Abstract
Practical relevance: Feline idiopathic inflammatory bowel disease (IBD) denotes one form of chronic enteropathy that is immunologically mediated and characterized by persistent or recurrent gastrointestinal (GI) signs and histologic inflammation. Signs of vomiting, diarrhea and weight loss generally predominate, and mucosal inflammation may occur in any portion of the GI tract (especially the small intestine). Affected cats may also have concurrent inflammation in other organs, such as the pancreas and liver, which may impact clinical disease severity.
Clinical challenges: The exact etiologies of this heterogeneous group of disorders have yet to be determined, though results from basic science and clinical studies suggest that interplay between genetic factors and enteric bacteria is crucial for disease development. The diagnosis is one of exclusion and requires intestinal mucosal biopsy to characterize the type and severity of the inflammatory infiltrate, and to differentiate IBD from other disorders, including alimentary lymphoma. Controversy exists concerning the relative diagnostic accuracy of endoscopic versus full-thickness specimens for the diagnosis of IBD and its differentiation from alimentary lymphoma.
Audience: This article is intended to provide veterinary practitioners with a comprehensive clinical update on idiopathic IBD in cats. It reviews the current evidence-based data, the diagnostic approach, the evolving histologic criteria, and treatment options and outcome for feline patients with this syndrome.
Current understanding of etiology and pathogenesis
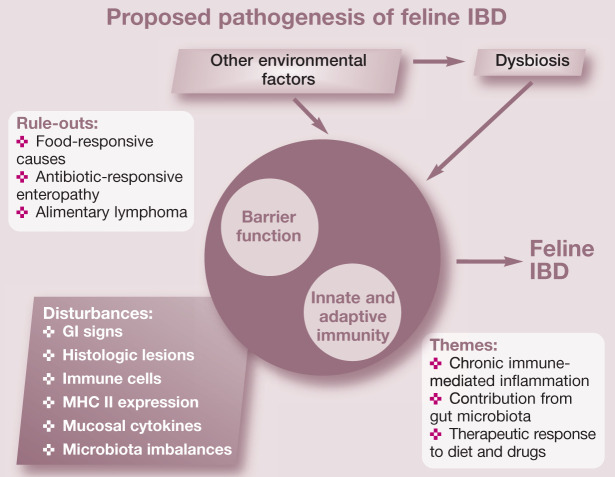
While the exact cause of inflammatory bowel disease (IBD) remains unknown, current hypotheses suggest that feline IBD, similar to IBD in humans and dogs, involves complex interactions between environmental factors (ie, intestinal microbial imbalances, dietary components) and the mucosal immune system, resulting in chronic inflammation in susceptible cats (Figure 1). 1
Figure 1.
Chronic intestinal inflammation in feline IBD involves a complex interplay between the mucosal immune system and the enteric microbiota in a genetically susceptible host. Potential genetic factors affecting barrier function or innate and adaptive immunity may predispose susceptible cats to gastrointestinal (GI) signs, aberrant host responses and microbial imbalances (dysbiosis). Environmental factors (dietary constituents, exposure to enteropathogens, NSAID or antibiotic administration, etc) likely govern inflammation onset or reactivation (disease flares)
Genetic defects in the recognition of commensal versus pathogenic bacteria by the innate immune system play a pivotal role in disease pathogenesis in humans and dogs with IBD. Mutations in innate immune receptors of humans (NOD2/CARD 15) and dogs (TLR4, TLR5) have now been linked to IBD susceptibility; and in the presence of an enteric microbiota may lead to upregulated proinflammatory cytokine production (eg, IL-17, TNF-α) and reduced bacterial clearance, thereby promoting chronic intestinal inflammation.1–3 Whether or not similar pathomechanisms come into play in the development of idiopathic feline IBD has not been fully determined. However, antigens derived from commensal bacteria are likely to be important in driving disease pathogenesis, as increased populations of mucosa-associated bacteria (ie, Enterobacteriaceae) have been linked to clinical signs, cytokine mRNA and histopathologic lesions in cats with IBD. 4
Other studies have reported increased lamina propria myeloid/histiocyte antigen-positive macrophages, upregulated epithelial major histocompatibility complex (MHC) class II molecule expression, and increased antibody reactivity to components of the commensal microbiota associated with chronic intestinal inflammation. 5 Moreover, a potential role for dietary constituents is suggested by the clinical benefit of dietary therapy in some cats with IBD. While a genetic basis for IBD in some cat breeds is suspected, causal genetic defects have not been identified to date.
Taken together, these studies suggest that chronic intestinal inflammation of IBD may be due to overly aggressive T cell responses to enteric bacteria (or fungi) 6 in hosts with genetic defects that regulate microbial killing, mucosal barrier function or immune responses. Environmental factors likely govern inflammation onset or reactivation and modulate genetic susceptibility to disease. Future studies are needed to identify possible genetic predispositions in certain feline breeds that contribute to IBD development.
Further unraveling of the pathogenesis of IBD should facilitate the development of novel and more specific treatment options (eg, use of prebiotics or probiotics) for cats. This will require additional clinical trials (and more research funding) to elucidate which therapies are most efficacious in the prevention and treatment of chronic IBD.
Signalment, history and clinical presentation
The clinical manifestations of IBD are diverse and are influenced by the organ(s) involved, the presence of active disease, and nutritional (cobalamin) deficiencies.7–9 Feline IBD predominantly affects middle-aged animals but may also occur in cats 2 years of age or less. 10 Certain breed predispositions (Siamese and other Asian breeds) for IBD are recognized but any breed may be affected. 11
Vomiting and small intestinal diarrhea are most commonly observed and are often accompanied by decreased appetite and weight loss. Gastric and duodenal inflammation is usually associated with vomiting and small bowel diarrhea, while colonic involvement causes large bowel diarrhea with blood, mucus and straining. Cats with both small and large bowel involvement are usually evaluated for diffuse GI disease. Some cats exhibit concurrent inflammatory disease involving the liver or pancreas (feline inflammatory disease [FID], ‘triaditis’) that also contributes to clinical findings. 12
The clinical course of IBD is generally cyclical and is characterized by spontaneous exacerbations and remissions. Triggers for recurring signs are rarely identified but may include dietary indiscretion, transient exposure to intestinal pathogens or drug (eg, steroids, NSAIDs, antibiotics) administration. 8 Importantly, the clinical signs of IBD are not disease specific and share numerous overlapping features with other feline disorders.
Diagnostic approach to idiopathic feline IBD
A diagnosis of IBD is one of exclusion and requires careful elimination of IBD mimics. The possible causes for chronic intestinal inflammation may be excluded through the integration of history, physical findings, clinicopathologic testing, diagnostic imaging and histopathology of intestinal biopsies (see box on page 446). Importantly, a baseline complete blood count (CBC), biochemistry profile, serum total T4, urinalysis and survey abdominal radiographs are useful in eliminating the most common systemic and metabolic disorders (eg, renal disease, hepatopathy, hyperthyroidism) causing chronic GI signs in cats.
After the exclusion of infectious/parasitic agents, non-GI disorders, exocrine pancreatic insufficiency and intestinal structural abnormalities requiring surgery, the most common groups of intestinal diseases associated with chronic small bowel diarrhea include food-responsive enteropathy (FRE), idiopathic feline IBD and alimentary lymphoma (Table 1). A dietary trial using an antigen-restricted diet fed exclusively for at least 7 days will effectively rule out adverse food reactions causing vomiting and diarrhea in cats. 13 Differentiation of severe IBD from well-differentiated lymphoma may be especially problematic in cats. Diagnostic strategies that employ the endoscopic collection of ileal mucosal biopsies, the procurement of multi-organ biopsies during laparotomy, and/or the use of immunohistochemistry for B cell/T cell markers or polymerse chain reaction (PCR) for clonal expansion of lymphocytes may help to distinguish IBD from alimentary lymphoma.14–16
Table 1.
Comparative features of feline IBD, food-responsive enteropathy and alimentary lymphoma
| Feline IBD | Food-responsive enteropathy | Alimentary lymphoma | |
|---|---|---|---|
| Signalment | Predominantly middle-aged cats | Young cats | Middle-aged to older cats |
| Siamese breed at risk? | No breed predisposition | No breed predisposition | |
| Clinical signs of illness | Lethargy, weight loss, inappetence, vomiting, diarrhea | Large bowel diarrhea, ± weight loss, ± cutaneous lesions (alopecia) | Lethargy, weight loss, inappetence, vomiting, diarrhea, ± icterus |
| Clinical course | Progessive signs or cyclical flares | Progessive signs or cyclical flares | Progessive signs |
| Physical examination findings | May be normal, ± thickened bowel loops, abdominal pain (cholangitis/pancreatitis) | Often normal, ± alopecia | May be normal, thickened bowel loops, ± palpable masses |
| Diagnostic evaluation | Rule out non-GI causes for clinical signs; FNA cytology of mesenteric lymph nodes or masses; intestinal biopsy for definitive diagnosis | Rule out GI parasites, perform dietary trial | Rule out non-GI causes for clinical signs; fine-needle aspirate (FNA) cytology of mesenteric lymph nodes or masses; intestinal biopsy for definitive diagnosis |
| Potential pitfalls of diagnostic testing | False negatives when enlarged lymph nodes are present; differentiation from alimentary lymphoma may be difficult; cholangitis or pancreatitis may be concurrent | None; clinical response to elimination diet confirms diagnosis | False negatives when enlarged lymph nodes are present; differentiation from feline IBD may be difficult; may require immunophenotyping or PCR for confirmation |
Defining the presence and extent of inflammatory disease in other organs, such as the liver and pancreas, is aided by the performance of baseline laboratory tests (showing increased liver enzyme activities), specialized serologies (eg, increased feline pancreatic lipase immunoreactivity [fPLI] or thyroxine [T4], abnormal bile acids) and diagnostic imaging, especially abdominal ultrasound. There is no specific formula for the detection of these sometimes elusive disorders; rather, clinicians must rely on the completeness of their physical examination and the presence of laboratory abnormalities that are localizing to individual organs. Tissue biopsy is then the most definitive means of confirming a specific diagnosis.
Abdominal radiography is most helpful in defining extra-alimentary tract disorders causing gastroenteritis. Survey radiography might detect organomegaly (liver, kidney) unrelated to IBD or intestinal obstruction that might cause similar GI signs. Abdominal ultrasound is superior to radiology in defining focal versus diffuse mucosal disease, loss of layering, intestinal thickening and mesenteric lymphadenopathy seen with IBD as well as other infiltrative (eg, lymphoma) disorders. 17 Older cats with ultrasonographic evidence of muscularis propria thickening are more likely to have lymphoma than IBD. 18
Cats with severe small intestinal disease (ie, frequent severe diarrhea, decreased activity/appetite, excessive weight loss) often have decreased serum cobalamin concentration.19,20 Serum concentrations of both folate and cobalamin should be measured as they may identify the need for supplementation and guide disease localization (ie, folate is absorbed in the duodenum and cobalamin is absorbed in the ileum). Failure to recognize and correct hypocobalaminemia can delay clinical recovery even with specific therapy for IBD. 9 A subset of cats with chronic enteropathy (ie, IBD or FRE) will have mild-to-moderate elevations in fPLI suggestive of pancreatitis; however, this observation does not appear to be associated with a negative outcome. 21 Pancreatitis is an elusive diagnosis and clinicians should integrate all clinical data (including repeat measurements of fPLI if possible) to arrive at the most accurate diagnosis.
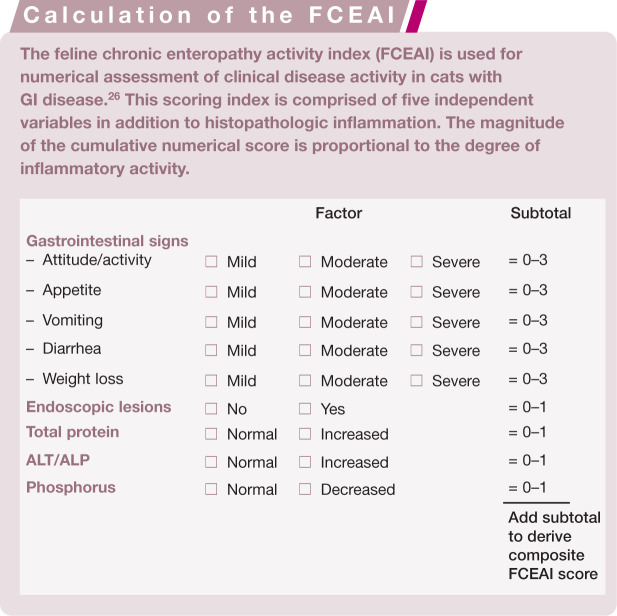
Index of inflammatory activity
The measurement of clinical disease activity by means of quantifiable indices is well established in humans22,23 and dogs24,25 with IBD. An index for the assessment of inflammatory activity in cats with chronic enteropathy has been recently designed. The feline chronic enteropathy activity index (FCEAI) is comprised of five independent variables (along with histopathologic inflammation) which can be temporally assessed and compared when collected at different times (see box above). 26 Similar to other indices, the magnitude of the numerical score is proportional to the degree of inflammatory activity. This index serves as the principal measure of response to a therapeutic trial and may be used to tailor medical therapy for an individual patient’s need. Recent controlled studies indicate that the FCEAI is of value in defining disease activity in cats with either IBD or FRE.
Intestinal biopsy and histopathologic evaluation
Intestinal biopsies are required to confirm histopathologic inflammation and to determine the extent of mucosal disease. The decision about which organs to biopsy is largely dictated by clinical signs – with anorexia, weight loss, vomiting and small bowel diarrhea suggesting the need for gastric and small intestinal mucosal biopsies, while large bowel signs (ie, straining, fresh blood and mucoid feces) indicate the need for colonic mucosal biopsies. Cats with diffuse enteric disease will exhibit mixed bowel signs and require biopsy of both the small and large intestines for diagnosis. The author’s preference is for diagnostic endoscopy, as this technique allows for direct assessment of mucosal abnormalities and the acquisition of targeted biopsy specimens. Biopsy guidelines for maximizing diagnostic yield of endoscopic specimens in cats have recently been published (Table 2).27,28 Celiotomy with collection of mucosal biopsies from the stomach and intestines may be performed when GI endoscopy is not available. Caution is urged when full-thickness biopsies of the colon are collected due to the risk of bacterial contamination and subsequent infection.
Table 2.
Endoscopic guidelines for cats with chronic enteropathy
| Site | Minimum number of biopsies required | |
|---|---|---|
| Stomach | Mild cellular infiltrate | 4 |
| Moderate cellular infiltrate | 6 | |
| Duodenum | Blunt villi | 6 |
| Mild cellular infiltrate | 4 | |
| Moderate cellular infiltrate | 4 | |
| Ileum | Increased cellular infiltrate | 3 |
| Colon | Increased cellular infiltrate | 6 |
Clinicians should obtain this minimum number of samples per organ when performing GI endoscopy. It is assumed that tissue samples are at least marginal for histopathologic interpretation. 28 Increased mucosal cellularity is graded as described elsewhere. 30 Multiple colonic regions should be biopsied to maximize diagnostic yield
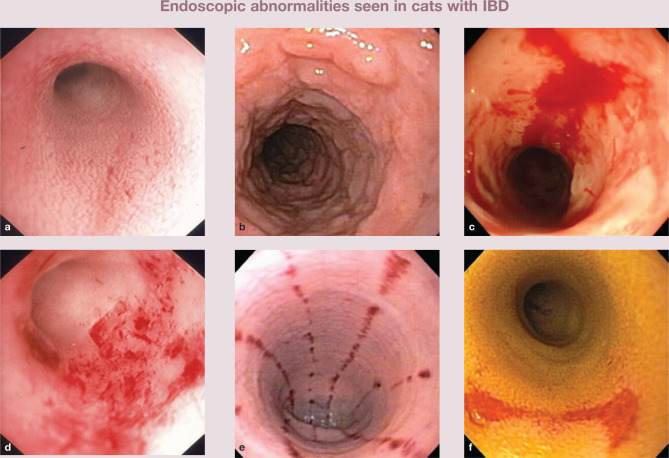
The association between endoscopic lesions and disease activity has been investigated to only to a limited extent. While endoscopic abnormalities may or may not correlate with outcome in dogs with IBD,24,29 in cats with IBD endoscopic abnormalities do correlate with both clinical activity and histopathologic lesions at diagnosis (Figure 2).4,26 Standard mucosal biopsies of the stomach and duodenum alone may occasionally miss more distal sites (eg, ileal mucosa) of cellular infiltration. Therefore, it is suggested that ileal biopsies should be obtained in cats to increase diagnostic yield whenever gastroduodenoscopy or colonoscopy is performed, especially since lymphoma is an important differential diagnosis.30,31 The need to perform ileoscopy may also be guided by the presence of hypocobalaminemia as cobalamin is absorbed in the ileum.
Figure 2.
Increased granularity (a,b), mucosal friability (c) and erosions (d,e,f) are associated with GI signs and histopathologic lesions in affected cats
Histopathology
The rationale for performing intestinal biopsy with microscopy is to arrive at a definitive diagnosis with the potential for assessing long-term outcome. For example, the prognosis with IBD is generally good to excellent for control of GI signs and a normal life span. Conversely, cats with alimentary lymphoma, mast cell tumors or infectious causes for their GI signs will have a guarded-to-poor prognosis, which may be aggravated by a delayed or even missed correct diagnosis. A general treatment approach using prednisolone/metronidazole/prednisolone with chlorambucil (for potential lymphoma) might result in short-term remission but may mask more aggressive and potentially costly (ie, multiple clinician visits and repeat testing) disease.
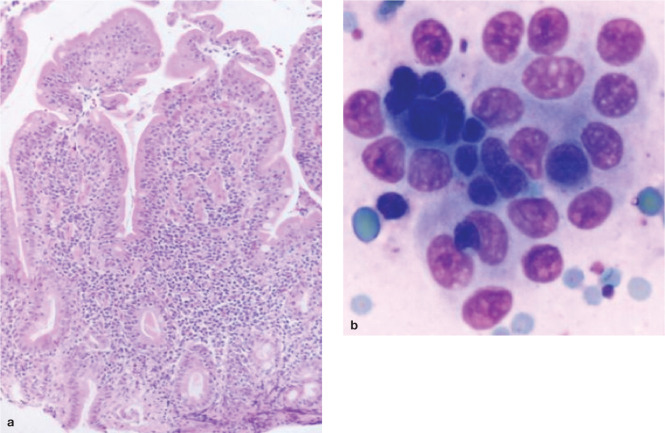
The microscopic findings in IBD consist of minimal to pronounced inflammatory cell infiltration of the gastric and/or intestinal mucosa accompanied by varying degrees of mucosal architectural disruption (Figure 3). Unfortunately, biopsy interpretation is notoriously subjective and suffers from extensive interobserver variability, as well as technical constraints of specimen size and procurement/processing artefacts inherent in evaluation of endoscopic specimens.32,33
Figure 3.
(a) Histopathologic inflammation in cases of feline IBD is predominantly lymphoplasmacytic in character, as seen in this endoscopic duodenal specimen. (b) Exfoliative cytology performed in the same cat shows a cluster of small lymphocytes embedded within a raft of normal small intestinal (epithelial) cells. This cytologic appearance is consistent with lymphocytic enteritis
Several grading systems for evaluation of endoscopic biopsies have been proposed but no standardized evaluation of intestinal histopathologic findings has been validated with respect to disease severity or outcome. One attempt to standardize interpretation of GI inflammation between pathologists has resulted in the design of a histology monograph that defines numerous morphologic and inflammatory features in endoscopic biopsies. 30 However, even with this standardized scheme there has been considerable disagreement between pathologists; moreover, simply summing unweighted numerical severity scores did not accurately reflect the microscopic severity in dogs with severe IBD (granulomatous colitis). 34 A subsequent analysis of these parameters has resulted in development of a ‘simplified model system’ for defining intestinal inflammation of IBD that is presently being tested in a separate clinical trial.
Histopathologic lesions of IBD are subjectively classified based on the predominant cellular infiltrate within the lamina propria. Intestinal infiltration with macrophages or neutrophils raises the possibility of an infectious process, and culture, special stains and/or fluorescence in situ hybridization (FISH) are indicated. While FISH is limited in its availability to a few academic/diagnostic laboratories, this molecular technique may be of clinical value by confirming the presence of increased mucosa-associated bacteria (with or without accompanying neutrophilic infiltration), which are best treated with antibiotics and/or probiotics. The presence of moderate to large numbers of eosinophils in intestinal biopsies (which, in the author’s experience, may be accompanied by mild circulating eosinophilia) suggests possible parasitic infestation or dietary intolerance. Increased numbers of lymphocytes and plasma cells –so-called ‘lymphoplasmacytic enteritis’ (LPE) – is the most frequently reported form of feline IBD.10,35,36 However, the appropriateness and clinical relevance of the term lymphocytic/plasmacytic enteritis is a contentious issue. For example, LPE may also be associated with intestinal parasites, dietary sensitivity and feline hyperthyroidism. 8 Moreover, cats with and without signs of intestinal disease have similar numbers of lymphocytes and plasma cells in tissues. 5 In hyperthyroid cats with LPE, successful treatment (via I131) of the hyperthyroidism has resulted in remission of clinical signs but follow-up biopsies were not performed to assess for eradication of mucosal inflammation (author’s unpublished observations).
Recent studies indicate that changes in mucosal architecture, such as villous morphology and fibrosis, are related to the presence and severity of GI disease. These studies have used quantitative observer-independent variables (eg, proinflammatory cytokines, mucosal bacteria) to identify histopathological correlates of disease. In cats with signs of GI disease, villus atrophy and fusion correlated with severity of clinical signs and degree of proinflammatory cytokine upregulation in the duodenal mucosa. In a separate investigation, histopathologic inflammation was correlated with clinical signs, endoscopic lesions (ie, mucosal friability, granularity and/or erosions), and clinicopathologic abnormalities (ie, increased total protein concentration, elevated ALT/ALP activities, hypophosphatemia, hypocobalaminemia, and/or increased fPLI) in cats with IBD. 26
Surgical versus endoscopically obtained biopsies
Biopsies for histopathologic diagnosis of IBD are obligatory and they may be obtained endoscopically (mucosal sample) or by laparoscopy or exploratory laparotomy (full-thickness sample). Controversy exists concerning the relative diagnostic accuracy of endoscopic versus full-thickness specimens for the diagnosis of IBD and alimentary lymphoma. Making a correct diagnosis is further complicated by the fact that these disorders share a variety of overlapping features (eg, clinical signs, physical examination findings, abdominal ultrasonographic abnormalities and histopathologic lesions) and that chronic mucosal inflammation (eg, gastric Helicobacter heilmannii infection) may progress to lymphoma in some instances. 40
Endoscopic biopsy specimens of the stomach and duodenum were considered inadequate compared with full-thickness biopsies for differentiating IBD from lymphoma in one study. 14 However, duodenal (endoscopic) assessment was limited to only 50% of the cats and mucosal biopsy was performed blindly (with only three specimens obtained per cat) in 8/22 (36%) of the cats. Because none of the cats in this study had endoscopic biopsy of the ileum performed, malignant infiltrates in this organ could only be confirmed in full-thickness specimens obtained by laparotomy. In another study, the likelihood for diagnosis of alimentary lymphoma was greatest in cats undergoing multi-organ biopsy from all segments of the intestine and the mesenteric lymph nodes. 15 Unfortunately, comparative data describing endoscopic biopsy results from different intestinal segments in these cats was not provided.
Overall, these studies emphasize that the quality of the endoscopic procedure and the adequacy of intestinal biopsies significantly impact accuracy in the diagnosis of feline lymphoma. Suboptimal endoscopic examination and failure to obtain adequate numbers of good quality specimens does not critically evaluate the diagnostic accuracy of thorough duodenoscopy and ileoscopy. 16 The ileum seems to be a consistently affected organ and should always be biopsied when lymphoma or IBD is suspected.14,31 Biopsy samples may be obtained by either ileoscopic examination (most ideal) using a smaller diameter endoscope or by passing the biopsy forceps blindly through the ileocolic sphincter (Figure 4). Histopathologic lesions may vary considerably between ileal and duodenal samples within the same animal.31,41 Finally, laparoscopy may be a useful procedure when full-thickness tissues are required, as this procedure is less invasive and has a shorter recovery time compared with laparotomy.
Figure 4.
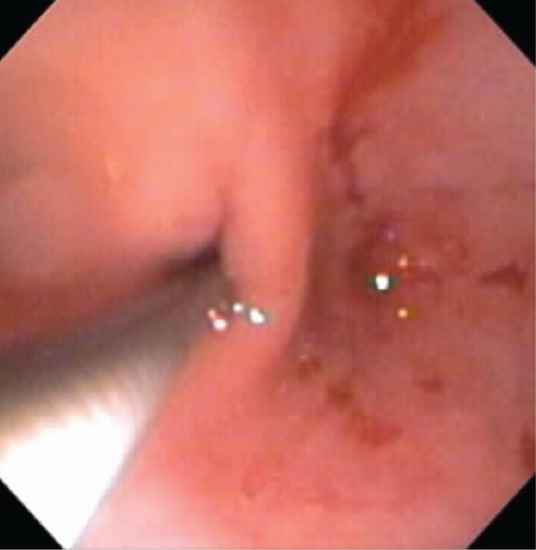
The ileum is a common site for GI inflammation in cats. Whenever possible, ileal biopsies should be procured by either direct mucosal assessment (ie, ileoscopy) or by passing the endoscope forceps blindly through the ileocolic sphincter
Cats in which there is a suspicion of multi-organ disease (see below) may be managed by celiotomy or laparoscopy, with biopsies of the liver and/or pancreas obtained along with full-thickness intestinal biopsies. Alternatively, GI endoscopy with procurement of hepatic biopsies aided by ultrasound guidance should detect inflammatory lesions affecting these organs.
Multiple organ inflammatory disease
Multi-organ inflammatory disease (ie, FID, triaditis) involving the liver, pancreas and possibly the kidneys has been previously reported with IBD. 12 Cats are at increased risk of the development of pancreatitis and cholangitis by virtue of the fact that the pancreatic duct enters the common bile duct before it opens into the proximal duodenum. This unique feline anatomy allows for reflux of bacteria (eg, Escherichia coli or other intestinal bacteria) and intestinal contents (eg, bile, pancreatic enzymes) into the pancreatic and biliary system. Moreover, small intestinal inflammation (ie, IBD) may also ascend the common bile duct and affect the pancreas and biliary tree due to the high bacterial numbers present in the duodenum. 42
In one retrospective study of 78 cats that had necropsy examinations of their liver, intestine, pancreas and kidney, the prevalence of IBD (15/18; 83%) and pancreatitis (9/18; 50%) was greater for cats with cholangiohepatitis compared with cats with and without lymphocytic portal hepatitis. 12 Thirty-nine percent of cats (7/18) with cholangiohepatitis had IBD and pancreatitis. Other investigators using similar retrospective approaches have shown that concurrent hepatic lipidosis and pancreatitis may be correlated with negative outcome, 43 and that subnormal serum cobalamin concentrations are common in cats with GI disorders affecting the small intestine, liver and/or pancreas. 20 A separate investigation evaluated histologic sections of 40 cats with acute pancreatitis and showed that numerous biochemical abnormalities suggestive of hepatic disease were present, including increased ALT and ALP activities and increased concentrations of bilirubin and cholesterol. 44 However, only mild histopathologic changes were observed in the intestine (13/40; 33%) and liver (5/40; 13%) of these cats.
Taken together as a whole, these studies indicate a potential causal association between small intestinal inflammation, pancreatitis and hepatic disease in cats. However, these earlier data were derived from retrospective studies, have focused on distinct subsets of cats, have used different indices for diagnosis of organ-specific disease, and have not confirmed a distinct temporal relationship between pancreatic, hepatic and intestinal inflammation (namely, that they occurred simultaneously). Furthermore, alterations in tissue morphology do not always indicate disturbances in organ function or the presence of clinically significant inflammation.45,46
More contemporary clinical reports indicate that some cats with IBD have pancreatic inflammation, as evidenced by increased fPLI concentrations and abnormalities in the pancreas detected on diagnostic imaging. While cats with IBD might have increased serum fPLI concentrations, this association does not appear to influence clinical outcome based on a recent report. 21 Clinicians should assume that there is a reasonable association between inflammatory diseases of the liver and pancreas in cats, while the association with intestinal inflammation is more tenuous.
Treatment options for cats with IBD
Nutritional therapy
The rationale for dietary therapy using an antigen-restricted or hydrolyzed diet is that restricting exposure to dietary antigens known to evoke sensitivity will reduce exaggerated host responses and attenuate intestinal inflammation. Other indications for specialized nutritional therapies include managing cats with a decreased appetite, impaired nutrient absorption or specific nutrient (eg, cobalamin) deficiencies.

The benefits of nutritional therapy (alone or in conjunction with pharmacologic therapy) in the clinical management of IBD are well documented. In one study, dietary therapy alone was successful in approximately 50% of referred cats with idiopathic IBD. 13 Moreover, most cats in this study responded quickly (within 2–3 days), suggesting that shorter dietary trials (minimum 7 days’ duration) are quite acceptable to gauge clinical response. While evidence-based observations indicate that most cats respond favorably to dietary intervention, the superiority of one novel protein source versus another, or the advantage in feeding an intact protein elimination diet versus a hydrolyzed protein elimination diet, has not been shown to date.
Characteristics of an ideal diet for IBD are that it should contain a novel intact (white fish, duck, venison, etc) or hydrolyzed protein source and a highly digestible carbohydrate, and be gluten-free, low in lactose and fiber, nutritionally balanced and highly palatable. 11 Traditionally, low-fat diets have been recommended in cats with chronic diarrhea in the belief that fat absorption is impaired in these patients and can aggravate clinical signs. The role of dietary fat in cats with chronic enteropathy has recently been questioned since low-versus high-fat diets resulted in a similar improvement in fecal scores. 49 A variety of commercial diets fulfills these requirements and is readily available.
Soluble fiber supplementation (ie, psyllium dosed at ¼ teaspoon at each meal) or the use of fiber-enriched diets such as Iams (Eukanuba) Low Residue cat food, which contains beet pulp, is useful in cats with colitis. Modifying the diet with omega-3 polyunsaturated fatty acids (PUFAs) may also modulate inflammatory responses by reducing the production of pro-inflammatory metabolites, such as leukotriene B4.50,51 Note that dosing is empirical in cats (ie, eicosapentaenoic acid at 17–25 mg/kg/day and docosahexaenoic acid at 8–18 mg/kg/day) and is extrapolated from dosages of enteric-coated PUFAs used in the treatment of human IBD. 52 Supplementation with parenteral cobalamin (250 µg/cat given subcutaneously weekly for 4–6 weeks while treatment for IBD is ongoing) is advised if serum concentrations are subnormal. A positive response to cobalamin treatment will include improved appetite, weight gain and a reduction in vomiting/diarrheic episodes in affected cats. 9
Pharmacologic therapy
Drug therapy for IBD includes the use of corticosteroids, antibiotics and various immunosuppressive agents. Practical drug treatment recommendations are determined by the clinical severity of disease, the segments of the GI tract involved, the character of the histopathologic lesions, and micronutrient (cobalamin) status. While some clinicians utilize a sequential therapeutic approach with diet and drugs, others propose concurrent therapy with diet, antibiotics, steroids and/or immunosuppressive drugs in cats with severe disease. The optimal drug or drug combination, as well as duration of therapy for induction and maintenance of remission of clinical signs, have not been determined for most protocols. 8

Glucocorticoids
Clinical data evaluating the efficacy of drug therapy for feline IBD is derived from only a few large case-based investigations. In separate studies it was shown that prednisolone alone (n = 14 cats) or used in combination with tylosin or sulfasalazine (n = 14 cats) resulted in resolution of clinical signs in cats with gastroenteritis or IBD colitis.35,36 Prednisone used alone or in combination with another drug resulted in partial or complete resolution of clinical signs in 39/47 (83%) of diseased cats in a different study. 54 A more recent trial reported excellent clinical responses with attenuation of GI signs and disease activity (ie, FCEAI scores) in cats with IBD treated with prednisolone as a single drug induction agent. 26
Taken together, these observations would suggest that corticosteroids such as prednisone and prednisolone are effective pharmacologic agents for treating IBD.
Metronidazole
Metronidazole has been advocated as a single drug agent or in combination with glucocorticoids for treatment of IBD. 8 The mechanisms of action of metronidazole in reducing intestinal inflammation might include antiprotozoan and antibacterial (including efficacy against mucosa-associated Clostridium species) activities, 4 and possibly immunomodulatory effects. 55 Metronidazole may be associated with poor patient compliance due to its bitter taste and tendency to promote inappetence. An alternative formulation is metronidazole benzoate, which is better tolerated by cats possibly due to a marked difference in taste. 52 As this latter formulation is only about 60% metronidazole by weight, it is dosed at a higher oral concentration to reach an equivalent amount of drug to 15 mg/kg of metronidazole, as shown in humans. 56
Caution is urged when using metronidazole as a long-term drug for feline IBD since it has been associated with the development of neoplasms in rodents 57 and in a subset of Crohn’s disease patients. 58
Chorambucil
Chlorambucil (an alkylating agent that crosslinks DNA) may also be used as an adjunct drug for IBD that is refractory to standard (ie, diet and prednisolone) therapy, for cats with severe lymphoplasmacytic IBD, or for cats with severe lymphocytic IBD that is difficult to differentiate from well-differentiated lymphoma. Chlorambucil is very well tolerated by most cats and is minimally myelosuppressive. It is ideally dosed at 2 mg/cat, administered q48–72h, although an alternative dosing regimen of 20 mg/m2 given every 14 days has also been used. 16 Clinicians should perform periodic monitoring (CBCs every 3 months) of cats receiving this 20 mg/m2 protocol.
Ciclosporin
Ciclosporin, an immunosuppressive agent used to treat steroid-refractory IBD in dogs, may also be effective in treating cats with refractory IBD. This drug’s actions include inhibition of T cell function and IL-2 production, which may reduce chronic intestinal inflammation. 59 Unfortunately, only anecdotal reports of clinical efficacy in feline IBD (at a dosage of 5 mg/kg q24h) are available.
Prebiotics and probiotics
Increasing evidence supports a potential therapeutic role for prebiotic and probiotic agents in human IBD. 60 If IBD in cats is similarly driven by impaired tolerance to components of the intestinal microbiota then prebiotics and probiotics may prove beneficial as primary therapies or in combination with dietary and drug therapy. A comparison of prebiotic and probiotic preparations is outlined in Table 3.
Table 3.
Prebiotics and probiotics presently in use for humans and cats with GI disorders
| Prebiotics | Probiotics | |
|---|---|---|
| Definition | Non-digestible carbohydrates that stimulate growth of protective enteric bacteria when fed | Live microorganisms which, given in adequate amounts, confer health benefits to the host |
| Examples | Fructo-oligosaccharides | E coli Nissle 1917 |
| Galacto-oligosaccharides | VSL #3 | |
| Inulin | Lactobacillus species | |
| Lactulose | Bifidobacterium species | |
| Psyllium | Saccharomyces boulardii | |
| Bran | Prostora Max (Iams)* | |
| Beet pulp, pumpkin | FortiFlora (Purina)* | |
| Resistant starch | Proviable-DC (Nutramax)* | |
| Protective role |
|
|
NB Controlled clinical trials attesting to the efficacy of either prebiotic or probiotic nutraceutical preparations for treatment of cats with idiopathic IBD have not been reported
Commercial products currently on the US market
There are only two published studies evaluating the effects of prebiotics on the intestinal microbiota in healthy dogs and cats. In one study, fructo-oligosaccharides (FOSs) supplemented at 0.75% dry matter produced qualitative and quantitative changes in the fecal flora of healthy cats. 61 Compared with samples from cats fed a basal diet, increased numbers of lactobacilli and Bacteroides species and decreased numbers of E coli were associated with the FOS diet. However, bacteriologic examination of the duodenal juice in these same cats showed wide variation in the composition of the duodenal flora across sampling periods, which was not affected by FOS supplementation. 62 Moreover, healthy Beagle dogs fed a 1% FOS diet over a 3-month trial showed inconsistent fecal excretion of Lactobacillus species and Bifidobacterium species. 63
Very little has been reported to date on the use of probiotics to treat IBD in dogs and cats. Recent in vitro studies have confirmed the capacity of a lyophilized probiotic cocktail (eg, three different Lactobacillus species strains) to modulate the expression of regulatory versus pro-inflammatory cytokines in dogs with chronic enteropathies. 64 However, a clinical trial using this same probiotic cocktail in dogs with food-responsive diarrhea failed to induce consistent patterns of regulatory (eg, beneficial) cytokine expression in spite of obvious clinical improvement. 65 One commercial probiotic (ie, FortiFlora – Enterococcus faecium strain SF68) was reported to be beneficial in controlling diarrhea in cats housed in an animal shelter. 66 Recently, a second probiotic (ie, Prostora Max – consisting of Bifidobacterium animalis strain AHC7) was shown to provide more rapid resolution of acute diarrhea than placebo in dogs. 67
Importantly, results from controlled clinical trials evaluating the efficacy of prebiotic or probiotic therapy in cats with IBD have not been reported. The author judiciously uses probiotics as an adjunctive agent along with diet and drug therapy in cats with severe clinical disease. A trial of at least 6–8 weeks’ duration is recommended and probiotic therapy may be continued indefinitely to maintain remission and to prevent relapse of signs.
Outcome
Little information is available regarding long-term outcome for cats with chronic intestinal inflammation. While many cats with IBD respond favorably to dietary and immunosuppressive therapy, treatment failures may still occur. Some of the more common reasons for inadequate clinical response include failure to adhere to dietary recommendations, the presence of severe intestinal inflammation, the possibility of concurrent disease (eg, hepatopathy, pancreatitis, occult hyperthyroidism, or hypocobalaminemia), and a missed diagnosis of GI lymphoma. 68 Low serum cobalamin concentration is one variable that has been associated with refractoriness to treatment in cats with chronic enteropathy. Both the FCEAI and serum acid glycoprotein (an acute phase protein indicative of inflammation) concentration have been shown to decrease in cats successfully treated for IBD or FRE, suggesting that acid glycoprotein may be suitable for laboratory evaluation of the effect of therapy in affected cats. 69
Footnotes
Funding: The author received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this review article.
The author declares that there is no conflict of interest.
Key Points
Feline idiopathic IBD denotes a heterogeneous group of chronic, relapsing inflammatory disorders of the GI tract that are immunologically mediated.
While their exact etiologies remain unknown, results from basic science and clinical studies suggest that interplay between genetic factors and enteric bacteria is crucial for disease development, owing to abnormal host responses directed against the commensal microbiota.
Key clinical signs include vomiting, diarrhea and weight loss, and histopathologic lesions of inflammation may involve the stomach, small intestine or colon. Affected cats may also have concurrent inflammation in other organs, such as the pancreas and liver, which may impact clinical disease severity.
Clinicians should assume there is a reasonable association between inflammatory diseases of the liver and pancreas in cats; the association with intestinal inflammation is more tenuous.
Controversy exists concerning the relative diagnostic accuracy of endoscopic and fullthickness specimens for diagnosis of IBD and alimentary lymphoma. The ileum seems to be a consistently affected organ and should always be biopsied when lymphoma or IBD is suspected.
Treatment principles for biopsy-proven feline IBD are empirical and consist of combination therapy using dietary and pharmacologic interventions. In general, an approach using sequential treatment with diet, select antibiotics and/or glucocorticoids has proven successful in most case studies.
References
- 1. Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis 2009; 22: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007; 448: 427–434. [DOI] [PubMed] [Google Scholar]
- 3. Kathrani A, House A, Catchpole B, Murphy A, German A, Werling D, et al. Polymorphisms in the TLR4 and TLR5 gene are significantly associated with inflammatory bowel disease in German Shepherd dogs. PLoS One 2010; 5: e15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janeczko S, Atwater D, Bogel E, Greiter-Wilke A, Gerold A, Baumgart M, et al. The relationship of mucosal bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet Microbiol 2008; 128: 178–193. [DOI] [PubMed] [Google Scholar]
- 5. Waly NE, Stokes CR, Gruffydd Jones TJ, Day MJ. Immune cell populations in the duodenal mucosa of cats with inflammatory bowel disease. J Vet Intern Med 2004; 18: 816–825. [DOI] [PubMed] [Google Scholar]
- 6. Suchodolski JS, Morris EK, Allenspach K, Jergens AE, Harmoinen JA, Westermarck E, et al. Prevalence and identification of fungal DNA in the small intestine of healthy dogs and dogs with chronic enteropathies. Vet Microbiol 2008; 132: 379–388. [DOI] [PubMed] [Google Scholar]
- 7. Simpson K, Jergens A. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet Clin North Am Small Anim Pract 2011; 41: 381–398. [DOI] [PubMed] [Google Scholar]
- 8. Jergens AE. Inflammatory bowel disease. Current perspectives. Vet Clin North Am Small Anim Pract 1999; 29: 501–521, vii. [PubMed] [Google Scholar]
- 9. Ruaux CG, Steiner JM, Williams DA. Early biochemical and clinical responses to cobalamin supplementation in cats with signs of gastrointestinal disease and severe hypocobalaminemia. J Vet Intern Med 2005; 19: 155–160. [DOI] [PubMed] [Google Scholar]
- 10. Jergens AE, Moore FM, Haynes JS, Miles KG. Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987–1990). J Am Vet Med Assoc 1992; 201: 1603–1608. [PubMed] [Google Scholar]
- 11. Guilford WG. Idiopathic inflammatory bowel diseases. In: Guilford WG. (ed). Strombeck’s small animal gastroenterology. 3rd ed. Philadelphia: WB Saunders; 1996, pp 451–486. [Google Scholar]
- 12. Weiss DJ, Gagne JM, Armstrong PJ. Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis, and nephritis in cats. J Am Vet Med Assoc 1996; 209: 1114–1116. [PubMed] [Google Scholar]
- 13. Guilford WG, Jones BR, Markwell PJ, Arthur DG, Collett MG, Harte JG. Food sensitivity in cats with chronic idiopathic gastrointestinal problems. J Vet Intern Med 2001; 15: 7–13. [DOI] [PubMed] [Google Scholar]
- 14. Evans SE, Bonczynski JJ, Broussard JD, Han E, Baer KE. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc 2006; 229: 1447–1450. [DOI] [PubMed] [Google Scholar]
- 15. Kleinschmidt S, Harder J, Nolte I, Marsilio S, Hewicker-Trautwein M. Chronic inflammatory and non-inflammatory diseases of the gastrointestinal tract in cats: diagnostic advantages of full-thickness intestinal and extraintestinal biopsies. J Feline Med Surg 2010; 12: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gieger T. Alimentary lymphoma in cats and dogs. Vet Clin North Am Small Anim Pract 2011; 41: 419–432. [DOI] [PubMed] [Google Scholar]
- 17. Gaschen L. Ultrasonography of small intestinal inflammatory and neoplastic diseases in dogs and cats. Vet Clin North Am Small Anim Pract 2011; 41: 329–344. [DOI] [PubMed] [Google Scholar]
- 18. Zwingenberger AL, Marks SL, Baker TW, Moore PF. Ultrasonographic evaluation of the muscularis propria in cats with diffuse small intestinal lymphoma or inflammatory bowel disease. J Vet Intern Med 2010; 24: 289–292. [DOI] [PubMed] [Google Scholar]
- 19. Reed N, Gunn-Moore D, Simpson K. Cobalamin, folate and inorganic phosphate abnormalities in ill cats. J Feline Med Surg 2007; 9: 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simpson KW, Fyfe J, Cornetta A, Sachs A, Strauss-Ayali D, Lamb SV, et al. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med 2001; 15: 26–32. [DOI] [PubMed] [Google Scholar]
- 21. Bailey S, Benigni L, Eastwood J, Garden OA, McMahon L, Smith K, et al. Comparisons between cats with normal and increased fPLI concentrations in cats diagnosed with inflammatory bowel disease. J Small Anim Pract 2010; 51: 484–489. [DOI] [PubMed] [Google Scholar]
- 22. Best WR, Becktel JM, Singleton JW, Kern F., Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976; 70: 439–444. [PubMed] [Google Scholar]
- 23. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980; 1: 514. [DOI] [PubMed] [Google Scholar]
- 24. Allenspach K, Wieland B, Grone A, Gaschen F. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med 2007; 21: 700–708. [DOI] [PubMed] [Google Scholar]
- 25. Jergens AE, Schreiner CA, Frank DE, Niyo Y, Ahrens FE, Eckersall PD, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med 2003; 17: 291–297. [DOI] [PubMed] [Google Scholar]
- 26. Jergens AE, Crandell JM, Evans R, Ackermann M, Miles KG, Wang C. A clinical index for disease activity in cats with chronic enteropathy. J Vet Intern Med 2010; 24: 1027–1033. [DOI] [PubMed] [Google Scholar]
- 27. Washabau RJ, Day MJ, Willard MD, Hall EJ, Jergens AE, Mansell J, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010; 24: 10–26. [DOI] [PubMed] [Google Scholar]
- 28. Willard MD, Mansell J, Fosgate GT, Gualtieri M, Olivero D, Lecoindre P, et al. Effect of sample quality on the sensitivity of endoscopic biopsy for detecting gastric and duodenal lesions in dogs and cats. J Vet Intern Med 2008; 22: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 29. Garca-Sancho M, Rodriguez-Franco F, Sainz A, Mancho C, Rodríguez A. Evaluation of clinical, macroscopic, and histopathologic response to treatment in nonhypoproteinemic dogs with lymphocytic-plasmacytic enteritis. J Vet Intern Med 2007; 21: 11–17. [DOI] [PubMed] [Google Scholar]
- 30. Day MJ, Bilzer T, Mansell J, Wilcock B, Hall EJ, Jergens A, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008; 138: S1–S43. [DOI] [PubMed] [Google Scholar]
- 31. Scott KD, Zoran DL, Mansell J, Norby B, Willard MD. Utility of endoscopic biopsies of the duodenum and ileum for diagnosis of inflammatory bowel disease and small cell lymphoma in cats. J Vet Intern Med 2011; 25: 1253–1257. [DOI] [PubMed] [Google Scholar]
- 32. Willard MD, Moore GE, Denton BD, Day MJ, Mansell J, Bilzer T, et al. Effect of tissue processing on assessment of endoscopic intestinal biopsies in dogs and cats. J Vet Intern Med 2010; 24: 84–89. [DOI] [PubMed] [Google Scholar]
- 33. Willard M, Jergens A, Duncan R, Leib MS, McCracken MD, DeNovo RC, et al. Interobserver variation among histopathologic evaluations of intestinal tissues from dogs and cats. J Am Vet Med Assoc 2002; 220: 1177–1182. [DOI] [PubMed] [Google Scholar]
- 34. Mansfield CS, James FE, Craven M, Davies DR, O’Hara AJ, Nicholls PK, et al. Remission of histiocytic ulcerative colitis in Boxer dogs correlates with eradication of invasive intramucosal Escherichia coli. J Vet Intern Med 2009; 23: 964–969. [DOI] [PubMed] [Google Scholar]
- 35. Dennis JS, Kruger JM, Mullaney TP. Lymphocytic/plasmacytic colitis in cats: 14 cases (1985–1990). J Am Vet Med Assoc 1993; 202: 313–318. [PubMed] [Google Scholar]
- 36. Dennis JS, Kruger JM, Mullaney TP. Lymphocytic/plasmacytic gastroenteritis in cats: 14 cases (1985–1990). J Am Vet Med Assoc 1992; 200: 1712–1718. [PubMed] [Google Scholar]
- 37. Hendrick M. A spectrum of hypereosinophilic syndromes exemplified by six cats with eosinophilic enteritis. Vet Pathol 1981; 18: 188–200. [DOI] [PubMed] [Google Scholar]
- 38. Wilcock B. Endoscopic biopsy interpretation in canine or feline enterocolitis. Semin Vet Med Surg (Small Anim) 1992; 7: 162–171. [PubMed] [Google Scholar]
- 39. Leib MS, Sponenberg DP, Wilcke JR, Loar AS. Suppurative colitis in a cat. J Am Vet Med Assoc 1986; 188: 739–741. [PubMed] [Google Scholar]
- 40. Bridgeford EC, Marini RP, Feng Y, Parry NMA, Rickman B, Fox JG. Gastric Helicobacter species as a cause of feline gastric lymphoma: a viable hypothesis. Vet Immunol Immunopathol 2008; 123: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Casamian-Sorrosal D, Willard MD, Murray JK, Hall EJ, Taylor SS, Day MJ. Comparison of histopathologic findings in biopsies from the duodenum and ileum of dogs with enteropathy. J Vet Intern Med 2010; 24: 80–83. [DOI] [PubMed] [Google Scholar]
- 42. Johnston K, Lamport A, Batt RM. An unexpected bacterial flora in the proximal small intestine of normal cats. Vet Rec 1993; 132: 362–363. [DOI] [PubMed] [Google Scholar]
- 43. Akol KG, Washabau RJ, Saunders HM, Hendrick MJ. Acute pancreatitis in cats with hepatic lipidosis. J Vet Intern Med 1993; 7: 205–209. [DOI] [PubMed] [Google Scholar]
- 44. Hill RC, Van Winkle TJ. Acute necrotizing pancreatitis and acute suppurative pancreatitis in the cat. A retrospective study of 40 cases (1976–1989). J Vet Intern Med 1993; 7: 25–33. [DOI] [PubMed] [Google Scholar]
- 45. Weiss D, Gagne JM, Armstrong PJ. Characterization of portal lymphocytic infiltrates in feline liver. Vet Clin Pathol 1995; 24: 91–95. [DOI] [PubMed] [Google Scholar]
- 46. De Cock HEV, Forman MA, Farver TB, Marks SL. Prevalence and histopathologic characteristics of pancreatitis in cats. Vet Pathol 2007; 44: 39–49. [DOI] [PubMed] [Google Scholar]
- 47. Kuhbacher T, Fölsch UR. Practical guidelines for the treatment of inflammatory bowel disease. World J Gastroenterol 2007; 13: 1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jergens AE, Crandell J, Morrison JA, Deitz K, Pressel M, Ackermann M, et al. Comparison of oral prednisone and prednisone combined with metronidazole for induction therapy of canine inflammatory bowel disease: a randomized-controlled trial. J Vet Intern Med 2010; 24: 269–277. [DOI] [PubMed] [Google Scholar]
- 49. Laflamme DP, Xu H, Long GM. Effect of diets differing in fat content on chronic diarrhea in cats. J Vet Intern Med 2011; 25: 230–235. [DOI] [PubMed] [Google Scholar]
- 50. Park HJ, Park JS, Hayek MG, Reinhart GA, Chew BP. Dietary fish oil and flaxseed oil suppress inflammation and immunity in cats. Vet Immunol Immunopathol 2011; 141: 301–306. [DOI] [PubMed] [Google Scholar]
- 51. Li Q, Zhang Q, Wang M, Zhao S, Xu G, Li J. n-3 polyunsaturated fatty acids prevent disruption of epithelial barrier function induced by proinflammatory cytokines. Mol Immunol 2008; 45: 1356–1365. [DOI] [PubMed] [Google Scholar]
- 52. Trepanier L. Idiopathic inflammatory bowel disease in cats. Rational treatment selection. J Feline Med Surg 2009; 11: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lichtenstein G, Abreu M, Cohen R, Tremaine W; American Gastroenterological Association. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006; 130: 935–939. [DOI] [PubMed] [Google Scholar]
- 54. Hart JR, Shaker E, Patnaik AK, Garvey MS. Lymphocytic-plasmacytic enterocolitis in cats: 60 cases (1988–1990). J Am Anim Hosp Assoc 1994; 30: 505–514. [Google Scholar]
- 55. Arndt H, Palitzsch KD, Grisham MB, Granger DN. Metronidazole inhibits leukocyte–endothelial cell adhesion in rat mesenteric venules. Gastroenterology 1994; 106: 1271–1276. [DOI] [PubMed] [Google Scholar]
- 56. Alestig K, Freij L, Arnold E. Absorption and excretion of metronidazole after administration of metronidazole benzoate mixture. Scand J Infect Dis 1980; 12: 149–152. [DOI] [PubMed] [Google Scholar]
- 57. A-Kareem AM, Fleiszer DM, Richards GK, Senterman MK, Brown RA. Effect of long-term metronidazole (MTZ) therapy on experimental colon cancer in rats. J Surg Res 1984; 36: 547–552. [DOI] [PubMed] [Google Scholar]
- 58. Krause JR, Ayuyang HQ, Ellis LD. Occurrence of three cases of carcinoma in individuals with Crohn’s disease treated with metronidazole. Am J Gastroenterol 1985; 80: 978–982. [PubMed] [Google Scholar]
- 59. Allenspach K, Rfenacht S, Sauter S, Gröne A, Steffan J, Strehlau G, et al. Pharmacokinetics and clinical efficacy of cyclosporine treatment of dogs with steroid-refractory inflammatory bowel disease. J Vet Intern Med 2006; 20: 239–244. [DOI] [PubMed] [Google Scholar]
- 60. Ewaschuk JB, Dieleman LA. Probiotics and prebiotics in chronic inflammatory bowel diseases. World J Gastroenterol 2006; 12: 5941–5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sparkes AH, Papasouliotis K, Sunvold G, Werrett G, Gruffydd-Jones EA, Egan K, et al. Effect of dietary supplementation with fructo-oligosaccharides on fecal flora of healthy cats. Am J Vet Res 1998; 59: 436–440. [PubMed] [Google Scholar]
- 62. Sparkes AH, Papasouliotis K, Sunvold G, Werrett G, Clarke C, Jones M, et al. Bacterial flora in the duodenum of healthy cats, and effect of dietary supplementation with fructo-oligosaccharides. Am J Vet Res 1998; 59: 431–435. [PubMed] [Google Scholar]
- 63. Willard MD, Simpson RB, Cohen ND, Clancy JS. Effects of dietary fructooligosaccharide on selected bacterial populations in feces of dogs. Am J Vet Res 2000; 61: 820–825. [DOI] [PubMed] [Google Scholar]
- 64. Sauter SN, Allenspach K, Gaschen F, Gröne A, Ontsouka E, Blum JW. Cytokine expression in an ex vivo culture system of duodenal samples from dogs with chronic enteropathies: modulation by probiotic bacteria. Domest Anim Endocrinol 2005; 29: 605–622. [DOI] [PubMed] [Google Scholar]
- 65. Sauter SN, Benyacoub J, Allenspach K, Gaschen F, Ontsouka E, Reuteler G, et al. Effects of probiotic bacteria in dogs with food responsive diarrhoea treated with an elimination diet. J Anim Physiol Anim Nutr (Berl) 2006; 90: 269–277. [DOI] [PubMed] [Google Scholar]
- 66. Bybee SN, Scorza AV, Lappin MR. Effect of the probiotic Enterococcus faecium SF68 on presence of diarrhea in cats and dogs housed in an animal shelter. J Vet Intern Med 2011; 25: 856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kelley RL, Minikhiem D, Kiely B, O’Mahony L, O’Sullivan D, Boileau T, et al. Clinical benefits of probiotic canine-derived Bifidobacterium animalis strain AHC7 in dogs with acute idiopathic diarrhea. Vet Ther 2009; 10: 121–130. [PubMed] [Google Scholar]
- 68. Jergens AE. Managing the refractory case of feline IBD. J Feline Med Surg 2003; 5: 47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jergens AE, Crandell JM, Morrison JA, et al. Serum acute phase proteins in feline inflammatory bowel disease [abstract]. J Vet Intern Med 2007; 21: 612. [Google Scholar]