Abstract
Practical relevance: The feline eosinophilic granuloma complex (EGC) comprises a group of clinically well recognised but poorly understood dermatoses that are common in cats. In many cases, lesions are severe and can be accompanied by varying degrees of (and sometimes considerable) pruritus and/or pain. In addition, lesions can be chronic and recurrent. It is, therefore, important to achieve a prompt and accurate diagnosis in order to provide optimal, often life-long, treatment for affected cats.
Patient group: There is no age predisposition or well documented breed predilection for the development of EGC lesions in cats. Some studies have reported a possible female predisposition, but this has not been consistently documented.
Clinical challenges: The clinical diagnosis of EGC lesions is usually straightforward, but investigation of the potential underlying aetiology can pose a challenge for the clinician. Information on the indication for various diagnostic tests and their interpretation is lacking, and the tendency for these cases to be managed with chronic medical intervention prior to achieving a definitive diagnosis can further complicate the interpretation of any diagnostic investigation. In addition, successful therapeutic management of these cases can be challenging. Some cats suffer only a single episode of disease that resolves with treatment, while others have recurrent lesions and some of these can be refractory to treatment. The individual variation in both the clinical nature of the disease and the response to therapy could be related to disease severity, but could also be explained by differences in the underlying aetiopathogenesis.
Evidence base: This article reviews the published literature to discuss the complex aetiology of the EGC and present an overview of the different clinical presentations and diagnosis. A further and particular aim has been to provide some evidence-based recommendations for the management of this unusual group of dermatoses.
Component lesions of the EGC
The feline eosinophilic granuloma complex (EGC) presents as a number of distinct clinical lesions, although their histopathological features are similar. Importantly, the EGC does not represent a specific dermatological diagnosis and numerous aetiological factors have been proposed as potential causes.1,2 The lesions are thought to represent a group of cutaneous reaction patterns to primary, underlying disease processes, most commonly hypersensitivity disorders. The lesions included in the EGC are indolent (or eosinophilic) ulcers, eosinophilic plaques and eosinophilic granulomas.
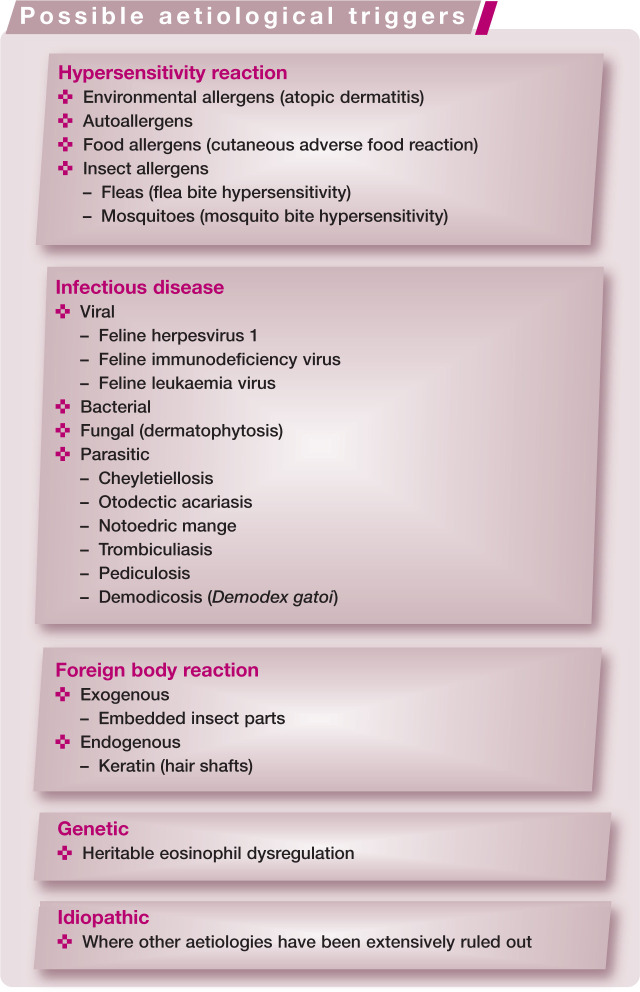
Aetiology and pathogenesis
A number of studies have attempted to elucidate the aetiopathological mechanisms underlying the development of the EGC. Most authors now recognise the EGC as a manifestation of feline allergic disease,2–9 but alternative aetiologies have also been investigated (see box on page 472).

It has been suggested that some eosinophilic granulomas affecting the oral cavity are due to embedded insect parts, 3 but this is not a commonly recognised histological feature. Mosquito bite hypersensitivity, a well recognised eosinophilic dermatosis that occurs at the site of mosquito bite inoculation,3,5,10 is generally considered a separate disease entity from the EGC. 11
Other reports have suggested that infectious agents are involved in the aetiology of the EGC. The evidence includes cytological and histological identification of bacteria within eosinophilic granulomas of the oral cavity, eosinophilic plaques and indolent ulcers. 12 EGC lesions, however, predispose tissue to bacterial colonisation and infection, and it is likely that the role of bacteria is secondary. Nevertheless, it is important to perform cytology in all cases and, where necessary, bacterial culture and antibiotic sensitivity testing, to identify the presence of bacterial infection. In one recent study of 16 cats with indolent ulcers and/or eosinophilic plaques, all lesions had cytological evidence of infection. Treatment with 62.5 mg of clavulanate–amoxicillin for 21 days resulted in a significant improvement in plaque lesions, with a mean reduction in lesion size of 96.2% (mean 65%). There was a mean 42.6% decrease in lesion size for the indolent ulcers, but this was not significant. 13
The inability to autologously transmit eosinophilic plaques to different locations on the body in five cats led one study to conclude that these lesions are not transmissible and not caused by an infectious agent. 14 Clinical and histological similarities between EGC and feline viral dermatoses have generated suspicion of an underlying viral aetiology, and a single case report of a cat with eosinophilic granuloma lesions identified virus particles. 15 However, two recent retrospective studies showed that feline herpesvirus 1 (FHV-1) was rare in EGC lesions;16,17 where it was identified it probably represented a histological misdiagnosis. FHV-1 is, therefore, unlikely to be an aetiological factor in the EGC.
A retrospective study of 19 cats with EGC lesions revealed that 68% had circulating antibodies to components of normal cat epithelium. This could suggest that the EGC has an autoimmune pathogenesis; however, the autoantibodies could also be secondary to epithelial damage. 18 Another study documenting the presence of anti-tissue antibodies came to the same conclusion. 14
EGC lesions occur commonly with allergic skin diseases in cats. These include flea bite hypersensitivity, cutaneous adverse food reaction and atopic dermatitis. In a study of experimentally induced flea bite hypersensitivity, 5/8 cats that developed clinical signs consistent with flea bite hypersensitivity also developed indolent ulcers. 9 Also, flea bite hypersensitivity is considered the most common cause of eosinophilic plaques in areas where fleas are present. 5 In 1995, Roosje and Willemse suggested an allergic aetiology by showing that a heat-stabile cytophilic antibody is involved in the pathogenesis of EGC lesions. 19 Further studies have documented similar immunopathological changes within lesional skin to those seen in canine and human atopic dermatitis.8,20,21
In addition to flea saliva, food proteins and environmental allergens, feline self-allergens have been proposed as a cause of EGC lesions. In human atopic dermatitis, specific IgE against human proteins (autoallergens) and exogenous (environmental) allergens can be demonstrated. 22 One study suggested that Felis domesticus allergen 1 (Fel d 1; the major allergen in human cat allergy) is an autoallergen in cats with the EGC. 23 The study demonstrated a T lymphocyte response, similar to that seen in human allergic inflammation, following exposure of cats with EGC lesions to Fel d 1. Autoallergens in human atopic dermatitis, however, are intracellular proteins that are released from cells following inflammation and self-trauma. Fel d 1, in contrast, is a protein found in cat saliva. It may be possible that Fel d 1 is implanted into deeper skin layers following excessive grooming of abnormal skin and is able to act in a similar way to exposed intracellular autoallergens in human disease. Nevertheless, further investigations are needed to verify the role of Fel d 1 in the EGC.
What can we conclude?
To date, most evidence points towards the EGC having an underlying allergic aetiology. There are, however, lesions that do not respond to parasite control trials or elimination diets and where allergen-specific IgE cannot be demonstrated serologically or by intradermal allergy testing. It may be possible that these lesions represent similar conditions to canine atopic-like dermatitis or human intrinsic atopic dermatitis.
Single episodes of EGC lesions and those that spontaneously resolve seem unlikely to be caused by allergic disease, as most hypersensitivity disorders cause recurrent, long-term disease. A heritable eosinophil dysregulation, predisposing cats to the development of eosinophilic granuloma and indolent ulcer, was suggested by a study in a colony of related, specific pathogen-free cats that frequently developed lesions. 24 Investigations for allergic disease, including intradermal allergy testing, were negative, but lesions were reported to wax and wane and had seasonal exacerbations, which may suggest the presence of an environmental trigger. Another report of eosinophilic granuloma and indolent ulcer in 17 related Norwegian Forest cats further supported a genetic background to the EGC. 25 More genetic studies are required to confirm these preliminary findings.
The exact aetiopathogenesis of the EGC remains unclear. However, enough evidence exists to warrant investigation of an underlying hypersensitivity disorder in affected cats, and it is important to approach these cases as such until proven otherwise.
Diagnosis
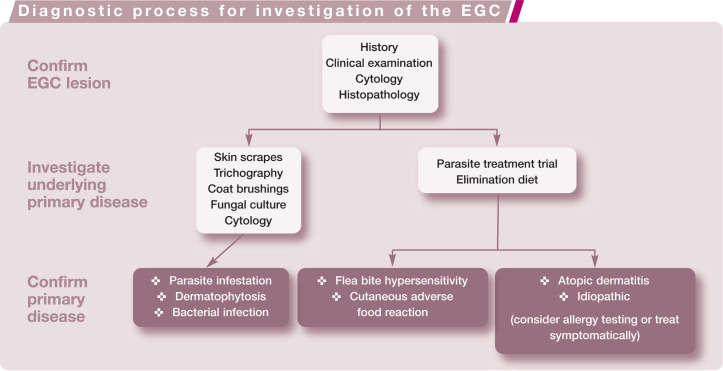
The investigation should focus on confirming the presence of an EGC lesion and investigating the primary underlying disease (see box below). Treatment of EGC lesions may need to be started before definitive diagnosis of the underlying disease is confirmed, but it is very important to pursue this diagnosis, particularly in recurrent cases.
Confirming the EGC lesion
Clinical signs and a cytological evaluation demonstrating large numbers of eosinophils (Figure 5) are highly suggestive of an EGC lesion. As the lesions are usually associated with an underlying allergy, differentiation between the individual lesions is of little importance diagnostically and is unlikely to change the therapeutic plan.2,5 However, ulcers, plaques, nodules or tumours may need to be biopsied and submitted to a dermatohistopathologist to rule out the non-allergic differential diagnoses, particularly viral disease and neoplasia. If bacterial or fungal infection is suspected, additional tissue should be submitted for routine and/or specialist culture. Biopsies can be performed under sedation plus local anaesthesia or general anaesthesia. Ideally, each lesion should be biopsied using a 6 mm biopsy punch and submitted in individual formalin pots with accompanying history. In sites where closure could be a problem, 4 mm punches may be more appropriate.
Figure 5.
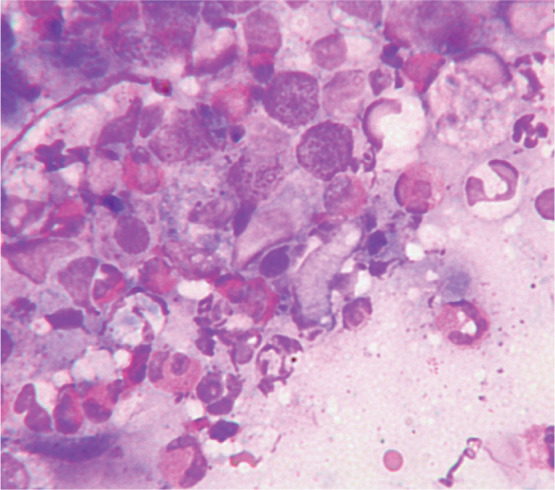
Cytology showing neutrophils, macrophages and numerous eosinophils from a cat with an eosinophilic granuloma. Rapi-Diff II stain; x1000 magnification
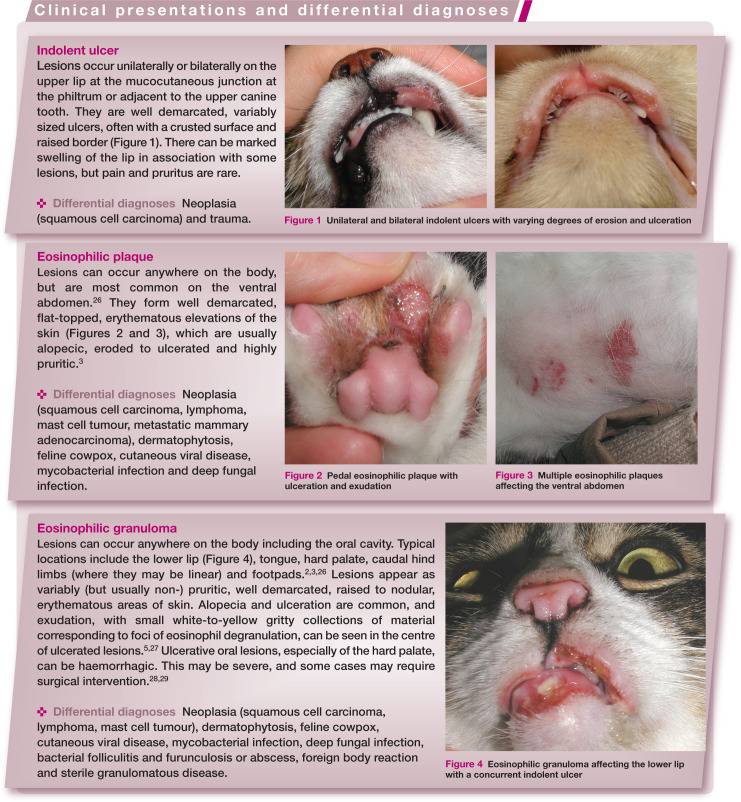
Figure 1.
Unilateral and bilateral indolent ulcers with varying degrees of erosion and ulceration
Figure 2.
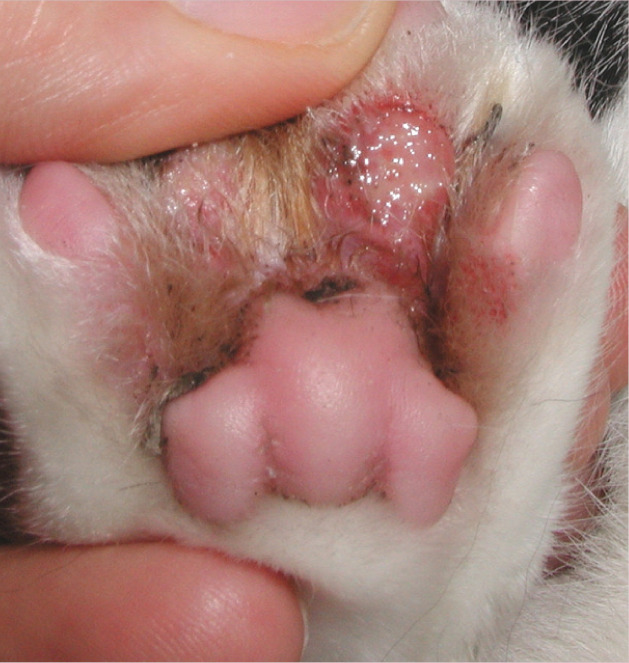
Pedal eosinophilic plaque with ulceration and exudation
Figure 3.
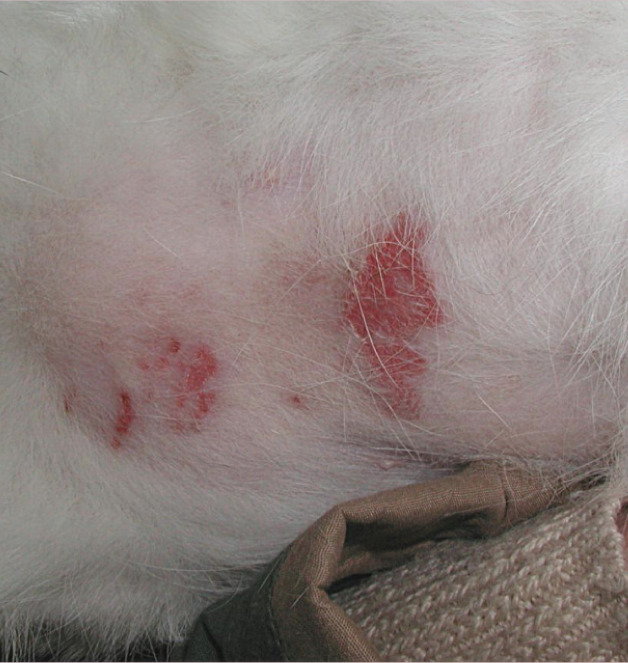
Multiple eosinophilic plaques affecting the ventral abdomen
Figure 4.
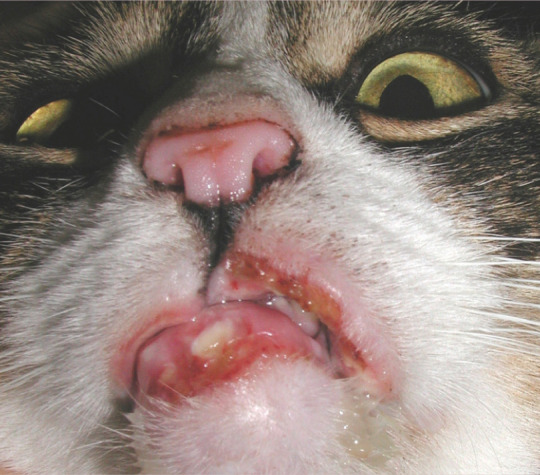
Eosinophilic granuloma affecting the lower lip with a concurrent indolent ulcer
While distinct histopathological features of each EGC lesion are documented, a previous study found that they are not reliably predictive of clinical lesions. 30 The main histopathological features are varying degrees of epidermal hyperplasia and erosion or ulceration (Figure 6), and a prominent eosinophilic dermal infiltrate (Figure 7).2,5 Small foci, known as flame figures, in which collagen fibres are surrounded by degranulated eosinophils, can be seen in all three lesions of the EGC.7,30
Figure 6.
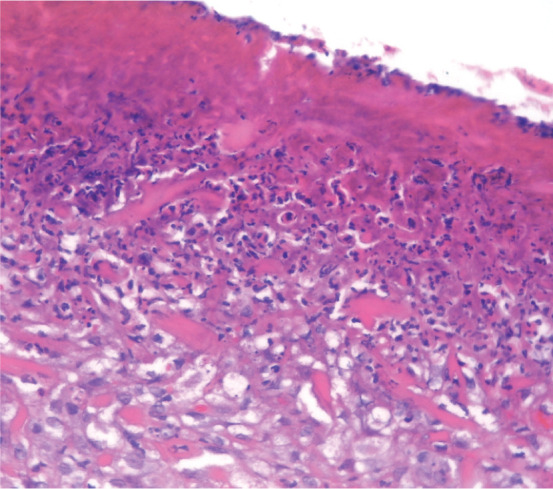
Histopathology of an eosinophilic plaque showing ulceration with a thick layer of surface inflammatory exudate in which there are large numbers of bacteria. Haematoxylin and eosin stain; x100 magnification
Figure 7.
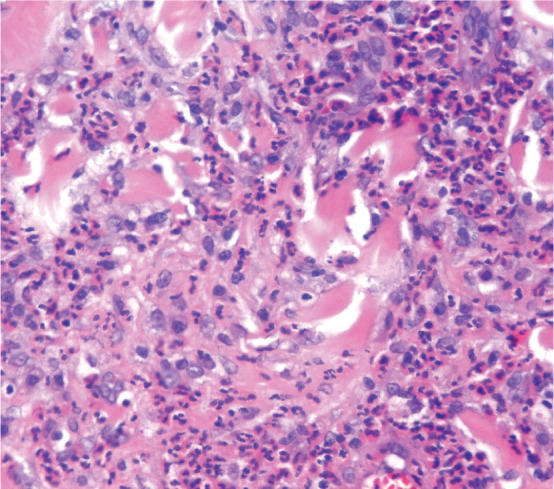
Histopathology of an eosinophilic plaque showing severe diffuse dermal infiltrate of eosinophils and mast cells. Haematoxylin and eosin stain; x400 magnification
Investigation of the primary disease
A thorough general and dermatological history is essential to identify possible aetiological factors. Good general health plus routine vaccination make FHV-1 and feline leukaemia virus (FeLV) infection less likely. A history of flea infestation, gastrointestinal disease and seasonal exacerbations of skin disease may increase suspicion, but are not diagnostic, for flea bite hypersensitivity, cutaneous adverse food reaction or atopic dermatitis, respectively. Additional clinical signs consistent with feline allergic disease may be noted alongside the lesions of the EGC (eg, miliary dermatitis, head and neck pruritus or symmetrical alopecia).
Coat brushings, trichography, adhesive tape impression and skin scrapings can be performed to evaluate for infestation with ectoparasites. However, the absence of fleas and flea faeces does not eliminate flea bite hypersensitivity as a potential underlying cause as cats are highly effective groomers. Fungal culture of coat brushings or hairs selected following Wood’s lamp examination is recommended for investigation of dermatophytosis.
Cytology via Diff Quik stained impression smears or adhesive tape impressions from the lesional surface should be performed for all EGC lesions to investigate the role of secondary bacterial infection and to confirm the inflammatory cell infiltrate. Small to moderate numbers of extracellular bacteria may not be relevant, as this could simply represent normal contamination or colonisation of the skin and oral cavity. Non-degenerate neutrophils, furthermore, are a common feature in many inflammatory conditions. The presence of degenerate neutrophils with intracellular bacteria (Figures 8 and 9), however, is diagnostic of colonisation and infection. Bacterial culture and antimicrobial sensitivity testing is not always necessary, as most staphylococcal isolates have a predictable pattern of antimicrobial sensitivity. However, culture should be performed if rod-shaped bacteria are seen on cytology (as their antimicrobial sensitivity is often limited) or if antimicrobial resistance is suspected (eg, nosocomial infections, history of multiple antimicrobial courses, failure of empirical therapy, and/or recent owner healthcare contacts).
Figure 8.
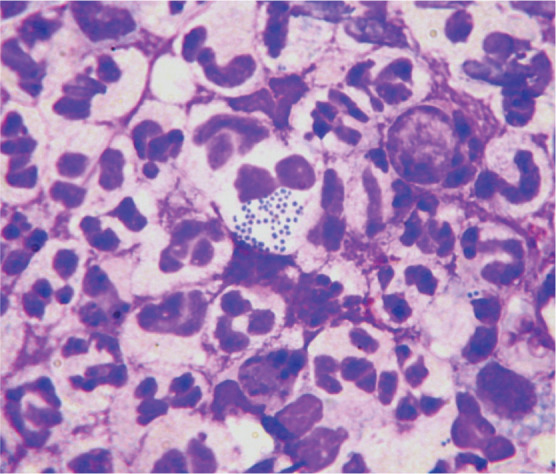
Cytology of an infected eosinophilic plaque showing neutrophils with phagocytosed intracytoplasmic coccoid bacteria. Rapi-Diff II stain; x1000 magnification
Figure 9.
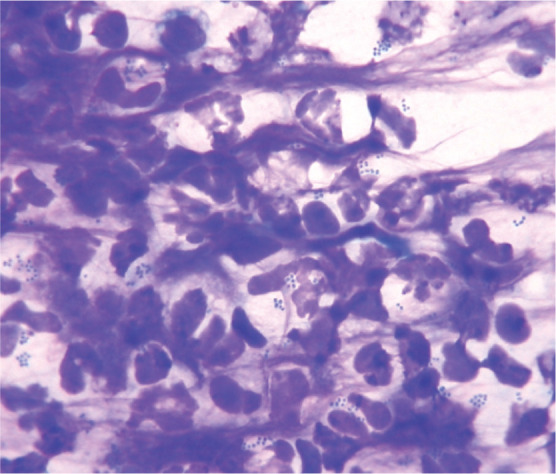
Cytology of a staphylococcal pyoderma showing degenerate neutrophils with intracytoplasmic coccoid bacteria. Rapi-Diff II stain; x1000 magnification
Testing for ectoparasites with a parasite treatment trial
In order to eliminate ectoparasites a strict 6–8 week parasite treatment trial should be performed using a topical adulticide such as selamectin (Stronghold; Pfizer Animal Health, UK) or imidacloprid and moxidectin (Advocate; Bayer, UK). These products kill fleas and are also miticidal, which helps to eliminate other ectoparasites. Other insecticides, however, may be more effective against fleas in certain locations. All in-contact animals (ie, cats, dogs, ferrets and rabbits) should also be treated for the duration of the trial. Potential sources of environmental contamination, including the home, outbuildings and cars, should be vacuumed (where possible) and treated with a combined environmental adulticide and insect growth regulator. The outdoor environment may need treating in warm climates. Written instructions and follow-up can improve compliance with flea control programmes.
It is not possible to investigate mosquito bite hypersensitivity using an ectoparasite treatment trial, as none of the ‘knock down’ repellent products are safe in cats. This diagnosis is usually made presumptively. Where it is suspected, most cases need to be kept indoors to prevent mosquito contact.
Testing for cutaneous adverse food reaction with an elimination diet
Adverse food reactions should be investigated by performing a strict 6–8 week elimination diet. 31 Options include a home-cooked novel protein diet, a commercial single novel protein diet or a commercial hydrolysed hypoallergenic diet. The diagnosis is based on resolution of the clinical signs on the trial food and recurrence on challenge with the cat’s usual diet. Given the severity of some lesions of the EGC it is often necessary to provide symptomatic treatment during diagnostic trials to ensure the cat is comfortable. In these cases it is necessary to withdraw symptomatic treatment prior to assessing the response, which may mean extending the trial beyond 6–8 weeks.
Problems with food trials in cats include cats that go outdoors having access to other foods (or resenting being kept indoors, which could worsen any behavioural component of their disease), palatability, and the risk of hepatic lipidosis if cats are starved. In practice, therefore, many food trials in cats are abandoned early.
Testing for hypersensitivity
In cases where all other primary diseases, especially ectoparasites and cutaneous adverse food reactions, have been excluded, atopic dermatitis is most likely. Positive IgE titres on serum allergy testing or positive reactions on intradermal skin testing support a diagnosis of atopic dermatitis, although doubts exist over the reliability and, therefore, benefit of allergy testing in cats. The authors routinely perform investigations for allergic disease, including serum and intradermal skin tests, in cats with EGC lesions consistent with atopic dermatitis to identify possible allergens for avoidance and allergen-specific immunotherapy. In cats with negative allergen tests it is impossible to determine whether they have idiopathic EGC or a disorder similar to canine atopic-like dermatitis.
Testing for other systemic disease
Cats presenting with EGC lesions should be systemically well, and those showing signs of systemic disease should be investigated for other problems, as appropriate. Where performed in cats with EGC lesions, haematology may reveal an eosinophilia, although this is non-diagnostic as it varies between different cats and different EGC lesions. Biochemistry and urinalysis are usually unremarkable. A peripheral lymphadenopathy can be seen in association with any EGC lesions. This is usually reactive, although it is prudent to perform fine needle aspirate cytology to ensure that affected lymph nodes are not neoplastic. FIV, FeLV and Toxoplasma serology may be considered prior to treatment with immunomodulatory drugs. The presence of endogenous and exogenous foreign bodies may be seen on histology, albeit rarely. However, small foreign bodies could easily be missed from a 4–6 mm punch biopsy.
Where previous biopsy has confirmed the presence of an EGC lesion but standard therapy is ineffective, repeat biopsy, or where possible, excision and histological examination for foreign bodies, viral disease and neoplasia is warranted.
Management
Glucocorticoids
EGC lesions usually show a good response to treatment with systemic glucocorticoids. Some lesions, however, require high dosages 26 and some can be refractory to treatment. Where possible, it is preferable to avoid depot corticosteroid preparations due to the inability to withdraw treatment if adverse effects are encountered and, conversely, the inability to increase the dose if insufficient response is seen. Oral treatment with prednisolone at starting doses of 1–2 mg/kg q24h can be effective, although in some cases higher doses of up to 4 mg/kg q24h can be required. Lesions should be reassessed after 7–14 days and daily doses should be tapered or alternate-day therapy used where possible. If no response is seen, additional therapy should be considered in order to avoid excessive use of glucocorticoids or, if the diagnosis is uncertain, further investigations should be performed.
If the owner struggles to administer tablets they may have success with dexamethasone solution. This can be given by mouth or added to food at 0.1–0.2 mg/kg q24h (induction dose) and 0.05–0.1 mg/kg q72h (maintenance dose).2,26,27 The aim is to maintain the cat on the lowest alternate-day (or less frequent) dose that keeps the lesions in remission.
Care must be taken with the long-term use of potent glucocorticoids due to the risk of adverse effects. A study of 14 cats treated daily with prednisolone or dexamethasone suggested that dexamethasone exhibits greater diabetogenic effects than equipotent doses of prednisolone. 32 Although cats seem to be more tolerant to systemic glucocorticoids than dogs, adverse effects including polydipsia, polyphagia, changes in weight, diabetes mellitus, urinary tract infection, iatrogenic hyperadrenocorticism, congestive heart failure, demodicosis and gastric ulceration can be seen.2,26,32
Ciclosporin
Ciclosporin is a calcineurin inhibitor that exerts an immunomodulating effect via, among other actions, suppression of T lymphocyte function. It is currently available in a capsulated form (Atopica; Novartis Animal Health UK) licensed for use in dogs, and a liquid form has recently been licensed for use in cats at 7 mg/kg q24h (Atopica Cat; Novartis Animal Health UK). Studies have shown that ciclosporin is very effective in the treatment of the feline EGC at doses ranging from 3.6–13.3 mg/kg q24h.33–35 Ciclosporin at 5 mg/kg q24h has been shown to be as effective as prednisolone (at 1 mg/kg) in feline allergic disease, 6 but it is likely that cats require higher doses than those licensed for use in dogs and daily doses of 7–7.5 mg/kg are recommended.26,36 As with dogs, once-daily treatment should be continued for 4 weeks and, if a good response is seen, treatment can be tapered to alternate-day and then twice-weekly therapy. Cats that relapse on alternate-day therapy can be managed on daily treatment, reducing the dose to the lowest that maintains remission.
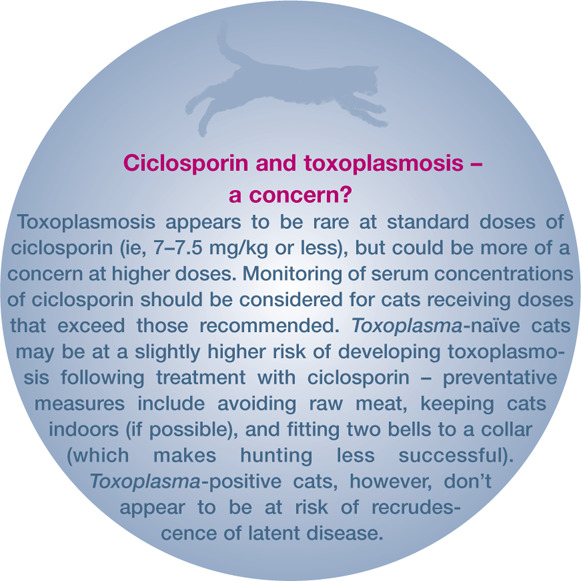
Ciclosporin, at the doses used to treat feline EGC, is well tolerated by most cats, 4 with the main adverse effects limited to mild gastrointestinal disturbances.6,33–35,37–39 Some reports have suggested a link between ciclosporin treatment and toxoplasmosis40,41 and neoplasia.42–44 The cited cases of neoplasia, however, all occurred following immunosuppression with a combination of ciclosporin and prednisolone prior to renal transplantation. Concurrent treatment with prednisolone and ciclosporin may also increase the risk of toxoplasmosis (see left). In the authors’ experience, prednisolone and ciclosporin is a potent immunosuppressive combination, and should be used with great care. Other, uncommon side effects include hypersalivation, hyperactivity, anorexia and gingival hyperplasia.6,38,39 The risk at the doses used to treat the feline EGC, however, seems to be small. 4
Ciclosporin can have a number of drug interactions. In particular concurrent treatment with some antibiotics (doxycycline and erythromycin) and antifungals (ketoconazole, itraconazole and fluconazole) inhibits the metabolism of ciclosporin, increasing plasma levels and the risk of adverse effects. 26
Hydrocortisone aceponate
Hydrocortisone aceponate (HCA) is a non-halogenated, double ester glucocorticoid licensed for topical use in dogs as a 0.0584% spray (Cortavance; Virbac, UK). Unlike conventional topical glucocorticoids, HCA is metabolised within the skin into a largely inactive form, allowing it to maintain local potency without the risk of systemic side effects.45,46
A recent study evaluated the efficacy of daily or alternate-day application of the commercially available 0.0584% HCA spray in 10 cats with presumed allergic disease. 4 Seven of these cats presented with eosinophilic plaques, and the remainder with combinations of miliary dermatitis and symmetrical alopecia. There were highly significant improvements in both clinical lesion and pruritus scores over the 56-day study period. Ease of application of the spray, as scored by owners in the study, increased significantly with time and most owners rated the drug’s efficacy as good or excellent. During the study, two sprays of HCA were applied to a 10 x 10 cm area of lesional skin daily for 28 days and reduced to alternate-day therapy if there was a greater than 50% improvement in clinical lesion and pruritus scores. However, the response to treatment was rapid and most of the clinical improvement was seen within 14 days. One cat was withdrawn from the study due to poor treatment efficacy and no adverse effects were reported in any of the cats. The study suggests that HCA is effective and safe for the treatment of EGC lesions, although further controlled studies are required.
Chlorambucil
Chlorambucil is a nitrogen mustard derivative, alkylating agent. It is not licensed for use in animals but may be considered in EGC cases that are refractory to steroid therapy. It is available in 2 mg tablets (Leukeran; GlaxoSmithKline, UK). Chlorambucil can be given concurrently with steroids at doses equivalent to 0.1–0.2 mg/kg daily or every other day (ie, one 2 mg tablet 2–3 times weekly for most cats – the tablets should not be crushed or split). Therapy has to be given for 4–8 weeks for the full effects to be appreciated. Once a clinical response is seen the steroid dose should be tapered first, followed by the chlorambucil dose if the case remains in remission.
Side effects are infrequent but may include vomiting, diarrhoea, anorexia, bone marrow suppression and hepatotoxicity.2,26 Haematology should be monitored every 2 weeks to assess for signs of bone marrow suppression. After 3–4 months haematology can be monitored every 3 months.
Interferon omega
Anecdotal reports suggest that twice-weekly or weekly subcutaneous injections of 2.5 MU interferon omega (Virbagen; Virbac Animal Health, UK) can be effective and well tolerated in some cats with EGC lesions. The mode of action is unknown, but is probably associated with the immunomodulating activity of interferon omega. However, only a few cats have been treated, no clinical trials have been reported, and the long-term safety and efficacy is unknown. Interferon omega should not therefore be used as a first-line treatment.
Progestagens
Megoestrol acetate (Ovarid; Virbac, UK) has been recommended for the management of EGC lesions. Due to the risk of severe adverse effects including weight gain, diabetes mellitus, adrenocortical suppression, pyometra and mammary hyperplasia, and the availability of safer, more efficacious drugs, it cannot be recommended other than as a last resort option in recalcitrant cases.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this review article.
The authors do not have any potential conflicts of interest to declare.
Key Points
EGC lesions are a widely recognised clinical presentation, but they are poorly understood.
Although grossly the lesions vary, they share similar histopathological features.
It is important to rule out differential diagnoses prior to symptomatic treatment.
Investigation and management of potential underlying aetiologies, particularly allergic disease, is indicated.
Idiopathic cases require symptomatic treatment.
Many cases require long-term treatment.
Case notes
Richard, a 2-year-old neutered male domestic shorthair cat, presented with recurrent exudative skin lesions and overgrooming. The skin disease had previously responded to treatment with depot steroid injections.
History
Richard lived with a dog and two other cats and had outdoor access. He was regularly vaccinated and wormed, and was fed a complete commercial diet. Richard first developed skin disease 6 months earlier when his owner noticed excessive grooming followed by patches of reddened, exudative skin. The skin lesions resolved following treatment with a depot steroid injection but Richard continued to overgroom. He had since had two further episodes with increasing severity, and he was currently severely pruritic.
Physical examination
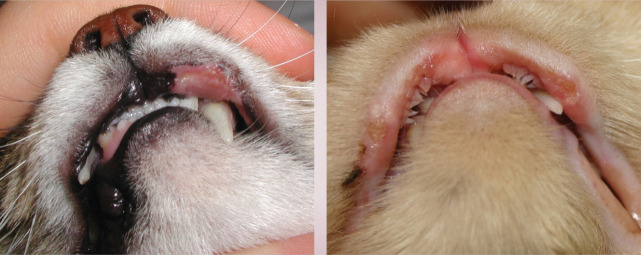
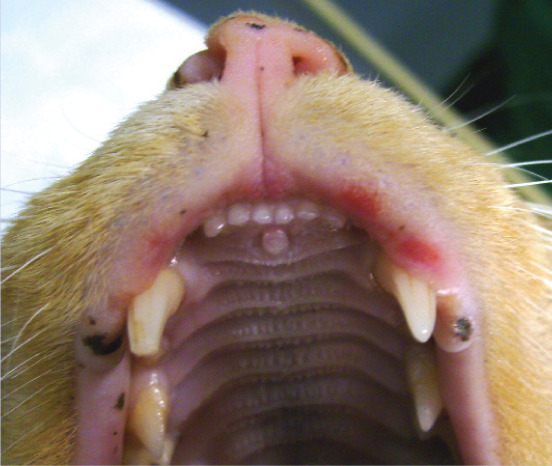
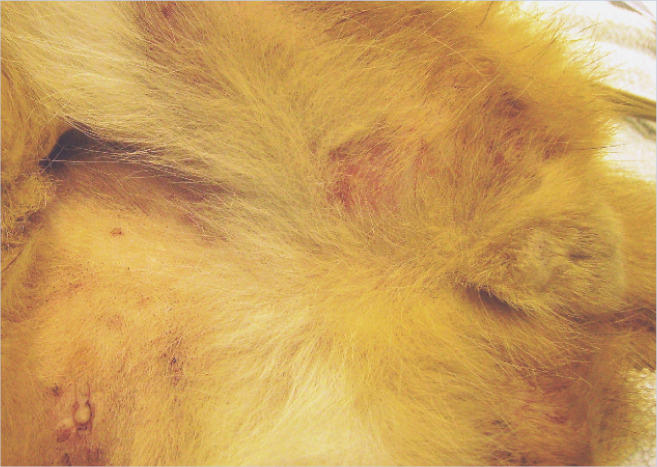
Richard was bright, alert, responsive and in good body condition. There was no evidence of anxiety or other behavioural problems in the consultation room or from the history. General physical examination was unremarkable. Dermatological examination revealed three areas of erosion to ulceration of the upper lip adjacent to the canine teeth (see right). There were also multiple partially haired, erythematous and exudative plaques and papular crusts affecting the medial and caudal aspects of the proximal hind limbs (see right).
Initial diagnostic tests
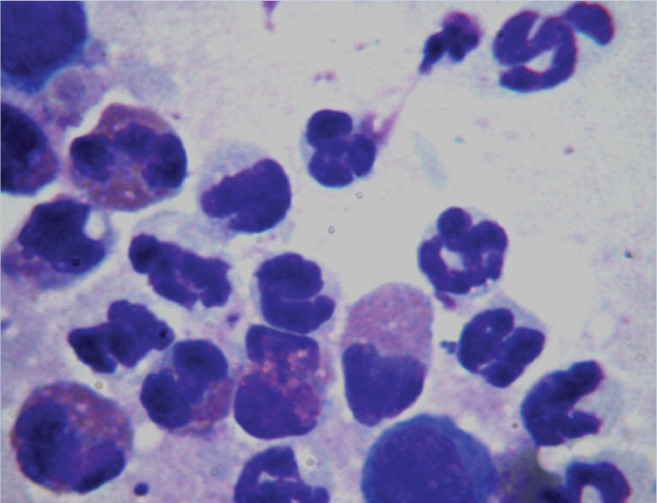
Cytology of Diff Quik stained direct impression smears taken from the surface of the exudative lesions revealed multiple eosinophils as well as neutrophils with intracytoplasmic coccoid bacteria (see right). Coat brushings, adhesive-tape examination and skin scrapes did not reveal any evidence of fleas or other ectoparasites. Trichography of hairs taken from around the lesional sites showed broken hair tips with a mixture of anagen and telogen hair bulbs.
What is Your Assessment?
- What is the most likely diagnosis based on the presentation and initial findings?
- (a) Indolent ulcers and flea bite hypersensitivity.
- (b) Feline cowpox.
- (c) FIV and bacterial pyoderma.
- (d) Indolent ulcers and esoinophilic plaques with secondary bacterial infection.
- (e) Feline herpesvirus-1 infection.
- What would you consider for initial treatment? Choose all options that apply
- (a) Enrofloxacin 5 mg/kg for 5–7 days.
- (b) Hydrocortisone aceponate applied to skin lesions q24h for 7 days.
- (c) Ciclosporin 7 mg/kg q24h for 4 weeks.
- (d) Prednisolone 1 mg/kg q24h for 7 days then q48h for 7 days.
- (e) Cefalexin 20 mg/kg q12h for 2 weeks.
- Which three of these diagnostic tests would you want to perform next?
- (a) Six-week elimination diet.
- (b) Skin biopsy and histopathology.
- (c) Fungal culture.
- (d) Six-week ectoparasite treatment trial.
- (e) Intradermal skin testing.
- When would allergen-specific serology or intradermal testing be appropriate?
- (a) In all cases of eosinophilic dermatitis.
- (b) After infections have been controlled, and ectoparasites and adverse food reactions have been eliminated.
- (c) As early as possible to permit treatment with glucocorticoids.
- (d) Never – allergen-specific tests are not suitable for cats.
- (e) If a cat fails to respond to anti-inflammatory treatment.
- Which treatment combination would be most appropriate for chronic relapsing eosinophilic plaques in association with atopic dermatitis?
- (a) Prednisolone and chlorambucil.
- (b) Hydrocortisone aceponate, interferon omega and cefalexin.
- (c) Alternate-day dexamethasone and allergen avoidance.
- (d) Ciclosporin, allergen-specific immunotherapy and flea control.
- (e) Depot steroid injections, topical chlorhexidine and essential fatty acids.
Answers to questions on page 478:
(d)
(b), (d) and (e)
(a), (c) and (d)
(b)
(d)
Further investigation and follow-up
A fungal culture was negative. Following 20 mg/kg cefalexin q12h for 20 days the lesions had slightly decreased in size, and were less exudative, with no cocci seen on cytology. There was no response to 6-week ectoparasite control (selamectin every 14 days for three applications for Richard and in-contact animals, and piperonyl butoxide/permethrin/pyriproxyfen for the house) or food trials. Methylprednisolone was given at 0.8 mg/kg q24h for 3–5 days if required to control severe pruritus during the trials.
At this stage the diagnosis would be consistent with feline atopic dermatitis or hypersensitivity dermatitis (ie, pruritus plus an eosinophilic dermatitis with elimination of other causes). Both intradermal skin testing and allergen-specific serology were negative. Ciclosporin was considered, as there had only been a partial response to glucocorticoids and the indolent ulcer was not suitable for treatment with hydrocortisone aceponate spray. Blood tests revealed that Richard was FIV-, FeLV- and Toxoplasma-negative. He was therefore started on 7 mg/kg ciclosporin q24h. The owners preferred to maintain his outdoor access, but avoided feeding him raw meat and fitted a collar with two bells to inhibit hunting. He responded well, with complete resolution of the lesions within 4 weeks, and was maintained on 7 mg/kg ciclosporin twice weekly.
References
- 1. Scott D. Observations on the feline eosinophilic granuloma complex of cats. J Am Anim Hosp Assoc 1975; 11: 261–270. [Google Scholar]
- 2. Bloom PB. Canine and feline eosinophilic skin diseases. Vet Clin North Am Small Anim Pract 2006; 36: 141–160, vii. [DOI] [PubMed] [Google Scholar]
- 3. Scott D, Miller WH, Griffin C. Skin immune system and allergic skin disease and miscellaneous skin diseases. Muller and Kirk’s small animal dermatology. Vol 1. 6th ed. Philadelphia: WB Saunders, 2001, pp 543–666, 1125–1283. [Google Scholar]
- 4. Schmidt V, Buckley LM, McEwan NA, Reme CA, Nuttall TJ. Efficacy of a 0.0584% hydrocortisone aceponate spray in presumed feline allergic dermatitis: an open label pilot study. Vet Dermatol 2012; 23: 11–16. [DOI] [PubMed] [Google Scholar]
- 5. Lee Gross T, Ihrke PJ, Walder EJ, Affolter VK. Spongiotic and vesicular disease of the epidermis. Nodular and diffuse diseases of the dermis with prominent eosinophils, neutrophils, or plasma cells. In: Skin diseases of the dog and cat: clinical and histopathologic diagnosis. Vol 1. 2nd ed. Oxford: Blackwell Science, 2005, pp 105–116 and 342–373. [Google Scholar]
- 6. Wisselink MA, Willemse T. The efficacy of cyclosporine A in cats with presumed atopic dermatitis: a double blind, randomised prednisolone-controlled study. Vet J 2009; 180: 55–59. [DOI] [PubMed] [Google Scholar]
- 7. Bardagi M, Fondati A, Fondevila D, Ferrer L. Ultrastructural study of cutaneous lesions in feline eosinophilic granuloma complex. Vet Dermatol 2003; 14: 297–303. [DOI] [PubMed] [Google Scholar]
- 8. Taglinger K, Day MJ, Foster AP. Characterization of inflammatory cell infiltration in feline allergic skin disease. J Comp Pathol 2007; 137: 211–223. [DOI] [PubMed] [Google Scholar]
- 9. Colombini S, Hodgin EC, Foil CS, Hosgood G, Foil LD. Induction of feline flea allergy dermatitis and the incidence and histopathological characteristics of concurrent indolent lip ulcers. Vet Dermatol 2001; 12: 155–161. [DOI] [PubMed] [Google Scholar]
- 10. Mason KV, Evans AG. Mosquito bite-caused eosinophilic dermatitis in cats. J Am Vet Med Assoc 1991; 198: 2086–2088. [PubMed] [Google Scholar]
- 11. Nagata M, Ishida T. Cutaneous reactivity to mosquito bites and its antigens in cats. Vet Dermatol 1997; 8: 19–26. [DOI] [PubMed] [Google Scholar]
- 12. Russell RG, Slattum MM, Abkowitz J. Filamentous bacteria in oral eosinophilic granulomas of a cat. Vet Pathol 1988; 25: 249–250. [DOI] [PubMed] [Google Scholar]
- 13. Wildermuth BE, Griffin CE, Rosenkrantz WS. Response of feline eosinophilic plaques and lip ulcers to amoxicillin trihydrate– clavulanate potassium therapy: a randomized, double-blind placebo-controlled prospective study. Vet Dermatol 2012; 23: 110–118. [DOI] [PubMed] [Google Scholar]
- 14. Moriello KA, Kunkle G, Miller LM, Crowley A. Lack of autologous tissue transmission of eosinophilic plaques in cats. Am J Vet Res 1990; 51: 995–998. [PubMed] [Google Scholar]
- 15. Neufeld JL, Burton L, Jeffery KR. Eosinophilic granuloma in a cat. Recovery of virus particles. Vet Pathol 1980; 17: 97–79. [DOI] [PubMed] [Google Scholar]
- 16. Lee M, Bosward KL, Norris JM. Immunohistological evaluation of feline herpesvirus-1 infection in feline eosinophilic dermatoses or stomatitis. J Feline Med Surg 2010; 12: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Persico P, Roccabianca P, Corona A, Vercelli A, Cornegliani L. Detection of feline herpes virus 1 via polymerase chain reaction and immunohistochemistry in cats with ulcerative facial dermatitis, eosinophilic granuloma complex reaction patterns and mosquito bite hypersensitivity. Vet Dermatol 2011; 22: 521–527. [DOI] [PubMed] [Google Scholar]
- 18. Gelberg HB, Lewis RM, Felsburg PJ, Smith CA. Antiepithelial autoantibodies associated with the feline eosinophilic granuloma complex. Am J Vet Res 1985; 46: 263–265. [PubMed] [Google Scholar]
- 19. Roosje PJ, Willemse T. Cytophilic antibodies in cats with miliary dermatitis and eosinophilic plaques: passive transfer of immediate-type hypersensitivity. Vet Q 1995; 17: 66–69. [DOI] [PubMed] [Google Scholar]
- 20. Kimura T, Kano R, Maeda S, Tsujimoto H, Nagata M, Hasegawa A. Expression of RANTES mRNA in skin lesions of feline eosinophilic plaque. Vet Dermatol 2003; 14: 269–273. [DOI] [PubMed] [Google Scholar]
- 21. Maeda S, Okayama T, Ohmori K, Masuda K, Ohno K, Tsujimoto H. Molecular cloning of the feline thymus and activation-regulated chemokine cDNA and its expression in lesional skin of cats with eosinophilic plaque. J Vet Med Sci 2003; 65: 275–278. [DOI] [PubMed] [Google Scholar]
- 22. Valenta R, Seiberler S, Natter S, Mahler V, Mossabeb R, Ring J, et al. Autoallergy: a pathogenetic factor in atopic dermatitis? J Allergy Clin Immunol 2000; 105: 432–437. [DOI] [PubMed] [Google Scholar]
- 23. Wisselink MA, van Ree R, Willemse T. Evaluation of Felis domesticus allergen I as a possible autoallergen in cats with eosinophilic granuloma complex. Am J Vet Res 2002; 63: 338–341. [DOI] [PubMed] [Google Scholar]
- 24. Power HT. Eosinophilic granuloma in a family of specific pathogen-free cats. Proceedings of the American Academy of Veterinary Dermatology/American College of Veterinary Dermatology. Vol 6. San Francisco; 1990, p 45. [Google Scholar]
- 25. Leistra WH, van Oost BA, Willemse T. Non-pruritic granuloma in Norwegian Forest cats. Vet Rec 2005; 156: 575–577. [DOI] [PubMed] [Google Scholar]
- 26. Foster A. Clinical approach to feline eosinophilic granuloma complex. In Pract 2003; 25: 2–10. [Google Scholar]
- 27. Power HT, Ihrke PJ. Selected feline eosinophilic skin diseases. Vet Clin North Am Small Anim Pract 1995; 25: 833–850. [DOI] [PubMed] [Google Scholar]
- 28. Bailey CJ, Tisdall PLC, Beatty JA, Lingard A, Barrs VR. Use of bipedicle mucoperiosteal flap to treat arterial haemorrhage from palatine ulcers in three cats. Aust Vet Pract 2007; 37: 6–7. [Google Scholar]
- 29. Corgozinho KB, Souza HJM, Ferreira AMR, Pereira AN, Souza RC. Hemorragia oral por lesao ulcerativa no palato duro em gatos domesticos. Clin Vet (Milano) 2007; 72: 24–26. [Google Scholar]
- 30. Fondati A, Fondevila D, Ferrer L. Histopathological study of feline eosinophilic dermatoses. Vet Dermatol 2001; 12: 333–338. [DOI] [PubMed] [Google Scholar]
- 31. Bryan J, Frank LA. Food allergy in the cat: a diagnosis by elimination. J Feline Med Surg 2010; 12: 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lowe AD, Graves TK, Campbell KL, Schaeffer DJ. A pilot study comparing the diabetogenic effects of dexamethasone and prednisolone in cats. J Am Anim Hosp Assoc 2009; 45: 215–224. [DOI] [PubMed] [Google Scholar]
- 33. Guaguère E, Prelaud P. Efficacy of cyclosporin in the treatment of 12 cases of eosinophilic granuloma complex. Vet Dermatol 2000; 11: S31. [Google Scholar]
- 34. Vercelli A, Raviri G, Cornegliani L. The use of oral cyclosporin to treat feline dermatoses: a retrospective analysis of 23 cases. Vet Dermatol 2006; 17: 201–206. [DOI] [PubMed] [Google Scholar]
- 35. Noli C, Scarampella F. Prospective open pilot study on the use of ciclosporin for feline allergic skin disease. J Small Anim Pract 2006; 47: 434–438. [DOI] [PubMed] [Google Scholar]
- 36. Nuttall T, Harvey RG, McKeever PJ. Feline eosinophilic granuloma complex. In: A colour handbook of skin diseases of the dog and cat. Vol 1. 2nd ed. London: Manson Publishing, 2009, pp 102–105. [Google Scholar]
- 37. Robson DC, Burton GG. Cyclosporin: applications in small animal dermatology. Vet Dermatol 2003; 14: 1–9. [DOI] [PubMed] [Google Scholar]
- 38. Latimer KS, Rakich PM, Purswell BJ, Kircher IM. Effects of cyclosporin A administration in cats. Vet Immunol Immunopathol 1986; 11: 161–173. [DOI] [PubMed] [Google Scholar]
- 39. Heinrich NA, McKeever PJ, Eisenschenk MC. Adverse events in 50 cats with allergic dermatitis receiving ciclosporin. Vet Dermatol 2011; 22: 511–520. [DOI] [PubMed] [Google Scholar]
- 40. Barrs V, Martin P, Beatty JA. Antemortem diagnosis and treatment of toxoplasmosis in two cats on cyclosporine therapy. Aust Vet J 2006; 84: 30–35. [DOI] [PubMed] [Google Scholar]
- 41. Last RD, Suzuki Y, Manning T, Lindsay D, Galipeau L, Whitbread TJ. A case of fatal systemic toxoplasmosis in a cat being treated with cyclosporin A for feline atopy. Vet Dermatol 2004; 15: 194–198. [DOI] [PubMed] [Google Scholar]
- 42. Gregory CR, Madewell BR, Griffey SM, Torten M. Feline leukemia virus-associated lymphosarcoma following renal transplantation in a cat. Transplantation 1991; 52: 1097–1099. [DOI] [PubMed] [Google Scholar]
- 43. Schmiedt CW, Grimes JA, Holzman G, McAnulty JF. Incidence and risk factors for development of malignant neoplasia after feline renal transplantation and cyclosporine-based immunosuppression. Vet Comp Oncol 2009; 7: 45–53. [DOI] [PubMed] [Google Scholar]
- 44. Wooldridge JD, Gregory CR, Mathews KG, Aronson LR, Kyles AE. The prevalence of malignant neoplasia in feline renal-transplant recipients. Vet Surg 2002; 31: 94–97. [DOI] [PubMed] [Google Scholar]
- 45. Schackert C, Korting HC, Schafer-Korting M. Qualitative and quantitative assessment of the benefit-risk ratio of medium potency topical corticosteroids in vitro and in vivo: characterisation of drugs with an increased benefit-risk ratio. BioDrugs 2000; 13: 267–277. [DOI] [PubMed] [Google Scholar]
- 46. Brazzini B, Pimpinelli N. New and established topical corticosteroids in dermatology: clinical pharmacology and therapeutic use. Am J Clin Dermatol 2002; 3: 47–58. [DOI] [PubMed] [Google Scholar]