Abstract
The clinical case records of 44 cats with distal aortic thromboembolism were reviewed. These detailed 49 separate episodes of thromboembolism. Of the 44 cats, 33 (75%) were neutered males, and 43 (98%) were domestic shorthairs. The mean age was 8.7 years (age range 2–16 years). Evidence of pre-existing heart disease had been noted in 23% of cases. Heart failure was a concurrent diagnosis in 51% of episodes and 36% of these cases survived their thromboembolic episodes. Overall, cats survived 39% of the episodes. Cats died during 28% of the episodes, while 33% of the episodes resulted in the cats being euthanased. The median survival time was 6 months. Seventeen per cent of cats on prophylactic aspirin therapy experienced an episode of re-embolisation. Forty-three per cent of episodes occurred in the spring.
Distal aortic thromboembolism is one of the most serious complications associated with cardiomyopathy in the cat and one of the most common causes of hindlimb paresis (Flanders 1986). Aortic thromboembolism can occur with any form of cardiomyopathy, but it is most commonly associated with hypertrophic cardiomyopathy (Laste & Harpster 1995, Sisson & Thomas 1995). Aortic thromboembolism is sometimes observed in cats without underlying heart disease (Pion & Kittleson 1989).
It is believed that aortic thromboembolism occurs when an embolus breaks free from a thrombus in the left atrium or left ventricle and enters the peripheral circulation. Although emboli most commonly lodge in the distal aortic trifurcation, they can also occlude a cerebral, renal, mesenteric, pulmonary or brachial artery. Feline distal aortic thromboembolism was first reported by Collet in 1930 (cited by Holzworth et al 1955). Since then much has been learnt about the pathophysiology of this condition. It has been shown by Imhoff (1961) that the condition cannot be reproduced by simple ligation or even double ligation of the aorta. Imhoff showed that collateral circulation was severely impeded if ligation of the aorta was combined with the presence of a clot. Other authors confirmed this work and found that vasoactive substances such as serotonin and prostaglandins released from the clot led to impaired collateral circulation and were crucial in the pathophysiology of this condition (Butler 1971, Olmstead & Butler 1977, Schaub et al 1982). The consequences of distal aortic thromboembolism are dependent on the completeness of the obstruction, the functional patency of collateral circulation and the duration of the obstruction (Sisson & Thomas 1995).
Aortic thromboembolism is reported to be more common in the neutered male (Buchanan et al 1966, Laste & Harpster 1995). Although the signalment and underlying cardiomyopathy are widely accepted, there is little conclusive evidence of other factors that might predispose an individual cat to a thromboembolic event and no reliable method to determine which cats with myocardial disease are at greater risk of such an event. Reported predisposing factors for thromboembolism include progestagen therapy, corticosteroid therapy and obesity (Pouchelon et al 1998). In a recent study from the Department of Cardiology, Angell Memorial Hospital, Boston, USA, a survival rate of 37% was reported (Laste & Harpster 1995). Given the high level of expertise and the facilities available to treat those reported cases, one might expect the survival rate to be significantly lower in first opinion practice.
Preventative treatments such as aspirin (Schaub et al 1982, Green 1985, Flanders 1986, Fox 1988) and warfarin (Harpster & Baty 1995) have been recommended, but these have not been evaluated by controlled studies. Recurrence of thromboembolism has been reported in 75% of cases on aspirin therapy in one study (Pion & Kittleson 1989), whereas another study reported five cats surviving more than 18 months on aspirin therapy without recurrence of thromboembolism (Flanders 1986). On the other hand, warfarin prophylaxis had a 43% recurrence rate and was associated with complications such as bleeding episodes (20%) and sudden death (12%) (Harpster & Baty 1995).
Only a small number of case series of aortic thromboembolism have been reported in the literature. Holzworth et al (1955) reported 11 cases. Another series of 14 cases was reported by Buchanan et al (1966). The only large series has come from Boston, USA (Laste & Harpster 1995). In the UK, Lucke & Sumner-Smith (1966) reported the surgical outcome of three cases in Bristol. A small series of seven cases in which nerve conduction and histopathology of the hindleg muscles were documented was reported from Glasgow (Griffiths & Duncan 1979).
The objective of this study was to evaluate retrospectively the records of a large number of cats that presented with distal aortic thromboembolism to a first opinion veterinary hospital over an 8-year period. This paper, presenting the signalment, history, clinical findings, results of diagnostic tests, treatment regimes and survival data, is the first large series of cats with aortic thromboembolism reported in Europe.
Materials and methods
The case records of cats with episodes of distal aortic thromboembolism (n=44) that presented to the Veterinary Hospital, Bishop's Stortford, between September 1990 and September 1998 were reviewed. Other forms such as cerebral or mesenteric arterial thromboembolism were not included, due to the difficulty in arriving at a definitive diagnosis. A diagnosis of complete distal aortic thromboembolism was based on the absence of both femoral pulses, cold hindlimbs, cyanosis of the nail beds, bilateral hindlimb paralysis and in most cases, severe pain. Episodes were classified as partial based on the acute onset of reduced (not absent) femoral pulses, proprioceptive deficits and unilateral or bilateral hindlimb paresis rather than bilateral paralysis, as was usually the case in the complete episodes. Pain was subjectively assessed by the attendant clinician based on resentment at palpation and vocalisation. Strict inclusion criteria were used and cats were deemed to have survived only if they lived for more than 36 hours after the start of the episode.
The medical records were analysed for details of signalment, history, clinical signs, clinical pathology, ultrasonographic, electrocardiographic and radiographic abnormalities, emergency treatment, prophylactic treatment, outcome and survival.
Clinical pathology was performed on QBC Vet Autoread, Vettest and Vetlyte (Idexx). The practice laboratory participated in regular monthly quality control. Radiography was performed using a Watson Roentgen R301 machine capable of 120 kV/300 mA and right lateral and dorsoventral projections of the thorax were obtained. Cardiomegaly was defined by a heart size of more than 2.5 rib spaces wide and an apical to basillary distance of more than 75% of the sternal to vertebral distance in the right lateral projection (Bonagura 1994).
Echocardiography was performed using a 7.5 mHz mechanical sector probe (Concept 2000, Dynamic Imaging) or with a 6 mHz VFI micro convex probe running at 7.5–10 mHz (Impact VFI; BCF Technology). Echocardiographic examination was performed from the right chest wall, using the parasternal long and short axis views, with the cats lying in right lateral recumbency on a purpose-built device. Hypertrophic cardiomyopathy was diagnosed if the left ventricular free wall and septal wall thickness was greater than 5 mm in diastole, with concomitant diminution in left ventricular lumen diameter.
Electrocardiography was performed with Cardiofax ECG 6101 (Nihon Kohden) employing the six standard limb leads. Measurements were performed in lead II (normal R wave less than 0.9 mV). Survival data were available in all the cases. Additional information about certain prodromal signs and the exact outcome was obtained from the owners by telephone.
Results
Signalment
Sex distribution showed that 75% of the cats were male and 25% were female. Forty-three of 44 cases (98%) were domestic short- or longhaired cats and one of 44 (2%) was a Japanese bobtail. The ages ranged from 2 to 16 years of age (mean age 8.7 years). The age distribution is shown in Fig 1.
Fig 1.
Age distribution of cats with distal aortic thromboembolism.
History
Most cats, 34 out of 44 (77%), presented without any evidence of pre-existing heart disease. The remaining 10 out of 44 (23%) cats had heart disease diagnosed from as little as 1 week to as much as 39 months prior to the thromboembolic episode. In seven out of these 10 cases (70%) the heart disease decompensated within the 3 weeks prior to the episode.
Possible predisposing factors were suggested in the history of 10 of 44 cats (23%). Two cats were given a depot corticosteroid preparation (Depo Medrone; Upjohn Ltd). One cat had two injections, 12 and 6 weeks prior to the episode. The other cat had 11 injections in the 15 months preceding the episode. Two cats were given megoesterol acetate (Ovarid; Mallinckrodt Veterinary Ltd) in the period prior to their episodes. One cat had 2.5 mg prescribed weekly for the preceding 6 months. The other cat was given 5 mg of megoesterol acetate every other day for the 15 days prior to the episode.
Five cats were anaesthetised or sedated prior to the episode. Three of these cats showed decompensation of their heart disease in the 2 weeks preceding their episodes. Of these, one cat had a general anaesthetic for dental treatment 3 weeks prior to the episode and showed signs of heart failure 1 week after the anaesthetic. Another cat was sedated for the lancing of an abscess 2 weeks prior to the episode and showed cardiac decompensation after the sedation. Another cat was anaemic (packed cell volume=0.22 l/l), pyrexic (40 °C), had a leucocytosis (white blood cell count=51 × 109/l) and was sedated for a radiographic study 5 days prior to the episode, after which its cardiac disease de-compensated. Two other cats were sedated for dematting procedures 2 and 4 days prior to their episodes, respectively. One other cat had pyrexia with a marked leucocytosis (white blood cell count=74 × 109/l) and hyperproteinaemia (total protein=107 g/l) found 9 days prior to the episode.
The histories of three cats showed a relationship between vomiting and the thromboembolic episode. In Cases 3 and 15, vomiting preceded the episode by a few seconds and in case 42, the owner considered the vomiting as a prodromal sign a few hours before both its thromboembolic episodes.
Clinical signs
Physical findings at presentation varied from acute onset unilateral hindlimb paresis, with little accompanying pain, to peracute onset bilateral hindlimb paralysis, with severe pain. Other concomitant findings included cold, rigid hindlimbs, absent or reduced femoral pulses, cyanotic pads, hypothermia, absence of deep pain in hindlimbs or tail, cardiac murmurs and arrhythmias, tachypnoea, dyspnoea and shock.
Fifteen out of 49 (31%) episodes were classified as partial. Cats survived 14 out of 15 (93%) of these episodes. Thirty-four of 49 (69%) were complete episodes. Cats only survived 5 out of 34 (15%) of these episodes.
Heart failure accompanied 25 out of the 49 episodes (51%) of thromboembolism. These cats presented with varying degrees of dyspnoea and oral mucosal cyanosis. Cats survived nine out of these 25 (36%) episodes.
Seasonal distribution
An analysis of the date of onset of each episode showed that five of the 49 (10%) episodes occurred in the summer months (June, July and August). The highest prevalence was noted in the spring (March, April, May), with 21 out of 49 (43%) episodes occurring this time of year.
Diagnostic tests
Of 25 cats examined radiographically, generalised cardiomegaly was noted in 11 cats (44%), pulmonary oedema in eight cats (32%) and pleural effusion in two cats (8%).
Electrocardiographic examination was performed in 11 cats. Abnormalities were noted in all but one of these cats and included second degree AV block, large R wave amplitude (>0.9 mV), deep S waves and ventricular premature contractions. Tall T waves (>1/4 of R wave amplitude) were noted in two cats.
Echocardiography was performed in 12 cats. A diagnosis of hypertrophic cardiomyopathy was made in nine out of these 12 cats. An enlarged left atrium (>20 mm) was noted in six out of these nine cats. Echocardiographic reports were not available for the other three cases.
Various laboratory tests were performed at the time of the episode in 13 cats. The most common laboratory abnormality, azotaemia, was detected in eight out of 10 cats. One cat had a urine sample taken concurrently and the azotaemia was classified as pre-renal. Four out of five cats had an elevated creatine kinase level. Two out of five cats had hyperglycaemia. Hyperkalaemia was noted in only one out of eight cats in which it was performed. The two remaining cats only had serum electrolyte concentrations measured and these were within normal limits.
Emergency treatment
Full data of the emergency treatment were available for 32 episodes. These cases were attended by different clinicians and treatments therefore varied from case to case. Aspirin (dispersible aspirin; CP Pharmaceuticals) was used in 21 cases at a dose of 75 mg per cat every third day. Frusemide (Lasix; Hoechst Roussel Vet Ltd) was used in 19 cases. This was used intravenously in the emergency setting at 1–4 mg/kg. Heparin (Multiparin; CP Pharmaceuticals) was used in 12 cases. Doses varied from 50–200 iu/kg three to four times daily.
Acepromazine (ACP; C-Vet Veterinary Products) was used in 10 cases, at a dose of 0.05–0.2 mg/kg twice daily. Nine cases had a combination of frusemide, heparin, aspirin and buprenorphine (Vetergesic, Animalcare). Buprenorphine was the most common analgesic used. The serotonin antagonist cyproheptadine (Periactin, Merck, Sharpe & Dohme) was used in eight cases at a dose of 2 mg twice daily per cat. Five of these eight cases (63%) were amongst the survivors. Other treatments included intravenous fluid therapy, oxygen therapy, the calcium channel blocker diltiazem (Tildiem-retard; Boots), various non-steroidal anti-inflammatory drugs other than aspirin and angiotensin converting enzyme (ACE)-inhibitors.
Prophylactic treatment
Aspirin prophylaxis was instituted in 12 out of the 17 cats that survived the episode. Of the four cats (cases 16, 31, 40, 42) that re-embolised, two (cases 16 and 42) were on aspirin therapy at the time. The re-embolisations were both complete episodes. Case 16 regained leg movement the next morning and lived a further 5 months, after which it re-embolised and died. Case 42 also regained leg movement by the third day, but euthanasia was performed due to worsening azotaemia. The other two cases (31, 40) had not received any anti-thrombotic therapy. Case 31 re-embolised 1 year later. This happened while the owner was on holiday, during which time frusemide therapy was temporarily stopped. It also had a complete episode, made a full recovery and is still alive 5 years later, despite being on frusemide therapy only. Case 40 re-embolised 1 month later and euthanasia was performed during the episode. Aspirin was administered in combination with heart disease treatment, which consisted of frusemide and either beta blockers, ACE-inhibitors or calcium channel blockers. None of the cats received other concurrent anticoagulant therapy.
Outcome
Overall, cats survived 19 out of 49 episodes (39%). Cats were euthanased during 16 of the 49 episodes (33%) and cats died during or within 36 hours of 14 of the 49 episodes (28%).
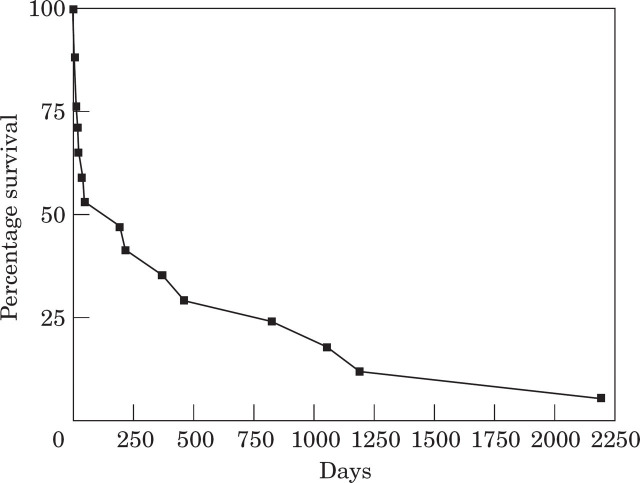
Data from the 17 cats that survived are summarised in Table 2. The mean survival time in these 17 cats is 13.4 months (range 3 days–73 months), while the median survival time is 6 months. If the four cats that died within 1 week after the episode are not included, then the mean survival time in the remaining 13 cats is 17.5 months (range 14 days–73 months). Four cats are still alive with a mean survival time of 36.8 months (range 12–73 months). The mean survival time in the nine remaining cats that died at least 1 week after the episode is 8.8 months (range 14 days–39.5 months). The two longest surviving cats are not on any anti-thrombotic therapy (Cases 12 and 31). The longest surviving cat (Case 31) has been on frusemide, as a sole therapy, for the past 6 years.
Table 2.
Signalment, treatment details and outcome in 17 cats that survived episodes of thromboembolism
| Case number | Age (years) | Sex | Prophylactic treatment | Treatment of underlying heart disease | Survival (days) | Outcome |
|---|---|---|---|---|---|---|
| 1 | 4.5 | Male | Aspirin | Diltiazem | 30 | Died at home |
| 4 | 10.5 | Male | Aspirin | None | 7 | Found dead at home |
| 5 | 2.5 | Male | Aspirin | Diltiazem | 365 | Still alive |
| 7 | 7 | Male | Aspirin | Enalapril | 14 | Died at home—heart failure |
| 8 | 10 | Male | Aspirin | None | 3 | Euthanased |
| 12 | 3 | Male | None | None | 1050 | Still alive |
| 15 | 13.5 | Male | Aspirin | None | 3 | Died at home |
| 16 | 6 | Male | Aspirin | Diltiazem, Enalapril | 184 | Re-embolised twice; died during third episode |
| 19 | 13 | Female | None | Frusemide, Hydralazine | 450 | Euthanased—old age |
| 25 | 2 | Male | Aspirin | Diltiazem | 820 | Still alive |
| 26 | 14.5 | Female | None | Frusemide | 255 | Euthanased—old age |
| 31 | 8.5 | Male | None | Frusemide | 2190 | Re-embolised 1 year later; still alive |
| 32 | 7 | Male | Aspirin | Frusemide | 210 | Euthanased—heart failure |
| 35 | 12 | Male | Aspirin | Diltiazem | 7 | Euthanased—heart failure |
| 40 | 10 | Male | None | None | 42 | Re-embolised; euthanased during second episode |
| 42 | 5.5 | Male | Aspirin | Prazosin | 1185 | Re-embolised; euthanased during second episode |
| 44 | 13 | Male | Aspirin | None | 18 | Died—heart failure |
Four out of 17 (24%) of the cats that survived the thromboembolism had an episode of re-embolisation. Euthanasia was performed on two of these cats (Cases 40 and 42) during their subsequent episodes and the third (Case 16) survived and underwent a third episode 5 months later, during which euthanasia was performed. The fourth cat (Case 31) underwent a second episode 1 year later and is still alive 5 years after this episode. The long-term survival is presented graphically in Fig 2.
Fig 2.
Long-term survival data in 17 cats that survived distal aortic thromboembolism.
Discussion
The history of peracute presentation, clinical signs and overall survival statistics of the cats in this study were similar to those in a series of 100 cases previously reported (Laste & Harpster 1995). Those authors reported a survival of 37% compared to 39% in this present study. The higher prevalence of males in this study was similar to that previously reported (Holzworth et al 1955, Buchanan et al 1966, Laste & Harpster 1995).
Since this study was undertaken in first opinion practice, extensive case histories were available. Careful evaluation of these histories have shown some speculative predisposing risk factors. Two of the cats received megoesterol acetate. Two other cats received depot corticosteroid preparations; one cat received relatively large doses over a long period of time. Although it would be tempting to speculate that corticosteroids and progestagens could be risk factors, this finding might be coincidental and further study is required in this field.
In a previous study, pre-existing heart disease was noted in only 11% of cases (Laste & Harpster 1995). This current study had a 23% prevalence of diagnosed pre-existing heart disease. In 70% of these cases, cardiac failure was diagnosed less than 3 weeks prior to their thromboembolic episodes. This suggests an increased risk of cats developing thromboembolism after decompensation of their heart disease. It was evident from the data that five cats had undergone either a general anaesthetic or sedation in the 3 weeks preceding their episodes. Three of these cats showed decompensation of their heart disease. Two cats decompensated 2 weeks prior and another cat decompensated 5 days prior to the episode. The other two cats were sedated 2 and 4 days prior to their episodes, respectively. These findings might indicate a previously unreported temporal relation between sedation or anaesthesia and episodes of thromboembolism. The deleterious effect of anaesthetic drugs on the pathophysiology of heart disease has been alluded to in a previous report (van der Linde Sipman et al 1992). It may be that the effect of the sedation in destabilising the cardiovascular system is responsible for the subsequent episodes. Factors implicated in thrombogenesis in cats are a hypercoagulable state, altered blood flow and endocardial injury (Harpster 1977, Flanders 1986, Fox 1988, 1991). These three factors are, however, the same as those stated nearly 150 years ago for predisposing humans to thromboembolism (Virchow 1856, as cited by Klein et al 1989). We are thus no closer to understanding an aetiopathogenesis.
Table 1.
Signalment, presence of heart disease, outcome and survival time in 44 cats experiencing distal aortic thromboembolism
| Case | Age | Sex | Clinical signs | Heart disease | Outcome | Survival |
|---|---|---|---|---|---|---|
| 1 | 4.5 | Male | Bilateral paresis | Detected 6 weeks prior to the episode, decompensated 3 weeks prior to the episode | Survived | 1 month |
| 2 | 14 | Female | Paraplegia, dyspnoea | Not detected prior to the episode, heart failure accompanied the episode | Euthanased | — |
| 3 | 1.5 | Male | Paraplegia | Not detected prior to the episode | Died | — |
| 4 | 10.5 | Male | Bilateral paresis, tachypnoea | Not detected prior to the episode, heart failure accompanied the episode | Survived | 7 days |
| 5 | 2.5 | Male | Paraplegia | Not detected prior to the episode | Survived | Alive 1 year later |
| 6 | 11 | Male | Paraplegia, dyspnoea | Detected and decompensated 10 days prior to the episode, heart failure accompanied the episode | Euthanased | — |
| 7 | 7 | Male | Left unilateral paresis, dyspnoea | Not detected prior to the episode, heart failure accompanied the episode | Survived | 14 days |
| 8 | 10 | Male | Paraplegia | Not detected prior to the episode | Survived | Owner requested euthanasia 3 days later |
| 9 | 4.5 | Male | Paraplegia | Detected 27 months prior to the episode, decompensated 11 days prior to the episode | Euthanased | — |
| 10 | 14 | Male | Paraplegia, hypothermia, dyspnoea | Detected and decompensated 2 weeks prior to the episode, heart failure accompanied the episode | Euthanased | — |
| 11 | 12.5 | Male | Paraplegia, dyspnoea | Not detected prior to the episode, heart failure accompanied the episode | Euthanased | — |
| 12 | 3 | Male | Bilateral paresis | Not detected prior to the episode | Survived | Alive 3 years later |
| 13 | 12 | Male | Paraplegia | Not detected prior to the episode | Euthanased | — |
| 14 | 9 | Male | Left unilateral paresis | Not detected prior to the episode | Euthanased | — |
| 15 | 13.5 | Male | Bilateral paresis, dyspnoea | Not detected prior to the episode, heart failure accompanied the episode | Survived | Died 3 days later |
| 16 (1st) | 6 | Male | Right unilateral paresis, dyspnoea | Not detected prior to the episode, heart failure accompanied the episode | Survived | Re-embolised 3 weeks later |
| 16 (2nd) | 6 | Male | Bilateral paresis | Detected three weeks prior to the episode | Survived | Re-embolised 5 months later |
| 16 (3rd) | 6.5 | Male | Paraplegia, dyspnoea | Detected 6 months prior to the episode, heart failure accompanied the episode | Died | — |
| 17 | 7.5 | Male | Paraplegia, dyspnoea | Not detected prior to the episode, heart failure accompanied the episode | Died | — |
| 18 | 14 | Female | Paraplegia | Not detected prior to the episode | Euthanased | — |
| 19 | 13 | Female | Bilateral paresis, dyspnoea | Detected 6 weeks prior to the episode, heart failure accompanied the episode | Survived | Euthanased 15 months later |
| 20 | 3 | Female | Paraplegia, dyspnoea | Not detected prior to the episode, heart failure accompanied the episode | Died | — |
| 21 | 15 | Female | Paraplegia | Not detected prior to the episode | Euthanased | — |
| 22 | 15 | Female | Paraplegia | Not detected prior to the episode | Euthanased | — |
| 23 | 12 | Female | Paraplegia, dyspnoea | Not detected prior to the episode, heart failure accompanied the episode | Died | — |
| 24 | 8.5 | Male | Paraplegia, dyspnoea | Detected and decompensated 2 weeks prior to the episode, heart failure accompanied the episode | Euthanased | — |
| 25 | 2 | Male | Paraplegia | Not detected prior to the episode | Survived | Alive 27 months later |
| 26 | 14.5 | Female | Bilateral paresis, dyspnoea | Not detected prior to the episode, heart failure accompanied the episode | Survived | Euthanased 8 months later |
| 27 | 3 | Male | Paraplegia, dyspnoea | Not detected prior to the episode, heart failure accompanied the episode | Died | — |
| 28 | 3.5 | Male | Paraplegia, dyspnoea | Detected and decompensated 6 days prior to the episode, heart failure accompanied the episode | Died | — |
| 29 | 11 | Male | Paraplegia, dyspnoea | Detected 29 months prior to the episode, heart failure accompanied the episode | Euthanased | — |
| 30 | 3.5 | Male | Paraplegia, hypothermia | Not detected prior to the episode | Died | — |
| 31 (1st) | 8.5 | Male | Paraplegia, dyspnoea | Not detected prior to the episode, heart failure accompanied the episode | Survived | Re-embolised 1 year later |
| 31 (2nd) | 9.5 | Male | Paraplegia, cyanosis | Not detected prior to the episode, heart failure accompanied the episode | Survived | Alive 5 years later |
| 32 | 7 | Male | Bilateral paresis | Not detected prior to the episode | Survived | Euthanased 7 months later |
| 33 | 11 | Male | Paraplegia, dyspnoea | Not detected prior to the episode, heart failure accompanied the episode | Died | — |
| 34 | 5 | Male | Paraplegia, dyspnoea | Detected and decompensated 2 weeks prior to the episode, heart failure accompanied the episode | Died | — |
| 35 | 12 | Male | Bilateral paresis, dyspnoea | Not detected prior to the episode, heart failure accompanied the episode | Survived | Euthanased 7 days later |
| 36 | 9 | Male | Paraplegia, tachypnoea | Not detected prior to the episode | Euthanased | — |
| 37 | 16 | Male | Paraplegia, intense pain | Detected 7 days prior to the episode | Euthanased | — |
| 38 | 6 | Female | Paraplegia, dyspnoea | Not detected prior to the episode, heart failure accompanied the episode | Died | — |
| 39 | 4 | Female | Paraplegia, intense pain | Not detected prior to the episode | Died | — |
| 40 (1st) | 10 | Male | Right unilateral paresis | Not detected prior to the episode | Survived | Re-embolised 6 weeks later |
| 40 (2nd) | 10 | Male | Paraplegia | Not detected prior to the episode | Euthanased | — |
| 41 | 10 | Female | Paraplegia | Not detected prior to the episode | Died | — |
| 42 (1st) | 4 | Male | Bilateral paresis | Not detected prior to the episode | Survived | Re-embolised 39 months later |
| 42 (2nd) | 7.5 | Male | Paraplegia, dyspnoea | Detected 39 months prior to the episode, heart failure accompanied the episode | Euthanased | — |
| 43 | 6.5 | Male | Paraplegia, cold hindlimbs | Not detected prior to the episode | Died | — |
| 44 | 13 | Male | Right unilateral paresis | Not detected prior to the episode | Survived | Died 2 weeks later |
It is apparent from these data that practitioners should be aware of the risk of thromboembolism associated with decompensating heart disease. Every effort should be made to avoid elective procedures in cats with significant heart disease. Increased vigilance and more thorough thoracic auscultation, especially for subtle murmurs or gallop rhythms, are required on the part of the veterinarian in identifying pre-existing heart disease in cats. Various authors have suggested an association between the thromboembolic episode and congestive heart failure (Bonagura 1977, Bond & Fox 1984). This study concurs in that cats had accompanying heart failure in 51% of episodes. It was suggested that the stress of the thromboembolic episode can cause cardiovascular decompensation (Flanders 1986). Case 16 in this study is an interesting example of a cat that presented with concomitant cardiac decompensation on both his first and third episode, but not during his second episode.
In three cases vomiting preceded the thromboembolic episodes. It is possible that the Valsalva manoeuvre, in which positive pressure in the chest is followed by a sudden negative pressure, could have led to the dislodgement of thrombi, as reported in humans by Kollef & Neelon-Kollef (1991). Holzworth et al (1955) reported that two out of the 11 cats in their study experienced vomiting episodes. Suter (1983) also mentioned vomiting as a prodromal sign.
Another feature, previously unreported, was the apparent seasonal prevalence noted. Only 10% of the thromboembolic episodes occurred in the summer months (June, July and August). The peak prevalence (43%) of the episodes was noticed during the spring (March, April and May). This is the season in which interaction amongst the cat population is high. Factors such as increased interaction associated with territorial aggression might have played a role. It was postulated previously by Buchanan et al (1966) that the higher incidence in males might be due to their propensity to be involved in cat fights. This apparent seasonal prevalence could however have been affected by many non-disease factors and further study is necessary in this field to evaluate this observation fully.
The diagnosis of aortic thromboembolism was made on clinical presentation and although corroborative data such as ultrasonographic demonstration of the thrombi were not available, there can be little doubt that a cat presenting with the constellation of previously described clinical signs was suffering from the disorder described. The progression of the partial episodes and the concomitant heart disease shown ruled out other differentials such as intervertebral disc disease and spinal neoplasia.
Radiological examination was the most common diagnostic test performed in these cases. This, in part, reflects the attending clinicians' familiarity with this modality. It was performed to rule out trauma (although scuffed toenails and bruising are usually apparent in these cases) and as an attempt to assess concomitant cardiovascular and pulmonary abnormalities. Radiology is currently the modality of choice for the assessment of pulmonary vascular status and an adjunct to echocardiography in assessing overall cardiac size. In one cat (Case 42) heart size was normal when the cat suffered its first episode and markedly enlarged when the cat suffered the second episode 3 years later. This underlines the fact that thromboembolism is not necessarily a feature of advanced heart disease.
In this study, only 17% of cats on aspirin therapy suffered recurrent episodes. The two cases that did re-embolise on aspirin therapy support the findings of Schaub et al (1982), which suggested that cats suffering a thromboembolic episode whilst on aspirin therapy, at doses high enough to inhibit platelet function, recover more quickly due to efficient collateral circulation. However, one cat on frusemide therapy also made a full recovery, and the author is not aware of any direct anti-thrombotic effect of frusemide. There would appear to be no real advantage in using warfarin rather than aspirin for prophylaxis. In a previous paper, 32% of cases died during warfarin treatment. This treatment was also associated with intensive monitoring (Harpster & Baty 1995). Furthermore, warfarin therapy was associated with a 43% recurrence rate.
The commitment of the owner is one factor that has not been alluded to in previous reports. The author has found that considerable home care, which can be required for prolonged periods, is necessary in some of these cases. Two cats (Cases 5 and 25) that survived complete bilateral paralysis episodes took months to recover. The neurological deficits persisted for more than 6 months. Both of these cats had to have external coaptation placed on both hindlimbs for the first 2 to 3 weeks. This was to prevent excoriative damage due to dragging of the dorsal aspects of the metatarsi on the ground. The cranial tibial reflex was the last of the local reflexes to return to normal in both cases and concurs with the findings of Griffiths & Duncan (1979). Inability to flex the hocks, due to gastrocnemius contracture, contributed to this. One of the cats (Case 25) suffered a fracture of the proximal diaphysis of the tibia, in the region of the nutrient foramen, a few months after the episode. Callus formation was very slow to form and it resembled a pathological fracture radiologically. It is postulated that the reduced blood supply and ischaemic damage probably contributed to this fracture.
The study reported here specifically addressed survival statistics, to give veterinary colleagues in first opinion practice accurate data to present to their clients. A marked difference was noted in the survival of the complete versus the partial episodes. Cats survived 93% of the partial episodes and only 15% of the complete episodes. The overall survival rate of 39% in this study compares very favourably with the 37% survival rate in a referral institution (Laste & Harpster 1995). The survival rate of 36% in cases that presented with concomitant heart failure was very similar to the overall survival rate, which indicated that concomitant heart failure does not necessarily worsen the prognosis in the acute stage. Only 28% of the cats in this study died naturally during the first 36 h after the onset of the episode. Although the author acknowledges that many of the cases where euthanasia was performed were either moribund or had severe signs, there is a tendency to view this as a fatal disorder and to recommend euthanasia prematurely. The overall survival rate could thus have been higher.
Experience with these cases would suggest that, provided suffering can be minimised with the proper use of analgesics, these cases may be treated successfully. As previously suggested by Bonagura (1994), and Holzworth et al (1955): ‘If the patient's primary heart lesion does not cause death, the peripheral disturbance is in time overcome’.
Conclusions
From this study, the following conclusions can be made: cases of partial thromboembolism carry a much more favourable prognosis than cases with complete episodes of thromboembolism. Prophylactic treatment with aspirin appeared to provide comparable survival times to those previously reported with warfarin treatment. Cats that suffered decompensation of their heart disease appeared to be at increased risk of thromboembolism. The most common potential risk for thromboembolism suggested by this study was the anaesthesia or sedation of a patient with underlying heart disease. Survival rates in this study from the UK were similar to those previously reported in the USA.
Acknowledgements
I would like to extend my gratitude to Mr ME Herrtage for his invaluable advice, proof-reading and encouragement in preparing the manuscript. I would also like to thank my colleagues at The Veterinary Hospital, Bishops Stortford for providing their part of the case material and in particular Mrs Fiona Robinson-Pelah MRCVS, whose diligent and extensive clinical notes were a great help.
References
- Bonagura JD. (1977) Feline cardiovascular emergencies. Veterinary Clinics of North America 7, 403. [DOI] [PubMed] [Google Scholar]
- Bonagura JD. (1994) Aortic thrombosis. In: Sherding RD. (ed.), The Cat: Diseases and Clinical Management, 2nd edn. New York, Churchill Livingstone, pp. 920–925. [Google Scholar]
- Bond BR, Fox PR. (1984) Advances in feline cardiomyopathy. Veterinary Clinics of North America 14, 1021–1038. [DOI] [PubMed] [Google Scholar]
- Buchanan JW, Baker GJ, Hill JD. (1966) Aortic embolism in cats: Prevalence, surgical treatment and electrocardiography The Veterinary Record 79, 496–506. [DOI] [PubMed] [Google Scholar]
- Butler HC. (1971) An investigation into the relationship of an aortic embolus to posterior paralysis in the cat. Journal of Small Animal Practice 12, 141–158. [DOI] [PubMed] [Google Scholar]
- Flanders J. (1986) Feline aortic thromboembolism. Compendium of Continuing Education for the Practicising Veterinarian 8, 478–484. [Google Scholar]
- Fox PR. (1988) Feline myocardial disease. In: Fox PR. (ed.), Canine and Feline Cardiology. New York, Churchill Livingstone, pp. 454–465. [Google Scholar]
- Fox PR. (1991) Evidence for and against efficacy of beta-blockers and aspirin for management of feline cardiomyopathies. Veterinary Clinics of North America: Small Animal Practice 21, 1011–1022. [DOI] [PubMed] [Google Scholar]
- Green CE. (1985) Aspirin and feline platelet aggregation. American Journal of Veterinary Research 46, 1820. [PubMed] [Google Scholar]
- Griffiths IR, Duncan ID. (1979) Ischaemic neuromyopathy in cats. The Veterinary Record 104, 518–522. [DOI] [PubMed] [Google Scholar]
- Harpster NK. (1977) Feline cardiomyopathy. Veterinary Clinics of North America 7, 355–360. [DOI] [PubMed] [Google Scholar]
- Harpster NK, Baty CJ. (1995) Warfarin therapy of the cat at risk of thromboembolism. In: Bonagura JD. (ed.), Kirk's Current Veterinary Therapy XII. Philadelphia, W.B. Saunders, pp. 868–873. [Google Scholar]
- Holzworth J, Simpson R, Wind A. (1955) Aortic thrombosis with posterior paralysis in the cat. The Cornell Veterinarian 45, 468–487. [PubMed] [Google Scholar]
- Imhoff RK. (1961) Production of aortic occlusion resembling acute aortic embolism syndrome in cats. Nature 192, 979–980. [Google Scholar]
- Klein MK, Dow SW, Rosychuk RAW. (1989) Pulmonary embolism associated with immune-mediated haemolytic anaemia in dogs: Ten cases (1982–1987). Journal of the American Veterinary Medical Association 195, 246–250. [PubMed] [Google Scholar]
- Kollef MH, Neelon-Kollef RA. (1991) Pulmonary embolism associated with the act of defecation. Heart and Lung 20, 451–454. [PubMed] [Google Scholar]
- Laste NJ, Harpster NK. (1995) A retrospective study of 100 cases of feline distal aortic thromboembolism: 1977–1993. Journal of the American Animal Hospital Association 31, 492–500. [DOI] [PubMed] [Google Scholar]
- Lucke VM, Sumner-Smith G. (1966) Aortic embolism in the cat. The Veterinary Record 79, 236–239. [DOI] [PubMed] [Google Scholar]
- Olmstead ML, Butler HC. (1977) Five-hydroxytryptamine antagonists and feline aortic embolism. Journal of Small Animal Practice 18, 247–259. [DOI] [PubMed] [Google Scholar]
- Pion PD, Kittleson MD. (1989) Therapy for feline aortic thromboembolism. In: Kirk RW. (ed.), Kirk's Current Veterinary Therapy X. Philadelphia, W.B. Saunders, pp. 295–302. [Google Scholar]
- Pouchelon J-L, Chetboul V, Devauchelle P, Delisle F, Mai W, Vial V. (1998) Diagnosis of pulmonary thromboembolism in a cat using echocardiography and pulmonary scintigraphy Journal of Small Animal Practice 38, 306–310. [DOI] [PubMed] [Google Scholar]
- Schaub RG, Gates KA, Roberts RE. (1982) Effects of aspirin on collateral blood flow after experimental thrombosis of the feline aorta. American Journal of Veterinary Research 43, 1647–1650. [PubMed] [Google Scholar]
- Sisson DD, Thomas WP. (1995) Myocardial diseases. In: Ettinger SJ, Feldman EC. (eds.), Textbook of Veterinary Internal Medicine, 4th edn. Philadelphia, W.B. Saunders, pp. 995–1032. [Google Scholar]
- Suter PF. (1983) Diseases of the peripheral vessels. In: Ettinger SJ. (ed.), Textbook of Veterinary Internal Medicine Diseases of the Dog and Cat, 2nd edn. Philadelphia, W.B. Saunders, p. 1062. [Google Scholar]
- Van der Linde-Sipman, Hellebrekers LJ, Lagerwey E. (1992) Myocardial damage in cats that died after anaesthesia. Veterinary Quarterly 15, 1–4. [DOI] [PubMed] [Google Scholar]