-guided lung fine-needle aspirates of a focal mass in Case 3 (Fig. 1a and b) yielded numerous inflammatory cells, of which the majority were neutrophils. A heavy pure growth of mycoplasmas was cultured from the sample and a mycoplasmal abscess was diagnosed. Lung fine-needle aspirate cytology in Case 10revealed numerous inflammatory cells, of which the majority were neutrophils. There were also clusters and sheets of relatively uniform epithelial cells and this was attributed to epithelial cellhyperplasia or well-differentiated neoplasia. A moderate growth of mycoplasmas wasobtained from a very small amount of diluted sample and heavy growth of mycoplasmas was also cultured from BAL fluid in this cat. As long-term follow-up eliminated the possibility of neoplasia, mycoplasmal LRTI was diagnosed. Fine needle aspirate cytology in the cat with mycobacterial LRTI (Case 20) revealed large numbers of inflammatory cells, the majority of which were intact anddegenerate neutrophils. Numerous Gram-positive, acid-fast bacteria, which tended to occur in lipid vacuoles, were also noted.
Figure 1.
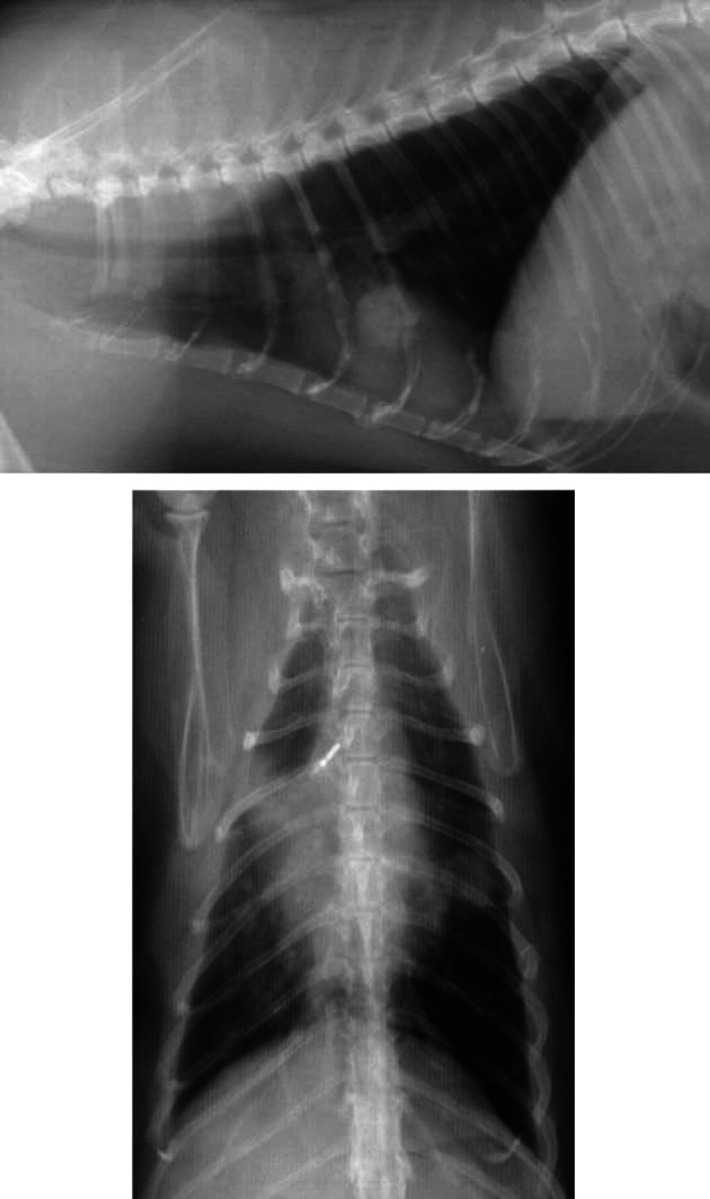
Right lateral and ventrodorsal radiographs from Case 3 (mycoplasmal abscess). A well circumscribed opacity is superimposed upon the cardiac silhouette at the level of the fifth and sixth intercostal space (Figure 1a). It has clearly defined margins and matches the soft tissue opacity visible in the caudal part of the left cranial lung lobe (Figure 1b). An ill-defined pulmonary opacity in the right cranial lobar region is also evident (Figure 1b).
Cytology on post-mortem squash preparations of lung in Case 21 (toxoplasmosis) revealed numerous inflammatory cells, mainly neutrophils. Epithelial cells were very pleomorphic with marked anisocytosis, anisokaryosis, large nuclei and prominent nucleoli (Fig. 2a). Intracellular tachyzoites were discovered in one oil immersion field (Fig. 2b). Histopathology on the necropsy samples was consistent with disseminated toxoplasmosis (subacute to chronic necrotising encephalitis and suppurative interstitial pneumonia with bradyzoites and tachyzoites in lung and brain and tachyzoites also in tracheobronchial lymph nodes) (Foster et al., 1998a).
Figure 2.
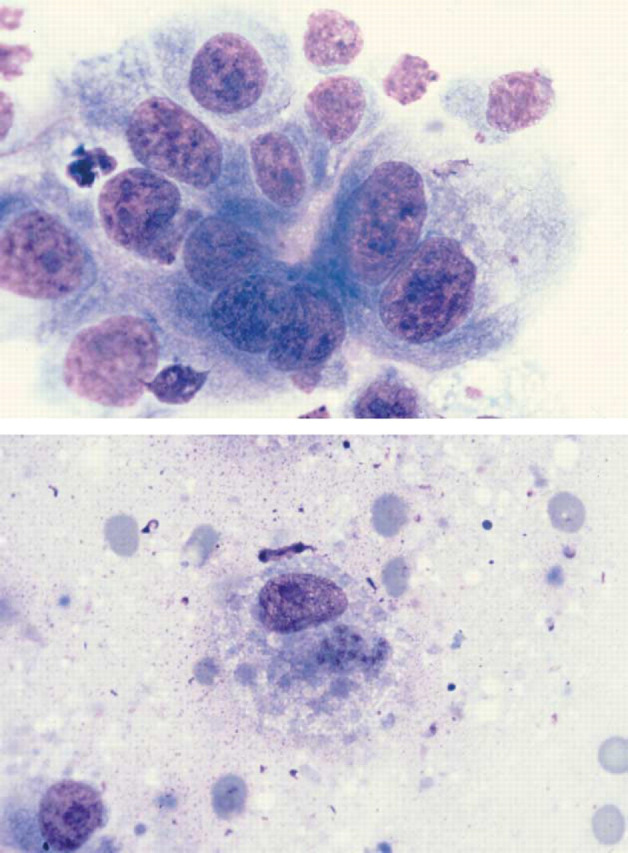
(a) Diff Quik-stained squash preparation of pulmonary parenchyma from Case 21 illustrating dysplastic bronchoalveolar epithelial cells. Note the variation in cell and nuclear size, the presence of prominent large nucleoli and one binucleate cell. Magnification ×735. (b) T. gondii tachyzoites within a macrophage in a squash preparation of pulmonary parenchyma from Case 21 (Diff Quik stain). Magnification ×735.
Therapy and outcomes
Therapy and outcome for each case are detailed in Table 1. Antibiotic therapy for the mycoplasmal LRTIs included fluoroquinolones (ciprofloxacin and enrofloxacin), doxycycline, gentamicin, azithromycin or combinations of these; bronchodilators were also used initially in five cases. Response to doxycycline (VibraVet and Vibra-Tabs 50; Pfizer) with doses approximating 5 mg/kg twice daily orally (length of treatment variable) appeared uniformly successful except in Case 3 which respondedinitially then required azithromycin (Zithromax; Pfizer). Enrofloxacin (Baytril; Bayer) was used in four cases and response was poor in two of these, one having heavy growth of mycoplasmas cultured from BAL whilst receiving enrofloxacin, prednisolone and terbutaline therapy.
Of the 13 cases of mycoplasmal LRTIs, two cases (1 and 2) resolved completely (both with three-year follow-up). Four cases have had occasional coughing episodes that require no treatment (cases 4, 5 and 6) or terbutaline (case 7); case 4 had a concurrent oesophageal motility disorder. Cases 5 and 6 had BALs performed 12.5 and 18.5 months later respectively, with numerous inflammatory cells noted in both cases. Inflammation was neutrophilic in Case 5 and mixed in Case 6. Neither sample had significant bacterial growth.
Case 8 had a three-year history of tylosin-responsive coughing every winter prior to having a BAL; there was no response to amoxycillin-clavulanate on the occasions it was administered. When the BAL was eventually performed there was a heavy pure growth of mycoplasmas and signs resolved after two-weeks of doxycycline therapy. Signs recurred again in the following winter and again, rapidly resolved with two weeks ofdoxycycline therapy. Case 9 also had recurrent doxycycline-responsive coughing when the weather was cold.
The cat with the mycoplasmal lung abscess (Case 3) was treated with a 10-week course of doxycycline (6 mg/kg twice daily orally (PO)). A repeat fine needle aspirate of the nodule 14 weeks after initial diagnosis did not yield inflammatory cells or mycoplasmas. Coughing recurred five months after this and a further course of doxycycline of unspecified duration was ineffective. A BAL performed six weeks after this course of doxycyline was prescribed (10 months after initial diagnosis) demonstrated the presence of mycoplasmas. An eight-week course of azithromycin (7 mg/kg twice weekly PO) led to complete resolution of the clinical signs and there has been no recurrence (18 month follow-up).
One cat with very severe clinical and radiographic signs that had mycoplasmas cultured both from lung fine-needle aspirate and BAL (Case 10), required gentamicin initially (2 mg/kg thrice daily intravenously for five days) as there was minimal clinical response to enrofloxacin (3 mg/kg twice daily subcutaneously (SC) for seven days) and clindamycin (Antirobe; Pharmacia and Upjohn; 15 mg/kg twice daily PO for four days). This cat was treated after discharge with gentamicin nebulisation and oral enrofloxacin (8 mg/kg once daily PO) for one month before being treated with azithromycin (5–6 mg/kg twice weekly PO). Continuous azithromycin therapy was required until its death from unrelated causes 29 months later; any withdrawal of the drug resulted in relapse.
One cat with mycoplasmal LRTI (Case 11) developed severe feline bronchial disease (FBD) that required continuous treatment with prednisolone, bronchodilators or both. A second BAL performed 16 months later demonstrated histiocytic inflammation and no significant bacterial growth. This cat was a FIV-positive stray and it is not known whether it had had FBD prior to its mycoplasmal LRTI. There was no bronchial pattern evident on the initial radiographs but radiographs taken at the time of diagnosis of FBD revealed a mild bronchointerstitial pattern. Throughout, there was consistent enlargement of the caudal lobar pulmonary arteries but heartworm infection was excluded on the basis of echocardiography and heartworm antigen and antibody testing.
Anthelminthic treatment was successful in the three cases of parasitic LRTI. Oral fenbendazole (Panacur; Intervet Australia; 50 mg/kg daily PO for three days) was used to treat one case of A. abstrusus (Case 16) and two doses of abamectin (Avomec Antiparasitic Injection for Cattle; Merial Australia), 300 μg/kg SC two weeks apart, were used for the other two parasitic LRTIs: A. abstrusus (Case 17) and E. aerophilus (Case 19).
Discussion
Published case reports of pneumonia are invariably restricted to case reports or small case series. An extensive literature search revealed only one detailed retrospective study of infectious causes of pneumonia in cats (Bart et al., 2000). That study reported microbiological, parasitic and histological findings from autopsied cats and identified bacteria, fungi, parasites and viruses as causes of feline LRTIs. It included no clinical data on the cats.
This study would appear to be the first clinical study of feline LRTIs. There is no “gold standard” that can be used to make a clinical diagnosis of LRTI (Peeters et al., 2000). Historical, haematologic and radiographic findings known to be compatible with LRTI are often non-specific or are inconsistently present (Hawkins, 2000). In addition, many cases of LRTI have concomitant, predisposing respiratory tract or systemic diseases, the presence of which does not preclude a diagnosis of LRTI (Peeterset al., 2000).
In this study, diagnosis of LRTI was not based solely on identification of an infectious agent. The infectious agent was only considered the aetiological agent if historical, clinical, radiographic and cytologic findings were supportive and if there was an unambiguous response to appropriate therapy. Each case was considered individually and then the group examined as a whole in an attempt to provide useful clinical data about both diagnosis and management of feline LRTIs.
The most common presenting complaint was coughing and the most common abnormalities detected during physical examination were dyspnoea, tachypnoea and coughing (or increased tracheal hypersensitivity). Pyrexia only occurred in five cats (24%) indicating it is an unreliable sign of LRTI. However, as mild pyrexia was only recorded in one of 25 cases (4%) of feline bronchial disease diagnosed during the same time (unpublished data), presence of pyrexia may be of assistance when trying to distinguish between the two disease categories if BAL microbiology is not feasible. Crackles on auscultation are reported as a clinical sign in cases of feline pneumonia (Henik and Yeager, 1994) but were only present in one case in this series (5%) and in three of 25 cases of FBD (12%) diagnosed during the same study period (Foster et al., 2004b). Twenty nine per cent of the cats, including 38% of cats with mycoplasmal LRTIs, had ocular or URT signs at the time of presentation. Mycoplasma felis is a recognised cause of URT and or ocular signs in cats (Campbell et al., 1973; Haesebrouck et al., 1990; Tan, 1974) and it is tempting to speculate that ocular discharge and URT signs in the cats with mycoplasmal infections may also have been due to mycoplasmosis. In another study of mycoplasmal respiratory infections in small animals, upperrespiratory tract signs were present in all three cats reported (Chandler and Lappin, 2002).
Although not statistically significant there was a trend towards a male sex predisposition. Two desexed male cats (three cases) and one desexed female cat were infected with FIV. Seroprevalence of FIV is reportedly two to three times higher in males than females (Sellon, 1998). As testing for FIV was not routine, it may be that FIV infection was underestimated in this group of cats.
Data on season of admission was not available for hospital cats in the years 1995–2000 which makes any conclusions about dates of presentation tenuous. Date of onset of signs is more useful than date of presentation but there were very few cats, for which this information was known. More cats presented in winter than the other seasons and for mycoplasmal infections, onset of clinical signs was frequently in autumn.
The most common haematological abnormalities were leucocytosis and neutrophilia, which would not be unexpected findings in bacterial infections. The most common biochemical abnormalities were hyperproteinaemia, hyperglobulinaemia increased ALT and increased CK. Hyperproteinaemia and hyperglobulinaemia, consistent with antigenic stimulation, were present in the majority of tested samples. Increased ALT in four of seven cats tested was unexpected, however, one of the cats was being treated with phenobarbitone and another had concurrent hyperthyroidism; the other two cases had very mild increases. Increased CK concentrations were also not expected. In one case, the increase was negligible and in Case 7 the cat had recently had seizures but the increased CK concentration was unexplained in the third cat (Case 19).
Radiographically, all lung patterns were represented and whilst 67% of cases had alveolar changes, 81% of cases had a bronchial pattern either alone or in combination with another pattern. This is consistent with the histological diagnoses of bronchitis, bronchointerstitial pneumonia or bronchopneumonia in non-septicaemic cases of bacterial pneumonia (Bart et al., 2000). A predominantly nodular pattern was noted in the cases of cryptococcosis, toxoplasmosis and mycoplasmal abscess.
All BALs except for those from one FIV-positive cat (cases 18 and 19), were neutrophilic. The three lung aspirate samples also had neutrophilic cytology suggesting that the normal response to pulmonary bacterial or protozoal infection is neutrophilic unless there is concurrent immunosuppression. None of the three cats with confirmed parasitic infections had an eosinophilic BAL although other factors may have influenced this: the presence of concurrent salmonellosis in two and FIV infection in the other.
Lung aspirate cytology has been reported in one study as 100% specific for neoplasia (De Berry et al., 2002). The lung cytology in the case of toxoplasmosis in our study, however, demonstrated that care needs to be taken with interpretation of hyperplastic and dysplastic epithelial changes when there is concurrent inflammation. As published previously, the dysplastic epithelial changes in the cat with toxoplasmosis were initially attributed to neoplasia as tachyzoites were sparse (Foster et al., 1998a). Cytological diagnosis of toxoplasmosis in both this cat, and another in the literature (Litster et al., 1999), was made retrospectively once a histological diagnosis was available and the slides reviewed.
The bacteria reported as occurring in the airways of healthy cats include Pasteurella spp., Pseudomonas spp., Staphylococcus spp., Streptococcus spp., E. coli and Micrococcus spp. (Padrid et al., 1991). Anaerobic bacteria and mycoplasmas have not been isolated from the lower airways of healthy cats (Padrid et al., 1991; Randolph et al., 1993). The most commonly reported bacterial causes of feline pneumonia include P. multocida, E. coli, K. pneumoniae, B. bronchiseptica, Streptococcus canis, mycobacteria and Eugonic Fermenter-4 (Bart et al., 2000; Henik and Yeager, 1994). In the present study infectious bacterial agents identified were mycoplasmas, Pasteurella spp., Salmonella typhimurium, B. bronchiseptica, Pseudomonas sp. and Mycobacterium thermoresistibile.
Mycoplasmas are known pulmonary pathogens in other species and have been recorded as causing pyothorax, pneumonia and pulmonary abscessation in cats (Crisp et al., 1987; Foster et al., 1998b; Malik et al., 1991; Wong and Noor, 1984). Mycopolasmas were identified in a recent study as the third most common cause of bacterial pneumonia in cats; the majority of cases in the study were kittens up to 12 weeks old (Bart et al., 2000). Mycoplasmas were the most common cause of LRTIs in our study, which included no patients younger than 14 weeks old. Mycoplasmas were also identified as the cause of pulmonary abscessation in one cat, the second such case in the feline veterinary literature (Crisp et al., 1987).
Response of the mycoplasmal LRTIs to appropriate antimicrobial therapy in this study would appear to be compelling evidence for the pathogenicity of mycoplasmas. However, the most commonly used antibiotic in these cases was doxycycline. In addition to its antibiotic effects, doxycycline has been shown to have immunomodulatory effects and a recent studydemonstrated suppression of in vitro induction of immunoglobulin E responses of peripheral blood mononuclear cells obtained from asthmatic humans (Smith-Norowitz et al., 2002). It is possible that the responses to doxycycline in our cases were, at least in part, due to the immunomodulatory effects of the drug, however, it is unlikely that the responses should have been so consistently dramatic and sustained if mycoplasmas had been incidental to FBD. In addition, no similar effects would be expected of the other agents employed: fluoroquinolones, aminoglycosides and azalides.
Mycoplasmal LRTIs are often considered to be a consequence of pre-existing pulmonary diseases although pulmonary pathology other than FBD would appear to be inadequate for mycoplasmal colonisation (Foster et al., 2004a). It is possible that the mycoplasmal infections in this study, whilst of clinical significance, were secondary to FBD especially as clinical signs only resolved completely in two cases. However, it is possible that the mycoplasmas caused serious pathology and resulted in bronchial inflammation and airway hyperresponsiveness similar to Mycoplasma pneumoniae in humans (Sabato et al., 1984). Certainly, LRTIs caused by other agents also caused clinical signs that persisted after the infections cleared, as evident in Cases 17 and 20.
The role of mycoplasmas is being increasingly examined in human asthma where there are strong associations between (i) mycoplasmal infection and exacerbation of asthma (ii) chronic mycoplasmal infections and asthma and (iii) induction of asthma subsequent to mycoplasmal infection (Gil et al., 1993; Kraft et al., 1998; Micillo et al., 2000;Petrovsky, 1990; Sabato et al., 1984; Seggev et al., 1986, 1996; Teo et al., 1986; Yano et al., 1994). We believe that mycoplasmal LRTIs in cats should be regarded as significant irrespective of any pre-existing pathology as even in cats with known FBD, there is the possibility of acute exacerbation by mycoplasmas. Evidence in the human literature also suggests that the role of mycoplasmas as causal agents of asthma/bronchial disease requires investigation.
The history in one case of mycoplasmal LRTI suggested recurrent infection every winter. Other owners noted recurrence of signs when the weather became cold. As known onset of clinical signs showed a trend for autumn, this would be consistent with the seasonal data and suggest that mycoplasmal LRTI may be more common in cold conditions. Alternatively, the cats may remainindoors in cold weather so signs are more obvious to owners.
Treatment of mycoplasmal infections in veterinary medicine is usually empirical. Antibiotic susceptibility profiles are not available for feline mycoplasmal isolates. Mycoplasmas are generally reported to be sensitive to macrolides (erythromycin and tylosin), fluoroquinolones (enrofloxacin and ciprofloxacin), tetracyclines, chloramphenicol and gentamicin (Greene, 1998). However, species differences are noted in human isolates with M. hominis being resistant to erythromycin and some of the other macrolides whilst M. pneumoniae is invariably sensitive. M. hominis is sensitive to ciprofloxacin whilst M. pneumoniae may or may not be (Taylor-Robinson, 1995). Doxycycline, used at approximately 5 mg/kg twice daily orally, appeared effective in most cases in this study, however, the cat with the mycoplasmal abscess responded initially and then required azithromycin, suggesting that resistance might have developed. Resistance of mycoplasmas can develop through chromosomal mutations or through acquisition of antibiotic resistance transposons. Tetracycline resistance mediated by the latter mechanism has been noted in some human mycoplasmal species (Taylor-Robinson, 1995).
Enrofloxacin did not appear as effective as doxycycline and one cat had mycoplasmas cultured whilst being treated with the drug, albeit with concurrent corticosteroid therapy. Enrofloxacin has the advantages of being bactericidal and requiring once daily administration but increasing reports of idiosyncratic retinopathy and blindness, even at normal dose rates (Gelatt et al., 2001; Wiebe and Hamilton, 2002), would suggest that this drug should be reserved for infections where it is specifically indicated. Azithromycin was used effectively in two cases and may be a better choice, especially in those cases where less frequent dosing is desirable.
It is difficult to comment on the role of Pasteurella spp. in feline LRTIs as in the three casesin this study, infections were mixed, one occurring shortly before death due to potassium bromide-induced FBD. P. multocida is a common inhabitant of the oral and upper respiratory mucous membranes in cats, with a carrier rate of over 30%, (Biberstein and Holzworth, 1987) and has been reported as a cause of pneumonia (Bart et al., 2000; Henik and Yeager, 1994).
Salmonellosis occurred twice in association with A. abstrusus (Fig. 3aand b) and it has been postulated that migrating lungworm larvae may act as carriers for intestinal bacteria (Barrs et al., 1999). Unlike the previously reported kitten (Case 16; Barrs et al., 1999), Case 17 was an apparently healthy adult cat with no signs of enteric salmonellosis. Pneumonia due to Salmonella choleraesuis has also been reported in a cat with no signs of gastrointestinal tract disease (Rodriguez et al., 1993).
Figure 3.
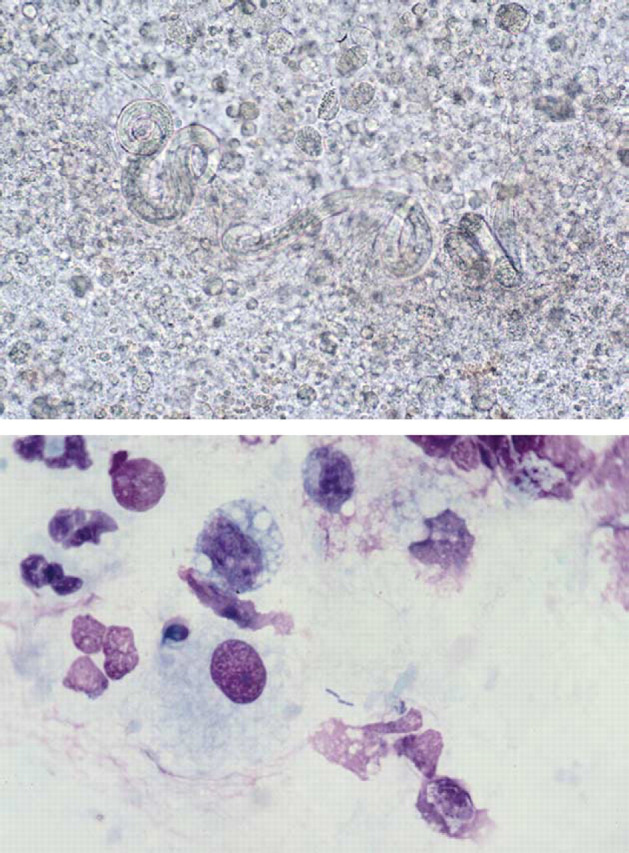
(a) Unstained wet preparation of the bronchoalveolar lavage from Case 17 illustrating larvae of A. abstrusus surrounded by inflammatory cells. Magnification ×147. (b) Diff Quik-stained smear of bronchoalveolar lavage from the same case illustrating bacterial rods of S. typhimurium surrounded by bronchoalveolar macrophages and intact and degenerate neutrophils. Magnification ×735.
In a previous Australian survey, A. abstrusus was found only in adult animals in autumn and winter (Wilson-Hanson and Prescott, 1982). The kitten in the present series would appear to be an exception to this with respect to both age and season; it presented in autumn, having shown signs in summer. The adult cat with A. abstrusus presented in winter and date of onset of signs was thought to be either summer or early autumn. The reason for this discrepancy may just be a result of insufficient sample size in the original survey or because itwas performed in Brisbane, which has different seasonal conditions than Sydney.
Ivermectin, a semi-synthetic analogue of avermectin B1, is often recommended for treatment of A. abstrusus as a single dose of 400 μg/kg orally or SC (Hawkins, 2000; Pechman, 1994). Presumably this recommendation is made because ivermectin administered at 200 μg/kg SC proved ineffective in treating A. abstrusus in one case report and a second dose of 400 μg/kg SC was required 2.5 weeks later (Kirkpatrick and Megella, 1987). Whilst the second dose was effective, it may be that two doses were in fact required for complete resolution. In another study, a single dose of oral ivermectin at 300 μg/kg was ineffective in three cats (Blagburnet al., 1987). Two doses of abamectin (a natural fermentation product of avermectin) at 300 μg/kg SC, two weeks apart, were used successfully for treatment of A. abstrusus and E. aerophilus in this study. Administration of ivermectin or abamectin is considerably easier than fenbendazole although use of these drugs is “off-label” and care needs to be taken in kittens (Plumb, 2002). The bioavailability of ivermectin may be lower in cats than dogs (Plumb, 2002) thus parenteral administration is preferable to oral.
Parasitic causes of LRTIs commonly cited include A. abstrusus and T. gondii (Bart et al., 2000). E. aerophilus is rarely mentioned despite the fact that in Australia at least, a similar prevalence of 3–5% has been reported for both E. aerophilus and A. abstrusus (Barrs et al., 2000). Presumably this is because prevalence of clinical disease due to E. aerophilus appears to be low (Pechman, 1994). Diagnosis of infection with this parasite should be straightforward as the ova are passed in the faeces and routine faecal flotation is adequate for detection. However, the double operculated ova of E. aerophilus may be mistaken for Trichuris spp. when found in faecal preparations (Barrs et al., 2000).
Diagnosis of T. gondii is probably the most difficult of the three parasites. The lung appears to be a target organ in both primary and reactivated toxoplasmosis in cats (Dubey and Carpenter 1993; Parker et al., 1981). Radiology and pulmonary cytology in both Case 21 and another cat in the literature (Litster et al., 1999) were consistentwith neoplasia. Case 21 was euthanased due to its poor neurological and systemic status but the decision was influenced by the radiographic changes. Diagnosis is possible by BAL (Brownlee and Sellon, 2001; Eddlestone et al., 1996) but both BAL and lung biopsy evaluation may fail to identify T. gondii in human patients. Immunohistochemistry and tissue culture have been recommended for lung biopsies and BAL specimens from human patients with AIDS (Derouin et al., 1989; Nash et al., 1994).
Fungal causes of feline LRTIs include Cryptococcus spp., Sporothrix schenkii, Aspergillus sp., Mucor sp., Candida sp., Histoplasma capsulatum, Coccidioides immitis and Blastomyces dermatitidis (Bart et al., 2000); the latter two are exotic to Australia. The lungs are considered the primary site of infection for cryptococcosis in humans (Maliket al., 2001) but pulmonary cryptococcal infections appear to be quite rare in cats (Gerds-Grogan and Dayrell-Hart, 1997; Malik et al., 1992; Medleauet al., 1995). In this study, cryptococcal LRTI was only identified in a single FIV-positive cat which had the hallmarks of AIDS-like disease: opportunistic infections and, terminally, neoplasia (Sellon, 1998).
Whilst this study has much information that may be pertinent only to cats in Sydney, it would appear to be the first clinical study of feline LRTIs. The typically cited bacteria in feline pneumonia were not identified commonly in these cats and the majority of LRTIs were caused by mycoplasmas. The diversity of causes even in this small number of cats, suggests that empirical therapy in coughing cats is not recommended and that BAL cytology and microbiology should be performed in all cases. If specimen transport and culture methods for mycoplasmal detection are sub-optimal, empirical therapy with two weeks of doxycycline at 5 mg/kg twice daily orally is recommended. Should BAL be impossible due to financial or patient constraints then therapy should at least address potential mycoplasmal and nematode infections before corticosteroids are administered. Bronchodilators such as terbutaline and theophylline may be needed as supportive therapy in any case of LRTI.
Acknowledgments
This study would not have been possible without the enthusiastic assistance of Rhian Foster and Karen Kilpatrick in medical records and Leanne Fitzsimmons from Veterinary Imaging Associates, Sydney. The clinicians who managed the cases are gratefully acknowledged as is Professor Ian Robertson, Murdoch University, for his assistance with statistics. Richard Malik is supported by the Valentine Charlton Bequest of the Post Graduate Foundation in Veterinary Science of the University of Sydney.
References
- Barrs V.R., Martin P., Nicoll R.G., Beatty J.A., Malik R. Pulmonary cryptococcosis and Capillaria aerophila infection in an FIV-positive cat, Aust Vet J, 78, 2000, 154–158. [DOI] [PubMed] [Google Scholar]
- Barrs V.R., Swinney G.R., Martin P., Nicoll R.G. Concurrent Aelurostrongylus abstrusus infection and salmonellosis in a kitten, Aust Vet J, 77, 1999, 229–232. [DOI] [PubMed] [Google Scholar]
- Bart M., Guscetti F., Zurbriggen A., Pospischil A., Schiller I. Feline infectious pneumonia: a short literature review and a retrospective immunohistological study on the involvement of Chlamydia spp. and distemper virus, The Vet J, 159, 2000, 220–230. [DOI] [PubMed] [Google Scholar]
- Biberstein E.L., Holzworth J. Bacterial diseases. Holzworth J. Diseases of the Cat, 1987, Saunders: Philadelphia, 279–319. [Google Scholar]
- Blagburn B.L., Hendrix C.M., Lindsay D.S., Vaughan J.L. Anthelminthic efficacy of ivermectin in naturally parasitized cats, Am J Vet Res, 48, 1987, 670–672. [PubMed] [Google Scholar]
- Brownlee L., Sellon R.K. Diagnosis of naturally occurring toxoplasmosis by bronchoalveolar lavage in a cat, J Am Anim Hosp Assoc, 37, 2001, 251–255. [DOI] [PubMed] [Google Scholar]
- Campbell L.H., Snyder S.B., Reed C., Fox J.G. Mycoplasma felis-associated conjunctivitis in cats, J Am Vet Med Assoc, 163, 1973, 991–995. [PubMed] [Google Scholar]
- Chandler J.C., Lappin M.R. Mycoplasmal respiratory infections in small animals: 17 cases (1988–1999), J Am Anim Hosp Assoc, 38, 2002, 111–119. [DOI] [PubMed] [Google Scholar]
- Crisp M.S., Birchard S.J., Lawrence A.E., Fingeroth J. Pulmonary abscess caused by a Mycoplasma sp in a cat, J Am Vet Med Assoc, 191, 1987, 340–342. [PubMed] [Google Scholar]
- De Berry J.D., Norris C.R., Samii V.F., Griffey S.M., Almy F.S. Correlation between fine-needle aspiration cytopathology and histopathology of the lung in dogs and cats, J Am Anim Hosp Assoc, 38, 2002, 327–336. [DOI] [PubMed] [Google Scholar]
- Derouin F., Sarfati C., Beauvais B., Iliou M.C., Dehen L., Larivière M. Laboratory diagnosis of pulmonary toxoplasmosis in patients with acquired immunodeficiency syndrome, J Clin Microbiol, 27, 1989, 1661–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P., Carpenter J.L. Histologically confirmed clinical toxoplasmosis in cats: 100 cases (1952–1990), J Am Vet Med Assoc, 203, 1993, 1556–1566. [PubMed] [Google Scholar]
- Eddlestone S.M., Hoskins J.D., Hosgood G., Dubey J.P. Take the Compendium challenge, Compend, 18, 1996, 774–779. [Google Scholar]
- Foster S.F., Martin P., Braddock J.A., Malik R. A retrospective analysis of feline bronchoalveolar lavagecytology and microbiology (1995–2000), JFMS, 2004a, in press. [DOI] [PMC free article] [PubMed]
- Foster S.F., Allan G.S., Martin P., Robertson I.D., Malik R. Twenty five cases of feline bronchial disease (1995–2000), JFMS, 2004b, in press. [DOI] [PMC free article] [PubMed]
- Foster S.F., Martin P., Davis W., Allan G.S., Mitchell D.H., Malik R. Chronic pneumonia caused by Mycobacterium thermoresistibile in a cat, J Sm Anim Pract, 40, 1999, 433–438. [DOI] [PubMed] [Google Scholar]
- Foster S.F., Charles J.A., Canfield P.J., Beatty J.A., Martin P. Reactivated toxoplasmosis in a FIV-positive cat, Aust Vet Pract, 28, 1998a, 159–163. [Google Scholar]
- Foster S.F., Barrs V.R., Martin P., Malik R. Pneumonia associated with Mycoplasma spp in three cats, Aust Vet J, 76, 1998b, 460–464. [DOI] [PubMed] [Google Scholar]
- Gabor L.J., Malik R., Canfield P.J. Clinical and anatomical features of lymphosarcoma in 118 cats, Aust Vet J, 76, 1998b, 725–732. [DOI] [PubMed] [Google Scholar]
- Gelatt K.N., van der Woerdt A., Ketring K.L., Andrew S.E., Brooks D.E., Biros D.J., Denis H.M., Cutler T.J. Enrofloxacin-associated retinal degeneration in cats, Vet Ophthlamol, 4, 2001, 99–106. [DOI] [PubMed] [Google Scholar]
- Gerds-Grogan S., Dayrell-Hart B. Feline cryptococcosis: a retrospective evaluation, J Am Anim Hosp Assoc, 33, 1997, 118–122. [DOI] [PubMed] [Google Scholar]
- Gil J.C., Cedillo R.L., Mayagoitia B.G., Paz M.D. Isolation of Mycoplasma pneumoniae from asthmatic patients, Annals of Allergy, 70, 1993, 23–25. [PubMed] [Google Scholar]
- Greene C.E. Mycoplasmal, ureaplasmal and L-form infections. Greene C.E. Infectious Diseases of the Dog and Cat, 1998, Saunders: Philadelphia, 174–177. [Google Scholar]
- Haesebrouck F., Devriese L.A., van Rijssen B., Cox E. Incidence and significance of isolation of Mycoplasma felis from conjunctival swabs of cats, Vet Microbiol, 26, 1990, 95–101. [DOI] [PubMed] [Google Scholar]
- Hawkins E.C. Pulmonary parenchymal diseases. Ettinger S.J., Feldman E.C. Textbook of Veterinary Internal Medicine, fifth ed, 2000, Saunders: Philadelphia, 1061–1091. [Google Scholar]
- Henik R.A., Yeager A.E. Bronchopulmonary diseases. Sherding R.G. The Cat: Diseases and Clinical Management, second ed, 1994, Churchill Livingstone: New York, 979–1052. [Google Scholar]
- Kirkpatrick C.E., Megella C. Use of ivermectin in treatment of Aelurostrongylus abstrusus and Toxocara cati infections in a cat, J Am Vet Med Assoc, 190, 1987, 1309–1310. [PubMed] [Google Scholar]
- Kraft M., Cassell G.H., Henson J.E., Watson H., Williamson J., Marmion B.P., Gaydos C.A., Martin R.J. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma, Am J Respir Crit Care Med, 158, 1998, 998–1001. [DOI] [PubMed] [Google Scholar]
- Litster A.L., Mitchell G., Menrath V. Pulmonary toxoplasmosis in a FIV-negative cat, Aust Vet Pract, 29, 1999, 154–158. [Google Scholar]
- Malik R., Jacobs G.J., Love D.N. Cryptococcosis:new perspectives on etiology, pathogenesis, diagnosis, and clinical management. August J.R. Consultationsin Feline Internal Medicine, fourth ed, 2001, Saunders: Philadelphia, 39–50. [Google Scholar]
- Malik R., Wigney D.I., Muir D.B., Gregory D.J., Love D.N. Cryptococcosis in cats: clinical and mycological assessment of 29 cases and evaluation of treatment using orally administered fluconazole, J Med Vet Mycol, 30, 1992, 133–144. [DOI] [PubMed] [Google Scholar]
- Malik R., Love D.N., Hunt G.B., Canfield P.J., Taylor V. Pyothorax associated with a Mycoplasma species in a kitten, J Small Anim Pract, 32, 1991, 31–34. [Google Scholar]
- Martin S.W., Meek A.H., Willeberg P. Veterinary Epidemiology. Principles and Methods, 1987, Iowa State University Press: Ames, 130. [Google Scholar]
- Medleau L., Jacobs G.J., Marks M.A. Itraconazole for the treatment of cryptococcosis in cats, J Vet Intern Med, 9, 1995, 39–42. [DOI] [PubMed] [Google Scholar]
- Micillo E., Bianco A., D'Auria D., Mazzarella G., Abbate G.F. Respiratory infections and asthma, Allergy, 55 (Suppl 61, 2000, 42–45. [DOI] [PubMed] [Google Scholar]
- Nash G., Kerschmann R.L., Herndier B., Dubey J.P. The pathological manifestations of pulmonary toxoplasmosis in the acquired immunodeficiency syndrome, Hum Pathol, 25, 1994, 652–658. [DOI] [PubMed] [Google Scholar]
- Padrid P.A., Feldman B.F., Funk K., Samitz E.M., Reil D., Cross C.E. Cytologic, microbiologic, and biochemical analysis of bronchoalveolar lavage fluid obtained from 24 healthy cats, Am J Vet Res, 52, 1991, 1300–1307. [PubMed] [Google Scholar]
- Parker G.A., Lanloss J.M., Dubey J.P., Hoover E.A. Pathogenesis of acute toxoplasmosis in specific-pathogen-free cats, Vet Pathol, 18, 1981, 786–803. [DOI] [PubMed] [Google Scholar]
- Pechman R.D. Respiratory parasites. Sherding R.G. The Cat: Diseases and Clinical Management, second ed, 1994, Churchill Livingstone: New York, 613–622. [Google Scholar]
- Peeters D.E., McKiernan B.C., Weisiger R.M., Schaeffer D.J., Clercx C. Quantitative bacterial cultures and cytological examination of bronchoalveolar lavage specimens in dogs, J Vet Intern Med, 14, 2002, 534–541. [DOI] [PubMed] [Google Scholar]
- Petrovsky T. Mycoplasma pneumoniae infection and post-infection asthma, Med J Aust, 152, 1990, 391. [DOI] [PubMed] [Google Scholar]
- Plumb D.C. Veterinary Drug Handbook, fourth ed, 2002, Iowa State Press: Iowa, 454–459. [Google Scholar]
- Randolph J.F., Moise N.S., Scarlett J.M., Shin S.J., Blue J.T., Corbett J.R. Prevalence of mycoplasmal and ureaplasmal recovery from tracheobronchial lavages and of mycoplasmal recovery from pharyngeal swab specimens in cats with or without pulmonary disease, Am J Vet Res, 54, 1993, 897–900. [PubMed] [Google Scholar]
- Rodriguez C.O., Moon M.L., Leib M.S. Salmonella choleraesuis pneumonia in a cat without signs of gastrointestinal tract disease, J Am Vet Med Assoc, 202, 1993, 953–955. [PubMed] [Google Scholar]
- Sabato A.R., Martin A.J., Marmion B.P., Kok T.W., Cooper D.M. Mycoplasma pneumoniae: acute illness, antibiotics and subsequent pulmonary function, Archives of Disease in Childhood, 59, 1984, 1034–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellon R.K. Feline immunodeficiency virus infection. Greene C.E. Infectious Diseases of the Dog and Cat, 1998, Saunders: Philadelphia, 84–96. [Google Scholar]
- Seggev J.S., Sedmak G.V., Kurup V.P. Isotype-specific antibody responses to acute Mycoplasma pneumoniae infection, Ann Allergy Asthma Immunol, 77, 1996, 67–73. [DOI] [PubMed] [Google Scholar]
- Seggev J.S., Lis I., Siman-Tov R., Gutman R., Abu-Samara H., Schey G., Naot Y. Mycoplasma pneumoniae is a frequent cause of exacerbation of bronchial asthma in adults, Annals of Allergy, 57, 1986, 263–265. [PubMed] [Google Scholar]
- Smith-Norowitz T.A., Bluth M.H., Drew H., Norowitz K.B., Chice S., Shah V.N., Nowakowski M., Josephson A.S., Durkin H.G., Joks R. Effect of minocycline and doxycycline on IgE responses, Ann Allergy Asthma Immunol, 89, 2002, 172–179. [DOI] [PubMed] [Google Scholar]
- Tan R.J.S. Susceptibility of kittens to Mycoplasma felis infection, Jpn J Exp Med, 44, 1974, 235–240. [PubMed] [Google Scholar]
- Taylor-Robinson D. Mycoplasma and ureaplasma. Murray P.R., Baron E.J., Pfaller M.A., Tenover F.C., Yolken R.H. Manual of Clinical Microbiology, sixth ed, 1995, ASM Press: Washington DC, 652–662. [Google Scholar]
- Teo J., Vellayappan K., Yip W.C.L., Doraisingham S. Mycoplasma pneumoniae and viral infections in childhood asthma, Journal of Tropical Pediatrics, 32, 1986, 87–89. [DOI] [PubMed] [Google Scholar]
- Wiebe V., Hamilton P. Fluoroquinolone-induced retinal degeneration in cats, J Am Vet Med Assoc, 221, 2002, 1568–1571. [DOI] [PubMed] [Google Scholar]
- Wilson-Hanson S.L., Prescott C.W. A survey for parasites in cats, Aust Vet J, 59, 1982, 194. [DOI] [PubMed] [Google Scholar]
- Wong W.T., Noor F. Pyothorax in the cat—a report of two cases, Kajian Vetrinar, 16, 1984, 15–17. [Google Scholar]
- Yano T., Ichikawa Y., Komatu S., Arai S., Oizumi K. Association of Mycoplasma pneumoniae antigen with initial onset of bronchial asthma, Am J Respir Crit Care, 149, 1994, 1348–1353. [DOI] [PubMed] [Google Scholar]


