Abstract
To determine whether there is a day-to-day variation in insulin sensitivity in cats, we subjected six clinically normal cats to four insulin-modified frequently sampled intravenous glucose tolerance tests (FSIVGTTs) over 7 days. The insulin-modified FSIVGTTs were analysed by the minimal model method. Minimal model insulin sensitivity (SI) averaged 2.9 ± 0.4 × 10−4 min−1/μU/ml (range 1.9–4.6 × 10−4 min−1/μU/ml), with a mean interday coefficient of variation (CV) of 35.4 ± 6.4% (range 12.8–58.5%). Glucose effectiveness (SG) averaged 0.029 ± 0.002 min−1 (range 0.024–0.037 min−1), and showed less interday variability with a mean CV of 24.7 ± 4.3% (range 7.9–39.3%). Insulin sensitivity was also measured after a short-term stressor (5-min spray bath) of sufficient magnitude to elevate blood glucose levels. The mean insulin sensitivity after the stressor was 3.6 ± 0.8 × 10−4 min−1/μU/ml (range 1.6–7.3 × 10−4 min−1/μU/ml), which was not significantly different to the mean insulin sensitivity before the short-term stressor (P=0.237). The mean glucose effectiveness after the stressor was 0.046 ± 0.004 min−1 (range 0.032–0.057 min−1), which was significantly different from mean glucose effectiveness before the short-term stressor (P=0.003). We conclude that insulin sensitivity is highly variable from day to day in normal cats, and that hyperglycaemia in response to short-term stressors is probably due to increased hepatic glucose production, rather than peripheral insulin resistance.
Insulin resistance, a pathological condition in which the magnitude of the biological response to insulin is decreased, is a feature of several metabolic disorders in humans and cats, including diabetes (Kahn 1978, Porte 1991, Feldhahn et al 1999). In humans, insulin sensitivity (the measure of insulin resistance) is also influenced by a variety of other factors, including obesity, diet, stimulants (tobacco and alcohol), exercise, stress, medication and interday variation (Yki-Järvinen & Nikkilä 1985, Facchini et al 1992, Lönnqvist et al 1992, Pandit et al 1993, Starke 1994, Steil et al 1994, Thorell et al 1994, Henriksson 1995, Walker 1995).
Numerous studies have documented a marked interday variation in insulin sensitivity in both normal and diabetic humans (Ferrari et al 1991, Abbate et al 1993, Steil et al 1994, Rey et al 1996). In addition, a diurnal variation in insulin sensitivity occurs in normal and diabetic humans, with decreased insulin sensitivity in the early morning (Waldhäusl 1989, Boden et al 1996). This is known as the ‘dawn phenomenon’, and results in increased insulin requirements in type 1 diabetic humans at this time. This may have implications for the diagnosis and the treatment of patients with type 2 diabetes (Boden et al 1996, Gautier & Cathelineau 1997). The dawn phenomenon results from decreased insulin sensitivity (primarily at the hepatic level) and the secretion of counter-regulatory hormones, such as cortisol and growth hormone, which increase hepatic glucose output (Perriello et al 1991).
In cats and humans, illness and psychological stress may cause hyperglycaemia, often termed ‘stress hyperglycaemia’ (DeFronzo et al 1980, Leidinger et al 1989, Kinnaird et al 1998). The major mediators of stress hyperglycaemia are thought to be catecholamines (Rolih & Ober 1995). Insulin resistance is one of the mechanisms by which the catecholamines induce hyperglycaemia (Rolih & Ober 1995). In cats, diagnosis of diabetes can be a problem because of their propensity to develop stress hyperglycaemia (Rand 1997). A combination of illness-associated stress and psychological stress following changes in the cat's environment, such as travel and handling, can result in blood glucose concentrations in the diabetic range (Leidinger et al 1989, Opitz 1990). In cats, the most frequent cause of apparent impaired glucose tolerance is stress (Link & Rand 1998).
The aim of the present study was to study interday variation and the effect of a short-term stressor on insulin sensitivity in clinically normal cats.
Research design and methods
Animals
This study was approved by the Animal Experimentation Ethics Committee of the University of Queensland. Six mixed-bred cats (three castrate males and three spayed females) were obtained from the University of Queensland veterinary research colony. The cats were adults and were estimated to be 1–2 years of age, but exact age was unknown. Mean bodyweight of the cats was 4.38 kg (range 3.7–5.7 kg). Normal body condition was determined using a 5-point body condition scoring system (Donoghue and Kronfeld 1993). Non-obese cats with a score of 2–4 were used in the study. For inclusion in the study, cats had to have fasting normoglycaemia and normal intravenous glucose tolerance test (IVGTT) results (T1/2 <86 min) (Link et al 1997). Clinical characteristics of the normal cats are shown in Table 1.
Table 1.
Clinical characteristics of normal cats
| Cat | Breed | Sex | Age (years) | Bodyweight (kg) | Body conditon score (1–5) |
|---|---|---|---|---|---|
| 1 | DSH | Mc | 1–2 | 4.7 | 3 |
| 2 | DLH | Fs | 1–2 | 3.9 | 3 |
| 3 | DSH | Mc | 1–2 | 4.5 | 3 |
| 4 | DSH | Fs | 1–2 | 3.8 | 2 |
| 5 | DSH | Fs | 1–2 | 5.7 | 4 |
| 6 | DSH | Mc | 1–2 | 3.7 | 2 |
DSH—Domestic short hair; DLH—Domestic long hair; Mc—Male castrate; Fs—Female spayed.
Experimental protocol
A jugular catheter was placed in the cats, followed 2 days later by an intravenous glucose tolerance test. The first insulin sensitivity test was performed 1 day after the glucose tolerance test. All tests began at 0800 hours after a 12-h fast. To minimise environmental influences on insulin sensitivity, the study was performed under conditions of fixed dietary intake and stable physical activity. The cats were fed a commercially available dry food for feline maintenance (protein 26%, fat 7%, fibre 5%) at a rate estimated to maintain stable bodyweight. To study day-today variation in insulin sensitivity, the insulin sensitivity test was performed on 4 separate days over a 7-day period. In order to study the effects of stress on insulin sensitivity, a fifth insulin sensitivity test was performed 2 days later, immediately after the cat was subjected to a short-term stressor (5-min spray-bath using water at room temperature). The stressor had previously been shown to cause a significant increase in blood glucose (Kinnaird et al 1998).
Jugular catheter placement, the intravenous glucose tolerance test and the insulin sensitivity tests were performed as previously reported (Feldhahn et al 1999). The insulin sensitivity test used was an insulin-modified frequently-sampled intravenous glucose tolerance test [intravenous injection of glucose (0.3 g/kg) at 0 min and human regular insulin (0.05 U/kg) at 20 min]. Insulin sensitivity and glucose effectiveness was determined from the test using the MINMOD computer program kindly donated by R.N. Bergman (Bergman et al 1979). Glucose effectiveness is the ability of glucose to enhance its own uptake and suppress glucose production at basal insulin levels (Finegood & Tzur 1996).
Bath challenge
Blood was taken prior to removal of the cat from its cage for measurement of plasma glucose before the stressor. A bathing harness (Cat Stay-N-Wash Noose, Pet Network Pty. Ltd, New Jersey, USA) was placed on each cat after removal from the cage. Cats were put into a carrier, moved to the bathing area, removed from the carrier, and the bathing harness fastened to the tub table with attached suction cup. Cats were thoroughly sprayed with water for 5 min. An assistant provided restraint as necessary to keep the cats in the tub. After bathing, the cats were towel-dried for 2 min, placed in the carrier, and transported back to their cage. The minimal model test (four baseline samples and 27 samples following glucose injection) was started immediately after the cat was replaced in its cage. The first of the four baseline blood glucose measurements in the insulin sensitivity test was used to determine the blood glucose concentration after the stressor.
Glucose and insulin measurements
Glucose was measured using a YSI glucose analyser (Yellow Springs Instruments, Yellow Springs, OH). Insulin was measured using the Pharmacia Diagnostics (Uppsala, Sweden) insulin radioimmunoassay kit which detects both human and feline insulin (Lutz and Rand 1993).
Statistical analysis
Data are reported as means and standard error of the mean (sem). Interday variation in fasting and peak plasma glucose concentration, insulin sensitivity and glucose effectiveness were expressed as coefficients of variation (CVs). Coefficients of variation for each parameter for each cat were calculated as the standard deviation (sd) divided by the mean for each set of four tests. The resultant value was multiplied by 100. To express the overall interday variation for a parameter, the mean value of the six individual cat CVs was used. The mean value was used rather than the square root of the average sum of square CVs, to allow for a non-constant CV within the population. Insulin sensitivity data for the six cats over the four tests were tested for outliers using the box and whiskas plot technique (Tukey 1977). One value was classed as a mild outlier but was retained in the calculation of CV. Statistical comparisons between the plasma glucose and insulin sensitivity before and after the short-term stressor were made using the paired Student's t-test, using a confidence level of P<0.05. The mean insulin sensitivity over the first four experiments was used as the before-stress (normal) value for comparison. The Student's t-test was performed using SigmaStat (SPSS Inc., Chicago, USA).
Results
Intravenous glucose tolerance test
The glucose half-life (T1/2) for the six clinically normal cats ranged from 49.84 to 65.27 min, (mean T1/2 53.82 min) and was within the reference range for our laboratory (<86 min; Link et al 1997).
Insulin-sensitivity test
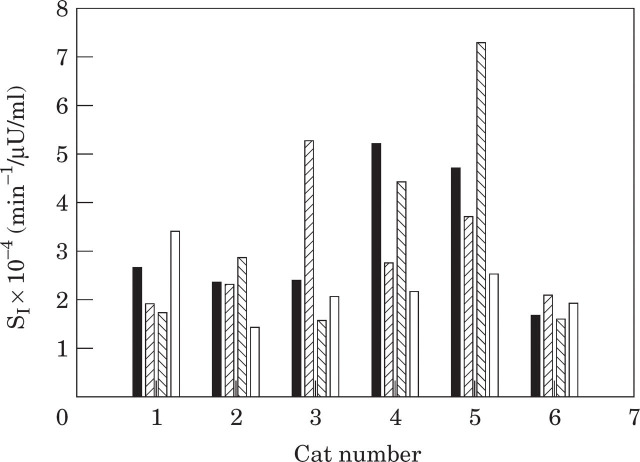
The overall interday variability in fasting plasma glucose was 9 ± 2%, and for peak plasma glucose was 13 ± 1%. The overall mean insulin sensitivity for the six cats over four tests was 2.9 ± 0.4 × 10−4 min−1/μU/ml (range 1.9–4.6 × 10−4 min−1/μU/ml). The mean of the individual CVs for insulin sensitivity was 35 ± 6% (range 13–59%) (Table 2, Fig 1).
Table 2.
Interday variation: means and CVs for insulin sensitivity test parameters
| Cat | SI × 10−4 (min−1/μU/ml) | SG (min−1) | ||
|---|---|---|---|---|
| Mean | CV(%) | Mean | CV(%) | |
| 1 | 2.44 | 31.7 | 0.032 | 28.7 |
| 2 | 2.26 | 26.7 | 0.029 | 39.3 |
| 3 | 2.84 | 58.5 | 0.024 | 25.6 |
| 4 | 3.66 | 38.9 | 0.025 | 27.4 |
| 5 | 4.59 | 44.0 | 0.037 | 19.0 |
| 6 | 1.86 | 12.8 | 0.027 | 7.9 |
| Mean | 2.94 | 35.4 | 0.029 | 24.7 |
| sem | 0.41 | 6.4 | 0.002 | 4.3 |
SI—Insulin sensitivity index; SG—Glucose effectiveness.
Fig 1.
Insulin sensitivity in six cats on 4 separate days over a 7-day period. (▪), Test 1; ( ), test 2; (
), test 2; ( ), test 3; (□), test 4.
), test 3; (□), test 4.
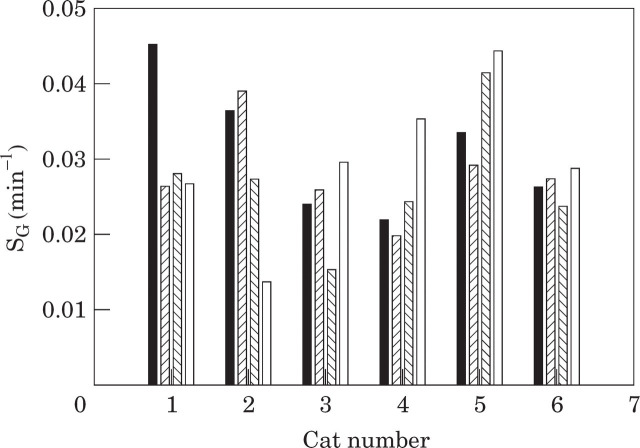
The mean glucose effectiveness was 0.029 ± 0.002 min−1 (range 0.024–0.037 min−1). The mean of the individual CVs for glucose effectiveness was 25 ± 4% (range 8–39%) (Table 2, Fig 2).
Fig 2.
Glucose effectiveness in six cats on 4 separate days over a 7-day period. (▪), Test 1; ( ), test 2; (
), test 2; ( ), test 3; (□), test 4.
), test 3; (□), test 4.
The mean plasma glucose before the stressor was 4.39 ± 0.16 mmol/l (range 3.94 ± 4.84 mmol/l), compared to a mean of 6.75 ± 0.87 mmol/l (range 4.02–10.1 mmol/l) after the stressor (Table 3). There was a statistically significant difference in plasma glucose before and after the short-term stressor (P=0.04). Plasma glucose did not increase during the stressor in 1 cat (4.0 mmol/l compared to 4.1 mmol/l pre-stress; cat 5), and this cat was the only one which did not struggle during the bath. A strong correlation between struggling and blood glucose has been reported by Kinnaird et al (1998) using the same bath model.
Table 3.
Effects of a short-term stressor (spray bath) on plasma glucose values
| Cat | Plasma glucose (mmol/l) | |
|---|---|---|
| Before bath (stressor) | After bath and replacement in cage | |
| 1 | 4.7 | 7.8 |
| 2 | 4.1 | 10.1 |
| 3 | 3.9 | 5.4 |
| 4 | 4.8 | 5.8 |
| 5 | 4.1 | 4.0 |
| 6 | 4.7 | 7.3 |
| Mean | 4.4 | 6.8 |
| SEM | 0.2 | 0.9 |
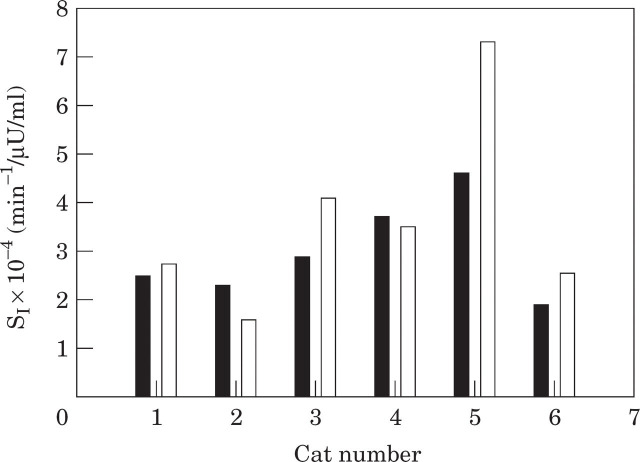
When the mean value for insulin sensitivity over the four pre-bath tests was compared to the post-bath value, there was no significant difference in insulin sensitivity (P=0.237) (Table 4, Fig 3). The mean insulin sensitivity in normal cats before the stressor was 2.9 ± 0.4 × 10−4 min−1/μU/ml (range 1.9–4.6 × 10−4 min−1/μU/ml), compared to a mean of 3.6 ± 0.8 × 10−4 min−1/μU/ml (range 1.6–7.3 × 10−4 min−1/μU/ml) after the stressor.
Table 4.
Effects of a short-term stressor: insulin sensitivity test parameters
| Cat | SI × 10−4 (min−1/μU/ml) | SG (min−1) | ||
|---|---|---|---|---|
| Normal | Stressed | Normal | Stressed | |
| 1 | 2.44 | 2.69 | 0.032 | 0.050 |
| 2 | 2.26 | 1.55 | 0.029 | 0.043 |
| 3 | 2.84 | 4.07 | 0.024 | 0.041 |
| 4 | 3.66 | 3.48 | 0.025 | 0.054 |
| 5 | 4.59 | 7.31 | 0.037 | 0.057 |
| 6 | 1.86 | 2.53 | 0.027 | 0.032 |
| Mean | 2.94 | 3.61 | 0.029 | 0.046 |
| sem | 0.41 | 0.82 | 0.002 | 0.004 |
Normal values are means of the parameters calculated from the four tests conducted prior to the stressor.
Fig 3.
Insulin sensitivity in six cats before (▪) and after (□) a short-term stressor (5-min spray bath).
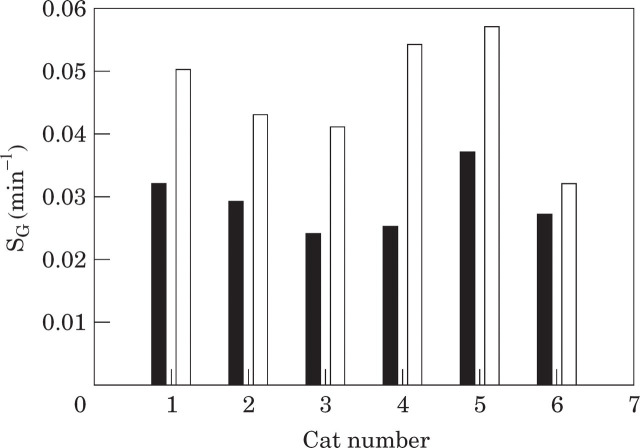
The mean glucose effectiveness in normal cats before the stressor was 0.029 ± 0.002 min−1 (range 0.024–0.037 min−1), compared to a mean of 0.046 ± 0.004 min−1 (range 0.032–0.057 min−1) after the stressor (Table 4, Fig 4). There was a statistically significant difference in glucose effectiveness before and after the short-term stressor (P=0.003).
Fig 4.
Glucose effectiveness in six cats before (▪) and after (□) a short-term stressor (5-min spray bath).
Discussion
The primary goal of this study was to determine the interday variation in insulin sensitivity in normal cats and to determine the effect on insulin sensitivity of a short-term stressor known to cause hyperglycaemia in cats.
The observed interday variance in insulin sensitivity (35%) may be separated into two parts: one that is associated with measurement error of glucose and insulin and one that is associated with true fluctuations in insulin-mediated glucose metabolism (Prigeon et al 1994). Measurement errors result from errors in the glucose and insulin assays, errors in sample timing and unmodelled glucose dynamics (eg, mixing) (Prigeon et al 1994). Some degree of measurement error is unavoidable and all methods for measuring insulin sensitivity are affected to various degrees (Prigeon et al 1994). While it is impossible to ascertain precisely the influence of the glucose and insulin measurement error on the insulin sensitivity estimate, Monte-Carlo simulation has shown that a 1.5% glucose assay error and an 8% insulin assay error (similar to the assay variance obtained in our laboratory) can only account for a CV in insulin sensitivity of about 12% (Prigeon et al 1994). When this is taken into account, the 35% CV obtained in our study suggests that 23% of the variation in insulin sensitivity in clinically normal cats is due to interday variation. No trend in insulin sensitivity was observed over the four experimental procedures. If the procedure itself was a sufficient stressor to lower insulin sensitivity, then acclimatisation to the procedure would have caused an increase in insulin sensitivity with each experiment, but this was not observed.
This is the first study in cats to document interday variation in insulin sensitivity. Similar studies have been conducted in non-diabetic humans. Abbatte et al (1993) reported a 17% variation in duplicate measures of SI (insulin sensitivity in Bergman's minimal model) in eight men (tests separated by 1 or 2 days), reported a 14% variation using duplicate measures (separated by 21 days) in 15 subjects, and Steil et al (1994) reported a 20% variation using triplicate measures (separated by 3 days) in 11 men. These studies used the tolbutamidemodified protocol, but the insulin-modified protocol produces similar results, with a 21% variation using duplicate measures reported in one study (Rey et al 1996). The CVs in those studies are lower than the 35% CV observed in our study. Even when correcting for the fact that CV measured with four tests is expected to be higher than CV measured with two or three tests, our CV may be significantly greater. When the first two tests were used to calculate the interday CV, our CV was 26%, and 30% when the first three tests were used.
An explanation for the difference may be that cats have a greater interday variability in insulin sensitivity than humans. It appears that changing insulin sensitivity is a potent mechanism for maintaining glucose homeostasis in cats, which are obligate carnivores. Resistance to the glucose-lowering effect of insulin is necessary in the post-prandial period for cats to maintain glucose supply to the brain, because insulin is secreted in response to a high protein–low carbohydrate meal (Brand Miller & Colagiuri 1994). In contrast, insulin sensitivity in fasting cats is similar to that in fasting humans (Feldhahn et al 1999). Another example of a possible change in insulin sensitivity in cats occurs after intravenous injection of arginine, where a four-fold increase in insulin concentration is associated with only a small decrease in glucose concentration (Link & Rand 1996). For blood glucose to be maintained, rapid and profound changes in carbohydrate metabolism are necessary.
Glucose effectiveness is also considered to be an important metabolic parameter (Ader et al 1985). Glucose effectiveness (SG) has been defined as the combined effect of glucose to enhance glucose uptake and suppress endogenous glucose production at basal insulin levels (Finegood and Tzur 1996). This parameter also displayed interday variation, with a CV of 25% (13% when measurement error is accounted for). This is lower than the 35% CV we observed for insulin sensitivity. The CV of glucose effectiveness calculated for the first three tests (19%) may be significantly lower than the 25% CV measured in triplicate by Steil et al (1994). Thus, while insulin sensitivity showed greater interday variation in the cats in our study than in humans, glucose effectiveness showed less interday variation. Glucose effectiveness is influenced by glucose concentration (Bergman et al 1985). Interday variation in both fasting (9%) and peak glucose (13%) values during the insulin sensitivity test were lower than the variation in glucose effectiveness (25%) and less than the variation in insulin sensitivity (35%). Thus, variability in fasting and peak glucose concentrations cannot account fully for the variation in glucose effectiveness.
The short-term bath stressor did not significantly change insulin sensitivity in clinically normal cats, even though glucose concentrations were significantly increased at the start of the insulin sensitivity test and, in Kinnaird's study, remained elevated to the end of the study 90 min after the bath (Kinnaird et al 1998). This suggests that hyperglycaemia resulting from short-term stress is due to increased glucose production rather than decreased utilisation from peripheral insulin resistance. Because most insulin sensitivity tests largely measure skeletal muscle uptake of glucose in response to insulin, the term ‘insulin sensitivity’ usually refers to peripheral insulin sensitivity (ie, in the muscles). However, the increased glucose production may represent insulin resistance at the level of the liver, resulting in increased hepatic glucose output. If this is the case, it suggests that, in cats, ‘insulin sensitivity’ may be more dependent on insulin action at the level of the liver, rather than in the periphery.
The conclusion that the hyperglycaemia of acute stress results from increased glucose production is supported by Kinnaird's findings in the spray-bath test. Blood glucose concentration was correlated with lactate concentration, a major substrate for hepatic gluconeogenesis, suggesting that hyperglycaemia resulted from lactate-based gluconeogenesis, rather than stress hormone-induced peripheral insulin resistance (Kinnaird et al 1998). This conclusion that hyperglycaemia secondary to an acute stressor is the result of increased gluconeogenesis rather than peripheral insulin resistance is physiologically logical. It would be expected that the flight or fight response to a short-term stressor would be facilitated by increased gluconeogenesis providing glucose for muscle and brain activity, rather than peripheral insulin resistance. However, there was no point-measure of insulin sensitivity and the test occured over 3 h, whereas this stressor was only over a 5 min period. It is possible that peripheral insulin resistance occurred during the 5 min bath, but insulin sensitivity had normalized after the stressor finished, despite persisting hyperglycaemia. It is, however, unlikely that muscle-based insulin resistance would change this quickly. An acute reduction in glucose uptake may also occur via reduced glucose oxidation, independent of changes in insulin sensitivity. However, glucose effectiveness (a measure of glucose uptake independent of insulin) increased after the bath rather than decreased, suggesting that this was not the cause of the hyperglycaemia. While increased hepatic glucose production associated with increased flux of gluconeogenic substrate to the liver seems the most likely mechanism for the hyperglycaemia, further studies need to be performed to rule out the possibility of reduced glucose oxidation. A euglycaemic insulin clamp combined with the infusion of labelled glucose (which allows measurement of hepatic glucose production and its sensitivity to insulin) and indirect calorimetry (which estimates intracellular glucose oxidation and storage) would need to be performed (Groop et al 1993).
Although some studies in humans have found decreased insulin sensitivity associated with stress, other studies have not supported this (Smith et al 1992, Rolih and Ober 1995). A study in healthy men showed that mild mental stress increased insulin sensitivity (Touma et al 1996). While there was a slight increase in insulin sensitivity after stress in our study (3.61 compared to 2.94 × 10−4 min−1/μU/ml), it was not statistically significant.
No study has been conducted in cats to document the effect of severe or chronic stressors on insulin sensitivity. It is possible that mechanisms producing hyperglycaemia in acute stress are different from those in chronic psychological or illness-associated stress. Many studies reporting insulin resistance with stress have been in illness, which is a more chronic stressor than the spray-bath used in this study (Smith et al 1992, Rolih and Ober 1995). In contrast, the ‘flight or fight’ response to acute stress is associated with increased metabolic rate (Guyton and Hall 1996), and normal or increased insulin sensitivity may be more advantageous than insulin resistance. While an increase in metabolic rate may be advantageous in acute stress, decreased muscle metabolism of glucose (resulting from peripheral insulin resistance) may be a more appropriate response to illness so that glucose is conserved for essential metabolic processes.
While insulin sensitivity did not change significantly, glucose effectiveness (the ability of glucose to enhance its own uptake and suppress its production) showed a significant increase after the stressor. Glucose effectiveness is determined by the combined effect of decreased glucose production and increased glucose uptake. As the stress hyperglycaemia was probably caused by increased endogenous glucose production, the increase in glucose effectiveness can only be accounted for by greatly enhanced glucose uptake into the tissues. Again, however, there is no point-measure of glucose effectiveness and we only measured glucose effectiveness after the stressor was over. Glucose production may increase during the stressor, but once the stressor is over it may greatly decrease, contributing to the increased glucose effectiveness. In Kinnaird's study, lactate concentration decreased more rapidly than glucose concentration, suggesting that factors causing hyperglycaemia may be different from those maintaining it.
In conclusion, insulin sensitivity varies from day to day in clinically normal cats (35 ± 6% CV), and stress hyperglycaemia is probably due to increased glucose production rather than muscle-based insulin resistance.
Acknowledgements
Funding for this study was provided by the Australian Companion Animal Health Foundation and the University of Queensland. Juanita Feldhahn was supported by an Australian Postgraduate Award and a University of Queensland Postgraduate Scholarship.
References
- Abbate SL, Fujimoto WY, Brunzell JD, Kahn SE. (1993) Effect of heparin on insulin-glucose interactions measured by the minimal model technique: Implications for reproducibility using this method. Metabolism—Clinical and Experimental 42, 353–357. [DOI] [PubMed] [Google Scholar]
- Ader M, Pacini G, Yang YJ, Bergmanr RN. (1985) Importance of glucose per se to intravenous glucose tolerance. Diabetes 34, 1092–1103. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Ider YZ, Bowden CR, Cobelli C. (1979) Quantitative estimation of insulin sensitivity. American Journal of Physiology 236, E667–E677. [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, Urbain JL. (1996) Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 45, 1044–1050. [DOI] [PubMed] [Google Scholar]
- Brand Miller JC, Colagiuri S. (1994) The carnivore connection: Dietary carbohydrate in the evolution of NIDDM. Diabetologia 37, 1280–1286. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Sherwin RS, Felig P. (1980) Synergistic interactions of counterregulatory hormones: A mechanism for stress hyperglycaemia. Acta Chirurgica Scandinavica 498(Suppl), 33–42. [PubMed] [Google Scholar]
- Donoghue S, Kronfeld DS. (1993) Feeding the hospitalised cat In: Handbood of Feline Medicine, Wills J, Wolf A. (ed.) Great Britain, Pergamon Press, pp. 35–47. [Google Scholar]
- Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM. (1992) Insulin resistance and cigarette smoking. Lancet 339, 1128–1130. [DOI] [PubMed] [Google Scholar]
- Feldhahn J, Rand JS, Martin G. (1999) Insulin sensitivity in normal and diabetic cats. Journal of Feline Medicine and Surgery, 1, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari P, Alleman Y, Riesen W, Weidmann P. (1991) Reproducibility of insulin sensitivity measured by the minimal model method. Diabetologia 34, 527–530. [DOI] [PubMed] [Google Scholar]
- Finegood DT, Tzur D. (1996) Reduced glucose effectiveness associated with reduced insulin release: An artifact of the minimal-model method. American Journal of Physiology 3, E485–E495. [DOI] [PubMed] [Google Scholar]
- Gautier JF, Cathelineau G. (1997) Insulin sensitivity and hepatic glucose production: Nycthemeral variations. Diabete et Metabolisme 23, 35–38. [PubMed] [Google Scholar]
- Groop LC, Widen E, Ferrannini E. (1993) Insulin resistance and insulin deficiency in the pathogenesis of type 2 (non-insulin-dependent) diabetes mellitus: Errors of metabolism or of methods? Diabetologia 36, 1326–1331. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Hall JE. (1996) ‘Alarm’ or ‘stress’ response of the sympathetic nervous system. In: Textbook of Medical Physiology, 9th edn. Philadelphia, WB. Saunders, p. 779. [Google Scholar]
- Henriksson J. (1995) Influence of exercise on insulin sensitivity. Journal of Cardiovascular Risk 2, 303–309. [PubMed] [Google Scholar]
- Kahn CR. (1978) Insulin resistance, insulin insensitivity, and insulin unresponsiveness: A necessary distinction. Metabolism—Clinical and Experimental 27 (Suppl 2), 1893–1902. [DOI] [PubMed] [Google Scholar]
- Kinnaird ER, Rand JS, Baglioni AJ, Jr, Blackshaw J. (1998) Stress hyperglycemia in cats. Journal of Veterinary Internal Medicine 12, 213. [DOI] [PubMed] [Google Scholar]
- Leidinger K, Nolte I, Eigenbrodt E. (1989) Klinische und labordiagnostische untersuchungen sum phanomen der hyperglykamie der katze. Kleintierpraxix 34, 457. [Google Scholar]
- Link KR, Rand JS. (1996) Arginine and phentolamine response tests in cats. Journal of Veterinary Internal Medicine 10, 185. [Google Scholar]
- Link KR, Rand JS. (1998) Reference values for glucose tolerance and glucose tolerance status in cats. Journal of the American Veterinary Association, 213: 492–496. [PubMed] [Google Scholar]
- Link KR, Rand JS, Hendrikz JK. (1997) Evaluation of a simplified intravenous glucose tolerance test and a reflectance glucose meter for use in cats. Veterinary Record 140, 253–256. [DOI] [PubMed] [Google Scholar]
- Lönnqvist F, Wennlund A, Wahrenberg H, Arner P. (1992) Effects of mental stress on lipolysis in humans. Metabolism—Clinical and Experimental 41, 622–630. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Rand JS. (1993) Comparison of five commercial radioimmunoassay kits for the measurement of feline insulin. Research in Veterinary Science 55, 64–69. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Rand JS. (1996) Plasma amylin and insulin concentrations in normoglycaemic and hyperglycaemic cats. Canadian Veterinary Journal 37, 27–34. [PMC free article] [PubMed] [Google Scholar]
- Opitz M. (1990) Stress hyperglycemia in cats. Berliner und Munchener Tierarzt Wochen 103, 151. [PubMed] [Google Scholar]
- Pandit MK, Burke J, Gustafson AB, Minocha A, Peiris AN. (1993) Drug-induced disorders of glucose tolerance. Annals of Internal Medicine 118, 529–539. [DOI] [PubMed] [Google Scholar]
- Perriello G, De Feo P, Torlone E, Fanelli C, Santeusanio F, Brunetti P, Bolli GB. (1991) The dawn phenomenon in Type 1 (insulin-dependent) diabetes mellitus: Magnitude, frequency, variability, and dependency on glucose counter-regulation and insulin sensitivity. Diabetologia 34, 21–28. [DOI] [PubMed] [Google Scholar]
- Porte D. (1991) β-cells in type II diabetes mellitus. Diabetes 40, 166–180. [DOI] [PubMed] [Google Scholar]
- Prigeon RL, Kahn SE, Porte D., Jr (1994) Reliability of error estimates from the minimal model: Implications for measurements in physiological studies. American Journal of Physiology 266, E279–E286. [DOI] [PubMed] [Google Scholar]
- Rand JS. (1997) Understanding feline diabetes. Australian Veterinary Practitioner 27, 17–26. [Google Scholar]
- Rey RH, Masnatta LD, Pirola D, Cuniberti LA, Maceira C, Werba JP. (1996) Repeatability of insulin sensitivity estimation using the Minimal Model and comparison with a modified short low-dose insulin tolerance test. Medicina Buenos Aires 56, 650–656. [PubMed] [Google Scholar]
- Rolih CA, Ober KP. (1995) The endocrine response to critical illness. Medicine Clinics of North America 79, 211–224. [DOI] [PubMed] [Google Scholar]
- Smith U, Attvall S, Eriksson J, Fowelin J, Lonnroth P, Wesslau C. (1992) The insulin antagonistic effect of the counterregulatory hormones—clinical and mechanistic aspects. New Concepts in the Pathogenesis of NIDDM. Advances in Experimental Medicine and Biology 334, 169–180. [DOI] [PubMed] [Google Scholar]
- Starke AR. (1994) The influence of diet and physical activity on insulin sensitivity. Wiener Klinische Wochenschrift 106, 768–773. [PubMed] [Google Scholar]
- Steil GM, Murray J, Bergman RN, Buchanan TA. (1994) Repeatability of insulin sensitivity and glucose effectiveness from the minimal model. Diabetes 43, 1365–1371. [DOI] [PubMed] [Google Scholar]
- Thorell A, Efendic S, Gutniak M, Häggmark T, Ljungqvist O. (1994) Insulin resistance after abdominal surgery. British Journal of Surgery 81, 59–63. [DOI] [PubMed] [Google Scholar]
- Touma T, Takishita S, Kimura Y, Muratani H, Fukiyama K. (1996) Mild mental stress increases insulin sensitivity in healthy young men. Clinical and Experimental Hypertension 18, 1105–1114. [DOI] [PubMed] [Google Scholar]
- Tukey JW. (1977) Box and whiskas plot. In: Exploratory Data Analysis. Philippines, Addison Wesley, pp. 39–47. [Google Scholar]
- Waldhäusl W. (1989) Circadiam rhythms of insulin needs and actions. Diabetes Research and Clinical Practise 6, S17–S24. [DOI] [PubMed] [Google Scholar]
- Walker M. (1995) Obesity, insulin resistance, and its link to non-insulin-dependent diabetes mellitus. Metabolism—Clinical and Experimental 44 (Suppl 3), 18–20. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H, Nikkilä EA. (1985) Ethanol decreases glucose utilization in healthy man. Journal of Clinical Endocrinology and Metabolism 61, 941–945. [DOI] [PubMed] [Google Scholar]