Abstract
A 12-year-old male neutered Siamese cat presented with a history of inappetance and lethargy and an enlarged left anal sac. The anal sac was surgically excised and histopathology confirmed the diagnosis of anal sac adenocarcinoma. Perianal tumours are rare in the cat and anal sac adenocarcinoma has not been previously reported. This is in contrast to the dog where anal sac adenocarcinoma is a well recognised albeit uncommon tumour.
The cat was referred to the Queen's Veterinary School Hospital, University of Cambridge, nine days after the initial presentation to the referring veterinarian. On presentation, the owner reported that the cat was generally well although the cat had not defecated or eaten during the previous two days. Physical examination revealed a colon distended by impacted faeces and confirmed the presence of 1 cm by 1 cm mass on the left side of the anus, ventral to the anal gland orifice. Thoracic radiography revealed no evidence of metastases and abdominal radiograph showed that the colon was distended with faeces. Abdominal ultrasonography demonstrated that the medial iliac lymph nodes were of normal size.
The cat was managed conservatively over the following five days by 118 mg of sterculia BPC (Peridale, Arnolds) bid (twice a day), 1.34 g oral lactulose (Lactulose, Neolab) bid and twice daily enemas (Micralax, Medeva). On day 6 the cat was pre-medicated with 0.1 mg acepromazine (ACP, C-Vet), 0.03 mg buprenorphine (Vetergesic, Alstoe Animal Health), 68 mg clavulanate-potentiated amoxycillin (Augmentin, Smith Kline Beecham) and 13.6 mg carprofen (Rimadyl, Pfizer) and anaesthetised with 20 mg propofol (Rapinovet, Schering Plough Animal Health) and maintained on isofluorane (Isoflo, Schering Plough Animal Health). The perianal region was clipped and prepared for aseptic surgery. A lateral approach was made to the left anal sac. The extent of the mass was identified by blunt dissection and was found to originate in the anal sac and appeared to invade the adjacent external sphincter. The mass was removed by blunt dissection which included the whole anal sac and part of the external sphincter. 2 metric poliglecaprone 25 (Monocryl, Ethicon) was used to repair the external sphincter and the subcutis was closed in 2 layers using 2 metric polyglactin 910 (Vicryl, Ethicon). The cat was discharged on day 7 on a five-day course of 50 mg metronidazole (Pharmaflex, Braun) bid and 50 mg synulox (Synulox, Pfizer) bid and a ten day course of 1.34 mg oral lactulose (Lactulose, Neolab) tid (three times a day) and 118 mg of sterculia BPC (Peridale, Arnolds) bid.
Table 1.
Serum biochemistry results
| Result | Normal range | |
|---|---|---|
| Urea (mmol/l) | 5.01 ** | 5.71–12.85 |
| Glucose (mmol/l) | 8.17 * | 4.22–8.06 |
| Creatinine (umol/l) | 128 | 71–212 |
| Total protein (g/l) | 76 | 57–89 |
| Albumin (g/l) | 27 | 26.0–39.0 |
| Calcium (mmol/l) | 2.27 | 1.95–2.83 |
| Phosphate (mmol/l) | 1.16 | 1.00–2.42 |
| ALKP (iu/l) | 36 | 14–111 |
| ALT (iu/l) | 36 | 12–130 |
| Cholesterol (mmol/l) | 3.92 | 1.68–5.81 |
| Sodium (mmol/l) | 153 | 150–165 |
| Potassium (mmol/l) | 3.74 | 3.50–5.80 |
| Chloride (mmol/l) | 116.1 | 112–129 |
above normal range
below normal range.
Histological examination of the resected tissue mass demonstrated a well demarcated expansile neoplastic proliferation of large cuboidal or polyhedral cells (Fig 1), forming multiple tubules and acini in a coarse desmoplastic stroma (Fig 2). In some areas cells were packed together to form solid cords or lobules. Examples of small groups of proliferating cells invading the stroma were identified and there was stromal haemorrhage. Groups of cells were seen proliferating in surrounding connective tissue. There were moderate to marked degree of anisokaryosis and the mitotic rate varied between 2 and 8 per high-powered field. Examination of levels through the submitted tissue revealed a close assocation with epithelial invagination consistent with an anal sac and in addition, the presence of proliferating cells in an endothelium lined vessel. Evidence for an apocrine morphology was sparse and equivocal amongst proliferating cells and Diastase resistant and Periodic Acid Schiff positive granules (consistent with an apocrine phenotype) were not demonstrated in proliferating cells. Whilst complete excision was attempted, the presence of neoplastic cells within endothelium lined spaces, considerably increased the likelihood of metastatic disease developing.
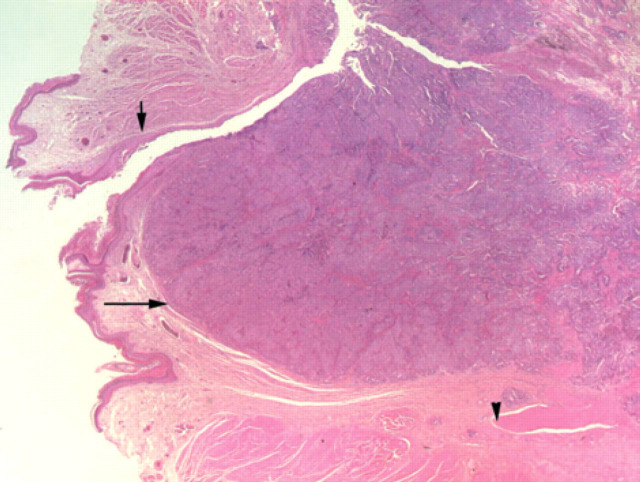
Fig 1.
Low power view (x20) of adenocarcinoma tissue (long arrow) associated with the collapsed anal sac (Hematoxylin and Eosin). The short arrow indicates epidermal lining of the anal sac remnant. There is infiltration into adjacent external anal sphincter muscle (arrowhead).
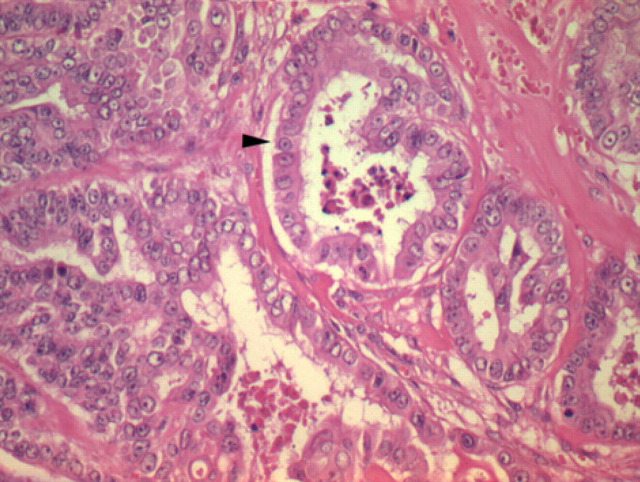
Fig 2.
High power view (x200) showing irregular acini containing degenerate cellular debris (arrowhead) and lined by a cuboidal epithelium exhibiting marked cellular pleomorpism (Hematoxylin and Eosin). The majority of the tumour comprised disorganised lobules and cords, shown on the left of the figure.
The cat was re-examined at the Queen's Veterinary School Hospital on day 18. The wound had healed uneventfully and the cat's appetite and demeanour was normal. Over the following three months the cat suffered from occasional bouts of constipation. Four months after the surgery, several cutaneous nodules were visible around the left perianal region. Cytology of fine needle aspirates taken from the cutaneous nodules were suggestive of a re-occurrence of the anal sac adenocarcinoma. The cutaneous nodules enlarged over the following two months and the cat was eventually euthanased six months after initial presentation. No post mortem was undertaken.
Perianal tumours are rare in the cat (Withrow 2001) and anal sac adenocarcinoma has not previously been reported in the cat. This is in contrast to the dog where anal sac adenocarcinoma is a well recognised albeit uncommon tumour. In dogs, anal sac adenocarcinoma tends to affect older bitches and perineal swellings, dyschezia and tenesmus are typical historical complaints (Goldschmidt & Zoltowski 1981, Ross et al 1991). Dogs with anal sac adenocarcinomas may develop humoral hypercalcaemia of malignancy and in some instances, the tumour is not detected until hypercalcaemia related problems such as polydipsia, polyuria, anorexia, vomiting and muscle weakness develop (Hause et al 1981, Meuten et al 1981, Ross et al 1991). One of the major causes of humoral hypercalcaemia of malignancy is the production of parathyroid hormone related protein (PTHrP). PTHrP has a similar effect to parathyroid hormone on bone and kidney leading to increased osteoclastic bone resorption and renal tubular calcium reabsorption (Grone et al 1994).
Canine anal sac adenocarcinomas tend to metastasise early in the course of the disease, frequently to the sublumbar lymph nodes (Ross et al 1991). Surgical excision of the primary tumour is the treatment of choice but complete excision can be difficult (Ogilvie & Moore 1996, DeNovo & Bright 2000). The follow up treatment of choice for incompletely excised tumours or tumours excised with a narrow margin of normal tissue remains unclear in both dogs and cats. In dogs, chemotherapy has been attempted on a small number of cases with limited success (Ogilvie et al 1989, Hammer et al 1994, Brearley 2000). Surgical removal of enlarged iliac lymph nodes has been described as a successful technique to manage regional lymph node metasteses from canine anal sac adenocarcinoma although the technique has been associated with serious post-operative complications (Ross et al 1991, Jeffery et al 2000). Radiotherapy has been used for canine perianal adenocarcinomas and rectal carcinomas (Turrel & Theon 1986, Vail et al 1990, Withrow 2001) but there is no published information on the use of adjuvant radiotherapy for the treatment of incompletely excised anal sac adenocarcinomas in dogs (DeNovo & Bright 2000, Withrow 2001). As in this case, recurrence is common in cases of canine anal sac adenocarcinoma regardless of treatment and the long term prognosis is poor for incompletely resected tumours (Goldschmidt & Zoltowski 1981, Ross et al 1991, DeNovo & Bright 2000).
Acknowledgements
Richard Mellanby would like to thank the Alice Noakes Trust for sponsoring his residency. The authors would also like to thank Andrew Torrance who performed the cytology, Kydd and Kydd Veterinary Health Centre for referring the case and the cat's owners for their help with the preparation of this report.
References
- Brearley MJ. (2000) Carboplatin chemotherapy in the treatment of perineal carcinomas in 10 dogs: 1993 to 1999 Journal of Small Animal Practice 41, 390. [Google Scholar]
- DeNovo RC, Bright RM. (2000) Recto-anal disease. In Textbook of Small Animal Internal Medicine. (eds). Ettinger SJ, Feldman EC. (5th Edn). Philadelphia: W.B. Saunders Company, pp. 1257–1270. [Google Scholar]
- Goldschmidt MH, Zoltowski C. (1981) Anal sac gland adenocarcinoma in the dog: 14 cases Journal of Small Animal Practice 22, 119–128. [DOI] [PubMed] [Google Scholar]
- Grone A, Werkmeister JR, Steinmeyer CL, et al. (1994) Parathyroid hormone-related protein in normal and neoplastic canine tissues: Immunohistochemical localization and biochemical extraction Veterinary Pathology 31, 308–315. [DOI] [PubMed] [Google Scholar]
- Hammer AS, Couto G, Ayl RD, et al. (1994) Treatment of tumor-bearing dogs with actinomycin D Journal of Veterinary Internal Medicine 8, 236–239. [DOI] [PubMed] [Google Scholar]
- Hause WR, Stevenson S, Meuten DJ, et al. (1981) Pseudohyperparathyroidism associated with adenocarcinomas of anal sac origin in four dogs Journal of American Animal Hospital Association 17, 373–379. [Google Scholar]
- Jeffery N, Phillips SM, Brearley MJ. (2000) Surgical management of metastases from anal sac apocrine gland adenocarcinoma of dogs Journal of Small Animal Practice 41, 390. [Google Scholar]
- Meuten DJ, Cooper BJ, Capen CC, et al. (1981) Hypercalcemia associated with an adenocarcinoma derived from the apocrine glands of the anal sac Veterinary Pathology 18, 454–471. [DOI] [PubMed] [Google Scholar]
- Ogilvie GK, Reynolds HA, Richardson RC, et al. (1989) Phase II evaluation of doxorubicin for treatment of various canine neoplasms Journal of American Veterinary Medical Association 195, 1580–1583. [PubMed] [Google Scholar]
- Ogilvie GK, Moore AS. (1996) Managing the veterinary cancer patient: A practice manual. In: Veterinary Learning systems. (1st edn) Ogilvie GK, Moore AS. (eds). New Jersey. pp 349–360. [Google Scholar]
- Ross JT, Scavelli TD, Matthiesen DT, et al. (1991) Adenocarcinoma of the apocrine glands of the anal sac in dogs: A review of 32 cases Journal of American Animal Hospital Association 27, 349–355. [Google Scholar]
- Turrel JM, Theon AP. (1986) Single high-dose irradiation for selected canine rectal carcinomas Veterinary Radiology 27, 141–145. [Google Scholar]
- Vail DM, Withrow SJ, Schwarz PD, et al. (1990) Perianal adenocarcinoma in the canine male: A retrospective study of 41 cases Journal of the American Animal Hospital Association 27, 329–334. [Google Scholar]
- Withrow SJ. (2001) Perianal tumors. In: Small Animal Clinical Oncology (2nd edn) Withrow SJ, MacEwen EG. Philadelphia: W.B. Saunders Company, pp. 346–353. [Google Scholar]