Abstract
Immune-mediated haemolytic anaemia associated with multicentric lymphoblastic infiltration is reported in two sibling cats. Both cats presented at under 16 months of age with clinical signs of acute anaemia. In each case there was autoagglutination, a positive Coombs’ test and the anaemia was regenerative. At presentation, both cats were negative for FeLV antigen. In each case, the disease proved fatal within 2 months of the initial diagnosis. In both cases, T-lymphoblastic infiltration of bone marrow, liver and spleen was found at post-mortem examination.
Primary immune-mediated haemolytic anaemia (IMHA) has been reported in many species, including man and dogs (Lewis et al 1965), but is uncommon in cats (Werner & Gorman 1984). Most cases of feline IMHA are secondary in nature, being associated with FeLV infection, FeLV-negative lymphoproliferative disease, Haemobartonella felis infection, or drug reactions (Scott et al 1973, Peterson et al 1984, Werner & Gorman 1984, Day 1996a). Immune-mediated, pure red cell aplasia (PRCA) has recently been documented in FeLV-negative cats (Stokol & Blue 1999). Given the wide range of aetiologies, feline IMHA may affect cats of any age (Werner & Gorman 1984, Day 1996a). However, in a recent report, four of five cases of FeLV-negative IMHA occurred in cats under 2 years of age (Person et al 1997), and two affected brothers were only 1 year of age (Utroska 1980). All nine of the cats with immune-mediated, PRCA were less than three years of age (Stokol & Blue 1999). A male predisposition to IMHA has been noted (Scott et al 1973, Day 1996a). This paper describes IMHA, associated with multi-centric lymphoblastic infiltration in two sibling cats.
Materials and methods
Cats
Four, 12-week-old, male sibling cats were obtained as part of a large group of specific pathogen-free (SPF) cats from a commercial supplier. The cats were maintained under SPF conditions, and castrated at 6 months of age. The two affected siblings were housed in separate rooms. The cats were subject to Home Office Regulations under licences issued to the University of Bristol.
Coombs' test and immunohistochemistry
Direct Coombs' tests were performed as described by Day (1996a), using a polyvalent feline Coombs’ reagent specific for IgG, IgM and complement C3 (ICN Biomedicals), rabbit-anti-cat IgG, and goat-anti-cat IgM (Nordic Laboratories, Tilburg, The Netherlands). Immunohistochemical staining was performed on paraffin-embedded sections of the liver, spleen and bone marrow of both cats using antisera specific for the pan T lymphocyte marker CD3, the pan B lymphocyte marker CD79a and Major Histocompatibility Complex (MHC) Class II molecules (Day 1998).
Results
Case reports
Case 1 was vaccinated subcutaneously, at 9 and 12 weeks of age, with a standard commercial vaccine against feline calicivirus, herpesvirus and panleukopenia virus. The cat was clinically healthy until 14 months of age, when it became quiet and developed pale mucous membranes. Examination of a blood sample demonstrated a moderately severe, regenerative anaemia (Table 1). Serum biochemistry revealed mild bilirubinaemia (8.1 μmol/l; reference range <5) and a mild polyclonal increase in gamma globulins. There was little clinical or haematological change over the next 3 weeks. No treatment was given. When the cat then became acutely depressed and weak, euthanasia was carried out. This occurred 3 weeks after the first clinical signs had been seen. A post-mortem examination was performed. The cat had not been exposed to FeLV, and was shown to be negative for FeLV p27 by a commercial ELISA test (Inochem, C. Lutz, CH).
Table 1.
Haematological changes seen in the two cases
| Case 1 | Case 2 | Ref. range | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 10 | Day 23 | Day 1 | Day 5 | Day 81 | ||
| Haematology | ||||||||
| Haematocrit % | 11.3 | 11.1 | 12.9 | 8.2 | 21.5 | 21.6 | 1.8† | 24–45 |
| * RBC × 1012/l | 1.34 | 1.26 | 1.32 | 0.91 | 3.24 | 3.19 | 0.31 | 5.5–7.5 |
| Haemoglobin g/dl | 3.0 | 2.9 | 3.6 | 2.1 | 6.7 | 6.5 | 1.0 | 10–13 |
| MCHC g/dl | 26.5 | 26.1 | 27.9 | 25.6 | 31.2 | 30.1 | 30–35 | |
| nRBC × 109/l | 2.4 | 1.8 | 3.8 | 3.5 | 0.4 | rare | 2.0 | |
| Anisocytosis | +++ | ++ | +++ | +++ | +++ | +++ | +++ | |
| Polychromasia | ++ | ++ | ++ | ++ | + | ++ | +++ | |
| Hypochromasia | ++ | ++ | ++ | |||||
| Macrocytosis | ++ | ++ | ++ | + | ||||
| Agglutination | + | + | + | + | + | + | + | |
| Platelets × 109/l | 51 | 60 | 31 | 81 | 80 | 57 | 10 | |
| clumped | macro | clumped/macro | clumped/macro | macro | macro | macro | ||
| WBC × 109/l | 5.3 | 9.7 | 10.1 | 12.7 | 12.6 | 14.2 | 2.7 | 9–18 |
| Neutrophils × 109/l | 2.44 | 3.4 | 4.65 | 6.48 | 7.18 | 10.9 | 1.05 | |
| Bands × 109/l | 0.16 | 0.19 | 0.4 | 0.38 | 0.13 | |||
| Lymphocytes × 109/l | 2.01 | 5.24 | 4.04 | 4.32 | 4.54 | 2.98 | 1.49 | |
| Monocytes × 109/l | 0.42 | 0.78 | 0.01 | 1.52 | 0.38 | 0.14 | 0.16 | |
| Eosinophils × 109/l | 0.21 | 0.10 | 0.25 | 0.14 | ||||
| Coombs' test‡ | ND | ND | 37°C Agglut. Haemol. | NP Agglut. Haemol. | ND | 37, 4°C Agglut. Haemol. | NP Agglut. Haemol. | |
RBC—red blood cells.
RBC indices were determined using a Baker 9000 Impedance Counter (Baker, USA). Due to the presence of RBC agglutination values for RBC number may be spurious so the mean cell volume and mean cell haemoglobin have not been included. nRBC—nucleated red blood cells. MCHC—mean cell haemoglobin concentration. WBC—white blood cells. Macro—macrothrombocytes present.
The extent of the RBC envelope haemolysis made RBC indices unreadable. ND—not determined. Agglut.—autoagglutinating. Haemol—haemolysing.
Coombs' test performed as per Day (1996a). NP—Coombs' test not possible because of autoagglutination.
Case 2 was not vaccinated. At 15 months of age it was challenged oro-nasally with feline herpesvirus B927. Within 4 days, the cat developed classical signs of cat ‘flu’, consisting of nasal ulceration, ocular and nasal discharge, sub-mandibular lymphadenopathy and an occasional cough. These signs had almost resolved 3 weeks later when the cat became acutely weak, pyrexic and displayed mucous membrane pallor. Examination of a blood sample demonstrated a moderate, regenerative anaemia (Table 1). Serum biochemistry revealed only mild bilirubinaemia (11.7 μmol/l; reference range <5). The cat's condition changed little over the next 5 days, after which it slowly improved. At 17 months of age, showing no signs of ill health, the cat was challenged oro-nasally with 106 FFU of FeLV-A, Glasgow-1 as part of an experimental study. Prior to this time, the cat had not been exposed to FeLV and was negative for FeLV p27 by a commercial ELISA test. Within 3 weeks, the cat developed fever and signs of acute anaemia, with marked jaundice, depression, dyspnoea and tachypnoea. By this stage it was viraemic for FeLV. The cat was found to have pancytopenia, bilirubinaemia (240.5 μmol/l, reference range <5), hepatocellular damage (alanine aminotransferase [ALT] 702 U/l, reference range 15–45; alkaline phosphatase [ALP], 104 U/l, reference range 15–60), and a moderate polyclonal increase in gamma globulins. Euthanasia was carried out and a post-mortem examination was performed.
In Case 1 Coombs’ tests were performed on days 10 and 23 after the start of clinical signs. On each occasion there was gross autoagglutination of the blood sample on cooling to 4°C and haemolysis when washing the erythrocytes. The Coombs’ test was therefore performed at 37°C only, using polyvalent feline Coombs’ reagent. On day 10, the test was positive (titre 80), but on day 23 there was autoagglutination in both test and control (saline) wells rendering the test invalid. In Case 2, Coombs’ tests were performed on days 5 and 81 after the onset of clinical signs. On the first occasion, there was no gross evidence of autoagglutination or haemolysis, so a full test (using polyvalent feline Coombs’ reagent, anti-cat IgG and anti-cat IgM) was performed in duplicate at 4°C and 37°C. The test was positive for polyvalent reagent (titre 320 at 37°C, 1280 at 4°C) and for IgG (titre 1280 at 37°C, 5120 at 4°C), but IgM antibody was not demonstrated. On the second occasion of testing (day 81), there was autoagglutination of the sample on cooling to 4°C, and haemolysis during washing. A full Coombs’ test was performed on both 4°C and 37°C, but there was agglutination in all wells, including controls, thus invalidating the test.
Post-mortem findings
Gross post-mortem examination of Case 1 revealed marked splenomegaly, moderate hepatomegaly and dark-red bone marrow. Case 2 was thin and markedly jaundiced. The liver, spleen and lymph nodes were enlarged, with red discoloration of the anterior mediastinal, anterior abdominal and mesenteric nodes. Marked pigmenturia was evident and the bone marrow was dark red.
Histopathological features were similar in both cases, although the lesions were more severe in Case 2. Examination of sections of the liver from each cat revealed moderate to severe hepatocyte vacuolation, with prominent portal infiltrates of mixed lymphoblastic and haematopoietic cells (Fig 1a). Occasional mitotic figures were present. The splenic red pulp of both cats also contained a mixed population of cells similar to those described in the liver (Fig 1b). Bone marrow from each cat was infiltrated by lymphoblastic cells (Fig 1c), and in Case 1, the marrow also contained obvious lymphoid follicles. The enlarged lymph nodes from Case 2 displayed follicular and paracortical hyperplasia, with congestion of cortical sinuses.
Fig 1.
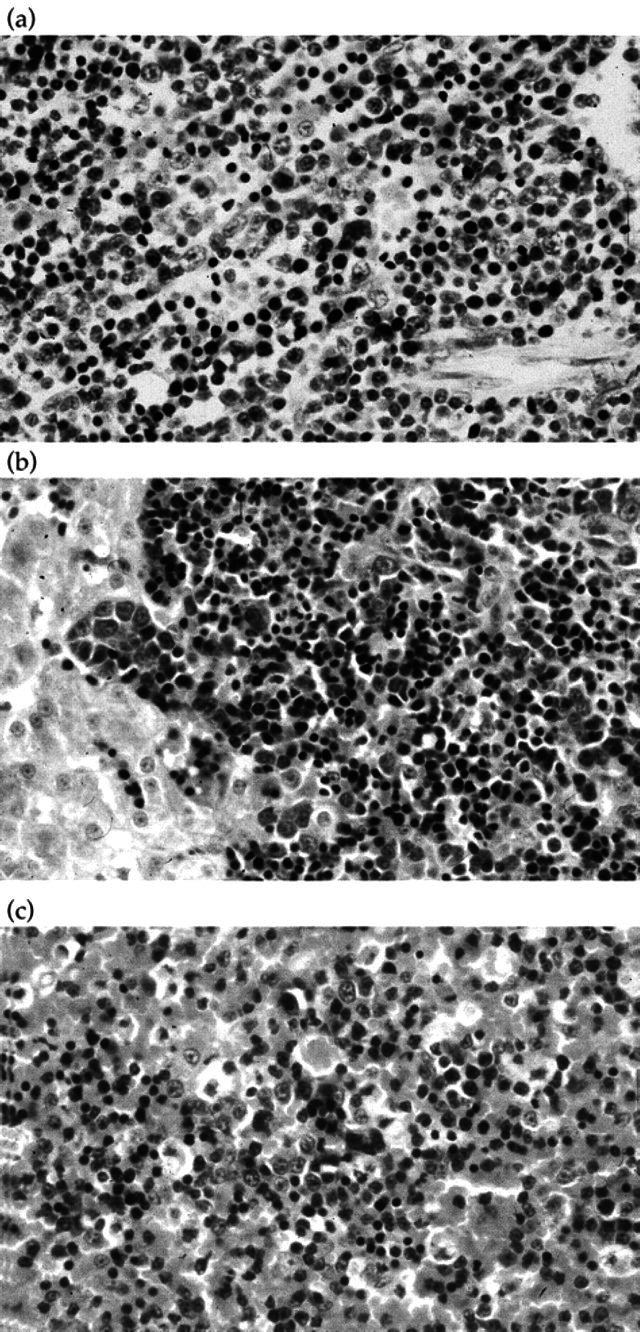
(a) Case 2, bone marrow, (H&E) × 125. The bone marrow is infiltrated by lymphoblastic cells. (b) Case 1, liver, haematoxlyin and eosin (H&E) × 125. The section shows moderate to severe hepatocyte vacuolation, with prominent portal infiltrates of mixed lymphoblastic and haematopoietic cells. (c) Case 2, spleen, (H&E) × 125. The splenic red pulp contains a mixed population of cells similar to those described in the liver (a).
Immunohistochemistry
In all tissues, a prominent population of CD3+ T lymphoblasts was identified (Fig 2), and these cells were mixed with a second, minor population of CD79a+ B lymphocytes. The follicular areas in the bone marrow of Case 1 also consisted of B cells. All lymphoid cells were positively stained for MHC Class II. Taken together, these findings are consistent with a diagnosis of IMHA with multicentric T-lymphoblastic infiltration and associated B-lymphocyte proliferation.
Fig 2.
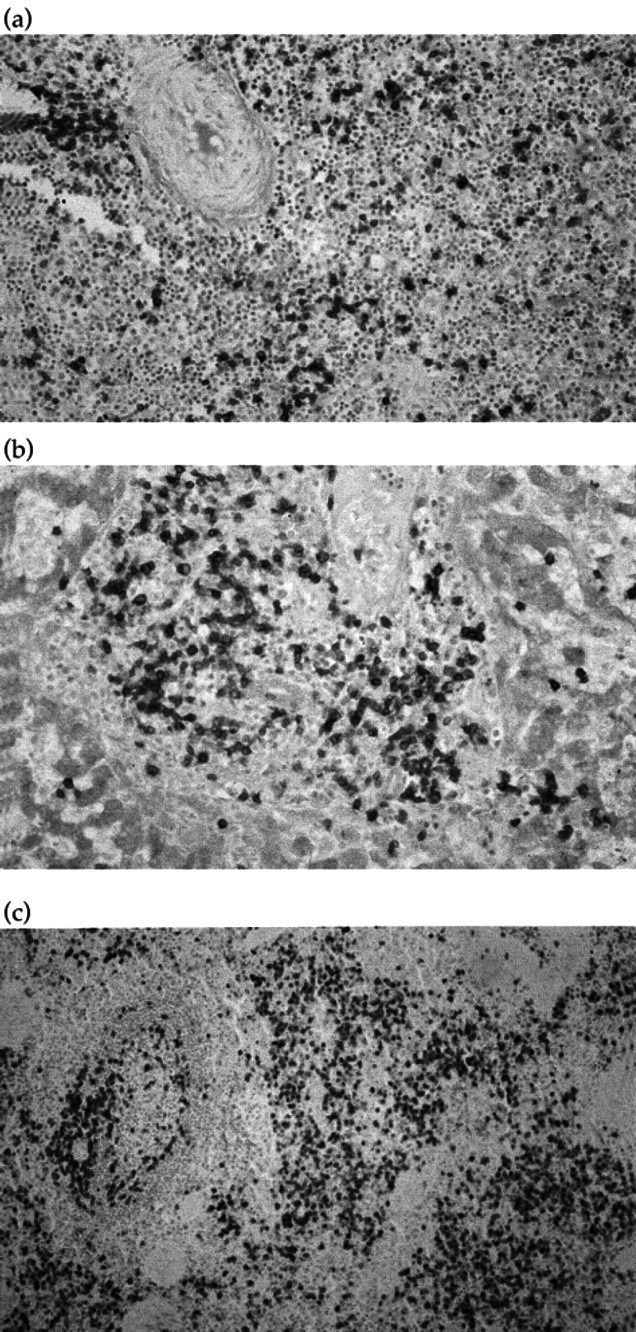
(a) Case 2, spleen, immunostaining, × 31.2; (b) Case 2, liver, immunostaining, × 62.5; (c) Case 2, bone marrow, immunostaining, × 50. All tissues show a prominent population of CD3+T lymphoblasts, mixed with a second, minor population of CD79a+B lymphocytes.
Discussion
The cats in this study presented with clinical signs similar to those previously reported for cats with IMHA, including pallor, lethargy, inappetence, tachycardia, icterus, hepatosplenomegaly, and lymphadenopathy. Less consistent abnormalities recorded in feline IMHA include tachypnoea, vomiting, fever, and other immune-mediated blood dyscrasias such as thrombocytopenia and leukopenia (Faircloth & Montgomery 1981, Werner & Gorman 1984, Day 1996a). Since complement-mediated intravascular haemolysis occurs less frequently than extravascular haemolysis in the cat, bilirubinaemia and bilirubinuria are seen less frequently (Werner & Gorman 1984, Day 1996a, Person et al 1997).
The cats in this study were both males of less than 16 months of age. This is consistent with the reported sex predisposition to IMHA (Scott et al 1973, Day 1996a), and supports initial reports suggesting that FeLV-negative IMHA may be seen more frequently in cats of less than two years of age (Utroska 1980, Person et al 1997).
In the current cases, IMHA was associated with multicentric, B-cell rich, T-lymphoblastic infiltration. The nature and distribution of the infiltration is unusual, and it could be argued that it may represent either an aberrant inflammatory response or an atypical lymphoproliferative disorder. Since a number of diseases can have overlapping clinical signs, histopathological changes, and cytological features, the differentiation of this type of disorder can be very difficult (Harris 1995, Hansmann & Kuppers 1996). Overall, from the histopathological nature, the distribution of the infiltration, and the lack of exposure to known inciting agents, a lymphoproliferative disorder appears to be most likely. While it is difficult to determine the exact nature of the cases in this study they are perhaps most similar to lymphoblastic leukaemia. Lymphoblastic leukaemia is characterised by the presence of lymphoblasts in the bone marrow, peripheral blood, liver, spleen and/or other organs (Couto 1985), and it may, on rare occasions, be aleukaemic (Handin et al 1995). The disease has an acute onset, rapid progression, and a poor response to therapy (Couto 1985). In this respect, both cats in this study had acute onset disease, and lymphoblasts were found in the bone marrow, liver and spleen. However, while both cats were dead within 2 months of the initial diagnoses, the pattern of disease progression was different in the two cases. In Case 1, the initial signs were followed by a 2 week period of relative stability, then an acute exacerbation which warranted euthanasia. In Case 2, the initial signs were followed by stabilisation and apparent remission for 3 weeks, after which the cat was challenged with FeLV and succumbed to acute and severe disease. The apparent remission would be atypical of lymphoblastic leukaemia. However, rather than a true remission it is perhaps more likely that the cat adapted to its anaemia, resulting in a resolution of clinical signs. However, on being challenged with FeLV, a relapse was precipitated.
While it is unclear whether the IMHA was a primary or secondary phenomenon, it is perhaps most likely that it occurred secondary to the observed lymphoblastic infiltration. By immunohistochemical staining, tissues from both cats were found to have a T-lymphoblastic infiltration associated with B-cell proliferation and extramedullary haematopoiesis. It is possible that release of cytokines from the T-lymphoblasts caused polyclonal B-lymphocyte activation and secondary IMHA (Hsu et al 1993). In man, IMHA occurs in association with neoplasia of CD5+ B lymphocytes (Sthoeger et al 1989) or may follow polyclonal activation of CD5+ B cells during infection (Casali & Notkins 1989). IMHA associated with lymphoma is also well documented in the dog (Keller 1992), but the phenotype of such tumours has not been reported.
The IMHA in the current cases was confirmed by macroscopic autoagglutination and positive Coombs’ tests. In Case 1, the results were consistent with the presence of a cold-reactive IgM antibody that eluted from the erythrocyte surface (at least on day 10), allowing detection of IgG antibody. The initial Coombs’ test performed on Case 2 demonstrated only an IgG antibody that reacted in vitro at both 4°C and 37°C. The auto-agglutination subsequently observed in this cat (day 81) suggests there was later development of a cold-reactive IgM antibody that did not fully elute from the erythrocyte surface. The findings are consistent with previous reports, where cats with IMHA have been found to have a high incidence of macroscopic autoagglutination and autoantibodies of the IgM class (Werner & Gorman 1984, Day 1996a).
Given the occurrence of an unusual lymphoblastic infiltration in sibling cats, the similarities in age of onset and disease presentation, and the apparent lack of a precipitating cause, it is possible that these two cats were genetically predisposed to the development of this disease. A genetic basis has previously been suggested for some cases of IMHA in cats (Utroska 1980), dogs (Day 1996b), and mice (Lewis et al 1965), and some cases of lymphoproliferative diseases in cats (Court et al 1997), dogs (Onions 1984, Teske et al 1994) and humans (Glaser & Jarrett 1996). While it cannot be ruled out that the cats had been exposed to an unknown precipitating factor, their maintenance within SPF conditions makes this unlikely. To the authors’ knowledge, this is the first report of IMHA with associated multicentric lymphoblastic infiltration in sibling cats.
References
- Casali P, Notkins AL. (1989) CD5+B lymphocytes, poly-reactive antibodies and the human B-cell repertoire. Immunology Today 10, 364–368. [DOI] [PubMed] [Google Scholar]
- Court EA, Watson AD, Peaston AE. (1997) Retrospective study of 60 cases of feline lymphosarcoma. Australian Veterinary Journal 75, 424–427. [DOI] [PubMed] [Google Scholar]
- Couto CG. (1985) Clinico-pathologic aspects of acute leukaemias in the dog. Journal of the American Veterinary Medicine Association 186, 681–685. [PubMed] [Google Scholar]
- Day MJ. (1996a) Diagnostic assessment of the feline immune system, Part 2. Feline Practice 24, 14–25. [Google Scholar]
- Day MJ. (1996b) Serological monitoring of clinical, haematological and immunological parameters in canine autoimmune haemolytic anaemia. Journal of Small Animal Practice 37, 523–534. [DOI] [PubMed] [Google Scholar]
- Day MJ. (1998) Immunohistochemical characterisation of the lesions of feline progressive lymphocytic cholangitis/cholangiohepatitis. Journal of Comparative Pathology 119, 135–147. [DOI] [PubMed] [Google Scholar]
- Faircloth JC, Montgomery JK. (1981) Systemic lupus erythematosus in a cat presenting with autoimmune haemolytic anaemia. Feline Practice 11, 22–26. [Google Scholar]
- Glaser SL, Jarrett RF. (1996) The epidemiology of Hodgkin's disease. Baillières Clinical Haematology 9, 401–416. [DOI] [PubMed] [Google Scholar]
- Handin RI, Lux SE, Stossel TP. (1995) Blood: Principles and Practice of Haematology. Philadelphia, PA, Lippincott-Raven, p. 750. [Google Scholar]
- Hansmann ML, Kuppers R. (1996) Pathology and ‘molecular histology’ of Hodgkin's disease and the border to non-Hodgkin's lymphomas. Baillières Clinical Haematology 9, 459–477. [DOI] [PubMed] [Google Scholar]
- Harris NL. (1995) A practical approach to the pathology of lymphoid neoplasms: A revised European-American classification from the International Lymphoma Study Group. Important Advances in Oncology, 111–140. [PubMed] [Google Scholar]
- Hsu SM, Hsu PL, Hough AR, Jr, Waldron JW., Jr (1993) Cytokines in malignant lymphomas: Review and prospective evaluation. Human Pathology 24, 1040–1057. [DOI] [PubMed] [Google Scholar]
- Keller ET. (1992) Immune-mediated disease as a risk factor for canine lymphoma. Cancer 70, 2334–2337. [DOI] [PubMed] [Google Scholar]
- Lewis RW, Schwartz RS, Gilmore CE. (1965) Autoimmune diseases in domestic animals. Annals of the New York Acadamy of Science 124, 178–200. [DOI] [PubMed] [Google Scholar]
- Onions DE. (1984) A prospective survey of familial canine lymphosarcoma. Journal of the National Cancer Institute 72, 909–912. [PubMed] [Google Scholar]
- Person JM, Sicard M, Pellerin JL. (1997) Les anemies hemolytiques auto-immunes chez le chat: Etude clinique et immunopathologique de cinq cas. Revue de Medecine Veterinaire 148, 107–114. [Google Scholar]
- Peterson ME, Hurvitz AI, Leib MS, Cavanagh PG, Dutton RE. (1984) Propylthiouracil-associated haemolytic anaemia, thrombocytopenia, and antinuclear antibodies in cats with hyperthyroidism. Journal of the American Veterinary Medicine Association 184, 806–808. [PubMed] [Google Scholar]
- Scott DW, Schultz RD, Post JE. (1973) Autoimmune haemolytic anaemia in the cat. Journal of the American Animal Hospital Association 9, 530–539. [Google Scholar]
- Sthoeger ZM, Wakai M, Tse DB, Vinciguerra VP, Allen SL, Budman DR, Lichtman SM, Schulman P, Weiselberg LR, Chiorazzi N. (1989) Production of autoantibodies by CD5-expressing B lymphocytes from patients with chronic lymphocytic leukaemia. Journal of Experimental Medicine 169, 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokol T, Blue JT. (1999) Pure red cell aplasia in cats: 9 cases (1989–1997). Journal of the American Veterinary Medicine Association 214, 75–79. [PubMed] [Google Scholar]
- Teske E, de Vos JP, Egberink HF, Vos JH. (1994) Clustering in canine malignant lymphoma. Veterinary Quarterly 16, 134–136. [DOI] [PubMed] [Google Scholar]
- Utroska B. (1980) Autoimmune haemolytic anaemia in sibling cats. Veterinary Medicine November, 1699–1701. [PubMed] [Google Scholar]
- Werner LL, Gorman NT. (1984) Immune-mediated disorders of cats. Veterinary Clinics of North America, Small Animal Practice 14, 1039–1063. [DOI] [PubMed] [Google Scholar]


