Abstract
A retrospective study was performed to investigate the frequency of identification and characteristics of oesophageal disease in cats, including assessment of the utility of diagnostic techniques and clinical outcome. Thirty-three cats met the inclusion criteria, giving an in-clinic frequency of 33/2894 (approximately 1%) of feline referral cases. Vomiting and/or regurgitation were the most common presenting signs described, although a number of cats (6/33) showed neither. Useful diagnostic modalities included plain radiography, fluoroscopy, barium radiography and endoscopy. A wide range of diseases was reported including congenital disease, oesophagitis, foreign body obstruction, neoplasia, extraluminal compression and hypomotility disorder. Five of six cats with acquired oesophageal strictures had recently received doxycycline per os.
Oesophageal disease is reported as rare in cats, but there have been few studies, to date, that document its frequency. 1,2 The majority of current literature consists of individual case reports or small case series with limited data on the range of clinical presentations and the utility of diagnostic testing modalities used to investigate diseases of the oesophagus in cats. 3–7 The largest retrospective study reviewed 44 cats with oesophageal motility dysfunction (diagnosed by video-fluoroscopy) and found it to represent 0.05% of their feline referral population. 2 Forty-three percent of these cats were diagnosed with idiopathic dysmotility and a significant proportion (45%) presented with respiratory signs (without concurrent gastrointestinal signs). The aim of the current study was, therefore, to characterise the frequency, presentation, investigation, treatment and outcome of cats with oesophageal disorders, regardless of diagnostic modality used.
Materials and methods
Clinical records of cats presented to Davies Veterinary Specialists between January 2000 and December 2007 were reviewed. A computer database word-search was performed for the terms ‘oesophagus’ and ‘oesophageal’, enabling selection of feline cases with oesophageal disease. Inclusion criteria were (i) a complete clinical record and (ii) definitive diagnosis of an oesophageal disorder. Data recorded included signalment, history, clinical examination, diagnostic tests applied and results thereof, treatment, complications, concurrent diseases and outcome. Descriptive terms used to identify regurgitation from the history included consistency of expelled material (undigested food, sausage-shaped, saliva), absence of abdominal effort, timing after eating (immediate – supportive, but not definitive/specific). Those used to support vomiting included nausea, abdominal effort and production of bile. Findings of investigations performed prior to referral, as well as at this hospital, were documented, including plain and contrast radiography. Frequency was calculated as the number of cases of oesophageal disease expressed as a percentage of the total number of new feline cases seen over the same period. For comparison only, a data search was also run for the term ‘vomiting’ for the same population/time period, and a separate frequency calculated for cases in which vomiting was part of the initial presenting complaint.
Results
Five cases were excluded due to either incomplete clinical records (one) or absence of definitive diagnosis due to owner reluctance for further investigations (four). Thirty-three cases met the inclusion criteria, representing approximately 1% (33/2894) of the hospital feline case load over the study period (January 2000–December 2007). This compared to a frequency of approximately 8% (221/2894) for vomiting.
Population demographic
Median age at presentation was 6 years (ranging from 3 months to 17 years). Twelve cats were neutered females and 21 were male (16 of which were neutered). Breeds represented included domestic shorthair (18), domestic longhair (three) and purebreds (12) – consisting of; Oriental (five – Siamese or unspecified), British Shorthair (two), Russian Blue, Devon Rex, Burmilla, Maine Coon, and Persian.
Clinical signs
Regurgitation and/or vomiting were the most common presenting clinical signs, reported in 27/33 cats. As determined from the clinical history or direct observation whilst hospitalised, true regurgitation was present in 16/27 cases and true vomiting present in 3/27. For the remaining eight cases, a distinction between the two could not be made or there was a suspicion that both were present. Five cats originally referred for perceived vomiting were subsequently discovered to be regurgitating. Of the six cats with no history of vomiting or regurgitation, some presented with signs still consistent with oesophageal disease (eg, hypersialism, dysphagia) and others with non-specific signs (eg, weight loss, anorexia and in two cats, respiratory signs alone).
Diagnostic tests
Plain thoracic or cervical radiography (PTCR) was performed in 26 cases, but in two of these it could not be confirmed that radiographs underwent specialist review so they were excluded from further analysis. In 15/24 cases, radiographs revealed evidence of an oesophageal abnormality: megaoesophagus (five, including one concurrent foreign body), foreign body alone (four), soft tissue opacity/mass (extra or intraluminal) (five) and an ill-defined increase in caudal oesophageal opacity (one). Two of these 15 cats showed concurrent radiographic respiratory abnormalities (lung patterns). Conversely, 4/24 cats showed radiographic signs of respiratory disease without oesophageal abnormalities (two had abnormal lung patterns, one an irregular dorsal tracheal margin, one a pleural effusion). Five cats had normal PTCR and the remaining seven had no record of PTCR being performed. Five cats had serial barium radiography (performed by the referring clinician, barium meal consistency not specified), four of which showed oesophageal abnormalities. When plain radiographs were obtained prior to referral (16/27), it was not always clear from clinical records whether chemical restraint had been used, and all cases with radiographic evidence of megaoesophagus were diagnosed from referred radiographs.
Twenty-six cats had an upper gastrointestinal endoscopic examination, nine of which also had barium fluoroscopy studies. In addition, two cats had fluoroscopy without endoscopy. Endoscopy revealed or confirmed an oesophageal abnormality in 22/26 cases including 4/5 foreign bodies (one, a sewing needle, was not seen at endoscopy). Oesophagitis was diagnosed based solely on the clinician's interpretation of the endoscopic appearance of the mucosa. Barium fluoroscopy (using a solid barium meal in all cases, but additional liquid study only mentioned in one) revealed abnormal oesophageal function in 9/11 cases.
Additional tests performed included: abdominal and thoracic ultrasound, bronchoscopy, angiography, fine needle aspirate or pinch biopsies (oesophagus, mediastinal mass, gastric, duodenal), urinalysis, haematology, biochemistry, serum cobalamin/folate concentrations, feline pancreatic lipase serum immunoreactivity, serum thyroxine, toxoplasma serology, anti-acetylcholine receptor antibody titre, cerebrospinal fluid (CSF) analysis, electromyography (EMG), muscle biopsy and pupillary pilocarpine response test. Surgical exploration was required for definitive diagnosis in three cases, angiography was performed in one case and had shown a vascular abnormality but did not correctly identify the anomalous vessels (an initial misdiagnosis of left aortic arch with right ductus arteriosus was made, instead of right aortic arch with aberrant left subclavian artery).
Diagnosis
Five cats had congenital oesophageal disease, including three vascular ring anomalies – two persistent right aortic arches (PRAAs) with a ligamentum arteriosum, one PRAA with a left subclavian artery, all having secondary focal megaoesophagus (proximal to the heartbase). There were also two cases of sliding hiatal hernia (both had secondary oesophagitis). All cases of congenital disease presented at, or earlier than, 2 years of age; 3/5 were purebreds (one Burmilla, two Siamese).
The remaining 28 cats had acquired disease. This included six cases of oesophageal stricture, five of which had received recent oral doxycycline (and, additionally, clindamycin in one case) for respiratory disease or haemolytic anaemia, the remaining cat had recently been anaesthetised for ovariohysterectomy. Five cats had oesophagitis as their primary oesophageal pathology (excluding the two cases of hiatal hernia with secondary oesophagitis), with underlying causes identified in four; vomiting (two – due to pancreatitis and gastritis), gastro-oesophageal reflux (one) and one secondary to a traumatic tracheal tear due to a cuffed endotracheal tube. Five cats had oesophageal foreign bodies (two bones, one staple within a hairball, one fish hook, one needle) (Fig 1). All received diagnostic or therapeutic endoscopy, but one (the sewing needle) was not diagnosed until exploratory surgery. Five had oesophageal neoplasia; cytologically confirmed in three cases (one squamous cell carcinoma, two unclassified carcinomas) and presumed in two cases which were not biopsied but behaved invasively (one intraluminal and one intramural mass). Three cats had oesophageal disorders caused by extraluminal compression (two mediastinal lymphomas, one mediastinal cyst). There were four cases of oesophageal hypomotility: one dysautonomia, one dystrophic myopathy, one secondary to gastric lymphoma and one idiopathic (see Table 1).
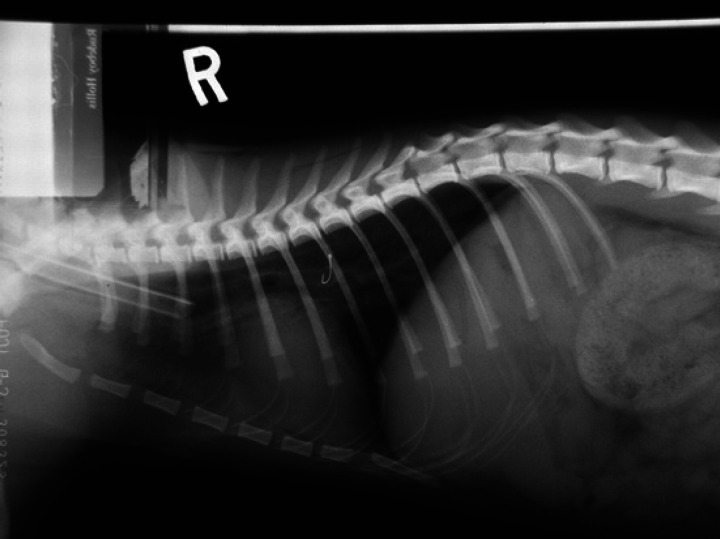
Fig 1.
Oesophageal foreign body: fish hook.
Table 1.
Summary of the main clinical findings in 33 cats with oesophageal disease.
| Final diagnosis (number) | Presenting clinical signs | Diagnostic tests performed | Test findings | Treatment | Outcomes |
|---|---|---|---|---|---|
| Hiatal hernia (2) | V+R, V, inappetence, weakness, weight loss | CXR, E, F, bronchoscopy, BAL, gastrointestinal biopsy, H/B, B12, folate, TLI, urinalysis, AUS | Hiatal hernia, secondary oesophagitis | Sucralfate, ranitidine, cephalexin, surgery | PTS (related) <1 year (1) Died (?related) <1 year (1) |
| Vascular ring anomaly (VRA) (3) | R, poor growth, heart murmur | CXR, Angiogram | VRA | Surgical ligation | Alive no ongoing signs (2) Alive mild signs (1) |
| Oesophageal stricture (6) | V/R; R; V+R, heart murmur, retching, dysphagia pyrexia, lethargy | E, F, Ba, CXR, biopsy, H/B, FIV/FeLV | Stricture | Balloon dilation, prednisolone, omeprazole/ranitidine, sucralfate, marbofloxacin, amoxyclavulanate, cisapride, adaptive feeding | PTS (related) <1 year (2) PTS (unrelated) >1 year (1) Alive no ongoing signs (3) |
| Oesophagitis (5) | R, V, inappetence, weight loss, dysphagia, lethargy, dyspnoea | CXR, AXR, E, AUS, bronchoscopy, H/B, urinalysis, fPLI, T4, BP, toxoplasma serology, faecal analysis | Oesophagitis, tracheal trauma/adhesion, gastritis, pancreatitis, hyperthyroid, lens luxation | Ranitidine, sucralfate, marbofloxacin, omeprazole, hypoallergenic or low fat diet, surgical debridement adhesion | Alive no ongoing signs (2) Alive mild signs (2) PTS (unrelated) <1 year (1) |
| Foreign body (5) | R, pyrexia, anorexia, hypersialism, dyspnoea | CXR, E | Bone (2), fish hook, furball (staple) | Endoscopic removal, co-amoxyclav, ranitidine, sucralfate | Alive no ongoing signs (3) Died (?related) <1 year (1) Died (unrelated) >1 year (1) |
| Oesophageal neoplasia (5) | R, V/R, weight loss, dysphagia, lethargy | H/B, CXR, E, F, BR, thoracic US, AUS, oesophageal biopsy, post-mortem, exploratory thoracotomy | Carcinoma, squamous cell carcinoma, unclassified/presumptive | PEG tube, liquid diet | PTS (related) <1 year (5) |
| Extraluminal compression (3) | R, dysphagia, weight loss, PUPD, stridor, dyspnoea, murmur, lymphadenopathy | H/B, FIV/FeLV, CXR, F, BR, thoracic US, FIV/FeLV, FNA | Mediastinal lymphoma, mediastinal cyst | COP, radiotherapy, aspiration | PTS (related) <1 year (1) PTS (?related) <1 year (1) Lost to follow-up (1) |
| Hypomotility (4) | Weight loss, R, V/R, dysphagia/malprehension, tachycardia, lethargy, wheezing, mydriasis, photophobia | CXR, E, F, BR, H/B, urinalysis, toxoplasma serology, AUS, EMG, nerve/muscle biopsy, CSF, pilocarpine response test, anti-acetylcholine receptor antibody | Oesophageal dysmotility ± gastric lymphoma, dystrophic myopathy, dysautonomia | COP, PEG tube, clindamycin, pilocarpine, postural feeding, ranitidine, sucralfate | PTS (related) <1 year (2) Alive no ongoing signs (1) Alive mild signs (1) |
V=vomiting, R=regurgitation, V/R=vomiting or regurgitation (unable to distinguish), V+R=both definitely present, CXR=thoracic radiography, AXR=abdominal radiography, AUS=abdominal ultrasound scan, US=ultrasound scan, BR=barium radiography, E=gastrointestinal endoscopy, F=fluoroscopy, BP=blood pressure, EMG=electromyelography, H/B=haematology/biochemistry, CSF=cerebrospinal fluid, Died/PTS (?related)=death/euthanasia suspected but not proven to be related to oesophageal disease, <1 year=event within 1 year of diagnosis, >1 year=event after 1 year of diagnosis. BAL=bronchoalveolar lavage, PUPD=polyuria/polydipsia, COP=cyclophosphamide, vincristine, prednisolone protocol, VRA
Aspiration pneumonia was diagnosed in 5/33 cases, based upon radiographic findings.
Treatment
Treatment consisted of medical management alone in nine cases (oesophagitis (five), neoplasia, dystrophic myopathy, dysautonomia, idiopathic hypomotility). This included ranitidine, sucralfate, omeprazole, prednisolone, antibiotics, cisapride, feeding an exclusion diet, and supportive feeding (feeding tubes and postural feeding). Two cases received surgery alone (both Vascular ring anomaly (VRA) ligation), and five received surgical and medical management combined (VRA ligation, hiatal hernia (two), tracheal adhesion, foreign body). Therapeutic endoscopy was performed in nine cases; foreign body retrieval (four) and stricture dilation (five, consisting of balloon dilation in two cases and bougienage in three). Repeated dilation of strictures was required for successful outcome (range 2–4 dilations), in keeping with previous studies. 8 Chemotherapy (a cyclophosphamide, vincristine, prednisolone (COP) protocol, with or without modification) was administered in the three cases of lymphoma, one of which, in addition, received a single dose of radiotherapy. Immediate euthanasia was performed in four cases (oesophageal neoplasia (three), severe stricture (one)). Complications arising from treatment included (one case of each): oesophageal rupture (during balloon dilation), laryngeal hemiplegia and forebrain dysfunction following anaesthesia (a presumed cerebrovascular accident).
Outcome
As no single disease was over-represented in our study population, accurate assessment of outcome and prognosis was not possible and the following information is included only as descriptive data. Outcome was available in 32/33 cases and was expressed as 1-year survival rate, as this was the maximum available follow-up time from date of diagnosis for some cases at the time of the study. The overall 1-year survival rate (from date of diagnosis) was 56%. Detailed outcome data for cases in each disease group are given in Table 1. Outcome is expressed as whether or not alive at end of study period (and if so, whether there were any ongoing clinical signs), or if deceased, whether survival time was greater or less than 1 year, and whether death was confirmed as related, unrelated or possibly related to oesophageal disease.
Discussion
The frequency of oesophageal disease identified in this referral population of cats was low, although higher than that reported elsewhere (0.05%). 2 This was probably due to the wider inclusion criteria (ie, not being restricted to only cases receiving fluoroscopy studies). A diverse range of age and breeds were recorded, with no definitive breed predisposition apparent from these data. Interestingly, the male:female ratio was 1.75:1, but there is no obvious clinical explanation for this and an odds ratio cannot be calculated as we do not know the gender ratio of all cats seen within this time period.
A large proportion of cases presented with either vomiting or regurgitation 27/33, and only 2/33 cats presented with respiratory signs in the absence of gastrointestinal signs. This contrasted with the findings of Moses et al where a higher proportion of cases with oesophageal dysmotility presented with respiratory signs alone (45%) than with gastrointestinal signs alone (36%). 2 The fact that some cats in the current study were originally referred for perceived vomiting but subsequently discovered to be regurgitating highlights the importance (and difficulty) of trying to distinguish between these processes to aid problem localisation. The possibility of regurgitation should be ruled out by close questioning in any cat presenting with perceived vomiting. The benefit of using the measurement of the pH of returned liquid to distinguish between vomiting and regurgitation is not proven and was not applied to any of these cases. The study also showed that oesophageal disease, although uncommon, should be a consideration in cats with non-specific signs of illness as well as those with gastrointestinal signs.
The insensitivity of plain radiography for detection of certain oesophageal diseases in cats (eg, oesophagitis, strictures) has been described previously. 9,10 Combinations of diagnostic imaging tools were often required to reach a definitive diagnosis in these cases.
A wide range of aetiologies were reported and in only one case of oesophageal disease were we unable to find an underlying cause (idiopathic dysmotility) which is less than Moses et al (43% of 44 cases). 2 Whilst not shown to be statistically significant, this difference may be due to a narrower range of diagnostic tools employed by the latter (although concurrent diagnostics were not specifically listed), or may represent a different incidence of dysmotility between populations because of temporal or geographic differences. Efforts should be made to identify an underlying cause for oesophageal dysmotility in order to optimise treatment and prognosis. Five out of six cats with strictures had received recent oral antibiotic treatment for either concurrent respiratory disease or anaemia (doxycycline, clindamycin), for as little as 48 h prior to onset of vomiting/regurgitation, highlighting the risks of drug-induced oesophageal disorders (DIOD) as previously reported. 7,10 This finding contrasted with that of another study in which gastro-oesophageal reflux under anaesthetic was reported as the most common cause of benign strictures in cats and dogs. 8 All cases of stricture were benign, consistent with previous reports of neoplasia being a rare cause of oesophageal stricture in cats. 9
This study had limitations in control due to its retrospective nature. Our method of case searching could not guarantee identification of all individuals with oesophageal disease within this population and search period and may have underestimated case numbers (eg, due to unidentified oesophagitis, cases excluded for failing to meet inclusion criteria, and the possibility of missed cases during our record search through a variety of recording errors). We, therefore, chose the non-specific term ‘frequency’ rather than more definitive epidemiological terms such as ‘incidence’, and conducted a comparative search for the term ‘vomiting’ to provide context. It is worth noting that both the overall frequency of disease and relative frequency of individual conditions recorded in this referral population may not be representative of a first opinion population. Without restricting interpretation of diagnostic studies to one individual, operator variation may have occurred, for example with endoscopic interpretation of oesophagitis. Another important criticism is that it was not always known if plain radiographs performed prior to referral had required chemical restraint. If not specified in the history, conscious studies were presumed, and therefore findings of megaoesophagus assumed to be genuine, rather than artefactual. This may have led to an over-estimation of the true frequency of megaoesophagus (MO) on PTCR (since 5/5 cases of MO were diagnosed on referred radiographs, with either no further radiographs taken (4/5), or no evidence of MO on repeat radiographs (1/5)). In 2/5 cases of oesophageal neoplasia, a definitive histological diagnosis was not reached because of owner's reluctance for pre- or post-mortem biopsies. Diagnosis was, therefore, presumptive, based on the aggressive appearance of lesions. Another significant limitation was small group size for any one disease, preventing any kind of statistical analysis of outcome/prognosis. Further studies are necessary to achieve this, however, given the evidence of low frequency of oesophageal disease, this will likely require a multi-centre collaboration.
Conclusion
Our results show that oesophageal disease is rare in cats and can manifest itself as non-specific clinical signs, such as weight loss and anorexia, as well as more typical localising signs. In addition, regurgitation can be mistaken for vomiting or may be accompanied by it, confusing body system localisation. Plain radiography appears to be relatively insensitive and a wide range of additional diagnostics may be required to detect and classify oesophageal dysfunction. A definitive diagnosis was reached in 32/33 cases, making idiopathic oesophageal dysfunction (in this case dysmotility) very rare. Recent oral medication (doxycycline, clindamycin) was the most common cause of oesophageal strictures and the need for careful administration of certain oral antibiotics was highlighted. Treatment and outcome were highly variable, reflecting a wide range of disease aetiology and severity and limiting meaningful data analysis given our small study population and disease sub-groups.
Acknowledgements
We would like to thank referring practitioners and owners for their assistance with follow-up data.
References
- 1. Guilford W.G., Strombeck D.R. Diseases of swallowing, Strombeck's small animal gastroenterology, 3rd edn, 1996, Saunders: Philadelphia, 211–238. [Google Scholar]
- 2. Moses L., Harpster N.K., Beck K.A., Hartzband L. Esophageal motility dysfunction in cats: a study of 44 cases, J Am Anim Hosp Assoc 36, 2000, 309–312. [DOI] [PubMed] [Google Scholar]
- 3. Gualtieri M., Monzeglio M.G., DiGiancamillo M. Oesophageal squamous cell carcinoma in 2 cats, J Small Anim Pract 40, 1999, 79–83. [DOI] [PubMed] [Google Scholar]
- 4. Kidder A.C., Johannes C., O’Brien D.P., Harkin K.R., Schermerhorn T. Feline dysautonomia in the midwestern United States: a retrospective study of nine cases, J Feline Med Surg 10, 2007, 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prymak C., Saunders H.M., Washabau R.J. Hiatal hernia repair by restoration and stabilization of normal anatomy. An evaluation in four dogs and one cat, Vet Surg 18, 1989, 386–391. [DOI] [PubMed] [Google Scholar]
- 6. White R.N., Burton C.A., Hale J.S. Vascular ring anomaly with coarctation of the aorta in a cat, J Small Anim Pract 44, 2003, 330–334. [DOI] [PubMed] [Google Scholar]
- 7. Beatty J.A., Swift N., Foster D.J., Barrs V.R. Suspected clindamycin-associated oesophageal injury in cats: five cases, J Feline Med Surg 8, 2006, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adamama-Moraitou K.K., Rallis T.S., Prassinos N.N., Galatos A.D. Benign esophageal stricture in the dog and cat: a retrospective study of 20 cases, Can J Vet Res 66, 2002, 55–59. [PMC free article] [PubMed] [Google Scholar]
- 9. Sellon R.K., Willard M.D. Esophagitis and esophageal strictures, Vet Clin North Am Small Anim Pract 33, 2003, 945–967. [DOI] [PubMed] [Google Scholar]
- 10. German A.J., Cannon M.J., Booth M.J., Pearson G.R., Reay C.A., Gruffydd-Jones T.J. Oesophageal strictures in cats associated with doxycycline therapy, J Feline Med Surg 7, 2005, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]