Abstract
This report describes the presenting signs, biochemical abnormalities, and radiographic changes in a 4-month-old kitten with vitamin D-dependent rickets type 2. Details of therapy are described and possible reasons for treatment failure are discussed.
A 4-month-old female domestic shorthair kitten had presenting complaints of a hunched appearance, bilateral forelimb swelling, reluctance to jump and a poor appetite. The kitten was significantly smaller and less active than its male sibling littermates. The litter of four kittens was found abandoned at approximately 6 weeks of age. Since that time the affected kitten had been fed on a commercial proprietary kitten diet.
Physical examination revealed bony swellings in the region of both carpi and stifles, palpation of these joints was resented. The kitten was small (1.6 kg) but proportionate for her age. It was reluctant to move around and when it did move it had a very stiff or stilted gait. The rest of the clinical examination was unremarkable.
Blood was taken for routine analysis and samples were submitted from one of the unaffected siblings for comparison, although not all parameters were tested in the sibling. No abnormalities were detected on routine haematological blood analysis (Table 1a). Alkaline phosphatase levels were elevated, and urea and phosphorus levels decreased on biochemical analysis of blood. The remainder of the parameters tested, including the total calcium levels, was normal (Table 1a).
Table 1.
Results of haematological and biochemical blood analysis
| Parameter | Reference range | Affected kitten | Unaffected sibling |
|---|---|---|---|
| (a) Routine biochemical and haematological analysis | |||
| Albumin | 22–39 g/l | 28 | |
| Alkaline phosphatase | 14–192 U/l | 955 (high) | 78 |
| Alanine transferase | 12–115 U/l | 32 | 37 |
| Amylase | 500–1400 U/l | 808 | |
| Urea | 5.71–11.78 mmol/l | 5.13 (low) | 8.51 |
| Total calcium | 1.98–2.83 mmol/l | 2.10 | |
| Cholesterol | 1.6–4.94 mmol/l | 3.35 | |
| Creatinine | 53–141 μmol/l | 79 | 150 |
| Glucose | 4.72–7.22 mmol/l | 7.07 | 11.19 (high) |
| Phosphate | 1.45–3.35 mmol/l | 1.38 (low) | |
| Total bilirubin | 0–15 μmol/l | <2 μmol/l | |
| Total protein | 52–82 g/l | 61 | 62 |
| Globulin | 28–48 g/l | 33 | |
| Haematocrit | 24.0–45.0% | 30.3 | 35.6 |
| Haemoglobin | 8.0–15.0 g/dl | 9.8 | 11.4 |
| Mean corpuscular haemoglobin concentration | 30.0–36.9 g/dl | 32.3 | 32.0 |
| Total leukocytes | 5.0–18.9×109/l | 11.9 | 10.5 |
| Granulocytes | 2.5–12.5×109/l | 4.9 | 5.9 |
| Lymphocytes/monocytes | 1.5–7.8×109/l | 7.0 | 4.6 |
| Platelets | 175–500×109/l | 451 | 366 |
| (b) Additional blood analysis results | |||
| 25(OH)D (vitamin D) | 5–30 ng/ml | 11.7 | 19.2 |
| 1,25(OH)2D (calcitriol) | 20–50 pg/ml | 590 (high) | 23 |
| Ionised calcium | 1.2–1.8 mmol/l | 1.14 (low) | |
| Parathyroid hormone | <23 pg/ml | 448 (high) | |
| Total thyroxine | 19–65 nmol/l | 40 | |
| Insulin-like growth factor-1 | Dwarf <50 ng/ml, Acromegaly >1000 ng/ml | 256 | |
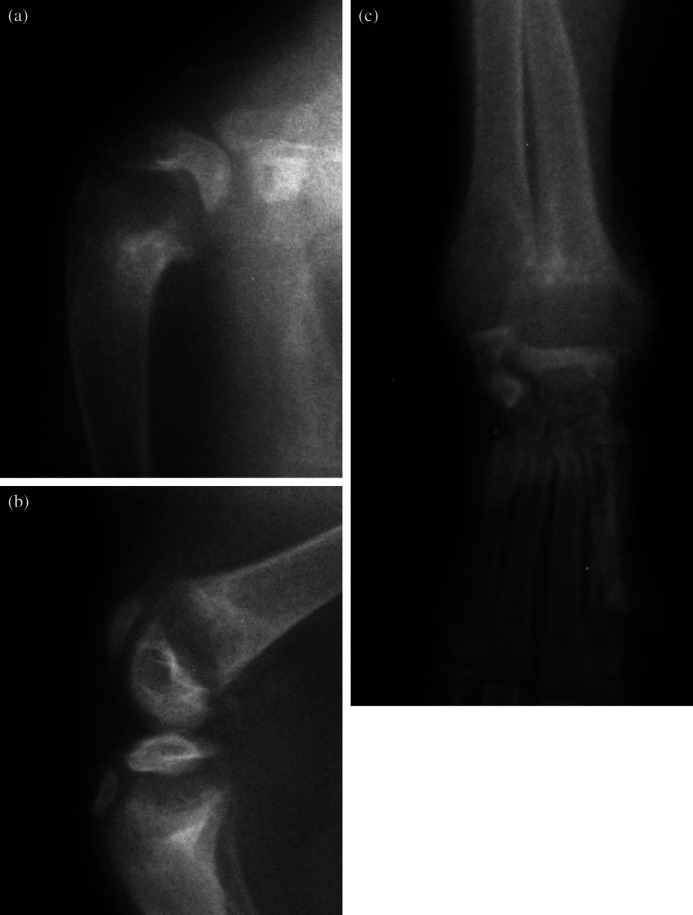
Anaesthesia was induced with a mixture of isoflurane (IsoFlo; Abbott) and oxygen administered via a close-fitting face mask. After intubation the kitten was maintained on the isoflurane/oxygen mixture and radiographs were taken of both thoracic and pelvic limbs. Generalised bone density was decreased; all long bone physes were significantly widened and flared (cup shaped) (Fig 1). Spinal radiographs showed mild generalised vertebral osteopenia, kyphosis and lordosis.
Fig 1.
Mediolateral radiographs of the shoulder (a) and stifle (b) and dorsopalmar radiograph of the carpus (c) of the affected kitten at four months of age. The physes are uniformly widened and flared; an appearance often described as ‘cup shaped’. The generalised bone density is decreased. All physes were similarly affected.
The signalement, blood test results and radiographic abnormalities in the physes were highly suggestive of rickets. Other differential diagnoses included pituitary dwarfism, congenital hypothyroidism, primary hypoparathyroidism, lysosomal storage disease and osteochondrodysplasia. Further blood tests were performed to try and establish a definitive diagnosis (Table 1b). At the request of the vitamin D laboratory (Vitamin D Research Group, University Department of Medicine, Manchester Royal Infirmary, Manchester M13 9WL) control samples were submitted from one of the unaffected siblings.
Normal levels of IGF-1 were determined by radioimmunoassay (Mediagnostics; Germany) and elevated intact PTH levels by immunoradiometric assay (iPTH IRMA) on a frozen EDTA plasma sample (Nicholls Institute, Europe). The radioimmunoassay for human IGF-1 has been validated for use in cats by Church et al (1994) and the use of an immunoradiometric assay (IRMA) for human intact parathormone has also been validated for use with feline samples (Bolliger et al 2002). These results ruled out pituitary dwarfism and hypoparathyroidism, respectively, as underlying causes for the kitten's presenting signs. Although levels of 25(OH)D were within normal ranges, reflecting normal levels of vitamin D nutrition, levels of 1,25(OH)2D (calcitriol) were markedly elevated when compared to samples submitted from the sibling. Ionised calcium levels were below the normal range, and parathyroid hormone levels were elevated. Results were diagnostic of vitamin D-dependent rickets type 2 (VDDR-2). This type of rickets was originally described as ‘calcitriol-resistant rickets’ (Hochberg et al 1985) and in humans it is caused by a functional mutation in the gene encoding the vitamin D receptor (Tiosano et al 2001). Calcitriol is unable to affect its target organs (primarily intestine and bone) due to this receptor dysfunction. This results in hypocalcaemia, which in turn leads to a secondary hyperparathyroidism. Both calcitonin (release of which is stimulated by hypocalcaemia) and hyperparathyroidism activate the enzyme 1-α-hydroxylase in the kidney, increasing calcitriol production from vitamin D (Allen and Weingand, 1985), hence the extremely high levels of calcitriol in cases of VDDR type 2. Normally calcitriol itself decreases 1-α-hydroxylase activity but this negative feedback pathway fails in VDDR type 2, as the pathway requires a functional vitamin D receptor (Murayama et al 1999).
Treatment consisted of calcium and vitamin D supplementation. Calcium supplementation was in the form of calcium lactate, initially at a dose of 75 mg PO qid (Schreiner and Nagode 2003) but increasing dosages were used as the ionised calcium levels, which were tested at 7–10 day intervals, remained low. At the start of treatment additional calcium was being administered as part of the vitamin D therapy in the form of calcium borogluconate (Zolcal-D, Vetark) 600 mg/day. Initially vitamin D3 (Zolcal-D, Vetark) was used as the vitamin D supplement at a dose rate of 37.5 IU PO sid. When the kitten's ionised calcium levels remained low despite therapy, this supplement was changed to calcitriol (Rocaltrol; Roche), 0.07 Ug/day (Gunn-More et al 1996). Calcitriol was used successfully in the treatment of a kitten suffering from VDDR-2 without radiographic evidence of bony changes (Schreiner and Nagode 2003).
Initially, there did seem to be a positive response to therapy with the owner reporting an improvement in both appetite and mobility. Repeat radiographs taken 8 weeks after calcium and vitamin D supplementation showed an increase in bone density and decrease in physeal lucency. The kitten's response to therapy was monitored by regularly rechecking her ionised calcium levels, which should normalise if supplementation is effective. However, the serum ionised calcium levels stayed persistently below the lower end of the normal range despite increasing doses of both calcium and vitamin D supplementation. The kittens gait remained stilted despite the owners perceived initial improvement and it failed to grow or gain weight despite a reasonable appetite. The kitten died at 13 months of age, 7 months after diagnosis. The body was not available for post mortem examination; cause of death was undetermined.
In humans vitamin D-dependent rickets type 2 (VDDR-2) is caused by one or more mutations in the vitamin D receptor (VDR) gene. The condition has autosomal recessive inheritance, and clinical signs include rachitic bone changes, hypocalcaemia, secondary hyperparathyroidism and frequently total body alopecia. Classic rachitic changes include widened growth plates, splaying of metaphyses and undermineralisation (Hochberg 2002). The diagnosis is not made in children until 3–6 months of age, because of the normal transplacental calcium flux from mother to fetus children are born with normal bones (Hochberg 2002).
Reports of similar cases in the veterinary literature are sparse. Gunn-Moore et al (1996) reported a metaphyseal abnormality in two kittens which was originally suspected to be a VDDR; however, the final diagnosis was of a metaphyseal chondrodysplasia. Schreiner and Nagode (2003) described a case of VDDR-2 in a 4-month-old cat. The main presenting signs in this cat were related to the hypocalcaemia with vomiting, diarrhoea, muscle tremors, and mydriasis. Classic radiographic abnormalities were not present. The presenting signs, lack of radiographic changes and the positive response to therapy in Schreiner and Nagode's (2003) case study led those authors to conclude that there most likely was only modest impairment of binding of calcitriol to the cat's vitamin D receptors. It is possible that kittens that survive VDDR-2, following treatment with calcitriol, have a mutation in the calcitriol binding site area, and that increasing the circulating calcitriol levels even further can eventually lead to an adequate functioning of the VDR.
Determining the appropriate dosage regime and formulation of both calcium and vitamin D to administer to the kitten was challenging given the lack of information available in the literature on VDDR-2 treatment in cats and the conflicting advice obtained from various sources. Schreiner and Nagode (2003) were successful in their treatment of a VDDR-2 kitten by effectively doubling the recommended dosage of calcium together with a high dosage of calcitriol (50 ng/kg) to supersaturate the vitamin D receptors. In humans, giving high doses of calcium alone (intravenous initially and then oral) can be successful (Hochberg 2002). In the kitten in this report the vitamin D supplement was changed to calcitriol following failure to normalise ionised calcium levels despite more than adequate calcium supplementation.
The kitten in this report failed to respond to calcium and vitamin D (calcitriol) supplementation. One possible explanation for this is that the kitten had a different mutation (or mutations) in the VDR to the kitten described previously (Schreiner and Nagode 2003). Other than the calcitriol binding site, the other sites at which mutations have been described in the human literature affected the DNA binding domain (Malloy et al 1994) interfered with coactivator binding (Malloy et al 2002) and caused multiple defects in receptor function (Malloy et al 2004). It is interesting to note that the patients in which these recent mutations were discovered, like the kitten currently under discussion, did not suffer from the whole body alopecia that commonly affects humans with VDDR-2; one could speculate, therefore, that one of these recently described receptor mutations could be present in this case.
In the human situation, the response to treatment of patients suffering from VDDR-2 is usually good. Depending on where the mutation is located, patients may or may not respond to treatment with vitamin D metabolites (Malloy et al 1999). Most, however, seem to respond to high dose intravenous calcium infusions (Malloy et al 1999, Hochberg 2002). This treatment is intensive, and the infusions are administered via a central catheter into the vena cava (Hochberg 2002). This therapy is unlikely to be an option in a veterinary setting, on either practical or ethical grounds. Treatment with oral calcium supplements is largely ineffective in children, as the lack of vitamin D means it cannot be absorbed from the gastrointestinal tract (Hochberg 2002). From the authors' experience with this single case the prognosis for a kitten affected with VDDR-2 that is unresponsive to vitamin D and calcium supplementation may be poor.
Acknowledgements
The authors would like to acknowledge Dr Larry Nagode, University of Ohio State University, and Dr Jacqueline Berry, from the vitamin D laboratory, for their helpful advice with this case.
References
- Allen T.A., Weingand K. The vitamin D (calciferol) endocrine system, Compendium of Continuing Education 7, 1985, 482–489. [Google Scholar]
- Bolliger A.P., Graham P.A., Richard V., Rosol T.J., Nachreiner R.F., Refsal K.R. Detection of parathyroid hormone-related protein in cats with humoral hypercalcemia of malignancy, Veterinary Clinical Pathology 31, 2002, 3–8. [DOI] [PubMed] [Google Scholar]
- Church D.B., Watson A.D., Emslie D.R., Middleton D.J., Tan K., Wong D. Effects of proligesterone and megestrol on plasma adrenocorticotrophic hormone, insulin and insulin-like growth factor-1 concentrations in cats, Research in Veterinary Science 56, 1994, 175–178. [DOI] [PubMed] [Google Scholar]
- Gunn-Moore D.A., Hagard G., Turner C., Duncan A.W., Barr F.J. Unusual metaphyseal disturbance in two kittens, Journal of Small Animal Practice 37, 1996, 583–590. [DOI] [PubMed] [Google Scholar]
- Hochberg Z. Vitamin-D-dependent rickets type 2, Hormone Research 58, 2002, 297–302. [DOI] [PubMed] [Google Scholar]
- Hochberg Z., Gilhar A., Haim S., Friedman R., Levy J., Benderly A. Calcitriol-resistant rickets with alopecia, Archives of Dermatology 121, 1985, 646–647. [PubMed] [Google Scholar]
- Malloy P.J., Pike J.W., Feldman D. The vitamin D receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-resistant rickets, Endocrine Reviews 20 (2), 1999, 156–188. [DOI] [PubMed] [Google Scholar]
- Malloy P.J., Weisman Y., Feldman D. Hereditary 1α,25-dihyrodxyvitamin D-resistant rickets resulting from a mutation in the vitamin D receptor deoxyribonucleic acid-binding domain, Journal of Clinical Endocrinology and Metabolism 78 (2), 1994, 313–316. [DOI] [PubMed] [Google Scholar]
- Malloy P.J., Xu R., Peng L., Peleg S., Al-Ashwal A., Feldman D. Hereditary 1,25-dihydroxyvitamin D resistant rickets due to a mutation causing multiple defects in vitamin D receptor function, Endocrinology 145 (11), 2004, 5106–5114. [DOI] [PubMed] [Google Scholar]
- Malloy P.J., Xu R., Peng L., Clark P.A., Feldman D. A novel mutation in helix 12 of the vitamin D receptor impairs coactivator interaction and causes hereditary 1,25-dihydroxyvitamin D-resistant rickets without alopecia, Molecular Endocrinology 16 (11), 2002, 2538–2546. [DOI] [PubMed] [Google Scholar]
- Murayama A., Takeyama K., Kitanaka S., Kodera Y., Kawaguchi Y., Hosoya T., Kato S. Positive and negative regulations of the renal 25-hydroxyvitamin D3 1α-hydroxylase gene by parathyroid hormone, calcitonin, and 1α,25(OH)2 D3 in intact animals, Endocrinology 140 (5), 1999, 2224–2231. [DOI] [PubMed] [Google Scholar]
- Schreiner C.A., Nagode L.A. Vitamin D-dependent rickets type 2 in a 4-month-old cat, Journal of the American Veterinary Medical Association 222 (3), 2003, 337–3398. [DOI] [PubMed] [Google Scholar]
- Tiosano D., Weisman Y., Hochberg Z. The role of the vitamin D receptor in regulating vitamin D metabolism: a study of vitamin D-dependent rickets, type II, Journal of Clinical Endocrinology and Metabolism 86 (5), 2001, 1908–1912. [DOI] [PubMed] [Google Scholar]