Abstract
An 8-year-old cat with recent-onset generalized seizures was diagnosed with a right forebrain mass using magnetic resonance imaging. The mass was excised and upon histologic and immunohistochemical examination shown to be a Toxoplasma gondii granuloma. Serology supported active T gondii infection. The cat was treated with phenobarbital to control seizures. After definitive diagnosis of toxoplasma granuloma, clindamycin was administered for approximately 1 month. Seizures recurred 8 months after initial presentation, and the cat was euthanased at the owner's request. This is a previously unreported manifestation of feline central nervous system toxoplasmosis. When a mass lesion is present in the brain of a cat and serologic test results support active infection with T gondii, toxoplasma granuloma must be a differential diagnosis. If the patient is suffering from clinical disease, surgical resection of the mass (if possible) can be complimented with medical treatment until definitive diagnosis is obtained. Immunocompromising factors should be identified and addressed if possible.
An 8-year-old castrated male domestic shorthair was referred to Long Island Veterinary Specialists for evaluation and management of recent-onset seizures. The cat had been owned since kittenhood and kept strictly indoors in a single-pet home. Focal facial seizures first occurred 2 days before presentation. According to the owner, frequency increased over that time period to approximately 1-min-long seizure every hour. Physical and neurological examinations at the time of presentation were unremarkable. Bloodwork and abdominal radiographs performed at a local emergency hospital were evaluated. Bloodwork abnormalities included mild lymphopenia (1.5×103 cells/ml, normal 1.8–5.5×103 cells/ml), mild hyperglycemia (170.5 mg/dl, normal 76.0–145.0 mg/dl), and mild hypernatremia (168 mmol/l, normal 150.0–165.0 mmol/l), but was otherwise within normal limits. Abdominal radiographs were unremarkable.
The cat was admitted to the hospital for a radiographic metastatic screening series of the thorax and magnetic resonance imaging (MRI) of the brain. No pulmonary masses or nodules were seen on thoracic radiographs, but cardiomegaly was noted. On MRI a small, right-sided, contrast-enhancing mass was present in the region of the olfactory bulb and rostral frontal lobe (Figs 1 and 2). A falcine meningioma was suspected. Cerebrospinal fluid was not collected because of potential for increased intracranial pressure due to the intracranial mass and the subsequent increased risk for brain herniation. Phenobarbital (generic, Excellium Pharmaceutical) therapy (2.75 mg/kg PO bid) was initiated and surgery to remove the mass scheduled. Seven days after MRI, the cat represented for lethargy and anorexia. Body temperature was 104.0° F and serous ocular discharge was present. Physical and neurological examinations were otherwise unremarkable. Prednisone (generic, Westward Pharmaceutical) (0.42 mg/kg PO bid) was added to the drug regimen.
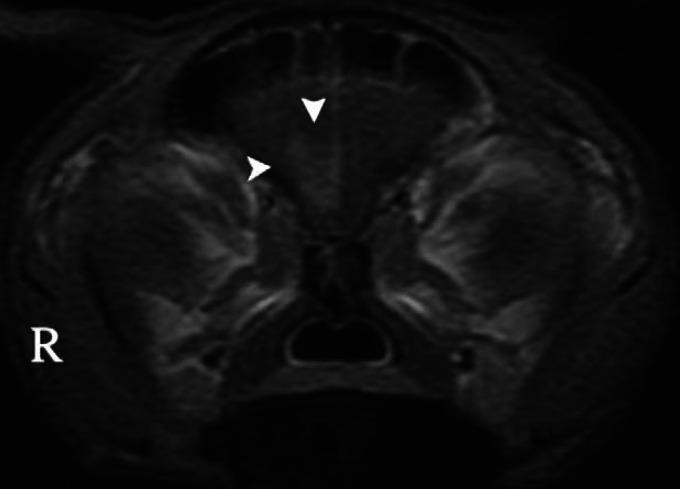
Fig 1.
Transverse T1-weighted image (post intravenous contrast medium [gadolinium] administration) at the level of the frontal lobes of the brain of a cat with recent-onset seizures. Note contrast enhancement in the right frontal lobe (arrowheads).
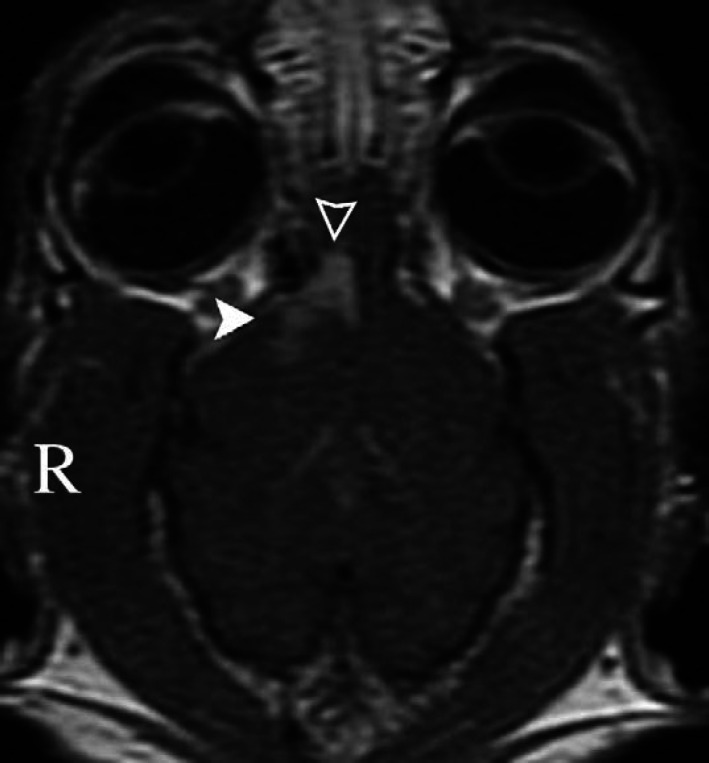
Fig 2.
Dorsal T1-weighted image (post intravenous contrast medium [gadolinium] administration) of the cat in Fig 1. Note contrast enhancement in the right olfactory bulb (empty arrowhead) and right frontal lobe (filled arrowhead).
Surgery was performed 5 days later. A rostrotentorial craniotomy with a transfrontal approach was performed on the right side. A 0.5 cm3 soft yellowish mass surrounded by a rim of apparently malacic brain tissue was present. All grossly abnormal tissues were resected and submitted for biopsy. The frontal sinus defect was closed with polymethylmethacrylate; soft tissue closure was routine. The cat recovered from surgery and was discharged from the hospital 5 days postoperatively on prednisone (0.84 mg/kg PO bid) and phenobarbital (2.75 mg/kg PO bid). Routine histologic examination was performed. Severe, subacute, locally extensive lymphohistiocytic encephalitis with intralesional protozoal tachyzoites and severe gliosis was present (Fig 3). Toxoplasmosis was suspected. Prednisone was discontinued and clindamycin (Antirobe; Pharmacia) therapy (12.7 mg/kg PO bid) initiated. Follow-up immunohistochemistry (Diagnostic Laboratory, College of Veterinary Medicine, Cornell University, Ithaca, NY) confirmed the presence of Toxoplasma gondii organisms. Subsequent serology for T gondii was performed (Antech Diagnostics, Lake Success, NY). Toxoplasma immunoglobulin G (IgG) and immunoglobulin M (IgM) antibody titers were 1:512 and 1:64, respectively, indicating recent exposure to or active infection with T gondii. Serum was negative for feline immunodeficiency virus (FIV) antibodies and feline leukemia virus (FeLV) antigen (Antech Diagnostics, Lake Success, NY).
Fig 3.
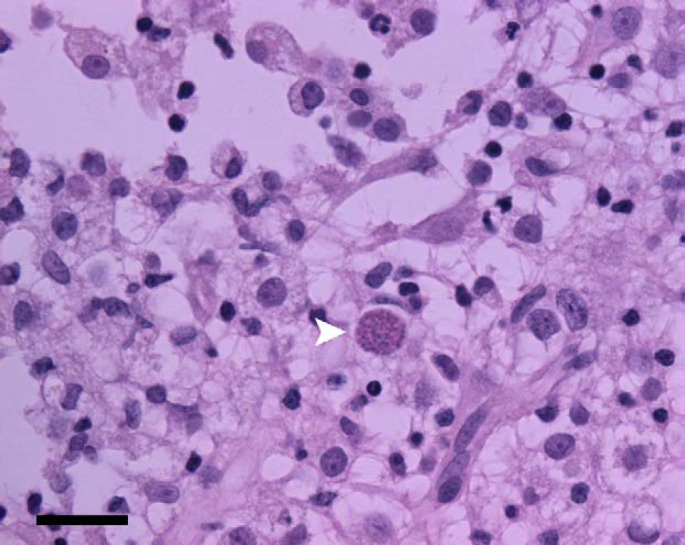
Photomicrograph of a section of the mass removed from the brain of the cat in Fig 1. Lymphohistiocytic inflammation is present. A protozoal organism is seen at the arrowhead. Immunohistochemistry later identified Toxoplasma gondii tachyzoites. H&E stain; bar=50 μm.
The cat presented twice for lethargy and inappetence within the first week following discharge from the hospital. Mild dehydration, dull mentation, decreased proprioception of the left thoracic limb, and chemosis, serous discharge, and blepharospasm of the left eye were present initially. Serous nasal discharge and sneezing developed. The cat was treated on an outpatient basis for dehydration and suspected upper respiratory viral infection on each occasion. The owner continued to medicate and force feed at home. At the 2-week postoperative recheck, sneezing and oculonasal discharge appeared to be resolving, but the cat remained inappetent, lethargic, and proprioceptive deficits were present in the left thoracic and pelvic limbs. Prednisone (0.57 mg/kg PO q48h) was started as an appetite stimulant at a dose that was not immunosuppressive and was not anticipated to interfere with medical treatment of central nervous system (CNS) toxoplasmosis.
Toxoplasma antibody titers were repeated 1 month postoperatively. IgG was unchanged at 1:512 and IgM was negative. One interpretation of these results in conjunction with previous serology is resolution of active infection. A second interpretation relates to the administration of prednisone before serologic evaluation. A study by Lappin et al (1992) demonstrated elevation of anti-T gondii IgM following the administration of methylprednisolone acetate. The explanation for increased IgM was release of negative feedback by markedly decreasing levels of IgG. The lowest dose administered in that study was 10-fold greater than the dose administered in this case. Here, the IgM titer did not remain persistently high during the course of prednisone administration, but rather demonstrated a decrease in IgM. In addition, the IgG titer remained high in this case; therefore, while the possibility of the IgM dynamics being related to prednisone administration cannot be ignored, this does not seem the most likely explanation. At this recheck the cat was exhibiting propulsive pacing and circling to the right, inappetence continued, and hyper-reflective retinal lesions indicative of resolved chorioretinitis were noted. A retinal examination had not previously been performed; therefore, the time course of ocular disease cannot be determined, but this may be evidence of multisystemic toxoplasmosis in this patient. Cardiomegaly noted on thoracic radiographs was not further characterized. This abnormality may represent unrelated feline cardiomyopathy, or less likely, the result of cardiac infection with T gondii. The persistent right forebrain signs were considered post-surgical changes. Clindamycin and prednisone were discontinued. Two months postoperatively the cat's appetite had returned to normal. The previous poor appetite was presumptively attributed to clindamycin administration, but may have represented a behavior change following brain surgery, or a clinical sign of toxoplasmosis. Propulsive pacing and circling to the right continued.
The owner chose not to return the patient for further evaluations until approximately 8 months after initial presentation. At that time, seizures had recurred, and the patient was euthanased at the owner's request.
Toxoplasmosis is the result of infection with the intracellular protozoal parasite T gondii. Cats are the only definitive hosts, but all mammals and birds can be intermediate hosts. One route of infection is by ingestion of tissues containing T gondii organisms (Turner 1978, Dubey and Lappin 1998). Another is ingestion of oocysts shed by cats into the environment in the feces. The oocysts subsequently sporulate and become infectious to all hosts. After ingestion, sporozoites are released into the gastrointestinal tract. Sporozoites penetrate the gastrointestinal mucosa and migrate to a variety of tissues and organs throughout the body (Turner 1978, Dubey and Lappin 1998). Feline tissues affected include liver, kidney, spleen, muscle, lung, heart, eye, brain, and spinal cord (Katsube et al 1969, Dubey and Carpenter 1993). Two forms exist in tissues. Tachyzoites are rapidly dividing and are responsible for tissue damage and necrosis. As a competent host immune response develops against the tachyzoites, transformation into bradyzoites occurs. The bradyzoite form (within tissue cyst) is slowly dividing and is responsible for latent infection.
Disease may occur with tissue destruction by tachyzoites in a novel infection without immunosuppression or may be the result of reactivation of a latent infection in a previously immunocompetent host that becomes immunocompromised (Heidel et al 1990, Dubey 1997, Dubey and Lappin 1998, Zenger 2000). Conditions in humans that are associated specifically with CNS toxoplasmosis are infection with HIV, post-transplant immunosuppression, and immunosuppression secondary to cancer chemotherapy (Ciricillo and Rosenblum 1990, Heidel et al 1990, Pruitt 1991, 2003). The majority of human CNS toxoplasmosis cases are the result of reactivation of a latent infection (Heidel et al 1990, Orefice et al 1992, Dietrich et al 2000). Occurrence of toxoplasma infection in cats is high, but most healthy cats develop immunity that prevents clinical disease. This immunity does not destroy tissue cysts and these cats remain latently infected (Nelson and Couto 2003). Two common feline diseases that can cause immunosuppression are FIV and FeLV, and any cat suspected or confirmed to have toxoplasmosis should be screened for these diseases as underlying predisposing factors. In the cat described in this report, serology supportive of active infection (novel or recrudescent) and lack of exposure history make reactivation of a latent infection the most likely scenario. The original infection may have been acquired during kittenhood (fecal-orally, lactogenically, or transplacentally), before current ownership, and the cat may have suffered an immunosuppressive stress other than FIV or FeLV. The nature of this immunosuppressive stress was not identified. There remains the possibility that the cat was recently exposed to T gondii by ingestion of rodents or cockroaches that came into the home, or through feeding of undercooked food, resulting in primary toxoplasmosis.
To the authors' knowledge, MRI of feline CNS toxoplasmosis has not been described. One study describes a mass lesion in a dog that was initially thought to be a meningioma but was later diagnosed as a toxoplasma lesion (Graham et al 1998). The MRI characteristics of human CNS toxoplasmosis have been described and are variable. Factors affecting MRI appearance include distribution of disease in the brain, stage of disease and treatment, and degree of host response to the infection. Lesions may be a single mass or multifocal. They may be hypointense, isointense, or hyperintense and are often ring-enhancing (Brightbill et al 1996, Vyas and Ebright 1996, Dietrich et al 2000, Nakazaki et al 2000, Chong-Han et al 2003). If treatment is effective, the radiologic characteristics improve, with lesions becoming less intense and smaller (Dietrich et al 2000, Nakazaki et al 2000). Host immune response may also affect appearance. If the host is severely compromised and does not mount a significant response to infection, less inflammation, and therefore edema, is present (Dietrich et al 2000). It is likely that MRI appearance of feline CNS toxoplasmosis is also variable.
Because of variability in clinical signs and likely variability in appearance with advanced imaging such as MRI, information beyond imaging is needed to make a diagnosis of CNS toxoplasmosis. As in human patients, a presumptive diagnosis can be made based on clinical signs in an immune-compromised host with serologic evidence of recent or active infection if there is clinical response to empirical treatment for toxoplasmosis (Piazza et al 1986, Ciricillo and Rosenblum 1990, Vyas and Ebright 1996, Pruitt 2003). Further supporting evidence would be resolution of lesions on MRI. Definitive diagnosis of toxoplasmosis as a cause for neurologic dysfunction requires biopsy. For focal mass lesions that are surgically accessible, biopsy can be achieved at the same time of surgical debulking or complete resection of the mass.
When a mass lesion is present on the MRI of a feline brain, the primary differential is neoplasia, with the most common being meningioma. Other primary brain neoplasms are gliomas, and rarely choroid plexus tumors and ependymomas. Metastatic neoplasia may also cause mass lesions. Bacterial and fungal (especially Cryptococcus neoformans) granulomas have been reported to cause mass lesions in the feline brain (Dewey 2003). In humans, histopathologic features of CNS toxoplasmosis are variable. Encephalitis or meningoencephalitis may be present or abscesses may form. Feline CNS toxoplasmosis typically causes an encephalitis or meningoencephalitis with lack of gross lesions. Histological findings are microscopic granulomas with intracellular tachyzoites and mononuclear perivascular cuffing (Turner 1978, Dubey and Carpenter 1993, Dubey et al 1996).
Extent and location of pathology determine the clinical presentation of CNS toxoplasmosis. Cats may present with signs indicative of a focal lesion or multifocal disease. The patient in this report developed a lymphohistiocytic granuloma as a consequence of CNS toxoplasmosis. Results of this granuloma formation were focal forebrain signs (seizures) and an MRI appearance that was similar to that of a falcine meningioma. Toxoplasmosis was not considered as a differential for this mass because of the lack of exposure history. Toxoplasma titers were performed retrospectively in this patient. For cats that present with focal CNS signs and have a mass lesion on MRI, toxoplasma granuloma should be a differential diagnosis, and toxoplasma titers should be run before performing surgery. As with this case, however, positive serology alone may not definitively implicate toxoplasmosis as the cause of clinical signs. A polymerase chain reaction (PCR) is another ante-mortem diagnostic that can be applied to various biological samples, including cerebrospinal fluid, to support the diagnosis of CNS toxoplasmosis (Stiles et al 1996, Schatzberg et al 2003). If toxoplasmosis is suspected, retinal examination should be routinely performed because of the potential for multisystemic disease, and the high incidence of ocular involvement (Lappin et al 1989, Stiles et al 1996).
IgG antibodies are initially absent during infection and may take several weeks to rise to protective levels. Once high levels are achieved, they may stay elevated for months to years following resolution of disease associated with infection. IgM antibodies are also initially absent during infection, but they rise to effective levels within days of exposure. These high levels decrease over a few weeks after resolution of disease. The dynamics of IgG and IgM can be used to characterize the chronicity of immune response and give a general time frame of exposure. Elevated IgG levels alone are characteristic of chronic immunity or historic exposure. Elevated IgM levels alone are usually characteristic of acutely developing immunity or recent exposure (Dubey and Lappin 1998). Elevated IgG levels in conjunction with elevated IgM levels may represent active infection in one of two ways: (1) chronic immunity (previous exposure) with acute re-challenge, either by re-exposure or by recrudescence or (2) serologic sampling a few weeks after exposure, at a time when IgG levels have risen and IgM levels have not yet fallen (Tizard 1996, Lappin 1996, Dubey and Lappin 1998). It should be noted that occasionally cats with chronic toxoplasmosis have persistently high IgM titers (Lappin 1996, Dubey and Lappin 1998).
Clinical and radiologic improvement have been seen in human patients with mass lesions attributable to toxoplasmosis that are treated medically without surgical debulking (Piazza et al 1986, Ciricillo and Rosenblum 1990, Vyas and Ebright 1996, Pruitt 2003). Because of the paucity of documented cases, the success rate of medical management alone for feline CNS toxoplasma granuloma is not known. Surgical treatment should not be withheld from a feline patient exhibiting clinical signs of a mass lesion of the brain. Based on success in humans, medical treatment may be added as an adjunctive therapy in cases where toxoplasmosis is suspected until definitive diagnosis is obtained. Feline CNS toxoplasmosis can be treated with clindamycin (8–17 mg/kg PO q8h or 10–12.5 mg/kg q12h for 2–4 weeks) or sulfonamide in conjunction with trimethoprim (Tribrissen; Schering-Plough) or pyrimethamine (Daraprim; Glaxo Wellcome). Clindamycin is preferred because sulfonamide, trimethoprim, and pyrimethamine can cause myelosuppression with prolonged administration (Dubey and Lappin 1998). Medical therapy can improve clinical signs associated with active infection, but organisms in tissue cysts survive, and clinical signs may recur (Lappin et al 1989, Lappin 1996, Dubey and Lappin 1998). This is a possible explanation for recurrence of seizures in this case, but diagnostics (repeat serology, PCR, and repeat MRI) were not permitted, and this hypothesis cannot be confirmed.
References
- Brightbill T.C., Post M.J., Hensley G.T.et al. MR of toxoplasma encephalitis: signal characteristics of T2-weighted images and pathologic correlation, Journal of Computer Assisted Tomography 20, 1996, 417–422. [DOI] [PubMed] [Google Scholar]
- Chong-Han C.H., Cortez S.C., Tung G.A. Diffusion-weighted MRI of cerebral Toxoplasma abscess, American Journal of Roentgenology 181, 2003, 1711–1714. [DOI] [PubMed] [Google Scholar]
- Ciricillo S.F., Rosenblum M.L. Use of CT and MR imaging to distinguish intracranial lesions and to define the need for biopsy in AIDS patients, Journal of Neurosurgery 73, 1990, 720–724. [DOI] [PubMed] [Google Scholar]
- Dewey C.W. Encephalopathies: disorders of the brain. Dewey C.W. A Practical Guide to Canine and Feline Neurology, 1st edn, 2003, Iowa State Press: Ames, IA, 127–140. [Google Scholar]
- Dietrich U., Maschke M., Dorfler A.et al. MRI of intracranial toxoplasmosis after bone marrow transplantation, Neuroradiology 42, 2000, 14–18. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Carpenter J.L. Histologically confirmed clinical toxoplasmosis in cats: 100 cases (1952–1990), Journal of the American Veterinary Medical Association 203, 1993, 1556–1566. [PubMed] [Google Scholar]
- Dubey J.P., Lappin M.R. Toxoplasmosis and neosporosis. Greene C.E. Infectious Diseases of the Dog and Cat, 2nd ed, 1998, WB Saunders Co.: Philadelphia, PA, 493–509. [Google Scholar]
- Dubey J.P., Mattix M.E., Lipscomb T.P. Lesions of neonatally induced toxoplasmosis in cats, Veterinary Pathology 33, 1996, 290–295. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Tissue cyst tropism in Toxoplasma gondii: a comparison of tissue cyst formation in organs of cats, and rodents fed oocysts, Parasitology 115, 1997, 15–20. [DOI] [PubMed] [Google Scholar]
- Graham J.P., Newell S.M., Voges A.K.et al. The dural tail sign in the diagnosis of meningiomas, Veterinary Radiology and Ultrasound 39, 1998, 297–302. [DOI] [PubMed] [Google Scholar]
- Heidel J.R., Dubey J.P., Blythe L.L.et al. Myelitis in a cat infected with Toxoplasma gondii and feline immunodeficiency virus, Journal of the American Veterinary Medical Association 196, 1990, 316–318. [PubMed] [Google Scholar]
- Katsube Y., Hagiwara T., Miyakawa H.et al. Studies on toxoplasmosis 2. Distribution of Toxoplasma in the organs of cat and dog cases of latent infection occurring naturally, Japanese Journal of Medical Science and Biology 22, 1969, 319–326. [PubMed] [Google Scholar]
- Lappin M.R. Feline toxoplasmosis: interpretation of diagnostic test results, Seminars in Veterinary Medicine and Surgery (Small Animal), 1996, 154–160. [DOI] [PubMed]
- Lappin M.R., Dawe D.L., Lindl P. The effect of glucocorticoid administration on oocyst shedding, serology, and cell-mediated immune response of cats with acute and chronic toxoplasmosis, Journal of the American Animal Hospital Association 27, 1992, 625–632. [Google Scholar]
- Lappin M.R., Greene C.E., Winston S.et al. Clinical feline toxoplasmosis: serologic diagnosis and therapeutic management of 15 cases, Journal of Veterinary Internal Medicine, 1989, 139–143. [DOI] [PubMed]
- Nakazaki S., Saeki N., Etoh S.et al. Toxoplasmic encephalitis in patients with acquired immunodeficiency syndrome—4 case reports, Neurologia Medico-Chirurfica 40, 2000, 120–123. [DOI] [PubMed] [Google Scholar]
- Nelson R.W., Couto C.G. Polysystemic protozoal infections: feline toxoplasmosis, Small Animal Internal Medicine, 3rd edn, 2003, Mosby: St. Louis, MO, pp. 1296–1299 [Google Scholar]
- Orefice G., Carrieri P.B., Chirianni A.et al. Cerebral toxoplasmosis and AIDS: clinical neuroradiological and immunological findings in 15 patients, ACTA Neurologica 14, 1992, 493–502. [PubMed] [Google Scholar]
- Piazza E., Condorelli A., Arcidiacono R.et al. Intracerebral mass lesions in patients affected by AIDS, Acta Neurochirurgica 83, 1986, 116–120. [DOI] [PubMed] [Google Scholar]
- Pruitt A.A. Central nervous system infections in cancer patients, Neurologic Clinics 9, 1991, 867–888. [PubMed] [Google Scholar]
- Pruitt A.A. Nervous system infections in patients with cancer, Neurologic Clinics 21, 2003, 193–219. [DOI] [PubMed] [Google Scholar]
- Schatzberg S.J., Haley N.J., Barr S.C.et al. Use of a multiplex polymerase chain reaction assay in the antemortem diagnosis of toxoplasmosis and neosporosis in the central nervous system of cats and dogs, American Journal of Veterinary Research, 2003, 1507–1713. [DOI] [PubMed]
- Stiles J., Prade R., Greene C. Detection of Toxoplasma gondii in feline and canine biological samples by use of the polymerase chain reaction, American Journal of Veterinary Research, 1996, 264–267. [PubMed]
- Tizard I.R. Antibodies: soluble forms of BCR, Veterinary Immunology; An Introduction, 5th edn, 1996, WB Saunders Co.: Philadelphia, PA, pp. 153–164 [Google Scholar]
- Turner G.V.S. Some aspects of the pathogenesis and comparative pathology of toxoplasmosis, Journal of the South African Veterinary Association 49, 1978, 3–8. [PubMed] [Google Scholar]
- Vyas R., Ebright J.R. Toxoplasmosis of the spinal cord in a patient with AIDS: case report and review, Clinical Infectious Diseases 23, 1996, 1061–1065. [DOI] [PubMed] [Google Scholar]
- Zenger E. Retroviral infection of the nervous system. Bonagura J.D., Kersey R. Kirk's Current Veterinary Therapy XIII: Small Animal Practice, 2000, Saunders: Philadelphia, PA, 288–291. [Google Scholar]